Abstract
The weight loss observed in consumers of extracts of Citrus aurantium (bitter orange) has been tentatively attributed to the lipolytic and thermogenic effects of the alkaloids abundant in the unripe fruit. Synephrine, octopamine, tyramine, and other alkaloids have been repeatedly identified and quantified in Citrus members of the Rutaceae family or in their extracts incorporated in dietary supplements for weight management. However, there are only scarce reports on their lipolytic action. This study aimed at comparing the acute lipolytic activity of synephrine, octopamine, tyramine, and N-methyltyramine in rat and human adipocytes. Maximal response to the prototypical β-adrenergic agonist isoprenaline was taken as reference in both species. In rat, octopamine was slightly more active than synephrine while tyramine and N-methyl tyramine did not stimulate—and even inhibited—lipolysis. In human adipocytes, none of these amines stimulated lipolysis when tested up to 10 μg/ml. At higher doses (≥100 μg/ml), tyramine and N-methyl tyramine induced only 20% of the maximal lipolysis and exhibited antilipolytic properties. Synephrine and octopamine were partially stimulatory at high doses. Since synephrine is more abundant than octopamine in C. aurantium, it should be the main responsible for the putative lipolytic action of the extracts claimed to mitigate obesity. Noteworthy, their common isopropyl derivative, isopropylnorsynephrine (also named isopropyloctopamine or betaphrine), was clearly lipolytic: active at 1 μg/ml and reproducing more than 60% of isoprenaline maximal effect in human adipocytes. This compound, not detected in C. aurantium, and which has few reported adverse effects to date, might be useful for in vivo triglyceride breakdown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citrus aurantium is a plant belonging to the Rutaceae family, the fruit of which (bitter orange) has been used for the preparation of extracts sold worldwide under the form of phytoproducts claimed to promote weight loss. The leaves, the peel, and the edible part of the fruits of C. aurantium contain elevated amounts of phenethylamine alkaloids (i.e. synephrine, octopamine, tyramine, N-methyltyramine, and hordenine). The synephrine content is negatively correlated with the maturity index of the fruit [10]. Since the content of such alkaloids is richer in unripe C. aurantium than in other fruits—such as mandarins (Citrus reticulata) or sweet oranges (Citrus sinensis)—extracts from the former are currently included in dietary supplements aiming at treating obesity.
Para-synephrine has been reported to be the main alkaloid of C. aurantium fruits and extracts [25], while the other alkaloids are present in much lower concentrations [18, 20, 23]. The contents found in dried extracts are approximately 3% synephrine, 0.3% octopamine, 0.06% tyramine, while the other alkaloids are at the limit of detection. Synephrine is an alkaloid that is similar in structure to ephedrine, which has been banned from dietary supplements inducing weight loss in view of its deleterious actions on the cardiovascular system. Synephrine is therefore a major component of “ephedra-free” products [25] and has been the subject of numerous analytical determinations in sweet oranges (∼16 μg/ml of juice), mandarins (80–160 μg/ml of juice), bitter oranges (56 μg/ml of juice, 1,120–3,000 μg/g in peel), or especially in multi-component formulations containing C. aurantium extracts (up to 92,000 μg/g in dried material) sold as weight-loss and athletic-performance-enhancement products [2, 10, 18–23]. Evidently, the amount of (+/−) synephrine, the major alkaloid found in such dietary supplements with supposed slimming effects, varies largely depending on different factors such as the grove, the extraction process, or the fact that the product respects what is on manufacturers' label or has been adulterated before reaching the consumer. Such aspects were reviewed elsewhere [12, 20, 22, 25] and will not be treated in the present study.
The aim of our comparison of the lipolytic activities of C. aurantium alkaloids was to detect which of them could be responsible for the fat mass reduction weakly documented in obese humans [12, 25]. A recent study has reported that the peel or segment wall extract from Satsuma mandarin orange is lipolytic in rat adipocytes via β-adrenergic activation [26]. Therefore, rat adipocytes were firstly used in our approach, but our attention was focused to delineate the lipolytic responses in human adipocytes, since there are interspecies differences regarding lipolysis control [8]. In fact, we have already reported that octopamine stimulates lipolysis in rodent white fat cells, activates oxygen consumption and thermogenesis in rat brown fat cells, but is almost inactive in human adipocytes [9]. Therefore, it appeared essential to test the putative lipolytic agents in human adipocytes too.
Thus, different protoalkaloids known to be present in C. aurantium [23], namely synephrine, octopamine N-methyltyramine, and tyramine (Fig. 1), were tested on human fat cell preparations highly responsive to the β-adrenergic receptor (AR) agonist isoprenaline, in the conditions in which we already investigated the influence of various pharmacological agents on adipocyte functions [7, 29]. Since inhibition of lipolysis is also an α2-adrenergic response highly developed in human fat cells [24], we compared the actions of the above-mentioned protoalkaloids to bromoxidine and methoxy-idazoxan (α2-agonist and antagonist of reference, respectively).
As there was a clear difference in the respective potency of isoprenaline and synephrine or octopamine, regarding lipolysis stimulation in rat adipocytes, an additional agent that can be considered as a structural intermediate between these amines was tested in human adipocytes, isopropylnorsynephrine, also named isopropyloctopamine or betaphrine (Fig. 1). This poorly documented synthetic amine [1] exhibited potent and effective stimulation of fat mobilization in human fat cells. Lastly, we tested the capacity of isopropylnorsynephrine and of the biogenic amines to be oxidized by the monoamine oxidase activity (MAO) present in adipose tissue.
Materials and methods
Chemicals
(−) Isoprenaline hydrochloride, (+/−) octopamine hydrochloride, tyramine hydrochloride, isobutylmethylxanthine (IBMX), collagenase (C-6885), and other reagents were obtained from Sigma-Aldrich (Saint Quentin Fallavier, F). 3H-2-deoxyglucose was from Perkin Elmer (Boston, MA, USA), while 14C-tyramine was from Sigma-Aldrich. N-methyltyramine hydrochloride, (+/−) synephrine hydrochloride and (+/−) isopropylnorsynephrine hydrochloride were synthesized and purified at Syntech SSPF Int'l Inc. (Montclair, CA, USA). 2-Methoxy-idazoxan (RX 821002) and bromoxidine (UK 14304) were generous gifts from Dr. H. Paris (INSERM U858, Toulouse).
Lipolytic activity of rat adipocyte preparations
The chosen index of lipolytic activity was the glycerol released by freshly isolated adipocytes with reference to the maximal response to (−) isoprenaline, a widely recognized lipolytic agent. Adipocytes were isolated from visceral fat pads of Wistar rats, and the cell suspension was distributed in incubation vials as previously described [11]. Briefly, pieces of adipose tissue were subjected to collagenase digestion at 37°C in the presence of 3.5% of serum bovine albumin in the following buffer: Krebs–Ringer salt solution containing 15 mM sodium bicarbonate, 10 mM HEPES, and 5.5 mM glucose (KRBHA). The mean body weight of the six Wistar rats used was 420 g, and the mean amount of fat cells distributed into the incubation vials was 17.3 ± 1.7 mg/400 μl. Tested amines were dissolved at 100,000 μg/ml in 10% DMSO (v/v), and subsequent 1/10 dilutions were done in distilled water. Of such working dilutions, 4 μl were added to 400 μl of fat cell suspension in KRBHA to reach the indicated final concentrations. The most concentrated assays contained 1,000 μg amine/ml and 0.1% DMSO at final concentration. All compounds were incubated with the fat cells during 90 min, and glycerol release was determined on 150 μl of medium by spectrophotometric measurement at 340 nm, as previously described [5].
Subjects and preparation of human adipose cells for lipolysis measurements
Samples of human subcutaneous adipose tissue were obtained from ten overweight women undergoing abdominal lipectomy at the plastic surgery department of Rangueil hospital (Toulouse, F). Their body mass index (BMI) was 24.6 ± 0.9 kg/m2. The surgical interventions were conducted under general anesthesia. The removed pieces of subcutaneous whitish adipose tissue (WAT) were transferred in less than 30 min to the laboratory. The ex vivo experiments were performed under the agreement of the ethics committee in accordance with the principles and guidelines established by the French National Institute of Medical Research (INSERM). Once isolated, the human adipocytes were immediately washed in KRBHA and used at 17.6 ± 1.0 mg lipid/400 μl (approx. 500,000 cells/vial) for lipolysis measurements, undergoing the same incubation conditions as described above for rat, while remaining small pieces of WAT where stored at −80°C before the assays of MAO activity on tissue homogenates.
Glucose transport assays in isolated adipocytes
3H-2-deoxyglucose (2-DG) uptake was determined for 10 min with freshly isolated human fat cells after 45 min preincubation with the indicated agents in 400 μl KRBHA (with 2 mM pyruvate replacing glucose as fuel supply) as previously described [15]. Separation of extracellular and internalized hexose was performed by centrifugation through dinonyl-phthalate layer, which allowed to separate buoyant intact fat cells [11]. Lipid content was determined as previously reported [4]. For these complementary determinations, the adipocyte preparations were obtained from six women undergoing lipectomy and having a mean BMI of 24.6 ± 1.3 and a mean age of 39 years.
MAO activity on WAT homogenates
Amine oxidation was determined in subcutaneous WAT homogenates, by incubating 0.5 mM 14C-tyramine during 30 min at 37°C together with approx. 100 μg proteins in the absence or the presence of reference MAO inhibitors as described [27]. For these determinations, mean BMI of the five female donors was 25.2, and mean age was 41 years.
Statistical analysis
Results are given as means ± S.E.M. Statistical significance was assessed by use of Student's t test (NS, non-significantly different from respective control).
Results
Assessment of the lipolytic effect of the main amines found in C. aurantium extracts
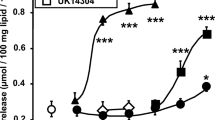
Since most of the reports related to the alkaloid content of Citrus fruits are giving concentrations as microgram per gram or microgram per milliliter, similar units were used in our functional studies. This allowed to directly compare with the amine richness in the fruits, juices, or extracts and did not alter the comparison of the pharmacological properties between the different amines tested since, with the used salts, 1 μg/ml corresponds to 4.9 μM synephrine, 5.3 μM octopamine, 5.3 μM N-methyltyramine, 5.8 μM tyramine, or 4.3 μM isopropylnorsynephrine. The DMSO vehicle used for the dissolution of these amines was without effect on lipolytic activity of rat adipocytes, even at the highest tested concentration (0.1%), basal 0.27 ± 0.05, vehicle 0.28 ± 0.05 μmol glycerol/100 mg lipid/90 min (n = 6, NS). As expected, the maximal lipolytic activation by isoprenaline reached a plateau. The maximal stimulation elicited by 10 μM isoprenaline (equivalent to 2.45 μg/ml) increased the basal activity by sixfold and was taken as 100% reference (Fig. 2). Tyramine and N-methyltyramine were not lipolytic. Synephrine was clearly stimulating the lipolysis in a dose-dependent manner, reaching around 80% of the maximal response to isoprenaline. The most lipolytic biogenic amine was octopamine, which reached (or even was greater than) the stimulation observed with isoprenaline. However, the two latter amines were only active at elevated doses and exhibited a very low potency when compared to isoprenaline: there was a difference of about four orders of magnitude in their EC50.
Comparison of the effects of the biogenic amines detected in Citrus aurantium on lipolysis in rat adipocytes. Rat adipocytes were incubated for 90 min with the indicated doses of the hydrochloride salts of isoprenaline (closed circles), octopamine (closed squares), synephrine (open squares), tyramine (open triangles) or N-methyl tyramine (closed triangles). Isoprenaline was used as the lipolytic agent of reference: its maximal effect (reaching 1.50 ± 0.15 μmol glycerol/100 mg cell lipids/90 min) was set at 100%. Mean ± SEM of six observations. Different from basal (open circle) at *p < 0.05; ***p < 0.001
Further analysis was performed by mixing increasing concentrations of the biogenic amines together with a submaximal dose of isoprenaline (10 nM, inducing a fivefold stimulation). Octopamine was clearly additive to isoprenaline, regarding lipolysis activation. This was not observed with synephrine (Fig. 3). Again, tyramine and N-methyltyramine behaved differently from the former amines, since they dose-dependently inhibited the submaximal effect of isoprenaline. They can be qualified as antilipolytic agents, which stimulate receptors negatively coupled to the adenylyl cyclase or act as partial agonists—or even antagonists—at β-ARs. Irrespective of their mechanism of action, these amines can hardly be suspected to promote the lipolytic action of C. aurantium and its related dietary supplements.
Effect of octopamine, synephrine, tyramine, and N-methyltyramine on lipolysis in rat adipocytes in the presence of 10 nM isoprenaline. Lipolytic activity was submaximally stimulated by 10 nM isoprenaline without or with the indicated amines. Results as percentage of lipolysis obtained with 10 nM isoprenaline alone (control), set at 100% with basal set at 0%. Mean ± SEM of six observations. Different from control at ***p < 0.001
Taken together, the observations obtained with rat adipocytes validated that octopamine and synephrine could be the components responsible of the claimed activation of lipid mobilization by C. aurantium extracts, if the case of their ingested dose is sufficient enough to reach 1 μg/ml in the interstitial fluid of fat depots. They also confirmed that tyramine exerts antilipolytic effects, as previously reported [28].
Then, we compared in human adipocytes the effects of the C. aurantium alkaloids, but we also investigated the effects of isopropylnorsynephrine, on the basis of its resemblance to isoprenaline, synephrine, and octopamine, as shown in Fig. 1.
Lipolytic effect of tyramine, synephrine, and related derivatives in human subcutaneous adipose cells
DMSO vehicle was without noticeable effect while isoprenaline provoked a dose-dependent stimulation of lipolysis in human adipocytes, which resembled to that obtained in rat, with a maximum of sevenfold increase over basal observed at 10 μM (Fig. 4). All the tested amines increased lipolysis but with clearly lower intrinsic activity compared with isoprenaline. According to the relative order of potency or to the ranking of maximal activity, the classification was (with maximal percent of isoprenaline-dependent activation given between parentheses): isoprenaline (100%) >> isopropylnorsynephrine (59%) ≥ octopamine (52%) > synephrine (33%) > tyramine (25%) = N-methyltyramine (20%). Tyramine and N-methyltyramine were poorly lipolytic at 100 μg/ml only, with an effect that decreased at the highest dose tested (1,000 μg/ml, approx. 5 mM). The effects of synephrine and octopamine were more impressive but only detected at concentrations above 10 μg/ml. The most lipolytic amine was isopropylnorsynephrine, characterized by a plateau of maximal effect between 1 and 1,000 μg/ml, equivalent in amplitude to one half of isoprenaline maximal stimulation. Notably, isopropylnorsynephrine elicited lipolytic responses with a high inter-individual variability but with an approximately 100-fold higher potency than synephrine.
Lipolytic responses of human adipose cells to the amines detected in Citrus aurantium and derivatives. Freshly isolated adipocytes were incubated for 90 min with increasing concentrations of isoprenaline (black circles) or the indicated amines. Results are expressed as percentage of 10 μM (2.4 μg/ml) isoprenaline-induced lipolysis. Mean ± SEM of five determinations. Different from basal (open circle) at *p < 0.05; **p < 0.01; ***p < 0.001
Synephrine, isopropylnorsynephrine, and the lipolysis control in human adipocytes
The amines were then compared under a condition of mild-stimulated lipolysis, in the presence of 10 μM adrenaline. None of the amines was able to amplify the response to adrenaline, but all of them tended to inhibit it, contrarily to the α2-AR antagonist methoxy-idazoxan, which clearly potentiated it (Table 1). Since only the latter was able to block the α2-adrenegic antilipolysis and to reveal the β-adrenergic component of adrenaline, it can be concluded that the tested alkaloids were unable to block α2-ARs. However, the amines could bind to the α2-ARs in an agonistic manner. This eventuality was tested by comparing their putative antilipolytic action to that of bromoxidine, a well-known α2-AR agonist. While the latter totally inhibited the IBMX-induced lipolysis in a manner that was reversed by the α2-AR antagonist methoxy-idazoxan (Table 2), tyramine and its methylated derivative exhibited partial—but significant—antilipolytic action. Such antilipolysis was not added to that of bromoxidine (Table 2), leading to suspect either a partial agonism at α2-AR or a completely different antilipolytic action. Again, isopropylnorsynephrine was the most lipolytic of the tested amines, exhibiting a tendency to enhance the IBMX lipolytic effect.
The effects of synephrine and isopropylnorsynephrine on glucose transport in human adipocytes
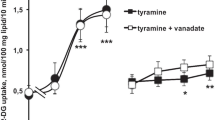
Then, we studied the capacity of synephrine and isopropylnorsynephrine to regulate glucose uptake in human fat cells since octopamine has been already described to partially activate (at 0.1 and 1 mM) glucose transport in such cells or in adipocytes from β3-AR KO mice [27]. Neither synephrine nor isopropylnorsynephrine were able to provoke a noticeable stimulation of glucose transport when incubated for 45 min with adipocytes, while 100 nM insulin enhanced the basal uptake by fourfold (not shown). However, a difference was found between synephrine and its derivative, regarding the acute influence on insulin responsiveness. Only the high dose (1 mM) of synephrine hampered the submaximal activation of uptake by 10 nM insulin (reducing it from 2.38 ± 0.27 to 1.22 ± 0.10 nmol 2-DG/100 mg lipid/10 min, n = 6, p < 0.01). Such inhibitory behavior was not observed with lower doses of synephrine (0.01–0.1 mM) or with isopropylnorsynephrine, since the uptake in the presence of 10 nM insulin plus 1 mM of this substituted amine was equivalent to 2.28 ± 0.24 nmol 2-DG/100 mg lipids/10 min (n = 6, NS).
Lack of isopropylnorsynephrine oxidation by MAO in human WAT
Tyramine and octopamine are MAO substrates in human adipocytes [27], which highly express MAO-A. We investigated whether the other amines interacted with MAO. While N-methyltyramine was clearly undergoing oxidation by MAO, isopropylnorsynephrine did not behave as a MAO substrate since, when tested from 100 nM to 10 mM on human WAT homogenates, it did not compete for 14C-tyramine oxidation. More precisely, the oxidation of 0.5 mM 14C-tyramine was producing 1.5 ± 0.1 nmol of labeled aldehyde per milligram protein per minute and was inhibited by approx. 90% by 10 mM cold tyramine or N-methyltyramine, while it was only inhibited by 20 ± 2% by 10 mM isopropylnorsynephrine. In this view, isopropylnorsynephrine was resembling to the β-AR agonist isoprenaline, which was also unable to inhibit 14C-tyramine oxidation (only 5 ± 4% inhibition, n = 5, NS).
Discussion
Our comparative study showed that in rat adipocytes, octopamine and synephrine exhibit lipolytic activities that may constitute the basis for an anti-obesity effect of Citrus extracts. When considering the numerous qualitative and quantitative determinations of the alkaloids present in the diverse multi-component preparations containing Citrus aurantium or in the fruits themselves, it appears that octopamine, which is only present in trace amounts [21], cannot be the major active component responsible for the lipid-mobilizing activity ascribed to such preparations [12, 25]. In spite of having a lower intrinsic activity than octopamine, synephrine can be considered as the predominant active component, since it is the most abundant alkaloid found in such preparations [20]. Unfortunately, synephrine (like octopamine) is clearly less lipolytic in human adipocytes than in rat, and the reduction in body weight gain observed without overtly declared alteration in oxidative stress in rodents after its subchronic administration [3] is probably not as evident in man. Since it is known that high doses of C. aurantium extracts (5,000 mg/kg) or of synephrine (2,000 mg/kg) exert in mouse toxic effects that are reversible and related to adrenergic overstimulation [2], the benefit/risk ratio of increasing the dietary intake of these biogenic amines in overweight or obese subjects to limit WAT extension remains to be established.
In our conditions, which can be considered as highly responsive to isoprenaline (sixfold stimulation), the lipolytic action of octopamine found in rat adipocytes is in complete agreement with its stimulatory action already reported in rodents [9, 27], including hibernators [5]. The mechanism of action of octopamine is related to the activation of β3-ARs, and a very low lipolytic response to this amine has been observed in guinea pig and in human adipocytes [9], known to express much less functional β3-ARs than the rat. A minor discrepancy appeared when comparing the maximal intrinsic activity of octopamine in human adipocytes observed in the present study (reaching 48% of maximal isoprenaline effect) to the faint activity previously reported (accounting for 12% of maximum) [9]. In spite of this difference which remains explained, the overall maximal octopamine-induced lipolysis remains much lower than that of isoprenaline in human adipocytes. Therefore, the capacity of repeated i.p. injections of octopamine to lower the body weight gain of obese rats [6] remains far for being extrapolated into a mere prediction of a lipid mobilization provocked by octopamine supplementation in obese patients.
The inhibition of lipolysis observed with tyramine in rat adipocytes is completely confirmatory of that already reported in the same model for millimolar doses of tyramine and other amine oxidase substrates [28]. This antilipolytic effect has been shown to be mediated by the hydrogen peroxide produced during amine oxidation occurring in fat cells [28]. The antilipolytic effect of N-methyl tyramine, which resembles to that of tyramine, does not seem to be mediated by an activation of α2-ARs since the α2-adrenergic antilipolytic component is very weak in rat adipocytes [27]. A very weak lipolytic effect of tyramine and of its methylated derivative was evidenced for the first time in human adipocytes. However, more remarkable was the capacity of these amines to exert a substantial antilipolysis in human cells when IBMX is present. A partial agonism at β-ARs could be excluded since IBMX activates lipolysis without stimulating adrenergic receptors. Because tyramine and its methylated derivative hardly added their effects to the antilipolytic α2-AR agonist bromoxidine, a partial agonism to α2-ARs cannot be excluded in human adipocytes. However, this hypothesis appears unlikely when considering that: (1) the amines were also antilipolytic in rat adipocytes lacking functional α2-ARs, (2) tyramine is readily oxidized by human adipocytes and may inhibit triglyceride breakdown in a hydrogen peroxide-mediated manner, as evidenced in rat adipocytes [28]. Whether the antilipolytic effect of tyramine exists under physiological situations deserves to be studied, since tyramine is widely distributed in numerous foods and beverages other than Citrus fruits (e.g., seafood, cheese, wine, beer).
Synephrine has been supposed to act as an antagonist on presynaptic α-receptors [17]. Our observations disagree with an α2-AR antagonist component since: (1) synephrine was unable to improve adrenaline-induced lipolysis in human fat cells, and (2) synephrine did not totally prevent bromoxidine-induced antilipolysis on IBMX stimulation. In the same conditions, the α2-adrenergic antagonist methoxy-idazoxan clearly potentiated the lipolytic effect of adrenaline (by blocking its α2-antilipolytic component, predominant in obese patients [24]) and totally reversed the α2-adrenergic antilipolytic effect of bromoxidine. A partial agonism at α2-ARs cannot be excluded but did not allow synephrine to notably inhibit IBMX-induced lipolysis. To verify whether such partial agonism exists for this amine, as for the other protoalkaloids, it should be necessary to test whether the addition of a β-blocker in the medium reveals a clearer antilipolytic behavior on IBMX. However, such verifications seem poorly mandatory in view of recent observations made on cells expressing the different subtypes of human α-ARs and showing the poor affinity of synephrine for α1-, α2A-, and α2C-ARs [17]. More informative can be to unravel how synephrine impairs insulin activation of glucose transport. One possible reason could be given by the fact that β3-ARs agonists, including octopamine, hamper the insulin action in rat fat cells [11, 27], but such event remains unlikely in human fat cells, which have only a weak β3-adrenergic responsiveness.
Another concern that can be raised is that (+/−) synephrine was used in our study while the naturally occurring amine is mainly R-(−)-synephrine. However, it has been recently shown that this natural form of synephrine undergoes high risks of racemization during the extraction procedure from the plant material as the result of an acceleration of this process by high temperatures and acidic or basic pH values [22].
The isopropyl derivative of demethylated synephrine or octopamine was the most lipolytic of the agents tested on human adipocytes. Its potency and intrinsic activitiy were more attractive than those of the protoalkaloids found in C. aurantium. However, this derivative has never been evidenced in bitter oranges (F. Pellatti, personal communication) and cannot be considered as a naturally occurring alkaloid. The lipolytic effect of isopropylnorsynephrine has been reported by Wenkeová and coworkers in their pioneering studies on pieces of human adipose tissue in vitro [30]. As in our present studies made on highly responsive isolated adipocytes, these authors found 35 years ago that isopropylnorsynephrine produced about 40% of the maximal lipolytic effect of isoprenaline on WAT pieces. A direct activation of β-ARs, but not of α-ARs by this amine has been evidenced on cardiovascular functional responses [1]. In the human adipocytes, the complete reversion of the bromoxidine-induced antilipolysis, observed with isopropylnorsynephrine was probably not due to an α2-AR antagonist property since at the same concentration, the agent did not potentiate the adrenaline effect. A more likely interpretation is that isopropylnorsynephrine has stimulated the β-ARs, leading to an activated lipolysis that was further enhanced by IBMX, resulted in a cAMP overproduction that cannot be lowered by bromoxidine. The impressive lipolytic capacities of isopropylnorsynephrine (half-maximal effect of isoprenaline reached with only 1 μg/ml) encourage to consider it as a good candidate to promote lipid mobilization. A drug-based approach using isopropylnorsynephrine rather than a diet supplementation may constitute a novel safe basis of managing body weight. However, facing to these lipolytic properties, the lack of serious adverse actions reported so far for this synephrine derivative have to be scrupulously verified before developing such novel lipid-mobilizing treatment.
Meanwhile, the fact that isopropylnorsynephrine does not undergo amine oxidation, as measured in our competition experiments of 14C-tyramine oxidation completely fits its lack of activation of glucose transport. It is also consistent with very ancient findings indicating that the lipolytic response to this adrenergic drug was not differing when using albumins of different origin (human vs. bovine) in the incubation medium, while the potency of catecholamines (e.g., adrenaline) was different from one preparation to another [16]. Our current interpretation of such findings is that, in this period, albumin preparations were less purified than at the present time, and the bovine one was more contaminated by soluble amine oxidase, the activity of which is more abundant in bovine than in human plasma [14]. Consequently, the catecholamines were more oxidized in the former preparation and their lipolytic properties were altered, when compared to incubations with human albumin. We therefore propose that the lipolytic action of isopropylnorsynephrine was the same irrespective of the source of albumine tested because isopropylnorsynephrine, like isoprenaline, was not oxidized during the incubations.
To conclude, synephrine, which is quantitatively the most represented alkaloid in C. aurantium extracts could be endowed of the putative lipid-mobilizing effects of such extracts in man. Only a direct in situ analyis of lipolysis, owing to the microdalysis technique [24] could definitively demonstrate such property. However, such investigations must be performed not only with multi-component dietary supplements but also with purified alkaloids. A recent clinical study showed that dietary supplements increased blood pressure and plasma glucose post-exercise, and modestly improved exercise tolerance [13], but it was impossible to make the part due to synephrine since such products contained synephrine and caffeine. Regardless of this issue, our observations have confirmed that isoprenaline, and to a lesser extent, isopropylnorsynephrine, are lipolytic drugs which are much more active than the dietary amines synephrine or octopamine, while tyramine and N-methyl tyramine exhibit antilipolytic actions.
References
Anderson WG (1983) The sympathomimetic activity of N-isopropyloctopamine in vitro. J Pharmacol Exp Ther 25:553–558
Arbo MD, Larentis ER, Linck VM, Aboy AL, Pimentel AL, Henriques AT, Dallegrave E, Garcia SC, Leal MB, Limberger RP (2008) Concentrations of p-synephrine in fruits and leaves of Citrus species (Rutaceae) and the acute toxicity testing of Citrus aurantium extract and p-synephrine. Food Chem Toxicol 46:2770–2775
Arbo MD, Schmitt GC, Limberger MF, Charão MF, Moro AM, Ribeiro GL, Dallegrave E, Garcia SC, Leal MB, Limberger RP (2009) Subchronic toxicity of Citrus aurantium L. (Rutaceae) extract and p-synephrine in mice. Regul Toxicol Pharmacol 54:114–117
Atgié C, Hadj-Sassi L, Bukowiecki L, Mauriège P (2009) High lipolytic activity and dyslipidemia in a spontaneous hypertensive/NIH corpulent (SHR/N-cp) rat: a genetic model of obesity and type 2 diabetes mellitus. J Physiol Biochem 65:33–42
Atgié C, Sauvant P, Ambid L, Carpéné C (2009) Possible mechanisms of weight loss of Siberian hamsters (Phodopus sungorus sungorus) exposed to short photoperiod. J Physiol Biochem 65:377–386
Bour S, Visentin V, Prévot D, Carpéné C (2003) Moderate weight-lowering effect of octopamine treatment in obese Zucker rats. J Physiol Biochem 59:175–182
Bour S, Iglesias-Osma MC, Marti L, Duro P, Garcia-Barrado M, Pastor M-F, Prévot D, Visentin V, Valet P, Moratinos J, Carpéné C (2006) The imidazoline I2-site ligands BU 224 and 2-BFI inhibit MAO-A and MAO-B activities, hydrogen peroxide production, and lipolysis in rodent and human adipocytes. Eur J Pharmacol 552:20–30
Carpéné C, Bousquet-Mélou A, Galitzky J, Berlan M, Lafontan M (1998) Lipolytic effects of beta1-, beta2-, and beta3-adrenergic agonists in white adipose tissue of mammals. Ann NY Sci 839:186–189
Carpéné C, Galitzky J, Fontana E, Atgié C, Lafontan M, Berlan M (1999) Selective activation of beta3-adrenoceptors by octopamine: comparative studies in mammalian fat cells. Naunyn-Schmiedeberg’s Arch Pharmacol 359:310–321
Dragull K, Breksa API, Cain B (2008) Synephrine content of juice from Satsuma mandarins (Citrus unshiu Marcovitch). J Agric Food Chem 56:8874–8878
Duffaut C, Bour S, Prévot D, Marti L, Testar X, Zorzano A, Carpéné C (2006) Prolonged treatment with the β3-adrenergic agonist CL 316243 induces adipose tissue remodeling in rat but not in guinea pig: 2) modulation of glucose uptake and monoamine oxidase activity. J Physiol Biochem 62:101–112
Haaz S, Fontaine KR, Cutter G, Limdi N, Perumean-Chaney S, Allison DB (2006) Citrus aurantium and synephrine alkaloids in the treatment of overweight and obesity: an update. Obes Rev 7:79–88
Haller CA, Duan M, Pr J, Benowitz N (2008) Human pharmacology of a performance-enhancing dietary supplement under resting and exercise conditions. Br J Clin Pharmacol 65:833–840
Holt A, Smith DJ, Cendron L, Zanotti G, Rigo A, DiPaolo ML (2008) Multiple binding sites for substrates and modulators of semicarbazide-sensitive amine oxidases: kinetic consequences. Mol Pharmacol 73:525–538
Iglesias-Osma MC, Bour S, Garcia-Barrado MJ, Visentin V, Pastor MF, Testar X, Marti L, Enrique-Tarancon G, Valet P, Moratinos J, Carpéné C (2005) Methylamine but not mafenide mimics insulin-like actvity of the semicarbazide-sensitive amine oxidase-substrate benzylamine on glucose tolerance and on human adipocyte metabolism. Pharmacol Res 52:475–484
Lincová D, Cepelík J, Cernohorský M, Elisová K (1977) Effect of different albumin media on the lipid-mobilizing action of sympathicotropic substances in vitro. Physiol Bohemoslov 2:165–172
Ma G, Bavadekar SA, Schaneberg BT, Khan IA, Feller DR (2010) Effects of synephrine and beta-phenethylamine on human alpha-adrenoceptor subtypes. Planta Med 76:981–986
Mercolini L, Mandrioli R, Trerè T, Bugamelli F, Ferranti A, Raggi MA (2010) Fast CE analysis of adrenergic amines in different parts of Citrus aurantium fruit and dietary supplements. J Sep Sci 33:2520–2527
Nelson BC, Putzbach K, Sharpless KE, Sander LC (2007) Mass spectrometric determination of the predominant adrenergic protoalkaloids in bitter orange (Citrus aurantium). J Agric Food Chem 55:9769–9775
Pellati F, Benvenuti S (2007) Chromatographic and electrophoretic methods for the analysis of phenethylamine alkaloids in Citrus aurantium. J Chromatogr A 1161:71–88
Pellati F, Benvenuti S (2007) Fast high-performance liquid chromatography analysis of phenethylamine alkaloids in Citrus natural products on a pentafluorophenylpropyl stationary phase. J Chromatogr A 1165:58–66
Pellati F, Cannazza G, Benvenuti S (2010) Study on the racemization of synephrine by off-column chiral high-performance liquid chromatography. J Chromatogr A 1217:3503–3510
Percy DW, Adcock JL, Conlan XA, Barnett NW, Gange ME, Noonan LK, Henderson LC, Francis PS (2010) Determination of Citrus aurantium protoalkaloids using HPLC with acidic potassium permanganate chemiluminescence detection. Talanta 80:2191–2195
Stich V, De Glisezinski I, Crampes F, Hejnova J, Cottet-Emard JM, Galitzky J, Lafontan M, Rivière D, Berlan M (2000) Activation of alpha2-adrenergic receptors impairs exercise-induced lipolysis in SCAT of obese subjects. Am J Physiol 279:R499–R504
Stohs SJ, Shara M (2007) A review of the safety and efficacy of Citrus aurantium in weight management. In: Bagchi D, Preuss HG (eds) Obesity: epidemiology, pathophysiology, and prevention. CRC, Boca Raton, pp 371–382
Tsujita T, Takaku T (2007) Lipolysis induced by segment wall extract from Satsuma mandarin orange (Citrus unshu Mark). J Nutr Sci Vitaminol (Tokyo) 53:547–551
Visentin V, Morin N, Fontana E, Prévot D, Boucher J, Castan I, Valet P, Grujic D, Carpéné C (2001) Dual action of octopamine on glucose transport into adipocytes: inhibition via b3-adrenoceptor activation and stimulation via oxidation by amine oxidases. J Pharmacol Exp Ther 299:96–104
Visentin V, Prevot D, Marti L, Carpéné C (2003) Inhibition of rat fat cell lipolysis by monoamine oxidase and semicarbazide-sensitive amine oxidase substrates. Eur J Pharmacol 466:235–243
Wanecq E, Prévot D, Carpéné C (2009) Lack of direct insulin-like action of visfatin/Nampt/PBEF1 in human adipocytes. J Physiol Biochem 65:351–360
Wenkeová J, Kuhna E, Wenke M (1975) Adrenergic lipolysis in human adipose tissue in vitro. Eur J Pharmacol 30:49–55
Acknowledgements
This work was partly supported by Communauté de Travail des Pyrénées and the DIOMED project (INTERREG IVB-SUDOE-FEDER, SOE1/P1/E178). The authors express gratitude to Virgile Visentin and Danielle Prévot for their help. They also acknowledge Philippe Valet (Univ. Toulouse, France) and Federica Pellati (Univ. Modena, Italy) for their respective knowledge on human adipocyte biology or Citrus biogenic amines and the staff of plastic surgery of Rangueil hospital for facilitating access to surgical wastes. In memoriam to Hervé Paris.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mercader, J., Wanecq, E., Chen, J. et al. Isopropylnorsynephrine is a stronger lipolytic agent in human adipocytes than synephrine and other amines present in Citrus aurantium . J Physiol Biochem 67, 443–452 (2011). https://doi.org/10.1007/s13105-011-0078-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-011-0078-2