Abstract
Given our previous findings that human cytomegalovirus (HCMV) nucleic acids and proteins are expressed in human malignant glioma in vivo, we investigated cellular signaling events associated with HCMV infection of human glioma and astroglial cells. HCMV infection caused rapid activation of the phosphatidylinositol-3 kinase (PI-3K) effector AKT kinase in human astro-glial and fibroblast cells, and induced tyrosine phosphorylation of phospholipase Cγ (PLCγ). Co-immunoprecipitation experiments revealed association of the p85 regulatory subunit of PI-3K with a high-molecular weight protein phosphorylated on tyrosine, following short-term exposure to HCMV. In contrast to a previous report, we were unable to confirm the identity of this high-molecular weight protein as being the epidermal growth factor receptor (EGFR). Stimulation of glioma and fibroblast cell lines over-expressing EGFR with HCMV (whole virus) or soluble glycoprotein B did not induce tyrosine phosphorylation of the receptor, as did the genuine ligand, EGF. Furthermore, we found that expression levels of the human ErbB1-4 receptors were not rate-limiting for HCMV infection. Dispensability of EGFR function during early HCMV infection was substantiated by demonstration of viral immediate early gene expression in cells lacking the EGFR gene, indicating that HCMV may promote oncogenic signaling pathways independently of EGFR activation. Among non-receptor cellular kinases, HCMV infection induced phosphorylation of focal adhesion kinase (FAK) Tyr397, which is indispensable for integrin-mediated cell migration and invasion. HCMV-induced FAK activation was paralleled by increased extracellular matrix-dependent migration of human malignant glioma but not normal astro-glial cells, suggesting that HCMV can selectively augment glioma cell invasiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human herpesvirus 5 [human cytomegalovirus (HCMV)] is ubiquitous worldwide, and persistent infection occurs in over 70% of adults. Association of HCMV infection with several human malignancies, including brain, prostate, and colon cancer has been reported [1], suggesting a potential role for HCMV in oncogenesis. We and others have recently demonstrated that HCMV is present in over 90% of human malignant gliomas [2, 3], while no viral gene products were detectable in the non-malignant brain tissue. These findings have important implications for glioma biology, since accumulating evidence indicates that HCMV viral gene products can alter signaling pathways underlying cellular apoptosis, proliferation, migration, and transformation [4–7]. For example, transcriptional activation of cellular oncogenes, including c-FOS, c-MYC, and c-JUN is induced by HCMV exposure [8], reminiscent of growth factor-mediated signaling events [9]. Intriguingly, this activation does not require viral infectivity nor de novo viral protein synthesis, suggesting that interaction of the virus with cell surface proteins elicits fast activation of cytoplasmic signaling cascades [8]. In efforts to exert cell cycle control and inhibit apoptosis, DNA viruses have acquired during evolution the capacity to subvert cellular signaling pathways, most notably by activation of the PI3-K/AKT axis, or interference with p53 and Rb cell cycle control functions [10, 11]. For example, activation of the phosphatidylinositol-3 kinase (PI-3K)/AKT pathway is central to the ability of human herpesvirus 4 [Epstein-Barr virus (EBV)] to establish viral latency and to induce transformation of B cells and of the oropharyngeal epithelium, leading to nasopharyngeal carcinoma [10, 12]. These data suggest that other members of the herpes virus family may utilize a similar mechanism, possibly engaging cellular receptors and promoting oncogenesis by modulating the PI3K-AKT pathway.
Given previous reports of epidermal growth factor receptor (EGFR) representing a critical cellular target for HCMV infection and initiation of downstream signaling [13–16], and considering the importance of EGFR signaling in malignant glioma biology, we sought to investigate early cellular signaling events associated with HCMV infection of astro-glial and glioma cells. Here we show that HCMV infection leads to prompt induction of cellular tyrosine kinase signaling, including tyrosine phosphorylation of a ∼180 kDa protein, associated with the p85 regulatory subunit of PI3-K, and distinct from EGFR. Additional oncomodulatory viral properties are supported by our data showing that short-term HCMV exposure induced recruitment of phospholipase Cγ (PLCγ) and FAK activation, while persistent HCMV infection promoted extracellular matrix-dependent migration of malignant glioma cells, but not normal astrocytes. Taken together, our data provide novel insights into HCMV-induced signaling pathways that modulate glioma cell growth, survival, and invasiveness.
Materials and methods
Cell lines and recombinant expression
Human embryonic lung fibroblasts (HEL) and U87 glioblastoma were obtained from ATCC. Intestinal fibroblasts of mice homozygous for targeted deletion of a functional EGFR gene (EGFR-/-) had been extensively characterized [17] and were obtained from Robert Whitehead. NR6 fibroblasts representing an EGFR-negative clone of NIH3T3 [18, 19] were provided by Harvey Herschman. Immortalized human astrocytes have previously been described [20]. U251 glioblastoma and RK3E epithelial cell lines [21] were kindly provided by G. Yancey Gillespie and J. Michael Ruppert, respectively. MDA-MB468 human breast carcinoma [22], NIH3T3 [23] and recombinant derivatives expressing human ErbB receptors including LTR-EGFR, LTR-ErbB2, LTR-ErbB3, LTR-ErbB4, and LTR-ErbB3 + ErbB2 have previously been characterized [24–27]. Recombinant human EGFR expression in RK3E was accomplished by retroviral transduction. Human EGFR open reading frame was cloned in SalI of pBABE-puro [28] LTR-based expression vector. Ten microgram BABE-EGFR or BABE-puro were transfected in BOSC23 packaging cell line using standard calcium phosphate technique [29] to obtain high-titer helper virus-free infectious particles. Selected mass populations of RK3E consisting of more than 100 individual colonies (cfu) were obtained following infection with similar titers of BABE-puro or BABE-EGFR retrovirus and puromycin selection at 2.5 μg/ml.
HCMV production
Human cytomegalovirus strains AD169 and Towne (ATCC) were propagated in human embryonic lung fibroblasts (HEL cells) for less than five passages. HEL cells were infected at a multiplicity of infection (MOI) of 1 and and virus collected over a period of 5–7 days following infection in serum free medium. Virus was pelleted by sucrose gradient centrifugation at 80,000 g and 4° and resuspended in DMEM. Mock controls were generated in parallel by conditioning and processing uninfected cultures identically. Virus titers were estimated by IE1 immunohistochemical staining. Recombinant, soluble glycoprotein B (S-gB680) from strain AD169, which was generated by truncation at codon 680 was kindly provided by Don J. Diamond and Zhongde Wang [30].
Immunoprecipitation and immunoblotting
Immunoprecipitation and Immunoblot analyses were conducted as previously reported [22, 26, 27]. Briefly, for protein-specific immunoprecipitation cell lysates were prepared in StaphA buffer containing 10 mM phosphate buffer 7.4, 0.1 M NaCl, 5 mM EGTA, 0.5% deoxycholate, 1% Triton, 0.1% SDS, 1 mM PMSF, 10 μg/ml aprotinin. Co-immunoprecipitation experiments were conducted on fresh protein lysates in mild detergent conditions preserving protein associations in vivo and containing 20 mM Tris–HCl 7.5, 100 mM NaCl, 5 mM EGTA, 1% NP40 as well as protease and phosphatase inhibitors [24]. For immunoprecipitation using uncoupled murine MAb except EGFR AB-1, protein G agarose was precoated with goat anti-mouse IgG secondary antibody, whereas immune complexes of AB-1 or rabbit polyclonal antibodies were directly precipitated. In immunoblots, bound antibodies were visualized by chemiluminescence using horseradish peroxidase-coupled secondary goat antibodies (Bio-Rad, Pierce, Rockford, IL, USA) and developed using ECL (Amersham, Piscataway, NJ, USA) or West Femto (Pierce). Antibody concentrations were adjusted according to manufacturers’ recommendation. Commercial antibody sources included Santa Cruz Biotechnology: AKT (sc-8312), pAKT (sc-16646-R), anti-phosphotyrosine pY99 and agarose conjugate (sc-7020 and sc-7020AC), pY576FAK (sc-16563-R), pY576/577FAK (sc-21831-R), pY397FAK (sc-11765-R), pS722FAK (sc-16662-R); Novocastra: NCL-CMVpp65; Chemicon: HCMV-IE1 (MAb810), FAK (MAb2156); Cell Signaling: pAKT (244F9); Upstate: p85 PI-3K (UBI 06-195), PLCγ (UBI 05-163), FAK (UBI 06-543); ICN: anti-phosphotyrosine (pY20); Oncogene Sciences: EGFR MAb (AB-1). ErbB receptor-specific peptide antisera that have been previously described [24–27] were used at dilutions up to 1:50,000 for chemiluminescent detection.
EGFR genotyping
Genomic DNA was purified by organic solvent extraction and ethanol precipitation. The EGFR genotype was confirmed in NIH3T3 and EGFR-/- fibroblasts by PCR as previously reported [25]. Allele-specific fragments of normal EGFR (350 bp) and targeted deletion EGFRtm1Mag (450 bp) were concurrently amplified using 100 ng genomic DNA and a mixture of three specific primers at 20 μM each: 5′-GCC CTG CCT TTC CCA CCA TA-3′, 5′-TTG CAG CAC ATC CCC CTT TC-3′ and 5′-ATC AAC TTT GGG AGC CAC AC-3′. PCR conditions were 3 min at 94°, 12 cycles: 20 s at 94°, 30 s at 64°–0.5°/cycle, 35 s at 72°, 25 cycles: 20 s at 94°, 30 s at 58°, 35 s at 72° followed by 2 min final extension at 72°. A 351 bp fragment of glyceraldehyde-3 phosphate dehydrogenase (GAPDH) was amplified as reference gene control under similar conditions using the following primers: 5′-TCT TGT GCA GTG CCA GCC T-3′ and 5′-GCC TTC TCC ATG GTG GTG AA-3′. PCR products were electrophoresed on 1.2% agarose gels and visualized by ethidium bromide staining.
Migration assay
Transwell-24 plates (Corning) with insets holding an 8 μm pore membrane were used to determine haptotactic migration. Membrane was coated with BSA or different extracellular matrix proteins (10 μg/ml). Following blocking of wells and filters in 1% BSA, 4 × 104 cells infected or mock-treated for 90 min 24 h earlier were seeded in each inset and allowed to migrate 5 h at 37°. Cells migrated to the bottom of the membrane were fixed and stained. Experimental conditions were tested in triplicate. Migrated cells were counted in 3 representative 1 mm2 areas of each well employing a hemocytometer grid and inverted microscope.
Results
HCMV induces rapid activation of PI3K-AKT signaling in human astrocytes
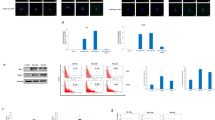
Given the documented presence of HCMV gene products in over 90% of human GBM samples [2, 3], we tested the susceptibility of human astrocytes to HCMV infection in vitro. For kinetic analysis, we used immortalized normal human astrocytes (NHA) [20]. NHAs were serum-starved for 48 h and exposed to HCMV at MOI of 1 for increasing periods of time, from 10 min to 48 h, prior to preparation of protein lysates. Since the structural pp65 HCMV tegument protein is detectable prior to viral gene transcription (due to virus entry into the cell) and its sustained expression requires de novo viral gene transcription, we monitored pp65 protein levels by immunoblot analysis of cell lysates up to 48 h following infection. As illustrated in Fig. 1, pp65 protein was readily detected 10 min following infection and protein levels were increased at 30 min in HCMV (Towne)-infected samples. At 2 and 6 h, pp65 detectable protein receded to levels observed at 10 min, followed by increasing levels (indicating de novo protein expression) at 24 and 48 h of infection. Detection of the HCMV immediate early protein (IE-1) p72 marks the onset of viral gene expression. IE-1 protein expression commenced 2–6 h following HCMV infection of human astrocytes, while the p80 splice variant (IE2) appeared at 48 h (Fig. 1A). Thus, the kinetic profiles of pp65 structural, as well as p72 and p80 immediate early viral protein expression confirmed the susceptibility of NHAs to HCMV infection and were similar to those measured in human fibroblasts, the most widely explored host cell [31, 32].
Rapid activation of PI 3-K effector AKT kinase by HCMV in human astro-glial cells and embryonic lung fibroblasts. (A) Time course of HCMV infection and AKT induction in NHAs; cells were serum-starved and mock treated (M) or infected with HCMV Towne strain (T). Immunoblot analysis shows the presence of HCMV pp65 protein 10 min following stimulation, while IE1/IE2 viral gene products become detectable 6 h following stimulation. pT308-AKT reaches maximum levels at 10 min following HCMV stimulation reminiscent of growth-factor-induced signaling. (B) Short-term (10 min) Towne triggering of HEL cells results in phosphorylation of AKT to a similar extent as the p-AKT induced by EGF
We next investigated the effects of HCMV infection on the PI3K downstream effector, AKT, in NHAs. Immunoblot analysis using a phosphor-specific antibody shows increased AKT activation between 10 min and 2 h following HCMV treatment of NHAs (Fig. 1A, third panel), while total cellular AKT levels remained constant throughout 48 h, in both Towne- and mock-treated cells.
To determine whether HCMV infection induces AKT activation in other cell types, we subjected HEL cells to short-term HCMV triggering in serum-free conditions; EGF and serum were used as positive control stimuli. As depicted in Fig. 1B (upper panel), 10 min exposure of serum-starved HEL cells to HCMV Towne (MOI-5) resulted in induction of AKT phosphorylation relative to mock treatment. The extent of AKT signal induction by HCMV appeared similar to that of 17 nM EGF and slightly stronger than the one elicited by 10% fetal bovine serum. Subsequent blotting of the membrane with an anti-EGFR polyclonal antibody revealed comparable levels of endogenous EGFR across all samples (Fig. 1B, lower panel). These findings demonstrate that prompt induction of AKT phosphorylation is a common property of HCMV infection, shared by different cell types, and indicate that HCMV exposure activates the cellular PI3K-AKT signaling pathway in a similar manner to the activation induced by growth factors binding to their cognate receptors.
HCMV induces tyrosine phosphorylation of a ∼180 KDa cellular protein and recruitment of the PI-3K and PLCγ signaling pathways
To obtain direct evidence for activation of cellular tyrosine kinase signaling by HCMV, we conducted co-immunoprecipitation experiments based on the high-binding affinity of PI-3K regulatory subunit p85 Src-homology-2 (SH-2) domains to sequence-specific, phosphotyrosine-containing epitopes of proteins recruited and activated upon stimulation [26]. Serum-starved HEL fibroblasts were mock treated or exposed to HCMV strains Towne and AD169 (MOI-5), for 10 min at 37°C. Cell lysates were immunoprecipitated using an antibody specific for the p85 regulatory subunit of PI-3K. Western blot analysis using an anti-pTyr antibody demonstrated considerable tyrosine phosphorylation of a protein in the 180–200 kDa range which became associated with p85 upon infection with either of the two HCMV strains (Fig. 2A, left panel). Direct immunoblotting of total cell lysates revealed a tyrosine phosphorylated protein of similar size present only the samples treated with HCMV (Fig. 2A, lysate lanes labeled T, A). Our data indicates that the 180–200 kDa protein was the principal moiety undergoing tyrosine phosphorylation upon infection with either Towne or AD169 strains of HCMV. When reversing co-immunoprecipitation using the anti-p85 antibody for immunoblot analysis, we noticed the presence of several cellular p-Tyr proteins associated with the PI3K regulatory subunit in the Towne-infected samples. Western blot of total cell lysates indicates that a fraction of cellular p85 co-immunoprecipitated with phosphotyrosine proteins, as p85 itself was not significantly phosphorylated on tyrosine (Fig. 2A, right panel). In addition, direct immunoblotting using anti-p85 antibody confirmed equivalent amounts of protein were present across all samples and demonstrated specificity of the p85 antiserum (Fig. 2A).
Recruitment of phospholipid substrate pathways PI-3K and PLCγ by HCMV-mediated activation of tyrosine kinase signaling. Serum-starved HEL cells were treated as indicated: M mock, T Towne, A AD169. (A) Immunoprecipitates using either anti-p85 antibody (UBI 06-195; left) or anti-phosphotyrosine MAb pY99 (sc-7020AC; right) were blotted using anti-pY99 (sc-7020; left panel) or anti-p85 (right panel), respectively. Towne and AD169 treated samples display the presence of a ∼180–200 kDa protein phosphorylated on Tyrosine, also present in the cell lysates (left panel). Several cellular p-Tyr proteins associated with p85 are detectable in the Towne-infected samples (right panel). (B) Four hundred μg fresh lysates were immunoprecipitated using anti-pY99 agarose conjugate and blotted using an anti-PLCγ MAb; association of PLCγ with cellular phosphotyrosine proteins is noticeable only in the Towne-infected samples
To assess whether additional phospholipid second messenger pathways of tyrosine kinase signaling became activated upon HCMV infection, we examined PLCγ. Anti-PLCγ immunoblot analysis of pTyr immunoprecipitates indicated association of PLCγ with cellular phosphotyrosine proteins upon HCMV infection, while direct anti-PLCγ immunoblotting confirmed similar PLCγ amounts in mock and HCMV treated fibroblasts (Fig. 2B). Taken together, these data demonstrate activation of cellular tyrosine kinase signaling and recruitment of the PI-3K, and PLCγ pathways upon short-term exposure to HCMV.
Lack of direct EGFR activation in vivo by HCMV
EGFR was reported to function as cellular receptor for HCMV, and its tyrosine kinase activity in vivo to be activated by HCMV with a kinetic and efficiency resembling that of its natural ligand [15]. We did not detect association of EGFR and p85 when re-probing the anti-pTyr blot of anti-p85 immunoprecipitates (shown in Fig. 2A, left panel) with EGFR peptide antiserum under conditions that distinctly visualized the 170 kDa EGFR product in the total cell lysates (Fig. 3A). Consequently, we investigated direct activation of EGFR upon short-term exposure to HCMV by measuring EGFR tyrosine phosphorylation as one of the most sensitive detection approaches for EGFR activity in vivo [26, 33, 34]. HEL cells were serum-starved and mock- or HCMV- (MOI ∼5) treated for 10 min at 37°C in serum-free conditions. Cell lysates were immunoblotted using anti-pTyr antibodies. An increase in tyrosine phosphorylation of a 180 kDa protein was noticed upon Towne infection in comparison to mock treatment (Fig. 3B), exceeding serum-dependent tyrosine phosphorylation of a similar size protein. EGF stimulation (17 nM) resulted in pronounced tyrosine phosphorylation of a 170 kDa protein. Immunoblot analysis of the anti-pTyr immunoprecipitates using anti-EGFR antibody identified the latter (∼170 kDa) as the phosphorylated EGFR. Furthermore, these data also demonstrated that the 180 kDa phosphotyrosine protein induced in HCMV-triggered HEL cells was distinct from EGFR (Fig. 3B). Within the time-frame of these experiments, we verified that Towne-infected cells expressed the pp65 HMCV protein, using Western blot analysis (Supplementary Fig. 1).
Lack of direct EGFR activation in vivo by HCMV or glycoprotein B. (A) Re-blotting of anti-p85 co-immunoprecipitates and lysates (Fig. 2 panel A) using EGFR peptide antiserum demonstrates the lack of association between p85 and EGFR, following either Towne or AD169 stimulation.(B) Cells were stimulated using HCMV Towne (MOI ∼5; T), EGF (E), and serum (S); lysates (200 μg) were immunoprecipitated using anti-pY20 phosphotyrosine MAb and evenly divided for immunoblot analysis using anti-pY20 and EGFR peptide antiserum E7. Only stimulation using EGF results in p-Tyr of EGFR, while Towne treatment induced p-Tyr of a ∼180–200 kDa protein, distinct from EGFR. (C) Immunoprecipitates of the EGFR extracellular domain were divided and subjected to immunoblot analysis using anti-pY20 (upper panel) and EGFR peptide antiserum E7 (lower panel). Again, only EGF stimulation (lane E) results in p-Tyr of EGFR, which is present at equivalent levels across all samples. (D) ErbB family members are not required for immediate early gene expression of HCMV. NIH3T3 fibroblasts over-expressing individual human ErbB receptors or an ErbB3-ErbB2 heterodimer [24–27] were mock treated (M) or infected (T) for 2 h. Protein lysates were subjected to immunoblotting using HCMV IE1 (Mab810) or ErbB receptor antibodies E7, M6, MK4, and E4 to confirm recombinant Erb1-4 over-expression in the respective transfectants [24–27]. Towne treatment resulted in robust IE1 expression on all the cell lines tested, including the NIH3T3 un-transfected, parental cells, demonstrating that human Erb1-4 receptors are not rate-limiting for HCMV infection. (E) HEL cells, NR6 cells representing an EGFR-negative derivative of NIH3T3 [18, 19], and fibroblasts from EGFR-/- mice [17] were mock treated (M) or infected with HCMV Towne (T) for 6 h (lane 4) or 24 h (lanes 2, 5, 7). IE1expression as determined by Western blot analysis using MAb810, shows that even in the absence of the EGFR gene, cells maintain their susceptibility to HCMV infection
We next analyzed EGFR-specific immunoprecipitates and total cell lysates by immunoblotting with anti-pTyr antibody (Fig. 3C). Direct immunoblotting of lysates with anti-pTyr illustrated in Fig. 3C, yielded similar results to those obtained upon enrichment of pTyr proteins by immunoprecipitation (Fig. 3B). In comparison to EGF treatment, HCMV Towne induced a subtle tyrosine phosphorylation of a protein in the 180–200 kDa range (Fig. 3C). Enrichment of EGFR by receptor-specific immunoprecipitation revealed activation of EGFR upon EGF stimulation, demonstrated by increased p-Tyr-EGFR levels (Fig. 3C, lanes 3 and 7, upper panel) relative to total EGFR (Fig. 3C, lanes 3 and 7, lower panel). The lack of phosphotyrosine proteins of similar molecular weight in the HCMV or serum treated samples clearly indicated that they were distinct from EGFR (Fig. 3C).
In additional experiments, we tested direct EGFR activation by HCMV in epithelial cells over-expressing the EGFR (BABE-EGFR) and in MDA-MB468 breast cancer cells known to harbor over-expression of normal human EGFR. Following serum starvation, cells were stimulated for 10 min at 37°C in serum-free medium with either EGF (17 nM), the HCMV virus or the purified HCMV glycoprotein B. While EGF caused potent tyrosine phosphorylation of the 170 kDa EGFR band in the BABE-EGFR and MDA-MB468 cells. Under these conditions, neither HCMV nor the HCMV glycoprotein B induced detectable EGFR tyrosine phosphorylation (Supplementary Fig. 2A). These data indicate that even at high EGFR expression levels facilitating more sensitive detection of EGFR activity, neither HCMV nor glycoprotein B was able to induce rapid activation of EGFR tyrosine kinase signaling.
We next tested the steady state activity of EGFR 24 h following infection of U251 human glioma cells, along with murine NIH3T3 fibroblasts and epithelial cells engineered to over-express human EGFR. Relative EGFR expression levels in various cell lines are shown by immunoblot analysis using EGFR peptide antibody (Supplementary Fig. 2B, lower panel). To attain maximal EGFR activation in different cell types, we exposed cells 10 min to 17 nM EGF (E). HCMV Towne (T) failed to induce EGFR-specific tyrosine phosphorylation 24 h following infection when compared to mock-treated cells. In contrast, robust receptor tyrosine kinase activity was elicited in vivo by EGF (Supplementary Fig. 2B). These data demonstrate that persistent HCMV infection fails to activate both endogenous EGFR in human glioma cells, as well as EGFR over-expressed in various cell types.
ErbB family members are not required for immediate early gene expression of HCMV
To test whether any of the human ErbB family members is required for HCMV infection of mammalian cells, we used NIH3T3 mouse fibroblasts engineered to stably over-express individual human ErbB receptors at high-receptor levels, including LTR-EGFR, LTR-ErbB2, LTR-ErbB3 or LTR-ErbB4 as well as an ErbB2-ErbB3 heterodimer (LTR-ErbB3 + ErbB2) [24–27]. Serum-starved cells were infected with Towne strain (T) or mock treated (M) for 2 h at 37°C in serum-free medium. HCMV IE1 protein was detected 12 h later in all Towne-infected but not mock treated NIH3T3 derivatives (Fig. 3D). IE1 expression did not appear up-regulated by over-expression of any of the four human ErbB receptors or the ErbB2-ErbB3 heterodimer (Fig. 3D). This finding was paralleled by indistinguishable morphologic appearance upon infection of control NIH3T3 and transfectants over-expressing the various human ErbB receptors, suggesting that HCMV infection and cytopathic effects were not dependent on the activity of any of the ErbB receptors tested.
To further explore EGFR receptor requirement during HCMV infection, NR6 cells, an EGFR-negative clonal derivative of NIH3T3 [18, 19] and intestinal fibroblasts derived from knockout mice homozygous for targeted deletion of the EGFR gene were subjected to HCMV infection at an MOI of ∼0.8. Effective infection of NR6 and EGFR-/- fibroblasts by HCMV Towne was demonstrated by expression of immediate early proteins IE1 and IE2 6 h (lane 4) and 24 h (lane 5) following virus exposure (Fig. 3E), indicating that mouse EGFR was not required for HCMV infection of rodent fibroblasts. By comparison to the actin loading control, immediate early gene expression appeared similar or possibly higher in EGFR-/- than in HEL cells (Fig. 3E). In concert, these observations unequivocally excluded requirement of EGFR function for HCMV immediate early gene expression.
HCMV induces activation of FAK by selective phosphorylation of Tyrosine 397
Based on the recent implication of integrins as cellular entry receptors for HCMV [13], we analyzed focal adhesion kinase (FAK), a cytoplasmic tyrosine kinase known to be involved in integrin-mediated transduction of signals emanating from the extracellular matrix [35]. Total FAK protein was immunoprecipitated from mock- or HCMV-treated cells and immunoblotted using anti-pTyr antibodies. A major 125 kDa tyrosine-phosphorylated band co-migrating with FAK was detected in immunoprecipitates of HCMV-infected but not mock-treated cells (Fig. 4A, left panel). Similar FAK expression level upon HCMV or mock treatment was confirmed by direct immunoblotting using total FAK antiserum (Fig. 4A, middle panel). These data demonstrated enhanced tyrosine phosphorylation of FAK in vivo, following short-term exposure to HCMV. To further substantiate these findings, we employed a set of phosphorylation site-specific antibodies directed toward pTyr397, pTyr576, pTyr576/577 or pSer722 of FAK, respectively. HCMV treatment caused increased FAK tyrosine phosphorylation in vivo at Tyr397 (Fig. 4A, right panel) and to a lesser extent at Tyr576/577, with marginal differences at Tyr576 (Supplementary Fig. 3). Serine phosphorylation at residue 722 appeared unaltered (Supplementary Fig. 3). Enhanced FAK tyrosine phosphorylation in vivo, especially around the consensus binding epitopes for Src and p85 SH2 domains surrounding Tyr397 implicate HCMV in activation of pathways responsible for cell motility and invasion.
HCMV induces FAK activation and enhances haptotactic migration of human glioblastoma cells. (A) Protein-specific immunoprecipitation of FAK (MAb2156) was conducted in the presence of ionic detergents using 100 μg lysates prepared in StaphA buffer. FAK immunoprecipitates were immunoblotted with pY20 phosphotyrosine MAb (left panel) and total cell lysates were analyzed by direct western blotting using a polyclonal FAK rabbit antibody (middle panel). Towne (T) treatment resulted in p-Tyr of FAK, as shown in the left panel. Immunoblot analysis using FAK phosphorylation-site specific antibody pY397 (right panel) revealed that HCMV(T)-induced p-Tyr of FAK detected by pY20 is mainly due to phosphorylation of Y397, essential for FAK-mediated cellular motility. (B) NHA and glioma cells (40,000/well) mock-treated (M) or HCMV-infected (T) were seeded in triplicate on inset membranes of Transwell-24 plates precoated with BSA, fibronectin (FN) or vitronectin (VN). Stained representative filter memebranes show cells that migrated within 5 h to the bottom of 8 μm pore membranes. (C) Number of migrated cells per mm2 (representing means of three representative 1 mm2 areas) counted for each of the triplicate wells (after background subtraction of cells migrated in the BSA condition) demonstrate that HCMV promotes U87 malignant glioma cell invasion, while inhibiting NHAs migration toward both VN and FN. (D) Immunoblot analysis (using MAb810) detected expression of HCMV IE1 (72 h following infection) in cells cultured in duplicate with those subjected to migration assays. M mock, T Towne; short (upper), and long (lower) film exposures demonstrate the presence of both IE1 and IE2 viral gene products in NHAs, while only IE1 is detectable in U87 glioma cells
HCMV infection enhances haptotactic migration of human glioblastoma cells
Given our findings that HCMV induces FAK activation, we next investigated whether HCMV can modulate glioma cell motility and invasion, which is a hallmark of these highly invasive tumors [13–15]. NHA immortalized human astrocytes and U87 glioma cells were exposed to sucrose-purified HCMV in serum-free DMEM (5 MOI) or mock infected for 90 min at 37°C. Subsequently, cells were maintained in growth medium and subjected to migration assays 24 h after infection. To determine haptotactic migration, 4 × 104 viable cells/well were allowed to migrate through 8 μm pore membranes of Transwell-24 plates toward the extracellular matrix proteins fibronectin or vitronectin for 5 h. Both non-malignant human astrocytes (NHA) and U87 glioblastoma cells revealed substantial migration toward fibronectin (FN) and vitronectin (VN) relative to 1% bovine serum albumin (BSA) serving as negative control that did not promote migration (Fig. 4B). Comparing mock (M) and HCMV-infected (T) cells, haptotactic migration appeared substantially altered by HCMV infection. HCMV inhibited migration of NHAs, while significantly promoting migration of U87 glioblastoma cells toward either substrate (Fig. 4B, C). As illustrated in Fig. 4C, mock-treated NHAs and U87 cells showed similar migration rates toward both fibronectin and vitronectin. However, while HCMV infection caused a reduction of approximately threefold in NHA migration, haptotactic migration was significantly increased (2.7-fold) by HCMV in U87 glioblastoma cells (Fig. 4C). To determine infection efficiency, we analyzed immediate early protein expression by Western blotting in the same cell populations seeded for migration. Seventy-two hours following infection, both NHA and U87 exhibited considerable IE1 expression (Fig. 4D). At 72 h post-infection, IE1 and IE2 showed similar prevalence in NHA, whereas IE1 was more abundant than IE2 in U87 glioma cells (Fig. 4D), suggesting possible differences in kinetics of infection or immediate early gene transcript processing between immortalized astro-glial and malignant glioma cells. Western blot analysis of lysates from NHA cells subjected to migration assays for 72 h did not reveal significant changes in the FAK pTyr397, levels (data not shown), most likely due to the short time course of FAK activation best captured within 10 min of stimulation as shown in Fig. 4A. These data indicate that, in addition to inducing rapid biochemical changes in cellular kinase activity, such as activation of FAK, sustained HCMV infection can selectively stimulate glioma cell invasion, and thus promote a more aggressive tumor phenotype.
Discussion
We and others have found a strong association between HCMV and malignant gliomas in vivo [2, 3, 36]. In efforts to delineate a possible role for HCMV in the neoplastic process, we investigated biological consequences of HCMV infection of immortalized and transformed astroglial cells. Here, we present evidence indicative of short-term induction of cellular tyrosine kinase signaling in human astro-glial cells, which is associated with recruitment of the PI-3K and PLCγ pathways akin to growth factor-mediated activation of intracellular signaling cascades. Both pathways have been implicated in the survival and proliferation of glioma cells, while PI-3K is also fundamental for inhibition of apoptosis [37, 38]. Our data documented activation of FAK kinase by phosphorylation on Tyr397, which represents a major SH2 domain binding site for the p85 regulatory subunit of PI3K. Thus, FAK activation might additionally contribute to short-term PI-3K recruitment upon HCMV infection. Since FAK represents an essential effector of integrin signaling [35], our observations are consistent with a previous report establishing a critical role for cellular integrins during virus attachment and entry [13] and warrant future studies toward identification of integrins that mediate HCMV-induced tyrosine kinase signaling specifically in human glioma cells.
Following HCMV exposure, we observed tyrosine phosphorylation of a high molecular weight protein that associated with the p85 regulatory subunit of PI-3K, which was distinct from EGFR. In stimulation experiments using either whole virus or HCMV gB, we did not detect HCMV-induced EGFR phosphorylation at efficiency comparable to the genuine ligand, EGF, in spite of documenting tyrosine kinase signaling, including PI-3K and PLCγ recruitment. This contrasts with earlier observations [14, 15] reporting EGFR as cellular receptor for HCMV and activation of EGFR signaling by HCMV resembling that elicited by EGF. Given the high percentage of human gliomas that display amplification and/or over-expression of EGFR, we conducted a series of experiments to unequivocally establish whether HCMV can activate this tyrosine kinase receptor. Employing model cells for stable over expression of human ErbB receptors or targeted EGFR deletion, we present genetic evidence indicating that none of the four human ErbB receptors was rate limiting or required for HCMV infection. These data are consistent with those of Isaacson and Compton (which were published during preparation of this manuscript) which also demonstrate that HCMV does not enter or signal through EGFR [39]. Utilizing EGFR blocking antibodies and small molecule inhibitors of the EGFR kinase activity the authors demonstrate lack of EGFR requirement during HCMV cellular infection. This study also showed that HCMV stimulation does not induce Tyr-phosphorylation of EGFR in several cell lines tested (including the HEL cells used by us). Thus, our data do not support a role for HCMV-induced conditionally transformed phenotype of EGFR, but rather suggest that distinct receptor(s) tyrosine kinases in conjunction with integrin heterodimers [13] may mediate HCMV-induced activation of cellular signaling cascades.
An important hallmark of malignant gliomas is their invasive behavior, a property linked to cell-extracellular matrix interaction involving integrin and PI-3K signaling [40–42]. The latter pathway is sensitized in gliomas by common abrogation of the PTEN tumor suppressor function [43]. While HCMV-induced FAK stimulation has been previously reported [16], our data documents selective phosphorylation of Tyr 397, which has been shown to play a pivotal role in mediating integrin-dependent glioma cell motility [44]. Based on evidence of HCMV inducing integrin [13], PI-3K and FAK signaling we investigated its effect on haptotactic migration of glioma cells, a biologic property critically controlled by FAK kinase signaling. Here, we present evidence that HCMV can significantly augment migration toward extracellular matrix proteins fibronectin and vitronectin of human glioma cells but not non-malignant astroglial cells in vitro. Since there was no notable difference in infectivity of immortalized and malignant glioma cells, these findings indicated that HCMV has the ability of potentiating the neoplastic phenotype of certain glioblastomas in vivo by enhancing cell migration toward extracellular matrix (haptotaxis). In this context, detection of HCMV in tumor tissue from glioblastoma patients might hold clinical relevance from a diagnostic and therapeutic perspective.
Mouse models of CMV infection demonstrated that virus reactivation in the adult occurs specifically in the neural stem cells of the subventricular zone [45]. Given the large body of evidence that currently implicates human adult resident neural stem cells as the “glioma cell of origin” [46], it is tempting to speculate that reactivation of HCMV within the same region may contribute to gliomagenesis by initiating oncogenic events in a particularly susceptible stem cell population. Data presented herein clearly demonstrate that HCMV can directly activate key cellular signaling mechanisms, specifically the PI3K/AKT, PLCγ and FAK pathways in astro-glial and glioma cells, thereby supporting an oncomodulatory role for this endemic human herpes virus.
References
Harkins L, Volk AL, Samanta M et al (2002) Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 360:1557–1563
Cobbs CS, Harkins L, Samanta M et al (2002) Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 62:3347–3350
Mitchell D, Xie W, Schmittling R et al (2007) Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro-Oncology (In press)
Yu Y, Alwine JC (2002) Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3′-OH kinase pathway and the cellular kinase Akt. J Virol 76:3731–3738
Zhu H, Shen Y, Shenk T (1995) Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J Virol 69:7960–7970
Streblow DN, Soderberg-Naucler C, Vieira et al (1999) The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511–520
Cinatl J, Scholz M, Kotchetkov R et al (2004) Molecular mechanisms of the modulatory effects of HCMV infection in tumor cell biology. Trends Mol Med 10:19–23
Boldogh I, AbuBakar S, Albrecht T (1990) Activation of proto-oncogenes: an immediate early event in human cytomegalovirus infection. Science 247:561–564
Aaronson SA (1991) Growth factors and cancer. Science 254:1146–1153
Cooray S (2004) The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J Gen Virol 85:1065–1076
O’Shea CC (2005) DNA tumor viruses-the spies who lyse us. Curr Opin Genet Dev 15:18–26
Dawson CW, Tramountanis G, Eliopoulos et al (2003) Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J Biol Chem 278:3694–3704
Feire AL, Koss H, Compton T (2004) Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci USA 101:15470–15475
Wang X, Huang DY, Huong SM et al (2005) Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat Med 11:515–521
Wang X, Huong SM, Chiu et al (2003) Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456–461
Streblow DN, Vomaske J, Smith P et al (2003) Human cytomegalovirus chemokine receptor US28-induced smooth muscle cell migration is mediated by focal adhesion kinase and Src. J Biol Chem 278:50456–50465
Threadgill DW, Dlugosz AA, Hansen LA et al (1995) Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269:230–234
Di Fiore PP, Pierce JH, Fleming TP et al (1987) Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell 51:1063–1070
Pruss RM, Herschman HR (1977) Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc Natl Acad Sci USA 74:3918–3921
Sonoda Y, Ozawa T, Hirose Y et al (2001) Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res 61:4956–4960
Ruppert JM, Vogelstein B, Kinzler KW (1991) The zinc finger protein GLI transforms primary cells in cooperation with adenovirus E1A. Mol Cell Biol 11:1724–1728
Kraus MH, Popescu NC, Amsbaugh SC et al (1987) Overexpression of the EGF receptor-related proto-oncogene erbB-2 in human mammary tumor cell lines by different molecular mechanisms. Embo J 6:605–610
Jainchill JL, Aaronson SA, Todaro GJ (1969) Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol 4:549–553
Alimandi M, Romano A, Curia MC et al (1995) Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 10:1813–1821
Baulida J, Kraus MH, Alimandi et al (1996) All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem 271:5251–5257
Fedi P, Pierce JH, di Fiore PP et al (1994) Efficient coupling with phosphatidylinositol 3-kinase, but not phospholipase C gamma or GTPase-activating protein, distinguishes ErbB-3 signaling from that of other ErbB/EGFR family members. Mol Cell Biol 14:492–500
Kraus MH, Fedi P, Starks V et al (1993) Demonstration of ligand-dependent signaling by the erbB-3 tyrosine kinase and its constitutive activation in human breast tumor cells. Proc Natl Acad Sci USA 90:2900–2904
Morgenstern JP, Land H (1990) Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res 18:3587–3596
Kraus MH, Yuasa Y, Aaronson SA (1984) A position 12-activated H-ras oncogene in all HS578T mammary carcinosarcoma cells but not normal mammary cells of the same patient. Proc Natl Acad Sci USA 81:5384–5388
Wang Z, La Rosa C, Maas R et al (2004) Recombinant modified vaccinia virus Ankara expressing a soluble form of glycoprotein B causes durable immunity and neutralizing antibodies against multiple strains of human cytomegalovirus. J Virol 78:3965–3976
Almeida-Porada G, Porada CD, Shanley JD et al (1997) Altered production of GM-CSF and IL-8 in cytomegalovirus-infected, IL-1-primed umbilical cord endothelial cells. Exp Hematol 25:1278–1285
Arbustini E, Grasso M, Diegoli M et al (1992) Histopathologic and molecular profile of human cytomegalovirus infections in patients with heart transplants. Am J Clin Pathol 98:205–213
Yarden Y, Schlessinger J (1987) Self-phosphorylation of epidermal growth factor receptor: evidence for a model of intermolecular allosteric activation. Biochemistry 26:1434–1442
Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103:211–225
Schlaepfer DD, Hunter T (1998) Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol 8:151–157
Sabatier J, Uro-Coste E, Pommepuy I, Labrousse F et al (2005) Detection of human cytomegalovirus genome and gene products in central nervous system tumours. Br J Cancer 92:747–750
Valius M, Kazlauskas A (1993) Phospholipase C-gamma 1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor’s mitogenic signal. Cell 73:321–334
Blume-Jensen P, Hunter T (2001) Oncogenic kinase signalling. Nature 411:355–365
Isaacson MK, Feire AL, Compton T (2007) The epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling. J Virol. doi: 10.1128/JVI.00169-07
Demuth T, Berens ME (2004) Molecular mechanisms of glioma cell migration and invasion. J Neurooncol 70:217–228
Ritchie CK, Giordano A, Khalili K (2000) Integrin involvement in glioblastoma multiforme: possible regulation by NF-kappaB. J Cell Physiol 184:214–221
Sansal I, Sellers WR (2004) The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol 22:2954–2963
Maher EA, Furnari FB, Bachoo RM (2001) Malignant glioma: genetics and biology of a grave matter. Genes Dev 15:1311–1333
Hsia DA, Mitra SK, Hauck et al (2003) Differential regulation of cell motility and invasion by FAK. J Cell Biol 160:753–767
Tsutsui Y, Kawasaki H, Kosugi I (2002) Reactivation of latent cytomegalovirus infection in mouse brain cells detected after transfer to brain slice cultures. J Virol 76:7247–7254
Singh SK, Hawkins C, Clarke et al (2004) Identification of human brain tumour initiating cells. Nature 432:396–401
Acknowledgments
We thank Robert Whitehead for EGFR-/- fibroblasts, Harvey Herschman for NR6 cells, Don J. Diamond and Zhongde Wang for purified gB protein, G. Yancey Gillespie for U251 glioma and J. Michael Ruppert for providing RK3E epithelial cell line. This study was supported by the UAB SPORE program in brain cancer (P50CA097247). Additional support by the Avon Breast Cancer Research Foundation is acknowledged (M.H.K.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cobbs, C.S., Soroceanu, L., Denham, S. et al. Human cytomegalovirus induces cellular tyrosine kinase signaling and promotes glioma cell invasiveness. J Neurooncol 85, 271–280 (2007). https://doi.org/10.1007/s11060-007-9423-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-007-9423-2