Abstract
Environmental abiotic and biotic factors are important in controlling soil CO2 efflux in forest ecosystems of different ages, as they play an important role in soil respiration. In understanding the spatial and temporal variation of soil CO2 efflux after several years of forest logging, there is a need to quantify the changing soil properties, environmental factors, and the total above and below ground biomass. This study was conducted in a 50-year old recovering tropical lowland forest in Peninsular Malaysia, measuring soil CO2 efflux using the continuous open flow chambers technique connected to a multi gas-handling unit and infrared gas analyser. The aim of this study was to determine the spatial and temporal variation of soil CO2 efflux in relation to changes in soil properties, environmental factors and forest carbon in a recovering forest. The efflux rates of about 389.20, 634.78, 564.81, 537.92 and 428.72 mg m−2 h−1, respectively, varied across the days and months, increasing from February and attaining the maximum in March and then gradually decreasing from April to June. The soil properties revealed a considerable amount of soil organic carbon, total organic carbon, and soil organic carbon stock, while the total above ground biomass, below ground biomass, soil pH, nitrogen to carbon ratio were found to provide nutrients for microbial activities in soil and to emit soil CO2. The multiple linear regression model indicated that the soil temperature and moisture explained the spatial and temporal variation in soil CO2 efflux; likewise, the changes in the soil properties and forest carbon significantly increased the soil CO2 efflux indicating a strong positive correlation (R 2 = 0.93).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil respiration usually refers to a suite of complex processes contributing to carbon dioxide (CO2) efflux from the surface of soils (Masyagina et al. 2006), which is directly related to the metabolic activities attributed to both heterotrophic respiration from soil microbes and autotrophic respiration from plant roots. Soil CO2 efflux is one of the major fluxes in the global carbon cycle; it has been estimated at 76.5 Pg C year−1 greater than terrestrial net primary productivity (Raich and Potter 1995), and varies with different ecosystems, climatic conditions and environmental factors (Raich and Schlesinger 1992). Small changes in environmental factors may strongly affect soil CO2 efflux and soil carbon sequestration on a global scale. Forest soil is one of the major carbon reserves in the biosphere. Therefore, understanding the contributing factors to soil CO2 efflux is a crucial challenge in research on global carbon cycles.
Many biotic and abiotic factors affect the rate of soil CO2 efflux, including soil temperature, microbial biomass, soil moisture, nitrogen, subtract supply, soil pH and carbon supply (Scott-Denton et al. 2003; Dilustro et al. 2005; Li et al. 2008). As a result of the changes in these factors, quantifying the contribution of the various environmental factors to total soil CO2 efflux is the key to estimate the spatial and temporal variation in soil CO2 efflux. The important mechanistic links of soil CO2 efflux with environmental factors and soil properties are poorly understood under forests of different ages after logging activity (Gong et al. 2012). Notwithstanding, various studies on soil CO2 efflux have been conducted in tropical lowland Peninsular Malaysia (Itoh et al. 2012; Saigusa et al. 2008; Adachi et al. 2006) with a clear focus on the spatial variation on CO2 efflux, carbon budget and the effect of deforestation on the primary forest of Pasoh reserved forest, Peninsular Malaysia, few studies exist concerning the effect of environmental factors, changes in the soil properties and forest biomass. The next important step is to determine the effects of change in the soil properties and environmental factors on soil CO2 efflux of recovering forests 50 years-old after logging. That to ascertain if the studied factors have any effect on soil CO2 efflux after several years from logging. The important aspect in understanding the factors responsible for the spatial and temporal soil CO2 efflux variation in a recovering forest is to quantify soil CO2 efflux rate in relation to the environmental factors, changes in soil properties and the biological activities in the tropical climate.
Studies have shown that increases in soil organic nutrients from carbon resulted in an increase in the biological activities of microbes and that root growth contributed greatly to soil CO2 efflux on a global scale (Bond-Lamberty et al. 2004). Soil organic matter is derived from forest biomass and distributed on the forest floor in layers (Kleber 2010), and, subsequently, followed by an accelerating rate of decomposition aided by carbon/nitrogen via litter fall. Hence, this is an important aspect of changes in the soil properties that need to be investigated in terms of its contribution to microbial activity and the variation in soil CO2 efflux (Cornwell et al. 2008). Furthermore, environmental factors, such as soil temperature, have also been found to increase soil respiration by 50–100 for every 10 °C temperature rise, while soil moisture, either dry or flooded, regulates soil respiration (Ito et al. 2010). In addition, Rustad et al. (2001) found that warming of the soil temperature by 0.3–6.0 °C increases soil CO2 efflux by an average of 20 %.
Previous studies have identified and highlighted the importance of forest stand age, vegetation development and soil disturbance history in terms of their impact on soil CO2 efflux (Campbell and Law 2005). Hence, it is an important aspect that needs to be considered in determining the factors responsible for the spatial and temporal variation in soil CO2 efflux in the tropical forest ecosystem, as less attention has been given in a recovering forest after several years of logging. The soil CO2 efflux rate in a recovering forest could differ from the primary forest as the environmental factors vary in relation to forest ages in both tropical and temperate forest climatic conditions.
The objectives of this study were (1) to understand the spatial and temporal variation of soil CO2 efflux in relation to the changes in soil properties, environmental factors, aboveground and below ground biomass in a 50 years old forest and (2) to quantify soil CO2 efflux in a 50 years forest after logging.
Materials and methods
Site description
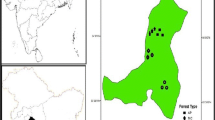
This study was carried out in a 50 years old recovering lowland forest of Sungai Menyala (27°47′99″N, 43°76′90″E), located in Port Dickson, Negeri Sembilan, Malaysia, approximately 93.1 km from Kuala Lumpur. The study area is located in Peninsula Malaysia and has a wet and humid tropical climate throughout the year that is characterized by high annual rainfall, humidity and temperature. The average temperature ranged between 23.7 and 32 °C, (Suhaila and Jemain 2008), while the average solar radiation was 17.00 MJ m−2 and the daily evaporation rate was 3.1 mm day−1 (MMD 2013). The soil was classified as the Serdang-Kedah series developed over mixed sedimentary rocks with a combination of local alluvium colluvium resulting from metamorphic rock (Paramananthan 1998, 2012). In the FAO/UNESCO Soil Map of the World—Revised Legend (FAO 1990) the Serdang series is classified as Haplic Nitisols. An experimental plot of 50 × 50 m with two replicates was designed for the field experiment. The period of study represents all the seasons of the tropics (post-monsoon, pre-monsoon- and monsoon period), and the located study area experiences the sea breeze from the Straits of Malacca.
Soil CO2 efflux and environmental factors measurement
Soil CO2 efflux data were obtained using two continuous open flow chamber systems of 64 cm in height and 50 cm width, with a volume of 3,250 cm3 and enclosed soil surface area of 2,500 cm2 to reduce the build-up of pressure on the soil interphase, and monitored with a barometer. The infrared gas analyser was calibrated in the laboratory using CO2 as zero standards (1,000 g). Soil collars were inserted at 3 cm from ground level into the soil, and installed 1 day before the measurement to allow the soil to recover from the disturbances and to account for within-plot variability, 30 soil collars were randomly established in the plot.
The measurement chambers were connected to a multi gas-handler (WA 161 model), which provides a channel to regulate the flow of CO2 from various chambers to a flow meter connected to a CO2/H2O gas analyser (Li-Cor 6262) for data output. The soil CO2 efflux was recorded every 5 s over a period of 5 min for each chamber. The coefficient of determination (R 2) of simple linear regression was typically better than 0.99.
To investigate the role of environmental factors in soil CO2 efflux, soil temperature was measured using the Watchdog data logger model 125 spectrum technology, soil moisture using the TDR Trime FM and water potential using Delmorst model KS-D1. All the measurements were conducted concurrently on a daily basis from 8:00 to 17:00 h. The forest canopy stand densities and light intensity distribution were determined based on the Leaf Area Index (LAI) using a Sunfleck Ceptometer (AccuPAR model sf-80, Decagon, Pullman, WA); over 188 trees were measured in the study plots. Leaves were collected to ascertain the carbon to nitrogen ratio from the litter trap net placed at 1 m above the forest floor, with ten in each plot, for the collection of leaves at 14-day intervals for the period of the study.
Total aboveground biomass, total below ground biomass, soil properties and total forest carbon stock.
The Estimation of forest biomass was carried out using allometric relationships obtained in the forest according to the International Biological Programme (Kira 1978). The total above ground biomass (TAGB) was determined using the diameter at breast height (DBH) of about 188 trees in the 50 × 50 m plot (Manokaran et al. 1990), all the trees >5 cm in DBH were identified, mapped and tagged, and their DBH were measured. If a tree had a large buttresses, its DBH was measured just above the buttresses (Niiyama et al. 1999). The DBH was measured using the DBH tape, 1.3 m above the forest floor for each tree and the TAGB was estimated using the model of Kato et al. (1978). The model estimates the tree stem, branch and leaf biomass. These components form the TAGB based on simple regression lines fitted for DBH and tree height:
where H is the tree height (m), D is DBH (cm), MaxHt is the maximum tree height (m), and ‘a’ is the coefficient with 2.0 for trees with DBH > 4.5 cm.
Weight (kg) of main stem (W s):
Weight (kg) of branches (W b):
Weight (kg) of leaves (W l):
The TAGB was calculated as:
The below ground carbon biomass was calculated using the model of Ogawa et al. (1963);
The total forest carbon stock was estimated based on the carbon content in the biomass data. The default value for the carbon content on biomass is 0.47 (Feldpausch et al. 2004), which varies among different countries; it was calculated as:
where Cb is the carbon content from the biomass, B is the total biomass, % C organic is the percentage value for carbon content, amounting to 0.47 default value or laboratory obtained value.
Soil sampling and analysis
Soil samples from 0 to 100 cm depth were collected from three sampling points using a soil auger, samples were placed in sterile plastic bags, sealed and returned to the laboratory and later oven-dried at 105 °C for 48 h to determine the soil water content (mass basis) (Gong et al. 2012). The standard method was used to analyse for soil organic carbon (SOC), soil moisture contents, bulk density (BD) and soil pH was measured in water (1:2.5 w/v) according to the Kjeldahl method (Bremner 1960), while the Walkley–Black wet oxidation technique was used to determine the total organic carbon (TOC) (Sollins et al. 1999). The soil carbon stock (SOCstock) was estimated using the model of Eleanor (2008) within a given depth of top soil range from 0 to 100 cm. The soil moisture content was estimated using the standard method based on the following equation:
where A is the mass of moist soil (g), B is the mass of oven dry soil (g).
The BD was estimated in accordance with the standard method (Nhantumbo et al. 2001):
where g is the oven dry mass of the sieve soil (g), v is the sample volume (cm−3).
Soil organic carbon was determined using the following equation:
where M is the molarities of ferrous ammonium sulphate solution (≈0.5 cm−3), V blank is the volume of ferrous ammonium sulphate solution required to titrate the blank (cm−3). Wt = weight of air dry soil (g) 0.3 = 3 × 10−3 × 100 where 3 is the equivalent weight of C.
The TOC was determined by the Walkley–Black method using a correction factor of 1.33 (Sollins et al. 1999) as it is appropriate for moisture analyses because of its simplicity.
where M is the molarities of ferrous sulphate solution (from blank titration) , V 1 is the cm−3 ferrous sulphate solution required for blank, V 2 is the cm−3 ferrous sulphate solution required for S = weight of air dry sample in grams, mcf is the 3 (equivalent weight of carbon) corrected factor.
The moisture corrected factor (mcf) is:
Soil carbon stock using the model of Eleanor (2008), where the given depth of soil was from 0 to 100 cm. The SOC based on the compacted soil was estimated by determining the BD. The equation is expressed as:
To measure the carbon to nitrogen (C/N) input due to litter fall, ten rectangular litter traps with surface area of 1 x 1 m were installed 1 m above the forest floor. Litter was collected at two weeks interval. The litter from each trap was transported to a laboratory and oven-dried at 65 °C for 48 h. All dried samples were separated into needle, bark, cones branches and miscellaneous components, and each component was weighed. The C/N ratio concentration was determined using a TruMac CNS Macro Analyser (LecoCorp), while the mass loss rates in the needle litter were estimate using the litterbag technique (Kim 2007).
Statistical analysis
Soil CO2 efflux, soil properties, TAGB, BGB, SOCstock and environmental properties data were analysed using a parametric one-way ANOVA, followed by a post hoc Dunn’s test and Turkey multiple comparison test (Mande et al. 2013; Müller et al. 2011). The analysis of variance (ANOVA) was used to test the difference of standard deviation and mean soil CO2 efflux, soil temperature, soil moisture and water potential in different months. The descriptive statistics was established to calculate and explain the normality of data distribution and also to quantify the correlations between soil CO2, TAGB, changes in soil properties as well as environmental factors. Exponential regression and the multiple linear regression models were employed to ascertain the significant effect of environmental properties on soil CO2 efflux in the study area, likewise the Pearson correlation was calculated to show the correlation of CO2 efflux variation with the environmental factors and changes soil properties. All the statistical tests were performed using SPSS version 21 software (SPSS Inc., Chicago, Illinois, USA). The techniques used were for both predictive and explanatory purposes within the experimental and nonexperimental designs while the multiple linear regressions can be expressed using the equation:
where thus, Y i is the ith observation of the dependent variable; X ij is the ith observation of the jth independent variable; and j = 1, 2,…, p. The β j values represent the parameters to be estimated, and ε i is the ith independent identically distributed normal error.
Results
Monthly variation in soil CO2 efflux
The total soil CO2 efflux in the 50 years recovering forest varied obviously at different months. Analysis of variance indicated that environmental factors significantly affected soil CO2 efflux. There was significant difference (p < 0.01) between the average mean of soil CO2 efflux in the months of measurement, and the minimum and maximum efflux rate was between 100.13 and 634.78 mg m−2 h−1 showing a variation in the flux pattern (Table 1). As soil CO2 efflux gradually increased after the monsoon period in the month of February to the peak in March and rapidly decreased from April to June with 100.18 to 389.20, 104.56 to 634.81, 100.13 to 564. 81,100.56 to 537.92, and 103.91 to 428.72 mg m−2 h−1 for the month of February, March, April, May and June, respectively (Table 1). Low efflux occurred in the morning hours between 8:00 and 11:00 h while high efflux rates were between 13:00 and 15:00 h. Significant (p < 0.01) higher values were recorded in March, April and May, respectively, while lower efflux was observed in February and June. The CO2 efflux variation pattern coincided with the increase and decrease in environmental properties as the correlation analysis showed that soil CO2 efflux was more correlated with soil temperature at 0.9, p < 0.01 compare to soil moisture and water potential (0.83, p < 0.01). This indicated that soil temperature controls the spatial and temporal variation in soil CO2 efflux. The environmental properties coincided with soil CO2 efflux due to variation in time and period in relation to microclimate condition as one-way ANOVA show significant variation between the months. Furthermore, the multiple regression model employed illustrated that environmental factors have significant influence on soil CO2 efflux (p < 0.01). Furthermore, the multiple regression model indicated a strong correlation at R 2 = 0.65, 0.78, 0.93, 0.68 and 0.86 for February, March, April, May and June, respectively. The relationship is equally at different months and relevance increased obviously from February to March and decrease from April to June.
Soil properties and forest biomass
To clarify the effects of soil properties and forest biomass on soil CO2 efflux, we also determined BD and analysed TOC, SOC, soil pH and carbon and nitrogen input. The finding showed that BD increased with soil depth between 0 and 100 cm (Fig. 1) given good porosity for soil water movement and cation exchange capacity to hold onto nutrients suitable for microbial activity. Likewise the soil analysis revealed a considerable amount of TOC and SOC of 2.20 and 4.96 %, respectively (Table 2), with a soil moisture content of 17.5 % and a corrective factor of 1.18 (Table 2) which are responsible for soil nutrients. The aforementioned parameters are being influenced by forest biomass in presence of soil temperature, moisture and water potential and positively and strongly related to soil CO2 efflux (R 2 = 0.8 at p < 0.01). The soil pH was found to be slightly acidic at 5.16 (Table 2). Furthermore, the carbon and nitrogen input from the litter fall contributed about 49.11–50.78 and 1.33–1.45 %, respectively (Table 2), and is responsible for the decomposition of organic matter by micro-organisms.
The 50 years old recovering lowland forest hosts an estimated forest biomass of total above ground biomass, below ground biomass and total forest carbon of 2.9 × 103, 1.0 × 103 and 3.0 × 103 mg, respectively (Table 2). The estimated soil carbon stock in the top 100 cm was 64.48 mg ha−1 (Table 2). The high percentage of occurrence of carbon input increases the soil nutrient and energy for microbial activity with a corresponding effect of releasing the soil CO2 efflux. The correlation coefficients for each variable do reflect the relationship between the affirmative variables and soil CO2 efflux (R 2 = 0.7, p < 0.01).
Environmental properties
The variations of soil CO2 efflux during the period of measurement occurred with the trend of environmental properties indicating a symmetrical parabola curve as both parameters are dependable. The multiple regression model was used to present the study in terms of the spatial and temporal variation of soil CO2 efflux with respect to soil temperature, moisture and water potential since it provided a better fit of R 2 (Table 3). The coefficient of the model of the environmental properties—soil temperature, moisture and water potential—had at a high value for February: 0.28, 0.25 and 0.44, respectively (Table 4), while in March, the beta coefficient was recorded as 0.42, 0.85 and −0.07, for soil temperature, moisture and water potential, respectively (Table 5). This indicated that soil temperature and moisture accounted for a significant effect in soil CO2 efflux compared to water potential (p < 0.01). Soil CO2 efflux was in response to increase in soil moisture as it was observed in the month of April to displaced a beta coefficient of soil temperature, moisture and water potential impact on soil CO2 efflux at −0.28, 1.02 and −0.39, respectively (Table 6), suggested a significant impact (p < 0.01) from the soil moisture, as the soil temperature and water potential were at a constant level. In May, the beta coefficient attributed effect of soil temperature on soil CO2 efflux compared to the moisture and water potential occurred at 0.51, −0.61 and −0.46, respectively (Table 7), and this is probably due to changes in the tropical climate. In addition, for the month of June, the soil temperature and water potential had a major effect on soil CO2 efflux with a beta coefficient of 0.23, −0.21 and 0.68 for soil temperature, moisture and water potential, respectively (Table 8). The correlation analysis indicated a strong to moderate relationship between soil CO2 efflux and soil temperature, and moisture with soil temperature varying across the day, gradually increasing in the morning and reaching the maximum in the afternoon before decreasing with time at 24.80–25.65 °C. The soil moisture remained relatively stable between 24.75 and 24.76 % and the water potential varies across the day, being higher in the morning, and decreasing over time with a rapid decrease towards evening at 97.45, 97.44 to 97.1 %. The fluctuation in soil temperature, soil moisture and water potential suggested their effect on the variation of soil CO2 efflux as both correlation and multiple regression indicated that soil temperature, moisture and water potential have a significant effect on soil CO2 efflux (p < 0.01). However, soil temperature was found to be the dominant controlling factor, and was followed closely by other factors, therefore, the combined effects of environmental factors proved to be the factors that account for the variation in soil CO2 efflux. This explains the monthly spatial and temporal variation in soil CO2 efflux across the recovering forest (Fig. 2).
To clarify the contribution of the entire affirmative factors to the observed changes in soil CO2 efflux rate, we performed a Pearson correlation analysis. With soil temperature as the control variable, the correlation between soil moisture and soil CO2 efflux was significant (p < 0.001) and positive (0.47) while water potential and soil CO2 efflux was significant (p < 0.001) and positive (0.43). During the entire period of measurement, soil temperature exerts a stronger control then the other environmental properties on soil CO2 efflux.
Discussion
Factors affecting the variation in soil CO2 efflux
The finding revealed soil CO2 efflux variation in forest ecosystem of the peninsular Malaysia was in response to environmental factors and changes soil properties which was similar to the poplar plantations of northwest China (Zhang et al. 2010), multiple ecosystems in central Japan (Inoue 2012) and successional forests in southern China (Huang et al. 2011). Likewise efflux variation in respect to the forest age was similar to the study reported by Yanai and Currie (2003) and Sartori et al. (2007). Furthermore, the results obtained from our measurement indicated that soil CO2 efflux spontaneously increased and varied during the period of measurement, which was attributed to forest carbon input, soil temperature, moisture, water variation and changes in soil properties.
The average soil CO2 efflux recorded ranges from 100.13 to 634.78 mg m−2 h−1 similar to what Jin et al. (2009), observed in the canopy of China, which ranges from 305.5 to 730.8 mg m−2 h−1. This is a little higher compared to the lowland tropical forest of Peninsular Malaysia. The month of February, being the end of the monsoon period, witnessed a rise in soil CO2 efflux to 389.20 mg m−2 h−1 similar to (Taylor and Hu 2011) of 307 mg m−2 h−1 for Central Hokkaido, Japan, which was strongly influenced by the availability of soil moisture and was significant correlated (p < 0.01), as reported by Howard and Howard (1993). There was a steady rise to 634.78 mg m−2 h−1 in the month of March parallel to the temperature of the post-monsoon indicated soil CO2 efflux was positively and significantly with soil temperature (p < 0.01; Table 3). A gradual decrease of 564.81 mg m−2 h−1 was also observed in the month of April, which was greater than that of the measurement recorded in the tropical forest of China (Jia et al. 2007). A steady decline continued for the months of May and June, as it experienced few showers with 537.92 and 428.72 mg m−2 h−1, respectively, greater and less than the open field measurement of 443.1 mg m−2 h−1 in woodland (Taylor and Hu 2011). The rise and gradual decrease in soil CO2 efflux indicated that soil temperature-water interaction explained the spatial and temporal variation of soil CO2 efflux (Janssens et al. 2001). The one-way ANOVA employed in soil CO2 efflux data analysis showed a significant difference at p < 0.01 and a normality of distribution aligned along a straight line without any outliers giving good skewness (Fig. 3).
The increase in the spatial and temporal variation in soil CO2 efflux was attributed to the change in soil temperature between the months, which was observed to be one of the important controlling factors. (Raich and Schlesinger 1992; Davidson et al. 1998). In addition, the soil moisture and water potential, which were relatively available during the measurement period, were found to be high and decreased with time within the day signifying a positive correlation with the spatial and temporal soil CO2 variation (p < 0.01). Furthermore, the forest carbon biomass influences soil nutrients, which explained the role of forest age and canopy cover of the 50 years old forest in decreasing the net radiation on the forest floor to provide a favourable condition for microorganisms and root growth to facilitate soil CO2 efflux, as was reported by McCarthy and Brown (2006), and Tanaka and Hashimoto (2006).
Input from litter fall (C:N), TAGB, BGB, SOCs, SOCstock, TOC and SOC
The significant input from the litter fall of the 50 year old forest was found to have a carbon and nitrogen ratio of 49.11–50.78 and 1.33–1.45 %, respectively. This is attributed to the stand density of the 50 year old forest, as was also reported by Asensio et al. (2012). This litter fall increased TOC, SOC, soil moisture content and moisture correction factor in a slightly acidic soil of 2.2, 4.96, 17.5, 1.18 and 5.16, respectively, as was also reported by (Ming and Ye 2001). This overall input from the forest biomass is related to forest age and management practices, which could also influence and change the rate of soil carbon stock, TOC and SOC (Johnson and Curtis 2001; Sartori et al. 2007; Teklay and Chang 2008; Jia et al. 2007) through litter fall, which could make more substrates available for decomposition (Jandl et al. 2007). The overall soil organic nutrients are the combined function of the high percentage of TAGB, BGB, SOCs and SOCstock recorded from the forest biomass (Saiz et al. 2006). The nutrients resulting from these activities, increase the food and energy for microbial activities as they respire in the process of decomposition of soil organic matter, serving as a prime factor to emit a considerable amount of soil CO2 (Adachi et al. 2006). However, logging and grazing could decrease the forest biomass as the recovering forest increases with an increase in the biomass (Li et al. 2000). The soil BD was found to increase with depth indicating the role that pore space plays in water movement, electric conductivity and microbial activity. Partial correlation and multiple regression analysis indicated a high and significant (p < 0.01, R 2 = 0.65–0.93) positive correlation between environmental factors and variation in soil CO2 efflux. The observed spatial and temporal variation of soil CO2 efflux might result from an enhancement of the soil temperature, moisture and water potential; likewise for TAGB, BGB, SOCs and SOCstock. Subsequent litter deposition and carbon–nitrogen ratio could supply more soil nutrients and decomposition for microorganisms to release CO2. Soil CO2 efflux is controlled by biotic and abiotic factors such as temperature, soil moisture, water potential, forest biomass, vegetation type, soil microbial biomass, photosynthetic characteristics of vegetation, SOC content and management of the forest (Raich and Schlesinger 1992; Fernandez et al. 1993; Neergaard et al. 2002; Adachi et al. 2006; Subke et al. 2006). In general, soil temperature, soil moisture and water potential are the key factors that affect the spatial and temporal variation in soil CO2 efflux (Rey et al. 2002; Subke et al. 2006; Iqbal et al. 2008). In the present study, we found similar correlations, but soil CO2 efflux was more strongly correlated with soil temperature then soil moisture and water potential. We also found a significant positive correlation between soil temperature, soil moisture, water potential, forest biomass and soil properties, which agree with the results of the previous studies (Tang et al. 2006).
The Pearson correlation analysis indicated that the strength of association between soil temperature and soil CO2 efflux was very high significantly different (p < 0.001) confirmed that soil temperature was significantly positively correlated with soil CO2 efflux compare to other environmental properties, suggesting that the contribution of soil temperature to soil CO2 efflux is stronger and played the dominant role. When other factors were added to the analysis, it was found that soil CO2 efflux was strongly influenced by the combined factors, as they were strongly positively correlated with soil temperature. Rising temperature can increase the physiological activity of soil micro-organism, leading to higher decomposition rates and higher soil CO2 efflux (Han et al. 2007). Therefore, these results suggested that soil temperature, soil moisture, water potential, changes in soil properties and forest biomass are major influencing factors on soil CO2 efflux in the 50 years old recovering forest of lowland Peninsular Malaysia.
Conclusions
The study demonstrated that soil CO2 efflux vary significantly as a function of environmental properties The spatial and temporal soil CO2 efflux results obtained showed a strong relationship (R 2 = 0.86, p < 0.001) with the contributing factors, such as forest biomass, changes in soil temperature, moisture and soil properties, as well as the forest age. The considerable amount of soil CO2 efflux was remarkable and varied on a daily basis over the months, inasmuch as the soil CO2 increased from February to the peak in March and then steadily declined from April to June. The contribution of the entire environmental factors to total soil CO2 efflux also varies with months, with increasing from February to March and decreases from April to June. The results indicate that soil temperature was the main factor influencing the soil CO2 variation (R 2 = 0.94, p < 0.001), followed by soil moisture, changes in the soil properties, high input of carbon from forest biomass and water potential as combined abiotic and biotic factors (R 2 = 0.83, p < 0.001). Therefore, the results suggested that the spatial and temporal variation in soil CO2 efflux is a function of the forest carbon input, soil properties and the microclimate condition. The results indicated that poor land management resulting from logging could have a negative effect on the global carbon cycle and alter the forest as a carbon sink and source.
References
Adachi M, Bekku YS, Rashidah W, Okuda T, Koizumi H (2006) Differences in soil respiration between different tropical ecosystems. Appl Soil Ecol 34(2–3):258–265. doi:10.1016/j.apsoil.2006.01.006
Asensio D, Yuste JC, Mattana S, Ribas À, Llusià J, Peñuelas J (2012) Litter VOCs induce changes in soil microbial biomass C and N and largely increase soil CO2 efflux. Plant Soil 360(1–2):163–174. doi:10.1007/s11104-012-1220-9
Bond-Lamberty B, Wang C, Gower ST (2004) Global relationship between the heterotrophic and autotrophic components of soil respiration? Glob Change Biol 10:1756–1766
Bremner JM (1960) Determination of nitrogen in soil by the Kjeldahl method. J Agric Sci 55:11–33
Campbell JL, Law BE (2005) Forest soil respiration across three climatologically distinct chronosequences in Oregon. Biogeochemistry 73:109–125
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pe´rez-Harguindeguy N, Quested HM, Santiago LSWDA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Dı´az S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Davidson EA, Belk E, Boone RD (1998) Soil water content and temperature as independent of confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob Chang Biol 4:217–227
Dilustro J, Collins B, Duincan L, Crawford C (2005) Moisture and soil texture effects on soil CO2 efflux components in south eastern mixed pine forests. For Ecol Manage 204:85–95
Eleanor M (2008) Soil organic carbon. In: Cleveland CJ (ed) Encyclopedia of earth. Environmental Information Coalition, National Council for Science and the Environment, Washington, DC. http://www.eoearth.org/article/Soil_organ. Accessed 13 June 2009
FAO (Food and Agariculture Organisazation. of the United Nation) (1990) FAO/UNESCO Soil map of the world: revised legend 1:5,000,000 Vol. 1–10 Paris: UNESCO
Feldpausch TR, Rondon MA, Fernandes ECM, Riha SJ, Wandelli E (2004) Carbon and nutrient accumulation in secondary forests regenerating on pastures in Central Amazonia. Ecol Appl 14:164–176
Fernandez IJ, Son Y, Kraske CR, Rustad LE, David MB (1993) Soil carbon dioxide characteristics under different forest types and after harvest. Soil Sci Soc Am J 57:1115–1121
Gong J, Ge Z, An R, Duan Q, You X, Huang Y (2012) Soil respiration in poplar plantations in northern China at different forest ages. Plant Soil 360(1–2):109–122
Han GX, Zhou GS, Xu ZZ, Yang Y, Liu JL, Shi KQ (2007) Biotic and abiotic factors controlling the spatial and temporal variation of soil respiration in an agricultural ecosystem. Soil Biol Biochem 39(418–425):418–425
Hong-Mei J, Osbert Jianxin Sun ZKL, Liu J (2009) Dynamics of soil respiration in sparse Ulmus pumila woodland under semi-arid climate. Ecol Res 24 731–739. doi:10.1007/s11284-008-0544-7
Howard DM, Howard PJA (1993) Relationships between CO2 evolution, moisture content and temperature for a range of soil types. Soil Biol Biochem 25(1537–1546):1537–1546
Huang Y, Zhou G, Tang X, Jiang H, Zhang D, Zhang Q (2011) Estimated soil respiration rates decreased with long-term soil microclimate changes in successional forests in southern China. Environ Manag 48(6):1189–1197. doi:10.1007/s00267-011-9758-5
Inoue T (2012) Seasonal variability of soil respiration in multiple ecosystems under the same physical—geographical environmental conditions in central Japan. For Sci Technol 8:37–41
Iqbal J, Hu RG, Du LJ, Lu L, Lin S, Chen T, Ruan LL (2008) Differences in soil CO2 flux between different land use types in mid-subtropical China. Soil Biol Biochem 40:2324–2333
Ito A, Ichii K, Kato T (2010) Spatial and temporal patterns of soil respiration over the Japanese Archipelago: a model intercomparison study. Ecol Res 25(5):1033–1044. doi:10.1007/s11284-010-0729-8
Itoh M, Kosugi Y, Takanashi S, Kanemitsu S, Osaka K, Hayashi Y, Rahim Nik A (2012) Effects of soil water status on the spatial variation of carbon dioxide, methane and nitrous oxide fluxes in tropical rain-forest soils in Peninsular Malaysia. J Trop Ecol, 28(06):557–570. doi:10.1017/S0266467412000569
Jandl R, Lindner M, Vesterdal L, Bauwens B, Baritz R, Hagedorn F, Johnson DW, Minkkinen K, Byrne KA (2007) How strongly can forest management influence soil carbon sequestration? Geoderma 137:253–268
Janssens IA, Lankreijer H, Matteucci G, et al. (2001) Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob Change Biol 7:269–278, 269–278
Jia B, Zhou G, Wang F, Wang Y, Weng E (2007) Effects Of grazing on soil respiration of leymus Chinensis steppe. Clim Change 82(1–2):211–223. doi:10.1007/s10584-006-9136-0
Johnson DW, Curtis PS (2001) Effects of forest management on soil C and N storage: meta analysis. For Ecol Manag 140(227–238):2001
Kato J, Tadaki Y, Ogawa H (1978) Plant. (1978) Biomass and growth increment studies in Pasoh Forest Malaysia. Nat J 30:211–224
Kim C (2007) Soil carbon storage, litter fall and CO2 efflux in fertilized and unfertilized larch (Larix leptolepis) plantations. Ecol Res 23(4):757–763. doi:10.1007/s11284-007-0436-2
Kira T (1978) Community architecture and organic matter dynamics in tropical lowland rain forests of southeast Asia with special reference to Pasoh Forest, West Malaysia. In: Tomlinson PB, Zimmermann MH (eds) Tropical trees as living systems. Cambridge Universit, Cambridge, pp 561–590
Kleber M (2010) What is recalcitrant soil organic matter? Environ Chem 7(320–332):320–332
Li LH, Wang QB, Bai YF, Zhou GS, Xing XR (2000) Soil respiration of a Leymus Chinensis grassland stand in the XiLin river basin as affected by over-grazing and climate. Acta Phytoecol Sin 24:680–686
Li HJ, Yan JX, Yue XF, Wang MB (2008) Significance of soil temperature and moisture for soil respiration in a Chinese mountain area. Agr For Meteorol 148:490–503
Malaysia Meteorological Department (MMD) (2013) http://www.met.gov.my
Mande KH, Ahmad AM, Ahmad ZA, Ahmad NA (2013) Soil carbon dioxide efflux and atmospheric impact in a 10-year-old dipterocarpus recovering lowland tropical forest, peninsular Malaysia. From source to solution, Proceedings of the IENFORCE 2013. Springer, Heidelberg, pp 165–169
Manokaran N, LaFrankie JV, Kochummen KM, Quah ES, Klahn JE, Ashton PS, Hubbell SP (1990) Methodology for the fifty-hectare research plot at Pasoh forest reserve. Res Pam For Res Inst Malaysia 104:1–69
Masyagina OV, Hirano T, Ji DH, Choi DS, Qu L, Fujinuma Y, Koike T (2006) Effect of spatial variation of soil respiration rates following disturbance by timber harvesting in a larch plantation in northern Japan. For Sci Technol 2(2):80–91
McCarthy DR, Brown KJ (2006) Soil respiration responses to topography, canopy cover, and prescribed burning in an oakhickory forest in southeastern Ohio. For Ecol Manage 237:94–102
Ming XU, Ye QI (2001) Soil-surface CO2 efflux and it spatial and Temporal variation in a young ponderosa pine plantation In northern California. Glob Change Biol 7:667–677
Müller E, Rottmann N, Bergstermann A, Wildhagen H, Joergensen RG (2011) Soil CO 2 evolution rates in the field—a comparison of three methods. Arch Agron Soil Sci 57(6):597–608
Neergaard A, Porter JR, Gorissen A (2002) Distribution of assimilated carbon in plants and rhizosphere soil of basket willow (Salix viminalis L). Plant Soil 245:307–314
Nhantumbo ADJ,. Da C, Bennie AT (2001) A procedure for determining the minimum bulk density of soils. South Afr J Plant Soil 18(1):44–46 doi:10.1080/02571862.2001.10634401
Niiyama K, Abdul Rahman K, Kimura K, Tange T, Iida S, Quah ES, Chan YC, Azizi R, Appanah S (1999) Design and methods for the study on tree demography in a hill dipterocarp forest at Semangkok forest reserve, Peninsular Malaysia. Forest Research Institute Malaysia, Kepong
Ogawa JM, Sandeno JL, Mathre JH (1963) Comparisons in development and chemical control of decay organism on mechanical and hand harvested stone fruits. Plant Das Rep 47:129–133
Paramananthan S (1998) Malaysian Soil Taxonomy (second Approximation): A Proposal for the Classification of Malaysian Soils. Malaysian Society of Soil Science, pp 121–156
Paramananthan S (2012) Keys to the identification of Malaysian soils using parent materials, 2rd Edn Param Agricultural Soil Surveys (M) Sdn. BHD Malaysia, pp 2–20
Raich JW, Potter CS (1995) Global patterns of carbon dioxide emissions from soils. Glob Biogeochem Cycles 9:23–36
Raich JW, Schlesinger WH (1992) The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 44B:81–99
Rey A, Pegoraro E, Tedeschi V, Parri I, Jarvis PG, Valentini R (2002) Annual variation in soil respiration and its components in a coppice oak forest in central Italy. Glob Chang Biol 8(851–866):851–866
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch JGN (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Saigusa N, Yamamoto S, Hirata R, Ohtani Y, Ide R, Asanuma J, Gamo M, Hirano T, Kondo H, Kosugi Y, Li SG, Nakai Y, Takagi K, Tani M, Wang H (2008) Temporal and spatial variations in the seasonal patterns of CO2 flux in boreal, temperate, and tropical forests in East Asia. Agric For Meteorol 148:700–713
Saiz G, Green C, Butterbach-Bahl K, Kiese R, Avitabile V, Farrell EP (2006) Seasonal and spatial variability of soil respiration in four Sitka spruce stands. Plant Soil 287(1–2):161–176. doi:10.1007/s11104-006-9052-0
Sartori F, Lal R, Ebinger MH, Eaton JA (2007) Changes in soil carbon and nutrient pools along a chronosequence of poplar plantations in the Columbia Plateau, Oregon, USA. Agric Ecosyst Environ 122:325–339
Scott-Denton LE, Sparks KL, Monson RK (2003) Spatial and temporal controls of soil respiration rate in a high elevation, subalpine forest. Soil Biol Biochem 35:525–534
Sollins P, Glassman C, Paul EA, Swanston C, Lajtha K, Heil JW, Elliott ET, Robertson PG (1999) Soil carbon and nitrogen: pools and fractions. Standard soil methods for long-term ecological research, pp 89–105 Oxford University Press, London
Subke JA, Inglima I, Cotrufo MF (2006) Trends and methodological impacts in soil CO2 efflux partitioning: a meta analytical review. Glob Chang Biol 12:921–943
Suhaila J, Jemain AA (2008) Fitting the Statistical Distribution for Daily Rainfall in Peninsular Malaysia Based on AIC. Criterion 4(12):1846–1857
Tanaka K, Hashimoto S (2006) Plant canopy effects on soil thermal and hydrological properties and soil respiration. Ecol Model 196:32–44
Tang X, Liu S, Zhou G, Zhang D, Zhou C (2006) Soil atmospheric exchange of CO2, CH4, and N2O in three subtropical forest ecosystems in southern China. Glob Chang Biol 12:546–560
Taylor P, Hu R (2011) Soil science and plant nutrition soil respiration and net ecosystem production in an onion field in Central Hokkaido, Japan Soil Respiration and Net Ecosystem Production in an Onion Field in Central Hokkaido, Japan, 37–41
Teklay T, Chang SX (2008) Temporal changes in soil carbon and nitrogen storage in a hybrid poplar chronosequence in northern Alberta. Geoderma 144:613–619
Yanai RD, Currie WS, Goodale CL (2003) Soil carbon dynamics after forest harvest: an ecosystem paradigm reconsidered. Ecosystems 56:197–212
Zhang J, Shangguan T, Meng Z (2010) Changes in soil carbon flux and carbon stock over a rotation of poplar plantations in northwest China. Ecol Res 26(1):153–161. doi:10.1007/s11284-010-0772-5
Acknowledgments
This research was jointly supported by the National Institute of Environmental Studies, Japan, and the Research Management Centre Universiti Putra Malaysia Grant Scheme (Project No. 0302122070) and Putra Grant (GPIPS/2013/9399600). We wish to thank the management staff of Negeri Sembilan Forest Department for approving the study area and the forest rangers for the security back up for the entire one year in the jungle. Our appreciation also goes to the staff of the Centre for Marine and Oceanographic Studies, Universiti Putra Malaysia, Port Dickson Centre, for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mande, H.K., Abdullah, A.M., Aris, A.Z. et al. Factors responsible for spatial and temporal variation of soil CO2 efflux in a 50 year recovering tropical forest, Peninsular Malaysia. Environ Earth Sci 73, 5559–5569 (2015). https://doi.org/10.1007/s12665-014-3810-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3810-8