Abstract
Human peripheral blood T lymphocytes are classified into alpha–beta T (αβΤ) cells and gamma–delta T (γδΤ) cells based on the difference in T cell receptors (TCRs). αβT cells are crucial for the acquired immune response, while γδΤ cells, though only a small subset, can recognize antigenic substances. These antigens do not need to be processed and presented and are not restricted by MHC. This distinguishes γδΤ cells from αβT cells and highlights their distinct role in innate immunity. Despite their small number, γδΤ cells hold significant significance in anti-tumor, anti-infection and immune regulation. Glioblastoma (GBM) represents one of the most prevalent malignant tumors within the central nervous system (CNS). Surgical resection alone proves to be an ineffective method for curing this type of cancer. Even with the combination of surgical resection, radiotherapy, and chemotherapy, the prognosis of some individuals with glioblastoma is still poor, and the recurrence rate is high. In this research, the classification, biological, and immunological functions of γδT cells and their research progress in anti-glioblastoma were reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Human peripheral blood T lymphocytes are classified into alpha–beta T (αβΤ) cells and gamma–delta T (γδΤ) cells based on the difference in T cell receptors (TCRs). αβΤ cells are vital for the acquired immune response. Human γδΤ cells were discovered in the 1980s. Given their distribution and absence of MHC (major histocompatibility complex) restriction in their immune response, human γδΤ cells serve a distinct role in innate immunity. γδΤ cells are made up of γ and δ chains, and they originate from the thymus. However, the peripheral tissues and organs are mature, accounting for about 0.5% of lymphocytes in the peripheral blood of healthy adults [1,2,3]. Recent research has demonstrated that γδ cells are crucial for anti-tumor, anti-infection, and immune regulation [4,5,6,7,8,9,10,11,12]. Glioblastoma (GBM) is one of the most prevalent malignant tumors in the central nervous system (CNS). The treatment of glioblastoma is particularly challenging, and surgical resection alone is rarely curative. Despite the combination of surgical resection, radiotherapy, and chemotherapy, the prognosis remains unfavorable for some patients, with a high rate of recurrence [13]. In the 2021 World Health Organization (WHO) classification of CNS tumors, low-grade gliomas (LGG) encompassed grades 1 and 2, while high-grade gliomas (HGG), which included certain types of CNS gliomas, were categorized into grades 3 and 4. Glioblastoma multiforme (GBM), classified as WHO grade 4, represents the most invasive and malignant primary brain tumor, with a mere 5% survival rate over 5 years [14]. Therefore, it is crucial to develop innovative strategies to effectively treat gliomas and significantly reduce mortality rates. The current article provides a review of the classification, biological and immunological functions of γδΤ cells, the expression characteristics of γδΤ cells in patients with GBM, and the progress of these cells against GBM.
2 Classification of γδT cells
2.1 Structural classification of γδT cells
Regulation of the delta chain of human γδT cells is carried out by three Vδ genes (1–3), which leads to their classification into Vδ1γδT cells, Vδ2γδT cells and Vδ3γδT cells based on the variation in their delta chains (Fig. 1) [15].
-
(1)
Vδ1γδT cells: Vδ1γδT cells are primarily found in the thymus, mucosa, and subcutaneous tissues, representing the most abundant subgroup present on the mucosal surface. This subgroup is crucial for maintaining the integrity of epithelial tissue. Moreover, it also secretes perforin and granzyme by producing interferon-γ (IFN-γ), IL-10, and small amounts of IL-4, IL-2, and other cytokines. These chemical substances, along with the secretion and expression of chemokines, exert a cytotoxic effect, thereby participating in the anti-tumor response. Moreover, this subgroup has an inhibitory effect on a variety of epithelial-derived tumors and certain leukemias. Vδ1γδT cells can participate in the resistance to microbial infections by secreting IL-17 and the pro-inflammatory cytokine IFN-γ. Vδ1γδT cells express the helper stimulator CD8 on the cell surface, playing an essential role in activating helper T cells. The mucosa and epithelial tissues are the first barrier against pathogen invasion, and they are also common sites for tumor development. The high proportion of γδT cells in these tissues suggests their crucial role in tumor immunity, as well as in protection against microbes and parasites [3, 8, 16].
-
(2)
Vδ2γδT cells: Vδ2γδT cells are primarily found in peripheral blood. During the TCRγδ recombination process, the Vδ2 chain almost exclusively combines with Vγ9, resulting in the formation of Vγ9Vδ2T cells [17]. Vγ9Vδ2T cells, being the predominant circulating cells, comprise 0.5% to 5% of adult peripheral blood. These cells can be specifically activated by phosphorylated antigens that are produced either by microorganisms or by abnormally transformed cells, causing exogenous infections and endogenous abnormal cell transformations [16, 18]. According to the different surface markers of Vγ9Vδ2γδT cells, they can be classified into four subgroups: CD45RA+CD27+ naive cells, CD45RA−CD27+ central memory cells, CD45RA−CD27-effector memory cells, and CD45RA+CD27-terminally differentiated cells. The first two types of cells are primarily located in secondary lymphoid tissues and proliferate under the stimulation of isopentenyl pyrophosphate. However, they typically do not exert direct effector functions. On the other hand, the last two types of cells are mainly distributed in infection and tumor sites, performing direct effector functions such as secretion of cytokine IFN-γ and tumor necrosis factor-α (TNF-α), as well as cytotoxicity [19].
-
(3)
Vδ3γδT cells: Vδ3γδT cells are abundant in the liver and are the least abundant subgroup in the body, accounting for only 0.2% of circulating γδT cells. CD56, CD161, and NK cell surface activation receptor D are expressed on their surface. Studies have shown that Vδ3γδT cells can not only secrete IFN-γ, TNF-α, and IL-4 to enhance the immune function of the body but also enhance the recognition of CD1d to act on CD1d+ target cells and induce dendrites. Cells (DCs) are transformed into antigen-presenting cells (APCs), and they are constantly detected and identified as cancerous cells [20, 21].
Classification and characteristics of human γδT cell subsets. A T cells are classified into αβT cells and γδT cells according to the differences in the types of their cell receptors (T cell receptor, TCR). B γδT cells can be divided into Vδ1γδT cells, Vδ2γδT cells and Vδ3γδT cells according to the difference of their δ chains. They play an important role in infectious disease and/or cancer
2.2 Functional classification of γδT cells
The structural heterogeneity among γδT cell subgroups leads to a wide range of functional diversity. As a result, based on their distinct functions, they can be classified into γδT cells that secrete IFN-γ (IFN-γ+γδT cells), γδT cells that secrete IL-17 (γδT17 cells), and regulatory γδT cells (γδTreg cells) [22], among others.
-
(1)
IFN-γ+γδT cells: IFN-γ+γδT cells are a type of γδT cells that highly express IFN-γ, which undergo functional differentiation in the thymus. Various factors in the thymus microenvironment, such as γδTCR and transforming growth factor β receptors, lymphotoxin β receptors, CD2, skint-1, intracellular molecule B lymphokinase, and promyelocytic leukemia zinc finger genes are all involved in this process [23]. IFN-γ+γδT cells play a crucial role in autoimmune diseases, tumor surveillance, host defense, and incision healing. Studies have found that their number in hepatitis B patients has increased significantly, suggesting functional IFN-γ+γδT cells also play an important role in controlling infection caused by the hepatitis B virus [24].
-
(2)
γδT17 cells: γδT17 cells belong to the subgroup of Vδ1γδT cells derived from thymus, which mainly secrete IL-17. They are capable of expressing aryl hydrocarbon receptors, retinoic acid-related nuclear orphan receptors γt, and IL-12 receptors such as Th17 cells, as well as CCR6 receptors. They can also directly act on pathogens through Toll-like receptors [25]. Among them, γδT17 cells with a terminally differentiated phenotype of CD27−CD45 RA+ can express tumor necrosis factor-related apoptosis-inducing ligands, granzyme B, FasL, and CD161. However, they do not produce IL-22 and IFN-γ. In terms of antigen activation, γδT17 cells can quickly trigger IL-8-mediated neutrophil migration and phagocytosis. Additionally, epithelial cells rely on IL-17 for the production of β defensins [18]. IL-17A produced by γδT17 cells also holds significant significance in the infection caused by the Mycobacterium BCG vaccine in the lungs, as well as in the development of granulomatous immune response induced by the BCG vaccine [26]. The above studies show that γδT17 cells play an important role in inflammation caused by microorganisms. Furthermore, γδT17 cells have been found to exert tumor-promoting effects. The IL-17 secreted by these cells can induce tumor angiogenesis. Furthermore, tumor-infiltrated γδT17 cells secrete IL-17, IL-8, TNF, and GM-CSF, which promote the proliferation of PMN-MDSC, forming an immunosuppressive microenvironment, thereby promoting tumor growth [27,28,29].
-
(3)
γδTreg cells: γδTreg cells mainly belong to the Vδ1 subgroup, with the Vδ1+CD27+CD25+ phenotype, and can express Foxp3 similar to the classic CD4 Treg cells. They mainly exert their inhibitory effect on the proliferation of CD4+ T cells through direct cell–cell contact. The cytokines secreted by γδTreg cells are mainly granulocyte–macrophage colony-stimulating factors and IFN-γ [30]. Moreover, γδTreg cells have a crucial role in various aspects such as anti-infection mechanisms, tumor immunotherapy, and graft-versus-host disease, among others. They exert these effects by regulating both innate and adaptive immune responses [31, 32].
3 The function of γδΤ cells
3.1 Biological function of γδΤ cells
Activated γδΤ cells exhibit various biological functions. Some of their notable functions include:
-
(1)
Cytokine production [33]: During intracellular bacterial infection, γδΤ cells have the ability to produce interferon-gamma (IFN-γ) and interleukin 2 (IL-2), exhibiting Th1-like effects similar to helper T lymphocyte type 1 cells. On the other hand, when infected by extracellular parasites, γδΤ cells produce IL-4, IL-5, and IL-10, which stimulate B cells and exhibit Th2-like effects similar to helper T lymphocyte type 2 cells. Additionally, the IL-10 produced during the aforementioned process can, in turn, inhibit the proliferation and secretion of cytokine IFN-γ by γδΤ cells [34].
-
(2)
Direct lysis of target cells: Activated γδΤ cells possess the ability to directly cleave target cells via the granzyme-perforin pathway. Moreover, they can trigger apoptosis of the target cells through Fas-FasL (transmembrane protein/transmembrane protein cytokines) and IFN-γ [35].
-
(3)
Recognition and killing of tumor cells: γδΤ cells are capable of recognizing stress-inducing molecules such as MICA, MICB, ULBP, and RAET1. Moreover, they can also recognize ectopic apolipoprotein A1 and Toll-like receptors present on the tumor surface [36]. MICA/B and ULBPs were expressed in various types of tumor epithelial cells. γδΤ cells, much like NK cells, recognize tumor cells unrestrictedly through NKG2D receptors. This suggests that even without the presence of human leukocyte antigen or tumor antigen, γδΤ cells retain their ability to eliminate target cells [37]. New immunotherapy strategies, such as chimeric antigen receptor (CAR) engineered γδT cells, can improve the efficacy of CAR-T cells, enhance anti-tumor effect and reduce its side effects [38,39,40,41].
-
(4)
Promoting wound healing: γδΤ cells are capable of responding rapidly to skin damage, and an increased presence of these cells can be observed at the wound site at 4 h [42]. A small quantity of vascular endothelial growth factor and fibroblast growth factor 2 were produced [43]. Activated γδΤ cells stimulate the proliferation of epidermal cells and the re-epithelialization of wounds by expressing KGFs and IGF-1 [44]. They also have the capacity to repair intestinal injury [45].
-
(5)
Mediate its recycling and homing: γδΤ cells, much like αβΤ cells, can bind to specific receptor molecules on endothelial cells using CD44, CD11a (LFA21) and MEL-14 (mouse CD62L APC labeled fluorescent monoclonal antibody). This binding facilitates γδΤ cells to adhere to endothelial cells, thus mediating their recirculation and homing (Fig. 2) [10].
The biological function of γδΤ cells. A Cytokine production. During infection, γδΤ cells can exhibiting Th1-like or Th2-like effects; in turn, the IL-10 can inhibit the proliferation and secretion of γδΤ cells. B γδΤ cells recognize and kill tumor cells through TCR and NKG2D receptors, or direct lysis of target cells. C Promoting wound healing: γδΤ cells stimulate the proliferation of epidermal cells and the re-epithelialization of wounds by expressing VEGF,FGF-2, KGFs and IGF-1. D γδΤ cells bind to specific receptor molecules on endothelial cells using CD44, CD11a (LFA21) and MEL-14, thus mediating their recirculation and homing
3.2 Immunological function of γδΤ cells
Activated γδΤ cells perform a wide range of immunological functions:
-
(1)
Antigen presentation: Partially activated γδΤ cells can differentiate into antigen-presenting cells (APCs) and show high expression levels of MHC-class II molecules and CD80, CD86, and CCR7 (chemokine receptors) on their surface. Moreover, they can process antigens and present them to αβΤ cells, triggering a specific immune response [46].
-
(2)
Non-specific immune response: In the absence of APCs, γδΤ cells can be directly activated via their TCR for recognizing a variety of antigenic components of bacteria and viruses. This process plays a significant role in non-specific immune responses [7].
-
(3)
Immune surveillance: Memory γδΤ cells can prevent the spread of viruses, combat opportunistic infections, and perform immune surveillance by over-expressing CCR7 and CD161 on their surface [47]. Cytomegalovirus (CMV) infection is usually associated with the development of GBM [48]. Human non-Vδ2T cells can directly bind endothelial protein C receptor (EPCR), which is a MHC-like molecule similar to antigen presentation molecule CD1d and can bind to lipid. Adrenergic receptor A2 (EphA2) is a stress-related molecule that also participates in the activation of non-Vδ2T cells. Both EPCR and EphA2 are expressed on endothelial cells infected by CMV and up-regulated during the development of GBM tumor [49, 50]. GBM tumor cells express BTN-like protein BTN3A, which mediates the recognition of PAg by γδTCR and contributes to the antigenic response of Vγ9Vδ2 T cells [51, 52].
-
(4)
Immunomodulatory function: Activated γδΤ cells have the ability to suppress the proliferation of Foxp3+Tregs (regulatory T cells) [53]. They can also generate IL-10 and TGF-β (transforming growth factor β) to perform an immunomodulatory function [54].
-
(5)
Stabilization of the internal immune environment: γδΤ cells can inhibit the overactivation of αβΤ cells, thus maintaining the relative balance between αβΤ and γδΤ cells [55].
-
(6)
Antibody-dependent cytotoxicity: Certain membrane receptors, such as FcγR (IgG Fc receptor), contribute to antibody-dependent cell-mediated cytotoxicity (ADCC) and enhance their cytotoxic effects through the secretion of IL-2 [56].
-
(7)
Bidirectional action on B cells: The majority of γδΤ cells are directly activated by antigens to produce IL-4, which in turn stimulates B cell proliferation and secretion of immunoglobulin (Ig). However, certain subsets of γδΤ cells suppress the production of Ig by B cells.
-
(8)
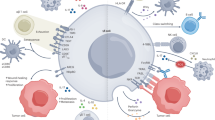
Immunological function: γδΤ cells play their immunological roles by activating, inhibiting, or recruiting other immune cells. Their interactions with immune cells, including dendritic cells, granulocytes, macrophages, Langerhans cells, αβΤ cells, and B cells, are closely related to their anti-infective function (Fig. 3) [57].
Immunological function of γδT cells. A Antibody-dependent cytotoxicity; B immunomodulatory function; C antigen presentation; stabilization of the internal immune environment; D immunesurveillance; E non-specific immune response; F γδΤ cells play their immunological roles by activating, inhibiting, or recruiting other immune cells. G Bidirectional action on B cells
4 Characteristics of γδΤ cell expression in patients with GBM
The proportion of total γδΤ cells in the peripheral blood of individuals with GBM was found to be similar to that of healthy individuals, but the absolute count showed a decreasing trend. Specifically, there was a decrease in double negative (CD4−CD8−) T γδ cells, an increase in immature γδΤ cells, a decrease in the expression levels of CD25 and CD279 (PD-1), and a significant increase in the expression levels of costimulatory markers CD27 and CD28 [58]. The balance between the two primary subsets, Vδ1 T cells to Vδ2 T cells, was disrupted. In the peripheral blood of individuals with GBM, Vδ1 T cells became the dominant subset of γδΤ cells. In individuals with GBM, there was a substantial increase in the proportion of Vδ1T cells, the expression of molecules associated with immunosuppression (Foxp3, CTLA-4), and immunosuppressive function. Conversely, the proportion of Vδ2T cells, the expression of perforin and TNF-α, and the activation of cytotoxicity-related signal pathways considerably decreased. Consequently, the lethality significantly decreased in these individuals. In terms of proliferation, γδΤ cells of untreated GBM patients still had a strong proliferative ability, while the proliferative ability of γδΤ cells decreased significantly after tumor resection or chemotherapy. Compared to healthy people, γδΤ cells in the peripheral blood of people with GBM displayed characteristics of cell depletion, functional impairment, reduced proliferation ability, and an imbalance between Vδ1T cells and Vδ2T cells. These characteristics might contribute to immunosuppression and enable tumors to evade immune surveillance, thus promoting the occurrence and development of tumors [59, 60]. Different researchers have different opinions on GBM-infiltrating γδΤ cells. Lee et al. found that γδΤ cells infiltrated in tumors, mainly Vγ9Vδ2T cell subtypes, and unique Vγ9Vδ2T cells controlled by Vγ9vγ2 sequence gave priority to infiltrating GBM. GBM infiltrating γδΤ cells exhibit high plasticity. Their activity is closely related to the activity of cytotoxic T lymphocytes and regulatory T cells, showing anti-tumor or pro-tumor activity. These findings, together with other studies, have confirmed that γδΤ cells can exhibit different phenotypes according to the surrounding microenvironment, including Th1 type, Th2 type, Th17 type, follicular Th2 type, or Treg characteristics [61,62,63,64]. However, Bryant et al. [60] found no infiltration of γδΤ cells in the tumor parenchyma. The emergence of these two different research outcomes may be linked to the timing of specimen selection, subtle differences in research methods, and other influencing factors. To sum up, γδT cells in peripheral blood of GBM patients are characterized by imbalance of Vδ1T cells and Vδ2T cells, decrease of cell killing function and proliferation ability, but activated GBM patients γδT cells still have cytotoxicity and dissolve GBM tumor cells in vitro [60]. GBM tumor infiltrating γδT cells have high plasticity. Their existence may be strongly associated with the onset and progression of gliomas (Table 1, Fig. 4).
Characteristics of γδΤ cells in the peripheral blood of patients with GBM. A Phenotypic characteristics, a decrease in CD4−CD8−T γδ cells, CD25 and CD279 (PD-1); a significant increase in CD27 and CD28; an increase in immature γδΤ cells. B In terms of proliferation, γδΤ cells of untreated GBM patients have strong proliferative ability, while the proliferative ability of γδΤ cells decreased significantly after tumor resection or chemotherapy. C The peripheral blood γδΤ cells of GBM displayed characteristics of cell depletion, functional impairment, reduced proliferation ability, and an imbalance between Vδ1T cells and Vδ2T cell, thus promoting the occurrence and development of tumors
5 Anti-GBM effect of γδΤ cells
Numerous reports have highlighted that γδΤ cells exhibit certain cytotoxic effects on GBM, although these effects vary across GBM cell lines. γδΤ cells display cytotoxicity towards GBM cell lines U87, U138, T70, U373, U251. Moreover, local injection of expanded γδΤ cells in vitro can slow down the tumor progression and improve the survival rate of human U251MG tumor xenografted non-thymic nude mice. However, it showed almost no cytotoxic effect on A172 cells. This difference might be associated with the expression of MICA/B, UL-16 binding protein (ULBP), intercellular adhesion molecule (ICAM-1), and PVR on the surface of tumor cells [62, 65, 66]. γδΤ cells have a broad capacity to recognize and immediately respond to various MHC-like stress-induced autoantigens. The majority of these autoantigens exhibit expression in human GBM cells but not in adjacent normal brain tissues [67,68,69]. When the expanded γδΤ cells were co-cultured with glioma cells, γδΤ cells recognized the related antigens expressed on the tumor cell surface through their surface TCR or natural killer receptor NKG2D and differentiated memory cells. These γδΤ cells then induced the tumor cells to undergo apoptosis by releasing substances such as perforin and granzyme B and by secreting Th1 cytokines IFN-γ and TNF-α [66, 70,71,72,73,74,75]. These provide a theoretical basis for adoptive immunotherapy of GBM with γδT cells [52, 76]. Nonetheless, the ability of γδΤ cells to suppress GBM tumor cells is limited and occurs in a dose-dependent manner [60, 66]. Research has demonstrated that nitrogen-containing phosphonates, including zoledronic acid (ZOL), minopronate (MDA), and chemotherapeutic drugs, can effectively improve the anti-GBM activity of γδΤ cells. ZOL and MDA can not only directly induce apoptosis of glioma cells but also enhance the production of IFN-γ and TNF-α by γδΤ cells and lead to the accumulation of intracellular IPP by interfering with the metabolic pathway of methoxylphosphonate. Additionally, γδΤ cells recognize and kill these cells containing phosphonate antigens through TCR γδ receptors [9, 18, 77].
Low-dose ZOL treatment not only significantly increased the cytotoxicity of γδΤ cells to GBM-sensitive strains but also strongly triggered the killing of γδΤ cells to resistant strain A172 cells. γδΤ cells recognized GBM cells that had been pretreated with ZOL using specific membrane surface receptors, and they killed these cells through a direct cytotoxicity mechanism. This enhanced cell-killing effect may be mediated by the expression of PVR on GBM cells and the existence of NK cell-activated receptor molecule (DNAM-1) on γδΤ cells [65]. Jarry et al. further confirmed the sensitizing effect of ZOL on GBM cells. Using 51Cr release assay, it was found that allogeneic Vγ9Vδ2T cells had no natural response to U-87MG cells and primary GBM-10 cells, while zoledronate pretreatment of GBM cells triggered significant dose-dependent antigen activation of Vγ9Vδ2T cells [51]. By stereotactic administration, they found that zoledronate or Vγ9Vδ2T cells alone did not significantly increase the median survival time of orthotopic implanted U-87MG or BMG-10 NSG mice. However, single and double administration of zoledronate and Vγ9Vδ2T cells significantly increased the survival rate of mice [51]. Primary GBM-10 is a kind of tumor cells that express high-level “stemness” markers CD133, CD90 and CD44, which are disseminated and invasive, and can reproduce the physiological characteristics of human GBM. The above results show that stereotactic administration of allogeneic human Vγ9Vδ2T cells combined with zoledronate can effectively eliminate not only low invasive tumors but also heterogeneous primary human GBM tumors characterized by “stemness” and invasive [51, 78].
The combination of MDA and γδΤ cells not only effectively induced the apoptosis of GBM cells in vitro but also significantly inhibited the growth of U87MG-derived tumors in NOG mice in vivo. Nakazawa et al. implanted U87MG cells subcutaneously into high immunodeficiency (NOG) mice and injected MDA/GDT intraperitoneally. It was found that MDA combined with GDT could inhibit the growth of unestablished U87MG-derived subcutaneous tumors, and NOG mice had good tolerance to systemic MDA/GDT therapy [75]. γδΤ cells are activated by TCR to recognize IPP metabolites in GBM cells exposed to MDA and induce apoptosis by releasing granzyme B and TNF-α in a cysteine protease (caspase) dependent manner. Therefore, the combination of ZOL or MDA and γδΤ cells produced in vitro may be an effective treatment for patients with GBM [51, 65, 74, 75].
IL-21, a nodular cytokine, is a sensitizing factor of Vγ9Vδ2T cells. It enhances their cytolytic activity by elevating the levels of granzyme B within Vγ9Vδ2T cells. The sensitization of IL-21 can last for at least 24 h in the absence of this factor, and does not affect the migration rate of Vγ9Vδ2T cells in vivo [79]. Joalland et al. established an invasive in situ GBM mouse model by stereotactic implantation of GBM-1 cells into NSG mice. After stereotactic administration, it was found that IL-21-sensitized Vγ9Vδ2T cells could eradicate GBM and significantly improve the survival rate of mice. These results show that IL-21-sensitized allogeneic Vγ9Vδ2T cells have natural cytotoxicity to heterogeneous invasive primary human GBM tumors [79].
Temozolomide (TMZ) is the main chemotherapeutic drug used in the treatment of GBM. It can temporarily upregulate a variety of emergency-induced NKG2D ligands, improve the immunogenicity of GBM, and make GBM cells sensitive to γδΤ cell-mediated lysis [80]. NKG2D ligand was also expressed in glioma stem cells, and its expression was significantly upregulated under the stimulation of TMZ [81]. However, TMZ also has a high cytotoxic effect on γδΤ cells. γδΤ cells modified by methylguanine DNA methyltransferase (MGMT) produce O6-alkylguanine DNA alkyltransferase (AGT), which allows γδΤ lymphocytes to play a role in the therapeutic concentration of TMZ and empowers them with resistance to TMZ. MGMT modified γδΤ cells were mainly effective memory phenotype, and gene modification did not change the proliferative ability and cytotoxicity of γδΤ cells. The combination of MGMT-modified γδΤ cells and TMZ can effectively improve the survival rate of primary GBM tumor xenotransplantation mice [82, 83]. γδΤ cells are genetically modified to resist the toxicity of chemotherapeutic drugs in order to realize the combined application of chemotherapy and immunotherapy. INB-200 is a genetically modified autologous γδT cell immunotherapy developed by IN8bio for the treatment of glioblastoma (GBM). Currently, an ongoing phase I clinical trial (NCT04165941) is testing the safety and tolerability of this therapy in combination with temozolomide (TMZ) in patients with newly diagnosed glioblastoma. Chauvin et al. [84] reported that allogeneic human Vγ9Vδ2T cells possess the ability to spontaneously recognize and clear human GBM mesenchymal cells without any treatment and significantly prolong the life span of tumor-bearing mice. This effect is mediated by γδΤCR and regulated by the stress-related NKG2D pathway (Table2, Fig. 5).
Killing effect of γδΤ cells on glioma cells. A γδΤ cells recognized the related antigens expressed on GBM cell surface through their surface TCR or NKG2D and differentiated memory cells. These γδΤ cells then induced the tumor cells to undergo apoptosis by releasing substances such as perforin and granzyme B and by secreting Th1 cytokines IFN-γ and TNF-α. B IL-21, ZOL, MDA and chemotherapeutic drugs, can effectively improve the anti-GBM activity of γδΤ cells
6 Concluding remarks
To sum up, γδΤ cells have the ability to suppress and kill GBM cells. Consequently, immunotherapy strategies based on γδΤ cells could potentially become a novel approach for treating GBM. It might be worthwhile to consider the development of drugs that can expand, activate and promote the function of γδΤ cells in targeting GBM. In summary, increasing the number of γδΤ cells and enhancing their functioning within the GBM microenvironment is crucial for GBM treatment strategies that are based on γδΤ. It is believed that with the deepening of the study, γδT cells will achieve ideal results in anti-GBM therapy.
References
Wang KQ, Hou YQ, Li QH, et al. Inhibitory effect of LY294002 on CD3mAb-activated T cells and Mtb-Ag-activated γδΤ cells via TCR signal transduction pathway. Int J Clin Exp Pathol. 2017;10:5538–44.
Wang KQ, Hou YQ, Gu CX, et al. Inhibitory effect of the mitogen activated protein kinase specific inhibitor PD98059 on Mtb-Ag-activated γδΤ cells. Int J Clin Exp Pathol. 2017;10:9644–8.
Wang KQ, Hou YQ, Wang XH, et al. Expression kinetics of CD69 molecule by CD3+ lymphocytes and γδΤ cells under three different activating modalities. Chin J Hematol. 2014;35(8):753–4. https://doi.org/10.3760/cma.j.issn.0253-2727.2014.08.020.
Wei L, Wang KQ, Ran ZS, Liu QH, Chen YY, Ji B, Meng L, Cao WW, An X. Auxiliary diagnostic value of γδΤ cell, IL-17, and IFN-γ levels in peripheral blood and bronchoalveolar lavage fluid for lung cancer complicated with chronic obstructive pulmonary disease. Int J Clin Exp Med. 2018;11(7):7183–91.
Chen ZW, Zhao YJ, Li XQ, Wang KQ. Study on the killing effect of γδT cells activated by Rukangyin on breast cancer MDA-MB-231 cells. Dis Mark. 2021. https://doi.org/10.1155/2021/5838582.
Zhu RH, Yan Q, Wang YS, Wang KQ. Biological characteristics of γδT cells and application in tumor immunotherapy. Front Genet. 2023;13:1077419. https://doi.org/10.3389/fgene.2022.1077419.
Wang YS, Zhou Y, Wang KQ. γδT cells in bacterium research progress in the mechanism of disease infection. Chin J Microbiol Immunol. 2016;36(7):555–60. https://doi.org/10.3760/cma.j.issn.0254-5101,2016.07.015.
Wang KQ, Hou YQ, Gu CX, et al. Western blotting was used to detect ZAP-70 molecule from γδΤ cells in peripheral blood. Int J Clin Exp Med. 2019;12(2):1785–90.
Wang YS, Bu WJ, Wang YR, et al. Increased values of peripheral blood γδT cells, Th17 cells, IL-17, ALT, AST, TB, and DB are closely related to the severity of chronic hepatitis B. Int J Clin Exp Med. 2019;12(6):7374–82.
Wang YR, Wang YS, Wang KQ. Research progress on the mechanism of γδT cells in pathogenic microbial infection. Int J Clin Exp Med. 2019;12(8):9597–606.
Zhao NG, Zhang JP, Zhang TT, et al. Expression of γδT and CD4+ CD25+ T cells in peripheral blood of HIV-infected patients/AIDS patients and their correlation. Chin J Microbiol Immunol. 2021;41(7):524–30. https://doi.org/10.3760/cma.j.cn112309-20200618-00322.
Zhao NG, Zhang TT, Zhao YJ, Zhang JP, Wang KQ. CD3+T, CD4+T, CD8+T, and CD4+T/CD8+T ratio and quantity of γδT cells in peripheral blood of HIV-infected/AIDS patients and its clinical significance. Comput Math Methods Med. 2021;2021: 8746264. https://doi.org/10.1155/2021/8746264.
Faustino AC, Viani GA, Hamamura AC. Patterns of recurrence and outcomes of glioblastoma multiforme treated with chemoradiation and adjuvant temozolomide. Clinics. 2020;75: e1553. https://doi.org/10.6061/clinics/2020/e1553.
Śledzińska P, Bebyn MG, Furtak J, et al. Prognostic and predictive biomarkers in gliomas. Int J Mol Sci. 2021;22(19):10373. https://doi.org/10.3390/ijms221910373.
Zhang M, Lu XL, Wei CR, et al. Association between αβ and γδT-cell subsets and clinicopathological characteristics in patients with breast cancer. Oncol Lett. 2020;20:3251–8. https://doi.org/10.3892/ol.2020.12188.
Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10(7):467–78. https://doi.org/10.1038/nri2781.
Chen ZW, Zhao YJ, Wang KQ. γδT cells: in-vitro expansion and its use in tumor therapy. Chin J Biomed Eng. 2022;28(1):98–104. https://doi.org/10.3760/cma.j.cn115668-20200616-00154.
Caccamo N, La Mendola C, Orlando V, Meraviglia S, Todaro M, Stassi G, et al. Differentiation, phenotype, and function of interleukin-17-producing human Vgamma9Vdelta2 T cells. Blood. 2011;118(1):129–38. https://doi.org/10.1182/blood-2011-01-331298.
Caccamo N, Meraviglia S, Ferlazzo V, et al. Differential requirements for antigen or homeostatic cytokines for proliferation and differentiation of human Vgamma9Vdelta2 naive, memory and effector T cell subsets. Eur J Immunol. 2005;35(6):1764–72. https://doi.org/10.1002/eji.200525983.
Mangan BA, Dunne MR, O’Reilly VP, et al. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vδ3 T cells. J Immunol. 2013;191(1):30–4. https://doi.org/10.4049/jimmunol.1300121.
Harly C, Peyrat MA, Netzer S, Déchanet-Merville J, Bonneville M, Scotet E. Up-regulation of cytolytic functions of human Vδ2-γ T lymphocytes through engagement of ILT2 expressed by tumor target cells. Blood. 2011;117(10):2864–73. https://doi.org/10.1182/blood-2010-09-309781.
Pang DJ, Neves JF, Sumaria N, Pennington DJ. Understanding the complexity of gammadelta T-cell subsets in mouse and human. Immunology. 2012;136(3):283–90. https://doi.org/10.1111/j.1365-2567.2012.03582.x.
Jensen KD, Su X, Shin S, et al. Thymic selection determines gammadelta T cell effector fate: antigen-native cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29(1):90–100. https://doi.org/10.1016/j.immuni.2008.04.022.
Conroy MJ, Mac Nicholas R, Taylor M, O’Dea S, Mulcahy F, Norris S, Doherty DG. Increased frequencies of circulating IFN-γ-producing Vδ1+ and Vδ2+γδT cells in patients with asymptomatic persistent hepatitis B virus infection. Viral Immunol. 2015;28(4):201–8. https://doi.org/10.1089/vim.2014.0133.
Umemura M, Yahagi A, Hamada S, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary mycobacterium bovisbacille Calmette-Guerin infection. J Immunol. 2007;178(6):3786–96. https://doi.org/10.4049/jimmunol.178.6.3786.
Okamoto Yoshida Y, Umemura M, Yahagi A, et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol. 2010;184(8):4414–22.
SilVa-Santos B. Promoting angiogenesis within the tumor microenvironment: the secret life of murine lymphoid IL-17-producing gammadelta T cells. Eur J Immunol. 2010;40(7):1873–6.
Wu P, Wu D, Ni C, et al. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40(5):785–800.
Agerholm R, Bekiaris V. Evolved to protect, designed to destroy: IL-17-producing γδ T cells in infection, inflammation, and cancer. Eur J Immunol. 2021;51:2164–77. https://doi.org/10.1002/eji.202049119.
Li X, Kang N, Zhang X, Dong X, Wei W, Cui L, Ba D, He W. Generation of human regulatory gammadelta T cells by TCR gammadelta stimulation in the presence ofTGF-beta and their involvement in the pathogenesis of systemiclupus erythematosus. J Immunol. 2011;186(12):6693–700. https://doi.org/10.4049/jimmunol.1002776.
Ye J, Ma C, Hsueh EC, et al. Tumor-derivedγδregulatory T cells suppress innate and adaptive immunity through the induction ofimmunosenescence. J Immunol. 2013;190(5):2403–14. https://doi.org/10.4049/jimmunol.1202369.
Hu Y, Cui Q, Gu Y, et al. Decitabine facilitates the generation and immunosuppressive function of regulatory γδT cells derived fromhuman peripheral blood mononuclear cells. Leukemia. 2013;27(7):1580–5. https://doi.org/10.1038/leu.2012.345.
Gong GG, Shao LY, Wang YQ, Chen CY, Huang D, Yao SY, Zhan XM, Sicard H, Wang R, Chen ZW. Phosphoantigen-activated V gamma 2V delta 2 T cells antagonize IL-2-induced CD4+CD25+Foxp3+T regulatory cells in mycobacterial infection. Blood. 2009;113:837–45. https://doi.org/10.1182/blood-2008-06-162792. (Epub 2008 Nov 3).
Kühl AA, Pawlowski NN, Grollich K, et al. Human peripheral γδT cells possess regulatory potential. Immunology. 2009;128:580–8. https://doi.org/10.1111/j.1365-2567.2009.03162.
Poonia B, Pauza CD. Gamma delta T cells from HIV+ donors can be expanded in vitro by zoledronate/interleukin-2 to become cytotoxic effectors for antibody-dependent cellular cytotoxicity. Cytotherapy. 2012;14(2):173–81. https://doi.org/10.3109/14653249.2011.623693.
Khatri M, Dwivedi V, Krakowka S, et al. Swine influenza H1N1 virus induces acute inflammatory immune responses in pig lungs: a potential animal model for human H1N1 influenza virus. J Virol. 2010;84(21):11210–8. https://doi.org/10.1128/JVI.01211-10.
Wen MJ, Liu M, Zhang XL, Cao B. Distribution of γδT17/Th17/Tc17 cells in lung of H1N1 infected mice and their relationship with immunologic injury of lung. Chin J Immunol. 2017;33(6):563–8. https://doi.org/10.3969/j.issn.1000-484X.2017.04.018.
Sánchez Martínez D, Tirado N, Mensurado S, Martínez-Moreno A, Romecín P, Gutiérrez Agüera F, et al. Generation and proof- of- concept for allogeneic CD123 CAR-delta one T (DOT) cells in acute myeloid leukemia. J Immunother Cancer. 2022;10(9): e005400. https://doi.org/10.1136/jitc-2022-005400.
Ang WX, Ng YY, Xiao L, Chen C, Li Z, Chi Z, et al. Electroporation of NKG2D RNA CAR improves Vgamma9Vdelta2 T cell responses against human solid tumor xenografts. Mol Ther Oncolytics. 2020;17:421–30. https://doi.org/10.1016/j.omto.2020.04.013.
Rozenbaum M, Meir A, Aharony Y, Itzhaki O, Schachter J, Bank I, et al. Gamma–delta CAR-T-cells show CAR-directed and independent activity against leukemia. Front Immunol. 2020;11:1347. https://doi.org/10.3389/fimmu.2020.01347.
Makkouk A, Yang XC, Barca T, Lucas A, Turkoz M, Wong JTS, et al. Off-the-shelf Vdelta1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J Immunother Cancer. 2021;9(12): e003441. https://doi.org/10.1136/jitc-2021-003441.
Nakajima J, Murakawa T, Fukami T, et al. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous γδT cells. Eur J Cardio Thorac Surg. 2010;37(5):92–103. https://doi.org/10.1016/j.ejcts.2009.11.051.
Beck BH, Kim HG, Kim H, et al. Adoptively transferred ex vivo expanded γδT cells mediate in vivo antitumor activity in preclinical mouse models of breast cancer. Breast Cancer Res Treat. 2010;122(1):135–44. https://doi.org/10.1007/s10549-009-0527-6.
Wen K, Bui T, Li G, et al. Characterization of immune modulating functions of γδT cell subsets in a gnotobiotic pig model of human rotavirus infection. Comp Immunol Microbiol Infect Dis. 2012;35(4):289–301. https://doi.org/10.1016/j.cimid.2012.01.010.
Nedellec S, Sabourin C, Bonneville M, Scotet E. NKG2D costimulates human Vγ9Vδ2 T cell antitumor cytotoxicity through protein kinase C theta-dependent modulation of early TCR-induced calcium and transduction signals. J Immunol. 2010;185(1):55–63. https://doi.org/10.4049/jimmunol.1000373.
Li Z. Potential of human γδT cells for immunotherapy of osteosarcoma. Mol Biol Rep. 2013;1:132–40.
Hanagiri T, Shigematsu Y, Kuroda K, et al. Antitumor activity of human γδT cells transducted with CD 8 and with T-cell receptors of tumor-specific cytotoxic T lymphocytes. Cancer Sci. 2012;103(8):232–9. https://doi.org/10.1111/j.1349-7006.2012.02337.x.
Cobbs CS, Soroceanu L, Denham S, et al. Human cytomegalovirus induces cellular tyrosine kinase signaling and promotes glioma cell invasiveness. J Neurooncol. 2007;85(3):271–80. https://doi.org/10.1007/s11060-007-9423-2.
Marlin R, Pappalardo A, Kaminski H, et al. Sensing of cell stress by human gammadelta TCR-dependent recognition of annexin A2. Proc Natl Acad Sci USA. 2017;114(12):3163–8. https://doi.org/10.1073/pnas.1621052114.
Day BW, Stringer BW, Boyd AW. Eph receptors as therapeutic targets in glioblastoma. Br J Cancer. 2014;111(7):1255–61. https://doi.org/10.1038/bjc.2014.73.
Jarry U, Chauvin C, Joalland N, et al. Stereotaxic administrations of allogeneic human Vgamma9Vdelta2 T cells efficiently control the development of human glioblastoma brain tumors. Onco Targets Ther. 2016;5(6): e1168554. https://doi.org/10.1080/2162402X.2016.1168554.
Chitadze G, Kabelitz D. Immune surveillance in glioblastoma: role of the NKG2D system and novel cell- based therapeutic approaches. Scand J Immunol. 2022;96: e13201. https://doi.org/10.1111/sji.13201.
Qin G, Mao H, Zheng J, et al. Phosphoantigen-expanded human γδT cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J Infect Dis. 2009;200:858–65. https://doi.org/10.1086/605413.
Kubota K. Innate IFN-γ production by subsets of natural killer cells, natural killer T cells and γδT cells in response to dying bacterial-infected macrophages. Scand J Immunol. 2010;71:199–209. https://doi.org/10.1111/j.1365-3083.2009.02366.x.
Wu Y, Wu W, Wong WM, Ward E, Thrasher AJ, Goldblatt D, Osman M, Digard P, Canaday DH, Gustafsson K. Human γδT cells: a lymphoid lineage cell capable of professional phagocytosis. J Immunol. 2009;183(9):5622–9. https://doi.org/10.4049/jimmunol.0901772.
Puttur FK, Fernandez MA, White R, Roediger B, Cunningham AL, Weninger W, Jones CA. Herpes simplex virus infects skin γδT cells before Langerhans cells and impedes migration of infected Langerhans cells by inducing apoptosis and blocking E-cadherin downregulation. J Immunol. 2010;185(1):477–87. https://doi.org/10.4049/jimmunol.0904106.
Chodaczek G, Papanna V, Zal MA, Zal T. Erratum: Body-barrier surveillance by epidermal γδTCRs. Nat Immunol. 2012;13(3):272–82. https://doi.org/10.1038/ni.2240.
Belghali MY, El Moumou L, Hazime R, Brahimi M, El Marrakchi M, Belaid HA, et al. Phenotypic characterization of human peripheral γδT-cell subsets in glioblastoma. Microbiol Immunol. 2022;66(10):465–76. https://doi.org/10.1111/1348-0421.13016.
Yue CB, Yang K, Wang QD, Hu FX, Zhao SM, Liu SQ. γδT cells in peripheral blood of glioma patients. Med Sci Monit. 2018;24:1784–92. https://doi.org/10.12659/MSM.905932.
Bryant NL, Suarez-Cuervo C, Gillespie GY, Markert JM, Nabors LB, Meleth S, Lopez RD, Lamb LS. Characterization and immunotherapeutic potential of γδT-cells in patients with glioblastoma. Neuro Oncol. 2009;11(4):357–67. https://doi.org/10.1215/15228517-2008-111.
Lee M, Park C, Woo J, Kim J, Kho I, Nam D-H, Park W-Y, Kim Y-S, Kong D-S, Lee HW, Kim TJ. Preferential infiltration of unique Vγ9Jγ2-Vδ2 T cells into glioblastoma multiforme. Front Immunol. 2019;10:555. https://doi.org/10.3389/fimmu.2019.00555.
Dunne MR, Mangan BA, Madrigal-Estebas L, Doherty DG. Preferential Th1 cytokine profile of phosphoantigen-stimulated human Vgamma9Vdelta2 T cells. Mediators Inflamm. 2010;2010: 704941. https://doi.org/10.1155/2010/704941.
Caccamo N, Todaro M, Sireci G, Meraviglia S, Stassi G, Dieli F. Mechanisms underlying lineage commitment and plasticity of human gammadelta T cells. Cell Mol Immunol. 2013;10(1):30–4. https://doi.org/10.1038/cmi.2012.42.
Casetti R, Agrati C, Wallace M, Sacchi A, Martini F, Martino A, et al. Cutting edge: TGF-beta1 and IL-15 induce FOXP3+ gammadelta regulatory T cells in the presence of antigen stimulation. J Immunol. 2009;183:3574–7. https://doi.org/10.4049/jimmunol.0901334.
Nakazawa T, Nakamura M, Park YS, Motoyama Y, Hironaka Y, Nishimura F, et al. Cytotoxic human peripheral blood-derived γδT cells kill glioblastoma cell lines: implications for cell-based immunotherapy for patients with glioblastoma. J Neurooncol. 2014;116:31–9. https://doi.org/10.1007/s11060-013-1258-4.
Bryant NL, Gillespie GY, Lopez RD, Markert JM, Cloud GA, Langford CP, et al. Preclinical evaluation of ex vivo expanded/activated γδT cells for immunotherapy of glioblastoma multiforme. J Neurooncol. 2011;101:179–88. https://doi.org/10.1007/s11060-010-0245-2.
Bryant NA, Rash AS, Woodward AL, Medcalf E, Helwegen M, Wohlfender F, et al. Isolation and characterisation of equine influenza viruses (H3N8) from Europe and North America from 2008 to 2009. Vet Microbiol. 2011;147(1–2):19–27. https://doi.org/10.1016/j.vetmic.2010.05.040.
Cherry ABC, Gherardin NA, Sikder HI. Intracellular radar: understanding γδT cell immune surveillance and implications for clinical strategies in oncology. Front Oncol. 2022;12:1011081. https://doi.org/10.3389/fonc.2022.1011081.
Poggi A, Carosio R, Fenoglio D, Brenci S, Murdaca G, Setti M, et al. Migration of V delta 1 and V delta 2 T cells in response to CXCR3 and CXCR4 ligands in healthy donors and HIV-1-infected patients: competition by HIV-1 Tat. Blood. 2004;103:2205–13. https://doi.org/10.1182/blood-2003-08-2928.
Correia DV, Lopes A, Silva-Santos B. Tumor cell recognition by gammadelta T lymphocytes: T-cell receptor vs. NK-cell receptors. Oncoimmunology. 2013;2(1): e22892. https://doi.org/10.4161/onci.22892.
Kong Y, Cao W, Xi X, Ma C, Cui L, He W. The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces cytotoxicity to tumor cells through both TCR gammadelta and NKG2D. Blood. 2009;114:310–7. https://doi.org/10.1182/blood-2008-12-196287.
Dai Y, Chen H, Mo C, Cui L, He W. Ectopically expressed human tumor biomarker MutS homologue 2 is a novel endogenous ligand that is recognized by human gammadelta T cells to induce innate anti-tumor/virus immunity. J Biol Chem. 2012;287(20):16812–9. https://doi.org/10.1074/jbc.M111.327650.
Vantourout P, Mookerjee-Basu J, Rolland C, Pont F, Martin H, Davrinche C, et al. Specific requirements for Vgamma9Vdelta2 T cell stimulation by a natural adenylated phosphoantigen. J Immunol. 2009;183(6):3848–57. https://doi.org/10.4049/jimmunol.0901085.
Cimini E, Piacentini P, Sacchi A, Gioia C, Leone S, Lauro GM, et al. Zoledronic acid enhances Vδ2T-lymphocyte antitumor response to human glioma cell lines. Int J Immunopathol Pharmacol. 2011;24(1):139–48. https://doi.org/10.1177/039463201102400116.
Nakazawa T, Nakamura M, Matsuda R, Nishimura F, Park YS, Motoyama Y, et al. Antitumor effects of minodronate, a third-generation nitrogen-containing bisphosphonate, in synergy with gammadelta T cells in human glioblastoma in vitro and in vivo. J Neurooncol. 2016;129:231–41. https://doi.org/10.1007/s11060-016-2186-x.
Lamb LS. γδT cells as immune effectors against high-grade gliomas. Immunol Res. 2009;45:85–95. https://doi.org/10.1007/s12026-009-8114-9.
Gober HJ, Kistowska M, Angman L, Jenö P, Mori L, De Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197(2):163–8. https://doi.org/10.1084/jem.20021500.
Oizel K, Chauvin C, Oliver L, Gratas C, Geraldo F, Jarry U, et al. Efficient mitochondrial glutamine targeting prevails over glioblastoma metabolic plasticity. Clin Cancer Res. 2017;23(20):6292–304. https://doi.org/10.1158/1078-0432.CCR-16-3102.
Joalland N, Chauvin C, Oliver L, Vallette FM, Pecqueur C, Jarry U, Scotet E. IL-21 increases the reactivity of allogeneic human Vγ9Vδ2 T cells against primary glioblastoma tumors. J Immunother. 2018;41(5):224–31. https://doi.org/10.1097/CJI.0000000000000225.
Chitadze G, Lettau M, Luecke S, Wang T, Janssen O, Fürst D, et al. NKG2D- and T-cell receptor-dependent lysis of malignant glioma cell lines by human γδT cells: modulation by temozolomide and A disintegrin and metalloproteases 10 and 17 inhibitors. OncoImmunology. 2015;5(4): e1093276. https://doi.org/10.1080/2162402X.2015.1093276.
Flüh C, Chitadze G, Adamski V, Hattermann K, Synowitz M, Kabelitz D, et al. NKG2D ligands in glioma stem-like cells: expression in situ and in vitro. Histochem Cell Biol. 2018;149:219–33.
Lamb LS Jr, Bowersock J, Dasgupta A, Gillespie GY, Su Y, Johnson A, Spencer HT. Engineered drug resistant gammadelta T cells kill glioblastoma cell lines during a chemotherapy challenge: a strategy for combining chemo- and immunotherapy. PLoS ONE. 2013;8(1): e51805. https://doi.org/10.1371/journal.pone.0051805.
Lamb LS, Pereboeva L, Youngblood S, Gillespie GY, Nabors LB, Markert JM, et al. A combined treatment regimen of MGMT-modified γδT cells and temozolomide chemotherapy is effective against primary high grade gliomas. Sci Rep. 2021;11(1):21133. https://doi.org/10.1038/s41598-021-00536-8.
Chauvin C, Joalland N, Perroteau J, Jarry U, Lafrance L, Willem C, et al. NKG2D controls natural reactivity of Vgamma9Vdelta2 T lymphocytes against mesenchymal glioblastoma cells. Clin Cancer Res. 2019;25:7218–28. https://doi.org/10.1158/1078-0432.CCR-19-0375.
Funding
This research was supported by the National Natural Science Foundation of China (Grant Numbers 82274538 and 81473687),the Natural Science Foundation of Shandong Province (Grant Numbers ZR2020MH312 and ZR2020MH357), and the Tai’an Science and Technology Plan (Grant Number 2020NS129).
Author information
Authors and Affiliations
Contributions
YZ, RZ and YW wrote the main manuscript text and KW prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Data availability
No data were involved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Y., Zhu, R., Wang, Y. et al. Classification and function of γδT cells and its research progress in anti-glioblastoma. Discov Onc 14, 150 (2023). https://doi.org/10.1007/s12672-023-00770-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-023-00770-8