Abstract
Aims
This study aims to test the effects of forest age on soil respiration in poplar ecosystems in northern China and to separate the contributions of root respiration (Rr) and soil microbes to the total soil respiration (Rs).
Methods
Rs in three poplar forests (5, 10, and 15 years old) were measured using an LI-6400-09 soil chamber connected to an LI-6400 portable infrared gas analyzer during the growing seasons in 2007 and 2008. Root respiration was measured using the root excision method. The soil micro-organisms were quantified using the dilution-plate method.
Results
The results show that Rs was the highest in the 5-year-old forest and lowest in 15-year-old forest. The contribution of Rr to Rs ranged from 29.4 to 81.0%. Rr/Rs tended to be significantly higher in the 15-year-old forest than that in the younger forests; but Rr was the highest in the 5-year-old forest. Temporal variation in Rs can be largely accounted by fine-root biomass (R = 0.718), while soil N was significantly negatively correlated with Rs (R = -0.646).
Conclusions
Rs, Rr and Rr/Rs vary significantly with the forest age. The lower Rs in the older forests increased their carbon use efficiency. Underground factors, dominated by fine-root biomass, affect Rs, Rr and Rr/Rs substantially. Soil microbial community structure is a particularly important underground factor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forests play a significant role in the global terrestrial carbon balance; the carbon fixed by forests accounts for 80% of global plant carbon and 40% of the soil carbon (Dixon 1994). In forest ecosystems, carbon sequestration results from the difference between photosynthetic carbon fixation and ecosystem respiration. In general, ecosystem respiration determines the net ecosystem carbon exchange (Valentini et al. 2000), so estimating the contributions of forest respiration to the atmosphere is a crucial challenge in research on carbon cycles and climate-change modeling.
Soil is the biggest carbon pool in the continental biosphere (Schimel 1995), and soil respiration is the primary path by which the CO2 fixed by plants returns to the atmosphere; these returns can amount to 40 to 90% of total respiration by the whole forest ecosystem (Granier et al. 2000; Schlesinger and Andrews, 2000). Thus, even small changes in soil respiration may greatly affect soil carbon sequestration and atmospheric carbon exchange (Raich and Schlesinger 1992). Therefore, it is important to obtain good estimates of soil respiration and to understand the response of soil respiration to global climate change and the consequences for the global carbon balance.
Soil respiration is mainly composed of respiration from roots and microbes, so quantifying the contributions of both components to total soil respiration is the key to calculating the carbon budgets of an ecosystem. The contribution of root respiration to total soil respiration ranges from 10 to 90%, depending on the vegetation type, season, and forest age (Boone et al. 1998; Hanson et al. 2000; Frey et al. 2006). Respiration by microbes is associated with microbial metabolic activities, the decomposition of litter, and the mineralization of soil organic matter. Many biotic and abiotic factors affect these processes, including soil temperature, substrate supply, soil moisture, vegetation, topography, nitrogen, soil texture, litter biomass, microbial biomass, carbon content, and soil pH (Lloyd and Taylor 1994; Buchmann 2000; Stoyan et al. 2000; Kang et al. 2003; Scott-Denton et al. 2003; Dilustro et al. 2005; Li et al. 2008). As a result of the temporal and spatial changes in these factors, soil respiration for a specific ecosystem shows temporal and spatial variability at both small and large scales, as well as variability among ecosystem types (Fang et al. 1998). To estimate soil respiration at long time scales and large spatial scales, a better understanding of these factors is therefore required.
Natural forests have suffered greatly from deforestation, so managed forests and plantations are increasing in importance. Poplar (Populus spp.) are species with fast growth and high biomass yield in short rotations under optimal conditions, and thus are an important afforestation or biomass production resource. Poplar plantations provide a source of timber to areas that are lacking natural forests, and their significance is likely to increase (Zsuffa et al. 1996). Poplar has proven to be an interesting research subject because of its fast responses to environmental changes and the growing importance of managed forests in the global carbon balance (Gielen and Ceulemans 2001). Although poplar is a major compartment for durable carbon sequestration, its aboveground biomass is harvested for transformation into wood products after a short growing period (often less than 20 years) compared with other forest types. The predicted responses of poplar to rising atmospheric CO2 will also have implications for future forest management. To guide future management decisions, detailed information on soil respiration and its control factors is critical for managing carbon budgets in poplar ecosystems.
In this study, we investigated soil respiration in poplar forests of different ages to determine the effects on soil respiration of biotic and abiotic factors, including root biomass, the total soil microbial population, soil temperature, soil moisture, substrate supply, nitrogen content, carbon content, and soil texture. Our main objectives were (i) to investigate the seasonal and annual variations in soil respiration in poplar ecosystems in northern China and address the relative effect of the biotic and abiotic factors on soil respiration, (ii) to distinguish the contributions of roots and microbes to soil respiration, and (iii) to determine the impact of forest age on soil respiration.
Materials and methods
Study site
The studies were conducted at a forestry station in the Qapqal plains in the Ili River Valley, Uygur Autonomous Region, of the Xinjiang region of northwestern China (43°86′N, 81°23′E). The region has a continental semi-arid temperate climate with a mean annual temperature of about 9.4°C, an annual precipitation of about 221.3 mm, most of which falls between May and September. The average altitude at the study site is 600 m, and the total number of sunlight hours per year averages 3049 annually, the soil type is a sandy loam with a pH value of 7.8.1
We selected three poplar forests of different ages for our study. The sites were located within 1 km of each other and had similar environmental conditions. Table 1 summarizes the characteristics of the forests. The main species at each site were Populus spp. in section Aigeiros. All three sites had similar management and planting histories. They all belonged to a forest farm and were used as seedlings growing bases prior to poplar planting. Table 2 showed the information on soil characteristics. During the growth stage of poplar, fertilizer, mainly nitrogen– based fertilizer, was applied during different growth periods. In the 2–4 years poplar plantation, Nitrogen fertilizer was applied with great amount (233–466gN/Plant/year). In the 5-6 years poplar plantation, the amount of fertilizer was reduced to 93–233gN/Plant/year. In the study area, leaves start their expansion from late April to mid-May, and leaf abscission starts in late October. We obtained our measurements during the growing season, from May to October, in 2007 and 2008. The ultimate target of this research was to discuss the total amount of carbon fixation of poplar plantation, which could be calculated directly through the biomass and carbon densities. The soil respiration was only one reference index of photosynthesis, microorganism, net primary productivity, etc. These indexes can only be obtained in growing season and explain the influence of temperature on the changing carbon pool. So the soil respiration outside growing season was not measured.
Soil respiration measurements
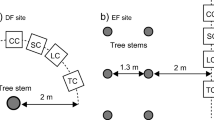
We randomly selected six 30 × 30 m permanent sample plots at each site. We then randomly selected two healthy sample trees on each measurement date in each plot. We obtained measurements around the sample trees at distances of 1 and 2 m to investigate the spatial variation of root and soil respiration. Diurnal and seasonal changes in soil respiration were measured monthly during the growing season in 2007 and 2008 using three LI-6400-09 soil chambers (Li-Cor, Inc., Lincoln, NE), each of which was connected to an LI-6400 portable infrared gas analyzer (Li-Cor). The soil chamber used soil collars (each covering 80 cm2 of soil surface area). The soil collars were inserted into the soil to a depth of 2 cm and installed 1 day before the measurements to allow the soil to recover from the disturbance. We used 6 soil collars in each sample plot. Soil respiration rates were measured every 2 h on each of the collars during the day from 08:00 to 20:00 on two cloud-free days per month.
Measurements of environmental factors
Soil temperature at a depth of 10 cm was measured at the same time as the soil respiration measurements using an LI-6400-09 TC thermocouple probe (Li-Cor) inserted in the soil near the collars. The volumetric soil water content (0 to 20 cm in depth) near the soil collars was monitored with a Diviner 2000 portable sensor (Sentek, Stepney, Australia). Soil temperature and moisture were measured simultaneously.
During the field measurements, we monitored a dynamic automatic weather station installed at the sites starting on 20 May to obtain a 24-hour description of the regional microclimate in the forest. We observed rainfall, air temperature, and soil temperature at a depth of 30 cm, total radiation, photosynthetically active radiation, relative humidity, wind speed, and wind direction.
Root respiration and biomass
In 2008, after we completed soil respiration measurements, root respiration was measured using the root excision method (Yi et al. 2007); After removing the PVC collar, we collected soil samples to a depth of 60 cm with the same diameter of Earth core (10 cm), and divided this into shallow (0 to 30 cm) and deep (30 to 60 cm) roots at the same location where soil respiration rates were measured using an auger that covered the same area as the respiration measurements. Roots were carefully removed from the soil core and then washed and blotted dry quickly. The live roots were separated from dead roots based on their color. We defined fine roots as white, unsuberized roots less than 4 mm in diameter. The root respiration was measured using the same instruments used for the soil respiration measurements. The root chamber comprised a sealed conical glass container with a wide base and narrow neck (10-cm diameter). All measurements of root respiration rates were made as soon as root excision was finished. After measurement of the root respiration rates, all the roots were collected and oven- dried at 75°C to constant weight to determine the dry weight of the root biomass. Root respiration was only measured in one of the years
Microbial abundance
After we finished measuring soil respiration, we obtained three soil samples in each plot that included the top 30 cm of the soil. The soil was sieved (<2 mm) and combined to produce a single homogenized sample. Samples were placed in sterile plastic bags, sealed, and returned to the laboratory for storage at 4°C until the soil micro-organisms and soil nutrients could be studied. All subsequent sample manipulations were performed aseptically. The soil micro-organisms were quantified using the dilution-plate method (Zhang et al. 1985). We used the following media to culture soil micro-organisms: bacteria were cultivated on Tryptone Soya Agar medium, fungi were cultivated using Martin’s medium, and actinomycetes were cultivated using casein-starch culture medium. The bacteria were cultured for 2 to 3 days, fungi were cultured for 5 to 7 days, and actinomycetes were cultured for 10 to 14 days until a single colony developed. (All culturing was done at 30°C). Each sample was diluted three times. The soil samples to a depth of 30 cm in each soil collar were also oven-dried at 105°C overnight to determine the soil water content (mass basis). Soil pH was measured in water (1:2.5 w/v). We measured total C according to the method of Walkley–Black wet oxidation technique (Nelson and Sommers 1982) and total N according to Kjeldahl method (Bremner 1960).
Statistical analysis
The soil and root respiration data were subjected to analysis of variance (ANOVA) using version 13.0 of the SPSS software (SPSS Inc., Chicago, Illinois, USA), followed by a multiple-comparison test to compare the means based on the least-significant-difference (LSD) method, with a significance level of P < 0.05. We also used correlation analysis (Pearson’s coefficient) and partial correlation analysis to analyze our experimental data using the SPSS software.
Results
Climate
Figure 1 presents the precipitation and the air and soil temperatures during the study period. Monthly precipitation varied throughout the year, with a total accumulation of 175 mm during the study period, which was lower than the mean annual precipitation of about 221.3 mm (Fig. 1). In 2007, the maximum precipitation occurred in July and August, and the minimum occurred in November. In 2008, the maximum precipitation occurred in April and July. The precipitation differed dramatically between July 2007 and July 2008, but the summer temperatures were comparable in both years. The mean daily air temperature showed a typical seasonal pattern for the study area, with the peak appearing from July to August in both years. The daily mean air temperature reached a maximum of 27.2°C in the summer and a minimum of -23.4°C in the winter. The daily mean soil temperatures reached a maximum of 26.8°C in the summer and minimum of -5.48°C in the winter.
Seasonal patterns of soil respiration at different forest ages
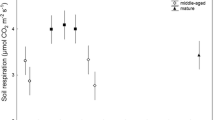
The total respiration varied obviously among the ecosystems at different forest ages. Analysis of variance indicated that forest age significantly affected soil respiration. There were significant differences between the 5-year-old forest and the 10-year-old forest (P < 0.05) and between the 5-year-old forest and the 15-year-old forest (P < 0.05), but not between the 10-year-old and 15-year-old forests (P > 0.05) (Fig. 2). In 2007, soil respiration in the 5-year-old forest ranged from 2.45 to 7.27 μmol m-2 s-1, versus ranges from 3.35 to 4.93 μmol m-2 s-1 in the 10-year-old forest and from 1.8 to 3.95 μmol m-2 s-1 in the 15-year-old forest. In 2008, soil respiration in the 5-year-old forest ranged from 2.75 to 7.93 μmol m-2 s-1, versus ranges of 1.87 to 4.74 μmol m-2 s-1 in the 10-year-old forest and 2.12 to 2.61 μmol m-2 s-1 in the 15-year-old forest. The soil respiration in the 5-year-old forest was significantly (P < 0.05) higher than those in the older forests in two years, but the 10-year-old forest had significantly higher soil respiration than the 15-year-old forest only in 2008.
Monthly changes in soil respiration for the three forests. The 5-year-old forest differed significantly from both the 10-year-old forest and the 15-year-old forest (P < 0.05), but the 10-year-old forest did not differ significantly from the 15-year-old forest (P > 0.05). Values represent means ± S.E. (n = 6)
Soil respiration showed a similar single-peak pattern in all three forests from May to October (Fig. 2). The mean daily soil respiration rate was low in May and increased sharply with increasing temperature and soil moisture, reaching a peak in August in most cases (except for the 10-year-old forest in 2008, for which respiration peaked in July). Soil respiration subsequently decreased into September and October. The magnitude of the seasonal fluctuation in the soil respiration rate was greatest in the 5-year-old forest, followed by the 10-year-old forest, but the 15-year-old forest showed much smaller fluctuations.
Effects of abiotic and biotic factors on soil respiration
Correlation analysis showed that soil respiration was more strongly correlated with soil temperature (2004, R = 0.622; 1999, R = 0.673; 1993, R = 0.818; P < 0.001) than with soil moisture (2004, R = 0.573; 1999, R = 0.596; 1993, R = 0.788; P < 0.0001) (Fig. 3a, b). The relationship is equally good at three ages and the relevance increased obviously with increasing forest age. We also found a significant positive correlation between soil temperature and soil moisture at three different forest ages (2004, R = 0.803; 1999, R = 0.848; 1993, R = 0.805; P < 0.0001) (Fig. 3c). The effects of soil temperature and soil moisture are therefore confounded. To clarify the contribution of these two factors to the observed changes in soil respiration rate, we performed a partial correlation analysis. With soil moisture as the control variable, the correlation between soil temperature and soil respiration was significant (P < 0.01) and positive (0.439). However, with soil temperature as the control variable, the correlation between soil moisture and soil respiration was small (0.047) and not significant (P > 0.05). Thus, during the poplar growing season, soil temperature exerts a stronger control than soil moisture on soil respiration.
To clarify the effects of biotic and abiotic factors on soil respiration, we also analyzed microbiological factors (total microbial amount, the ratio of bacteria to fungi), fine-root biomass, soil C, and soil N. Our correlation analysis between the mean monthly values of these factors and soil respiration (Fig. 4) showed that soil respiration was significantly and positively correlated with soil temperature (R = 0.724, P < 0.01), fine-root biomass (R = 0.666, P < 0.05), total soil microbiological content (R = 0.596, P < 0.05), and the bacteria/fungi ratio (R = 0.645, P < 0.05). However, there was no significant correlation with soil moisture (R = 0.438, P > 0.05), soil C (R = -0.178, P > 0.05), or soil N (R = -0.469, P > 0.05). Because of the interactions among various factors, such as the high and significant correlation between soil temperature and soil moisture (Table 3), the correlation coefficients for each variable do not reflect the real situation for the isolated relationship between each variable and soil respiration. Therefore, partial correlation analysis should be performed. Partial correlation analysis (Table 4) showed that the fine-root biomass and soil respiration had the strongest significant (P < 0.05) correlation (R = 0.718), followed by the bacteria/fungi ratio (R = 0.612), soil carbon (R = 0.600), and soil temperature (R = 0.501); however, soil moisture and total microorganisms were not significantly correlated with soil respiration, and soil N was significantly negatively correlated (R = -0.646)
Root respiration at different forest ages
Figure 5 shows the seasonal changes in the ratio of root respiration to total soil respiration (R r/R s; Fig. 5a) and in root respiration (Fig. 5b) in the three forests. The contribution of R r to R s differed among the three forests, and ranged from 29.4 to 81.0%. R r/R s ranged from 50.1 to 81.0% in the 15-year-old forest, from 39.2 to 48.7% in the 10-year-old forest, and from 29.4 to 46.7% in the 5-year-old forest. R r/R s was significantly (P < 0.05) higher in the 15-year-old forest than in the two younger forests, which did not differ significantly from each other.
Root respiration (Fig. 5b) ranged from 1.20 to 2.63 μmol m-2 s-1, with minimum values in June and increases to maximum values in July and August. Our ANOVA results showed that forest age significantly affected both root and soil respiration. There were obvious and significant differences, with higher root respiration in the 5-year-old forest than in the 10-year-old forest (P < 0.01) and the 15-year-old forest (P < 0.05), but there was no significant difference between the 10-year-old and 15-year-old forests (P > 0.05). Root respiration ranged from 1.95 to 2.63 μmol m-2 s-1 in the 5-year-old forest, from 1.24 to 2.31 μmol m-2 s-1 in the 10-year-old forest, and from 1.20 to 1.87 μmol m-2 s-1 in the 15-year-old forest.
Root biomass
There were significant differences in total root biomass and fine-root biomass among the three forest ages (Fig. 6). The 5-year-old forest had significantly higher fine-root biomass than the two older forests, but had significantly lower root biomass. The fine-root biomass decreased with increasing forest age, but total root biomass increased with increasing age. The fine roots were distributed mainly in the surface 30 cm, and were rarely found in the 30- to 60-cm horizon (data not shown).
Discussion
The seasonal patterns of soil respiration
Soil respiration in the 5-year-old forest was significantly higher than that in the older forests. The rate decreased as forest age increased. The magnitude of the fluctuations in soil respiration in response to changes in environmental factors also decreased with age. Few previous studies have examined the effect of forest age on soil respiration. A trend of decreasing soil respiration with increasing forest age was previously reported for fir, spruce, pine and oak (Law et al. 1999; Saiz et al. 2006; Tedeschi et al. 2006). In contrast, soil respiration was reported to increase with forest age for slash pine plantations in Florida (Ewel et al. 1987) and loblolly pine plantations in Virginia (Wiseman and Seiler 2004). In our poplar forests, there was no significant difference in soil respiration between the 10-year-old and 15-year-old forests. High soil respiration rates in the 5-year-old forest may result from the high physiological activity that is associated with root growth respiration. Studies have shown that increasing respiration is associated with the synthesis of new tissue (Chen et al. 2009). High temperature is another reason for the high soil respiration rates in the 5-year-old fores that is likely to have lower canopy cover than the older forests, the soils are likely to be warmer..With increasing forest age, respiration by soil micro-organisms and roots both decrease gradually and then tend to stabilize. At the same time, the bacteria/fungi ratio decreases with age. Fungal-dominated soil communities may enhance C storage and slow the turnover of soil organic matter because the fungi change the physical properties of the soil (Six et al. 2006). Fungi grow faster than filamentous bacteria and have a stronger ability to take up nutrients, and therefore use soil organic carbon and nitrogen more efficiently to form their own biomass storage and reduce soil CO2 emissions (Beare et al. 1992; Thiet et al. 2006). As a result, the lower soil respiration in the 10-year-old and 15-year-old forests would improve the efficiency of carbon sequestration. The change in soil respiration rates as these forests age may therefore affect the long-term carbon balance of the forests.
Root respiration
In this study, the excision method was used to determine root respiration. Some common techniques involve comparing soil respiration measurements; root respiration was based on root-free plots: roots are killed by digging of a trench around the plot (Epron et al., 1999). A disadvantage of these techniques is that the estimations of the component fluxes are bound to be correlated, as one is deduced from the other. Direct gas-exchange measurements on living roots and subsequent upscaling are one such option for the independent estimation of the autotrophic component. This is a standard approach for the quantification of above-ground woody respiration (Damesin et al., 2002). A disadvantage of these techniques is that the soil pore structure is destructed and Soil microbial activity is disturbed. Root respiration varied seasonally, with a peak in July and August, followed by a decrease until September. Root respiration is often significantly correlated with soil temperature and soil water content (Wiseman and Seiler 2004), and we obtained this result in the present study. Root respiration peaked in July and then decreased until September in all three forests. The peak of root respiration appeared at the time of year when temperature and rainfall were highest. With decreasing temperature and rainfall, root respiration also decreased.
The forest age significantly affected root respiration, which decreased with increasing age. Root respiration was significantly higher in the 5-year-old forest than in the older forests (P < 0.01; Fig. 5). Earlier studies also showed that root biomass significantly affected soil respiration (Klopatek 2002; Saiz et al. 2006).
Fine-root biomass was positively and significantly correlated with root respiration (R = 0.904, P < 0.001; Fig. 7a). This is in agreement with previous reports, in which fine roots had high physiological activity that was associated with root growth respiration and the synthesis of new tissue (Chen et al. 2009). In contrast, we found a significant negative correlation between the total root biomass and root respiration(R = -0.792, P < 0.05, Fig.7b). Thus, the coarse roots contributed relatively little to total root respiration.
Contribution of root respiration to total soil respiration
The contribution of root respiration to total soil respiration varies with vegetation type, species composition, soil type, season of year, the measurement methods employed, and environmental conditions (Hanson et al. 2000; Lee et al. 2003; Tang et al. 2005; Bond-Lanmberty et al. (2004). Hanson et al. (2000) summarized the results of 50 studies, in which root respiration accounted for 10 to 90% of total soil respiration. J.W. Tang et al. (2005) reported a range of 16 to 56% in a ponderosa pine plantation. Lee et al. (2003) reported a range of 27 to 71% for a temperate deciduous forest. Bond-Lanmberty et al. (2004) reported annual root respiration was < 5% of RS in the recently Burned stands, 40% in the 21-year-old stands and 5–15% in the oldest (152-year-old) stands. In our study, the R r/R s ratio ranged from 29.4 to 81.0%, but the contribution varied among the forests. R r/R s was significantly higher in the 15-year-old forest than in the younger forests. In the study area, the 15-year-old forest is already considered to be relatively mature. Nakane et al. (1996) reported that the contribution of root respiration to total soil respiration was 51% in a mature deciduous broad-leaved forest. Our estimates are slightly higher than those in previous studies. Although the fine-root biomass in the 15-year-old forest was less than that in the 5-year-old forest, its contribution to the total soil respiration was higher, indicating a lower contribution by microbial respiration. This is consistent with the lower total amounts levels of micro-organisms in the 15-year-old forest. The contribution of root respiration to total soil respiration in the 10-year-old forest ranged from 39.2 to 48.7%, versus values of 29.4 to 46.7% in the 5-year-old forest. The 10-year-old and 5-year-old forests were relatively young, and the root systems may therefore have had greater activity. If the soil microclimate was conducive to microbial activity, this would explain why the contributions of microbial respiration to the total respiration were slightly higher in these forests.
Factors affecting soil respiration
Soil respiration is controlled by biotic and abiotic factors such as temperature, soil water content, soil microbial biomass, vegetation type, photosynthetic characteristics of the vegetation, the management of the forests, and the soil organic carbon content (Raich & Schlesinger 1992; Fernandez et al. 1993; Neergaard et al. 2002; Adachi et al. 2006; Subke et al. 2006). In general, soil temperature and soil water content are the key factors that affect the temporal variation in soil respiration (Rey et al. 2002; Iqbal et al. 2008). In the present study, we found similar correlations, but soil respiration was more strongly correlated with soil temperature than with soil moisture. We also found a significant positive correlation between soil temperature and soil moisture, which agrees with the results of previous studies (Tang et al. 2006).
Our partial correlation analysis confirmed that soil temperature was significantly positively correlated with soil respiration, but we found no significant partial correlation between soil moisture and soil respiration, suggesting that the contribution of soil temperature to soil respiration is stronger than that of soil moisture. Therefore, temperature played the dominant role in soil respiration in the poplar community. As a result of the correlation between the two factors, the effect of soil moisture on soil respiration resulted primarily from the effect of temperature on soil respiration. When other factors were added to the analysis, we found that the soil respiration was strongly influenced by the fine-root biomass, total soil microbial production, and the bacteria/fungi ratio. High fine-root biomass and high soil micro-organism levels both caused high soil respiration. Our results also showed that the total soil micro-organisms and bacteria/fungi ratio were strongly positively correlated with soil temperature. Rising temperatures can increase the physiological activity of soil micro-organisms, leading to higher decomposition rates and higher soil respiration rates (Han et al. 2007).The lower degree of canopy cover in the 5-year-old forest than in the older forests led to increased soil temperatures, which would have increased microbial respiration. The respiration by soil microbes that decompose soil organic matter is a major factor that affects the soil respiration rate (Adachi et al. 2006).
The partial correlations in our analysis differed greatly among the factors. The major factors responsible for changes in the soil respiration rate were fine-root biomass, the bacteria/fungi ratio, total soil C, and soil temperature. Soil respiration was positively correlated with the fine-root biomass (R = 0.718, P < 0.0001), indicating that the fine-root component of root biomass significantly affects soil respiration in forests of different ages. The fine-root biomass was higher in the 5-year-old forest than in the older forests, and higher fine-root biomass caused a high respiration rate, which is similar to the results of previous studies (Wiseman and Seiler 2004; Yi et al. 2007). Fine roots have very strong physiological functions such as high metabolic activity, strong nutrient uptake and transport capacity, and high growth respiration (Wells and Eissenstat 2001; Pregitzer 2002; Chen et al. 2009). Root exudates and root litter enhance soil respiration by stimulating microbial growth and activity (Lohila et al. 2003). Thus, fine-root biomass is a major factor that affects soil respiration in our poplar forest system. Soil N was significantly negatively correlated (R = -0.646). High soil nitrogen is conducive to higher fungi and actinomycetes breeding (Laughlin and Stevens, 2002). Fungi have a stronger ability to take up nutrients, and therefore use soil organic carbon and nitrogen more efficiently to form their own biomass storage and reduce soil CO2 emissions (Thiet et al. 2006)
Some studies have reported that soil respiration was significantly correlated with microbial biomass (Adachi et al. 2006; Yi et al. 2007). In our study, the partial correlation analysis showed a moderate but significant (R =0.596, P < 0.05; Fig. 5) positive correlation between the total microbial production and soil respiration. However, soil respiration was more strongly positively correlated with the bacteria/fungi ratio (R = 0.645, P < 0.05; Fig. 4), indicating that the contribution of bacteria/fungi ratio to soil respiration is stronger than that of the total microbial production. The structure of the microbial community affects soil respiration, and in our poplar forests, this effect resulted primarily from the impact of the bacteria/fungi ratio on soil respiration. The balance between the inputs and losses of organic matter determine the net effect on soil C levels. Bacteria and fungi typically comprise more than 90% of the total soil microbial biomass and are responsible for the decomposition of soil organic matter, but their impact changes in response to changes in the microbial community composition and function (Frey et al. 1999; Six et al. 2006). Thus, these micro-organisms play an important role in determining the rates of C loss from the soil. As the forest aged, the bacteria/fungi ratio decreased. Bacteria have lower C assimilation efficiency than fungi, and therefore store less of the C that they metabolize (Bailey et al. 2002). Fungal-dominated soil communities may therefore enhance C storage and slow the turnover of soil organic matter because the fungi change the physical properties of the soil (Beare et al. 1992; Thiet et al. 2006).
Our partial correlation analysis revealed that the soil C content was significantly positively correlated with soil respiration and was the major contribution to the soil respiration rate. Soil organic C is the major energy source for soil microbes (Adachi et al. 2006), and is also used by microbes as the primary resource in litter decomposition (Singh and Gupta 1977). Mineral soil carbon also represents a potential carbon substrate for microbes and accordingly affects their activity (Wang et al. 2002). Thus, soil respiration varies with the soil C content.
Conclusions
Our study demonstrated that soil respiration and root respiration vary significantly as a function of forest age in the poplar ecosystems we studied. Soil respiration of the young forest was remarkably higher than that of the older forests as a result of high physiological activity by fine roots, a high bacteria/fungi ratio and higher soil temperature which leads to less C storage and more soil CO2 emissions than in older forests. Thus, soil respiration in middle-aged and mature poplar populations is likely to improve the efficiency of carbon sequestration. Forest age may therefore affect the carbon balance of the forest. Root respiration decreases with increasing forest age, since fine-root biomass contributes more than the coarse root biomass to root respiration, and there are more fine roots in the younger forest. The contribution of root respiration to total soil respiration also varies with forest age, increasing with increasing age, and varies during the growing season. Our results also indicate that fine-root biomass, soil temperature, the bacteria/fungi ratio (i.e., the microbial community structure), and total soil C content are the dominant factors that control the temporal variation in soil respiration. Therefore, to accurately estimate soil respiration, researchers must not only monitor abiotic environmental factors such as soil temperature and soil moisture, but must also monitor belowground abiotic and biotic factors such as fine-root biomass, total microbial production, and the microbial community structure. The microbial community structure appears to be a particularly important factor.
References
Adachi M, Bekku YS, Rashidah W, Okuda T, Koizumi H (2006) Differences in soil respiration between different tropical ecosystems. Appl Soil Ecol 34:258–265
Bailey VL, Smith JL, Bolton JH (2002) Fungal-to-bacterial ratios in soils investigated for enhanced C sequestration. Soil Biol Biochem 34:997–1007
Beare MH, Parmelee RW, Hendrix PF, Cheng W, Coleman DC, Grossley DA (1992) Microbial and faunal interactions and effects on litter nitrogen and decomposition in agroecosystems. Ecol Monogr 62:569–591
Bond-Lanmberty B, Wang CK, Gower ST (2004) Contribution of root respiration to soil surface CO2 flux in a boreal black spruce chronosequence. Tree Physiol 24:1387–1395
Boone RD, Nadelhoffer KJ, Canary JD, Kaye JP (1998) Roots exert a strong influence on the temperature sensitivity of soil respiration. Nature 396:570–572
Bremner JM (1960) Determination of nitrogen in soil by the Kjeldahl method. J Agric Sci 55:11–33
Buchmann N (2000) Biotic and abiotic factors controlling soil respiration in Picea abies stands. Soil Biol Biochem 32:1625–1635
Chen D, Zhang Y, Lin YB, Chen H, Fu SL (2009) Stand level estimation of root respiration for two subtropical plantations based on in situ measurement of specific root respiration. For Ecol Manage 257:2088–2097
Damesin C, Ceschia E, Le Goff N, Ottorini JM, Dufrene E (2002) Stem and branch respiration of beech: from tree measurements to estimations at the stand level. New Phytol 153:159–172
Dilustro J, Collins B, Duincan L, Crawford C (2005) Moisture and soil texture effects on soil CO2 efflux components in southeastern mixed pine forests. For Ecol Manage 204:85–95
Dixon RK (1994) Carbon pools and flux of global forest ecosystems. Science 263:185–190
Ewel KC, Cropper WP, Golz HL (1987) Soil CO2 evolution in Florida slash pine plantations. 1. Changes through time. Can J Forest Res 17:325–329
Epron D, Farque L, Lucot E, Badot PM (1999) Soil CO2 efflux in a beech forest: the contribution of root spiration. Ann Forest Sci 56:289–295
Fang JY, Wang GG, Liu GH, Xu SL (1998) Forest biomass of China: an estimate based on the biomass- volume relationship. Ecol Appl 8:1084–1091
Fernandez IJ, Son Y, Kraske CR, Rustad LE, David MB (1993) Soil carbon dioxide characteristics under different forest types and after harvest. Soil Science Society of American Journal 57:1115–1121
Frey SD, Elliott ET, Paustian K (1999) Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biol Biochem 31:573–585
Frey B, Hagedorn F, Giudici F (2006) Effect of girdling on soil respiration and root composition in a sweet chestnut forest. For Ecol Manage 225:271–277
Gielen B, Ceulemans R (2001) The likely impact of rising atmospheric CO2 on natural and managed Populus. Environ Pollut 115:335–358
Granier A, Ceschia E, Damesin C, Dufrêne E, Epron D, Gross P, Lebaube S, Le Dantec V, Le Goff N, Lemoine D, Lucot E, Ottorini JM, Pontailler JY, Saugier B (2000) The carbon balance of a young beech forest. Funct Ecol 14:312–325
Han GX, Zhou GS, Xu ZZ, Yang Y, Liu JL, Shi KQ (2007) Biotic and abiotic factors controlling the spatial and temporal variation of soil respiration in an agricultural ecosystem. Soil Biol Biochem 39:418–425
Hanson PJ, Edwards NT, Garten CT, Andrews JA (2000) Separating root and soil microbial contributions to soil respiration: a review of methods and observations. Biogeochemistry 48:115–146
Iqbal J, Hu RG, Du LJ, Lu L, Lin S, Chen T, Ruan LL (2008) Differences in soil CO2 flux between different land use types in mid-subtropical China. Soil Biol Biochem 40:2324–2333
Kang S, Doh S, Lee D, Lee D, Jin VL, Kimball J (2003) Topographic and climatic controls on soil respiration in six temperate mixed-hardwood forest slopes, Korea. Glob Chang Biol 9:1427–1437
Klopatek JM (2002) Belowground C pools and processes in different age stands of Douglas-fir. Tree Physiol 22:197–204
Laughlin J, Stevens KJ (2002) Evidence for fungal dominance of denitrification and codenitrification in a grassland soil. Soil Sci Soc Am J 66:1540–1548
Law BE, Ryan MG, Anthoni PM (1999) Seasonal and annual respiration of a ponderosa pine ecosystem. Glob Chang Biol 5:169–182
Lee MS, Nakane K, Nakatsubo T, Koizumi H (2003) Seasonal changes in the contribution of root respiration to total soil respiration in a cool-temperate deciduous forest. Plant Soil 255:311–318
Li HJ, Yan JX, Yue XF, Wang MB (2008) Significance of soil temperature and moisture for soil respiration in a Chinese mountain area. Agr Forest Meteorol 148:490–503
Lloyd J, Taylor JA (1994) On the temperature dependence of soil respiration. Funct Ecol 8:315–323
Lohila A, Aurela M, Regina K, Laurila T (2003) Soil and total ecosystem respiration in agricultural fields: effect of soil and crop type. Plant Soil 251:303–317
Nakane K, Kohno T, Horikoshi T (1996) Root respiration rate before and just after clear felling in a mature, deciduous, broad-leaved forest. Ecol Res 11:111–119
Neergaard A, Porter JR, Gorissen A (2002) Distribution of assimilated carbon in plants and rhizosphere soil of basket willow (Salix viminalis L). Plant Soil 245:307–314
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. American Society of Agronomy, Madison, pp 539–579
Pregitzer KS (2002) Fine roots of trees: a new perspective. New Phytol 154:267–270
Raich JW, Schlesinger WH (1992) The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 44:81–99
Rey A, Pegoraro E, Tedeschi V, Parri I, Jarvis P, Valentini R (2002) Annual variation in soil respiration and its components in a coppice oak forest in central Italy. Glob Chang Biol 8:851–866
Saiz G, Byrne KA, Bahl KB, Kiese R, Blujdea V, Farrell E (2006) Stand age-related effects on soil respiration in a first rotation Sitka spruce chronosequence in central Ireland. Glob Chang Biol 12:1007–1020
Schimel DS (1995) Terrestrial ecosystems and the carbon cycle. Glob Chang Biol 1:77–91
Schlesinger WH, Andrews JA (2000) Soil respiration and the global carbon cycle. Biogeochemistry 48:7–20
Scott-Denton LE, Sparks KL, Monson RK (2003) Spatial and temporal controls of soil respiration rate in a high-elevation, subalpine forest. Soil Biol Biochem 35:525–534
Singh JS, Gupta SR (1977) Plant decomposition and soil respiration in terrestrial ecosystems. Bot Rev 43:449–528
Six J, Frey SD, Thie RK, Batten KM (2006) Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J 70:555–569
Stoyan H, De-Polli H, Bohm S, Robertson P, Paul E (2000) Spatial heterogeneity of soil respiration and related properties at the plant scale. Plant Soil 222:203–214
Subke JA, Inglima I, Cotrufo MF (2006) Trends and methodological impacts in soil CO2 efflux partitioning: a metaanalytical review. Glob Chang Biol 12:921–943
Tang JW, Misson L, Gershenson A, Cheng WX, Goldstein AH (2005) Continuous measurements of soil respiration with and without roots in a ponderosa pine plantation in the Sierra Nevada Mountains. Agr Forest Meteorol 132:212–227
Tang X, Liu S, Zhou G, Zhang D, Zhou C (2006) Soil-atmospheric exchange of CO2, CH4, and N2O in three subtropical forest ecosystems in southern China. Glob Chang Biol 12:546–560
Tedeschi V, Rey A, Manca R (2006) Different developmental stages after coppicing. Glob Chang Biol 12:110–121
Thiet RK, Frey SD, Six J (2006) Do growth yield efficiencies differ between soil microbial communities differing in fungal:bacterial ratios? Soil Biol Biochem 38:837–844
Valentini R, Matteucci G, Dolman AJ, Schulze ED, Rebmann C, Moors EJ, Granier A, Gross P, Jensen NO, Pilegaard K, Lindroth A, Grelle A, Bernhofer C, Grünwald T, Aubinet M, Ceulemans R, Kowalski AS, Vesala T, Rannik Ü, Berbigier P, Lousteau D, Gudmundsson J, Thorgeirsson H, Ibrom A, Morgenstern K, Clement R, Moncrieff J, Montagnani L, Minerbi S, Jarvis PG (2000) Respiration as the main determinant of European forests carbon balance. Nature 404:861–865
Wang C, Bond LB, Gower ST (2002) Environmental controls on carbon dioxide flux from black spruce coarse woody debris. Oecologia 132:374–381
Wells CE, Eissenstat DM (2001) Marked differences in survivorship among apple roots of different diameters. Ecology 82:882–892
Wiseman PE, Seiler JR (2004) Soil CO2 efflux across four age classes of plantation loblolly pine (Pinus taeda L.) on the Virginia Piedmont. For Ecol Manage 192:297–311
Yi HG, Fu SL, Yi WM, Zhou GY, Mo JM, Zhang DQ, Ding MM, Wang XM, Zhou LX (2007) Partitioning soil respiration of subtropical forests with different successional stages in south China. For Ecol Manage 243:178–186
Zhang X, Wang HX, Hai Y (1985) Institute of Soil Science, Chinese Academy of Science. Methods for soil microbial research. Science, Beijing, pp 40–54
Zsuffa L, Giordano E, Pryor LD, Stettler RF (1996) Trends in popular culture: some global and regional perspectives. In: Stettler RF, Bradshaw HD, Heilman PE, Hinckley TM (eds) Biology of Populus and its Implications for Management and Conservation (Part II, Chapter 19). NRC Research Press, National Research Council of Canada, Ottawa, pp 515–539
Acknowledgments
We thank the staff of the Plain Forest Farm Nursery of the State Forestry Bureau of Ili Autonomous Prefecture in China’s Xinjiang Autonomous Region for field assistance. This study was supported by the National Natural Science Foundation of China (No. 40771069 and 41030535). The authors highly appreciate the comments of two anonymous reviewers, which greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
About this article
Cite this article
Gong, J., Ge, Z., An, R. et al. Soil respiration in poplar plantations in northern China at different forest ages. Plant Soil 360, 109–122 (2012). https://doi.org/10.1007/s11104-011-1121-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-1121-3