Abstract
Soil CO2 efflux (Fsoil) is a significant contributor of labile CO2 to the atmosphere. The Himalayas, a global climate hotspot, condense several climate zones on account of their elevational gradients, thus, creating an opportunity to investigate the Fsoil trends in different climate zones. Presently, the studies in the Indian Himalayan region are localized to a particular forest type, climate zone, or area of interest, such as seasonal variation. We used a portable infrared gas analyzer to investigate the Fsoil rates in Himalayan tropical to alpine scrub forest along a 3100-m elevational gradient. Several study parameters such as seasons, forest types, tree species identity, age of trees, distance from tree base, elevation, climatic factors, and soil physico-chemical and enzymatic parameters were investigated to infer their impact on Fsoil regulation. Our results indicate the warm and wet rainy season Fsoil rates to be 3.8 times higher than the cold and relatively dry winter season. The tropical forest types showed up to 11 times higher Fsoil rates than the alpine scrub forest. The temperate Himalayan blue pine and tropical dipterocarp sal showed significant Fsoil rates, while the alpine Rhododendron shrubs the least. Temperature and moisture together regulate the rainy season Fsoil maxima. Spatially, Fsoil rates decreased with distance from the tree base (ρ = − 0.301; p < 0.0001). Nepalese alder showed a significant positive increase in Fsoil with stem girth (R2 = 0.7771; p = 0.048). Species richness (r, 0.81) and diversity (r, 0.77) were significantly associated with Fsoil, while elevation and major edaphic properties showed a negative association. Surface litter inclusion presented an elevation-modulated impact. Temperature sensitivity was exorbitantly higher in the sub-tropical pine (Q10, 11.80) and the alpine scrub (Q10, 9.08) forests. We conclude that the rise in atmospheric temperature and the reduction in stand density could enhance the Fsoil rates on account of increased temperature sensitivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil and biotic carbon (C) pools together constitute the third largest global carbon pool, i.e., terrestrial carbon pool, with an estimated reserve of 1950–3050 petagrams (Pg) of C (Friedlingstein et al., 2020; Lal, 2008). Several other studies place the estimated reserve from 2860 to 3123 Pg C (Lal, 2008; Lal et al., 2021; Wani et al., 2022); such discrepancies are due to the uncertainties in the terrestrial carbon fluxes (Lovenduski & Bonan, 2017). The influx of carbon in terrestrial C pool is through the process of photosynthesis (108–120 Pg year−1), while the pool loses C to the atmospheric pool primarily through the process of soil respiration (Rs) (60–107 Pg year−1) and through terrestrial fires (Hari & Tyagi, 2022; Lal, 2008). Excessive carbon emission into the atmospheric pool is critical since the labile atmospheric carbon, principally in the form of carbon dioxide, is responsible for global warming (Lashof & Ahuja, 1990). Soil respiration (Rs) is the largest source of CO2 efflux to the atmosphere and even exceeds the CO2 release from fossil fuel combustion by seven times (Giardina et al., 2014). A slight change in Rs levels could cause significant variations in the atmospheric C pool (Trumbore, 2006). The global surface warming hiatus during 1999–2014 hints towards global temperature stabilization in the early twenty-first century. Its relapse, however, is imminent due to a rebounding increase in mean surface air temperatures (Lei et al., 2021). Estimating the levels of Rs is therefore crucial for developing climate policies.
Besides its role in the terrestrial C cycle, Rs is a prominent soil quality indicator, primarily due to its sensitivity towards vegetational changes, anthropogenic activities, soil contamination, and ecosystem degradation (Bastida et al., 2008; Cardoso et al., 2013). Soil respiration has two functional components or sources: autotrophic and heterotrophic respiration (Kuzyakov, 2006), and the terms Rs and soil CO2 efflux (Fsoil) are erroneously used interchangeably (Maier et al., 2011). Soil respiration under instantaneous estimation is limited up to 67% through several features such as soil pore spaces, dissolution with soil water, and biological or chemical reactions (Maier et al., 2011; Sánchez-Cañete et al., 2018). Therefore, for short-term measurements, the true soil respiration rate cannot be determined, and such measurements thus give an estimate of soil CO2 efflux. The Fsoil is a quantitative measure that describes the steady-state CO2 efflux dynamics and generally reflects the soil health (Bastida et al., 2008; Laishram et al., 2012).
Globally, Fsoil regulation is at the temporal and spatial levels, along with perturbations from natural and anthropogenic disturbances (Luo & Zhou, 2006). The temporal regulation of Fsoil is primarily determined by soil temperature and moisture variations (Raich & Potter, 1995). Soil temperature influences Fsoil by regulating microbial respiration, soil microfaunal populations, and root respiration levels, while soil moisture is regulatory only at very low (drought) or very high (waterlogging) levels (Epron et al., 1999; Fang & Moncrieff, 2001). Besides these abiotic factors, the vegetation type and substrate quality also impact Fsoil (Jenkins & Adams, 2011). Most global and regional studies have associated temperature and moisture influences on Rs and Fsoil, while the impact of vegetational characteristics and their spatial distribution remains largely understudied (Giardina et al., 2014; Metcalfe et al., 2011; Schurman & Thomas, 2021).
Within the terrestrial ecosystem, forests cover the most considerable portion, and trees in the forest ecosystem have the largest share of the forest C pool (Lorenz & Lal, 2010). Therefore, understanding the influence of tree species on Fsoil could help with regional C management goals. Impact of climate change is not uniform throughout the terrestrial ecosystem. The mountain ecosystems face the most significant climate change impact (Becker & Bugmann, 2001). Climate change greatly impacts the Rs in the cold and high-latitude regions of the world (Karhu et al., 2014; Lei et al., 2021). The Himalayan mountains, especially the central and western parts, have more significant warming patterns than the average global trend (Schickhoff et al., 2016). Apart from this, the Rs and Fsoil research in the Himalayan region remains limited, and detailed in situ studies have recently emerged using sensitive measurement techniques. Recent studies in the Himalayas focus on several aspects, viz., influence of different temperate tree species on Rs under different seasons (Kumar et al., 2023; Rawat et al., 2021); use of Fsoil as an indicator to understand the role of a particular species (Alnus nepalensis D.Don) in influencing soil properties (Joshi & Garkoti, 2020); role of soil macrofauna such as termites in regulating Fsoil (Chakraborty et al., 2021); impact of land-use systems on Rs (Bhuyan et al., 2014; Vikram et al., 2022); impact of different ecosystems on Rs (Byanjankar et al., 2020); determining the controlling factors such as temperature, precipitation, and slope aspect on Fsoil (Dhital et al., 2022; Kumar et al., 2020); large-scale assessment of vegetational, topographic, and edaphic drivers of Rs (Pandey et al., 2023; Wangdi et al., 2017); impact of soil erosion on terrestrial carbon loss through soil respiration (Mariappan et al., 2022); impact of forest fire on soil basal respiration (Singh et al., 2021); seasonal variation in night time Fsoil under conifer (Pinus roxburghii) plantation (Singh & Parida, 2019); experimental warming impact on alpine Rs and ecosystem respiration (Tiwari et al., 2021); and even net ecosystem exchange of CO2 including Fsoil (Singh et al., 2019).
In the IHR, older studies investigated the impact of seasons and vegetation on Fsoil primarily using the alkali-trap-based method for Fsoil determination (Bargali et al., 1992; Dar et al., 2015; Joshi, 1994; Ramesh et al., 2013). This method has lower sensitivity than infrared gas analyzer (IRGA)–based systems (Pongracic et al., 1997). Furthermore, studies incorporating the impact of vegetational characteristics on Fsoil in the Himalayas are primarily restricted to specific forest type/s, species, climate zones, or land use classes (Bargali et al., 1992; Joshi, 1994; Joshi & Garkoti, 2020; Joshi et al., 1991; Kumar et al., 2023; Pandey et al., 2023; Rawat et al., 2021; Vikram et al., 2022). The elevational gradients in mountain ecosystems mimic the latitudinal patterns, and the former prove advantageous because of their steep gradients, accommodating several climate zones in smaller geographic areas. This helps to reduce the spatial extent, thus enhancing replicates for ecological studies (Sanders & Rahbek, 2012). With immense topographic heterogeneity and being climate-sensitive, Himalayan forests are key in understanding the spatio-temporal determinants and vegetational characteristics influencing soil CO2 efflux trends along wider elevational transects.
We, thus, undertook this study to understand the impact of elevation on Fsoil in major forest types of Central Himalayas, India, covering an elevational span of 3100 m. Furthermore, we also investigated temporal, spatial, and tree species–associated features (species identity and diversity) to determine how each variable influences Fsoil at different elevational ranges. This study is probably a first-of-its-kind study in the Indian Himalayan region (IHR) along a broader elevational gradient.
We hypothesized that (1) forest types will have different Fsoil rates on account of the variation in species composition; (2) Fsoil will have temporal variations (seasons) at forest type and species level due to the seasonality of soil temperature and moisture trends; (3) forest floor litter will impact total surface CO2 efflux rates on account of litter respiration; and (4) spatial factors like distance from tree base, age class, topographic, climatic, species diversity, and edaphic factors will impact Fsoil rates. To test these hypotheses, the primary objectives of our study included the following:
-
(a)
Measure Fsoil along an elevational gradient at forest type and tree species level.
-
(b)
Estimate the temporal variation (seasonal) in Fsoil rates for forest types and tree species together with the temperature sensitivity (Q10).
-
(c)
Measure the influence of surface litter on the CO2 efflux rates.
-
(d)
Calculate the impact of distance from the tree base on Fsoil rates (spatial variation) and tree species age class (stem girth) on Fsoil rates.
-
(e)
Measure the impact of elevation, species diversity, topographic, climatic, soil physical, chemical, and enzymatic properties on Fsoil rates.
Materials and methods
Study area description
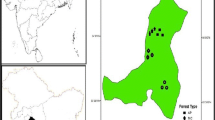
Nine forest stands were selected in the Garhwal region of Uttarakhand, India (Fig. 1). Uttarakhand represents the Indian portion of the Central Himalayas, referred to as the Indian Central Himalayas (ICH). The ICH has 44 recognized forest types (Champion & Seth, 1968). Based on site accessibility and protected area status (to reduce anthropogenic interference), nine forest stands were selected representing nine forest types (FT) (Table 1, Fig. 1). Forest types selected are major forest types, as they represent 63.42% of the total forest cover of the state (FSI, 2019). The study sites lie between the geographic bounds of 30° 00′ to 30° 46′ N latitude and 77° 48′ to 79° 13′ E longitude (Fig. 1), covering a wide elevational span from 360 to 3510 m (Table 1).
The forest type FT1 lie in the core zone of Rajaji National Park. Forest types FT2, FT4, and FT6 are reserve forests, while FT3, FT5, FT7, FT8, and FT9 lie in the core zone of the Kedarnath Wildlife Sanctuary. The climate of the study area has three distinct seasons: cold and relatively dry winter season (November–February), warm and dry summer season (April–June), and warm and wet rainy season (July–September). Short spring and autumn seasons are prevalent during March and October, respectively (Singh & Singh, 1987). The winter season in the sub-alpine to alpine regions may extend up to April (Rai et al., 2012). The forest types FT3–FT9 receive snowfall during the winter months, with FT5–FT9 receiving heavy snowfall, leading to high snow density and depth. Therefore, we collected the Fsoil measurements in these forest stands either after the first spell of light snowfall or during the first week of December, when the precipitation chances are lower (Fick & Hijmans, 2017). Overall, the study area experiences a monsoon climate with maximum rainfall during July and August and the least from October to December. Within the study area’s elevational range (Table 1), the average maximum monthly temperature ranged from 17.19 to 38.23 °C, while the average minimum monthly temperature ranged from − 5.44 to 6.97 °C (Fick & Hijmans, 2017).
The vegetational characteristics, community structure, and anthropogenic disturbance levels for each forest type (except FT9) have been detailed previously (Kaushal et al., 2021). The dwarf Rhododendron scrub forest (FT9) at Chandrashila is dominated by an alpine shrub species, Rhododendron anthopogon D.Don (Rai et al., 2012).
Along the elevational gradient, the forest types lie in the Cenozoic Himalayan Foreland Basin, Inner Lesser Himalayan sedimentary belt, and Higher Himalayan Crystallines belt. The major geologic constituents in the study sites are sandstones, shales, carbonates, mica schists, gneisses, and granites (Jain, 2020). Based on the digital FAO-UNESCO soil map of the world (FAO, 2007), the tropical forest types FT1 and FT2 have Calcaric Fluvisols soil types. Dystric Cambisols make up the FT4 and FT6 soil types, whereas Lithosols, Humic Cambisols, and Rankers combine to form the primary soil type for FT3, FT5, FT7, FT8, and FT9, respectively (Supplementary Fig. S1).
Study sampling design and acquisition of remotely sensed topographic and climate data
A preliminary reconnaissance survey for the analyses of Fsoil was carried out from September to December 2020. The standard quadrat method was used for vegetation sampling and Fsoil measurements. In FT1 to FT6, ten square quadrats of dimension 1000 m2 (31.6 m × 31.6 m) were randomly laid in each forest type to cover 1 ha of area (Kaushal & Baishya, 2021; Kaushal et al., 2021). In FT7 and FT8, due to undulating terrain and unpredictable weather conditions, five quadrats (1000 m2) were laid in each forest type. In alpine forest type FT9, five quadrats of 25 m2 (5 m × 5 m) were laid due to the limited terrain and being a tapering mountain peak (Chandrashila Peak). Therefore, the total area surveyed in this study for the in situ Fsoil measurements was 7.0125 ha. All the Fsoil measurements were conducted within the permanent plots established.
Site-specific topographic estimates of elevation, slope, and slope aspects were classified from the digital elevation model (DEM) data of the shuttle radar topography mission (SRTM) at 1-Arcsec spatial resolution (ca. 26 m at 30° latitude) (https://earthexplorer.usgs.gov/). The average monthly climate data, viz., minimum temperature (Tmin) (°C), maximum temperature (Tmax) (°C), precipitation (mm), solar radiation (kJ m−2 day−1), and water vapor pressure (kPa), was extracted from WorldClim global dataset at 30-Arcsec resolution (Ver. 2.1 released, 2020) (Fick & Hijmans, 2017). The topographic and climate raster layers were processed using ArcGIS (ver. 10.5) software.
Determination of in situ soil CO2 efflux (Fsoil)
The in situ Fsoil was measured from February 2021 to June 2022 using the CI-340 Portable Photosynthesis System (CID Bio-Science, Inc., USA) (Fig. 2a) equipped with a CI-301SR soil respiration chamber (Fig. 2b). The chamber has an area of 73.4 cm2 and a volume of 0.58 liters when inserted to a depth of 1 cm in the soil. CI-340 is an environmentally sealed, non-dispersive infrared gas analyzer (IRGA) unit coupled with a gas pump, flow regulators, and environmental analyzers. The system was operated in closed-system mode with 1-s sampling (IRGA detection) interval, 0.5-l min−1 gas flow rate, and 15 to 180 s of measurement time. The measurement time varied to accommodate the Fsoil range since a shorter sampling time (15 s) avoids the oversaturation of the analyzer (2200 ppm CO2) during measurement. An infrared (IR) temperature sensor attached to the soil chamber determines the soil surface (T0) temperature (°C), while a photosynthetically active radiation (PAR) sensor determines the incoming PAR values (μmol m−2 s−1) (Fig. 2b).
Quantification of in situ Fsoil rates. a CI-340 Portable IRGA with CI-301SR soil chamber in closed-system mode (see the looped connector tube on the top); b soil chamber with IR temperature sensor and PAR sensor; also notice a soil temperature probe inserted next to the chamber; distance effect from tree base under c Tectona grandis (FT1) and d Aesculus indica (FT5); temporal effect under rainy and winter season respectively at FT1 (e, f), FT3 (h, i), and FT9 (k, l); notice the effect of season on the ground flora; g Fsoil under forest type (note equidistant placement) at FT7; j Fsoil determination under tree species base (Cedrus deodara, FT6)
The Fsoil in the studied forest types was randomly analyzed at two points within each quadrat. Thus, for the 75 quadrats laid in the study, 150 Fsoil measurements were taken which represents 20 Fsoil measurements each for FT1–FT6 and 10 each for FT7–FT9 for a particular season. The measurements were taken at least 3–5 m from any tree or large shrub to avoid the undue effects of specific plant species. Before inserting the chamber, freshly fallen and surface litter was removed, and any herbaceous growth was clipped with field scissors to avoid CO2 capture due to photosynthesis (Fig. 2a and g). Forest type–specific Fsoil estimates were collected seasonally to determine the temporal (seasonal) influence. The Fsoil under forest types and dominant or major tree species (Fig. 2j) was measured separately in two seasons, i.e., rainy and winter (Fig. 2e, f, h, i, k, and l). These seasons were selected due to the maximum differential in their temperature and precipitation parameters. The summer season (May–June) and the rainy season were alike in terms of temperature parameters. It was thus not included and only used for exploratory measurements in climax forest types, viz., FT1, FT3, FT4, and FT6. For tree species–specific Fsoil measurements, around 15–20 replicates were collected per species per season and these measurements are independent of the seasonal forest-type Fsoil determination. The measurements were made during the peak period of the winter (December–January) and rainy season (July–August). Only one set of measurements was taken for each season to reduce the within-season variability.
Organic litter-associated CO2 efflux was estimated by placing the chamber on the ground without removing the surface litter, followed by a second measurement after clearing the surface litter. For the impact of litter on Fsoil, we randomly sampled 15 replicates within the permanent quadrats for each forest type. The effect of distance from the tree base (spatial variation) on Fsoil was determined for dominant or major tree species (Kaushal et al., 2021) at three distances from the tree base, viz., 0 cm (just under the tree trunk), 100 cm, and 250 cm (Fig. 2c, d). Around 5–10 individuals of each tree species were randomly selected for the distance effect. The influence of age class (determined using stem girth) on Fsoil was estimated at the base of dominant or major tree species under varying stem girths. The forest types studied had individuals of various girth classes (Kaushal & Baishya, 2021), and around 10–20 individuals were selected for each species, incorporating the maximum girth range available.
Quantification of soil physical, chemical, and enzymatic properties
Samples for soil analyses (FT1 to FT8 only; FT9 excluded owing to rugged terrain) were randomly collected from two points within each quadrat during the rainy and winter seasons (70 quadrats × 2 samples per quadrat × 2 seasons). Soil samples were collected from 0 to 10 cm soil depth using a soil hoe by excavating a 10 cm × 10 cm × 10 cm soil block. We selected the 0 to 10 cm soil depth since this layer contains the maximum amount of organic matter undergoing decomposition, the presence of surface litter with associated microbial faction, litter leachates, and the maximum contribution of nutrients from throughfall and stemflow. This soil layer is also directly in contact with the atmospheric temperature, moisture conditions, and plant roots. All replicate samples for a forest type were homogenized and reduced using the quartering method. Soil samples were sieved using a 2-mm sieve prior to soil analyses. Soil temperature was measured up to a depth of 0–15 cm (T15) using a soil probe thermometer (R-tek® digital thermometer) (Fig. 2b). Soil moisture was determined gravimetrically at 105 °C in a forced hot-air oven and expressed as a percentage of dry soil weight (Anderson & Ingram, 1993). Soil pHH2O was determined using field moist 2-mm sieved soil in a 1:2.5 (w/v) ratio of soil and de-ionized water solution (Anderson & Ingram, 1993). Soil electrical conductivity was determined in a 1:2 (w/v) solution of fresh sieved soil and de-ionized water using a handheld conductivity tester (PCSTestr™ 35, Eutech Instruments). Soil organic carbon (SOC) was determined in sieved air-dried finely ground soil samples using IRGA-based elemental analyzer (liquiTOC II, Elementar GmbH, Germany). Total Kjeldahl nitrogen (TKN) was determined by Kjeldahl digestion and steam distillation using an automatic distillation unit (UDK149, VELP Scientifica, Italy). Soil available phosphorous was determined using 2.5% acetic acid extraction and molybdenum blue chemistry (Allen et al., 1974). Soil exchangeable potassium was estimated using 1 M neutral ammonium acetate extraction and subsequent determination using flame photometry (Flame Photometer 128, Systronics, Ahmedabad). Soil dehydrogenase enzyme activity (DHA) was determined in fresh sieved soil by 2, 3, 5-triphenyltetrazolium chloride (TTC) reduction assay (Casida, 1977).
The effect of temperature dependence (temperature sensitivity—Q10) on Fsoil was determined using the Q10 coefficient (Mundim et al., 2020) at the forest-type level and for individual tree species. The Q10 value was calculated using a first-order exponential equation (Eq. 1) (Fang & Moncrieff, 2001):
Here, Q10 is the temperature sensitivity factor; R1 and R2 refer to Fsoil rates under two seasons with temperatures (in °C) T1 and T2, respectively, with consideration of T2 > T1. The average of Fsoil rates for a particular season and soil temperature (0–15 cm depth) were used for Q10 measurements.
Statistical analyses
The data were first tested for the assumption of normality using Shapiro–Wilk’s test and for homoscedasticity using Levene’s test. An Independent sample t-test was conducted to determine the significant difference between the means for the rainy and winter seasons among each forest type and tree species–specific Fsoil measurements. Welch’s one-way analysis of variance (ANOVA) with the Games-Howell post hoc test was used to test significant differences between the Fsoil means (for different forest types of a particular season) and the comparison of summer, rainy, and winter season Fsoil rates for the forest types. Parametric ANOVA with Tukey’s HSD post hoc test was used to determine the significant difference in assessing spatial variation, i.e., distance from tree base effect on Fsoil rates. A paired-sample t-test was used to assess the impact of surface litter on Fsoil rates. Linear and non-linear regressions were performed to determine the relationship between the tree stem girth (independent variable) and Fsoil rate (dependent variable) for different tree species under different forest types.
A two-tailed Pearson correlation analysis was used to determine the degree and direction of association between the Fsoil rates (under rainy and winter seasons); topographic, climatic, and tree species diversity (Kaushal et al., 2021); tree species biomass stock (Kaushal & Baishya, 2021); and soil properties. A factor reduction analysis as principal component analysis (PCA) was performed with forest-type Fsoil rates, climatic, topographic, species diversity, biomass, and soil properties. The PCA was performed using a correlation matrix. Components with Eigenvalues > 1 were retained as principal components (Kaiser’s rule) (Zuur et al., 2007). The PCA loading correlations and biplot were used to interpret the factor reduction results.
All statistical analyses were conducted at a 5% significance level either using SPSS ver. 23 (IBM, USA) or PAST ver. 4.11 software (Hammer et al., 2001). The graphs were plotted using GraphPad Prism ver. 9.5.0 for Windows (GraphPad Software, USA).
Results
In this investigation, ca. 1200 Fsoil measurements were recorded and further scrutinized to exclude erroneous observations due to inaccurate measurements, improper gas flow rate, and poor soil chamber fit.
Fsoil rates under different forest types and seasons (temporal effect)
Among all forest types, FT1 had the highest Fsoil rate (10.37 μmol m−2 s−1) during the rainy season and FT5 (4.42 μmol m−2 s−1) during the winter (Fig. 3). The FT9 had the least Fsoil value among all the forest types for both the seasons. All forest types showed a statistically significant difference between the means of Fsoil for rainy and winter seasons (Fig. 3 and Supplementary Table S1). Throughout the forest types, the rainy season had an average of 3.8 times higher Fsoil rates than the winter, with the increment level ranging from 1.8 (FT5) to 11.5 (FT9) times. The forest types showed a statistically significant difference in Fsoil rates during the rainy (Welch’s F(8, 28.3), 11.22; p < 0.0001) and the winter season (Welch’s F(8, 19.4), 36.08; p < 0.0001). However, the Games-Howell post hoc test showed the non-significance of Fsoil rates between several forest types for a particular season (Supplementary Table S1). FT5 and FT9 showed the least spread in the Fsoil rates for the rainy and winter seasons, respectively, with a smaller interquartile range (Fig. 3).
A significant difference was observed between the summer, rainy, and winter seasons’ Fsoil rates (Fig. 4 and Supplementary Table S2). The Games-Howell post hoc test showed that the tropical (FT2) and sub-tropical (FT3) forest types significantly differ for the rainy season Fsoil values from the other two seasons. However, the summer and winter seasons’ Fsoil did not differ significantly. This trend was not consistent for the temperate (FT4 and FT6) forest types, where all three seasons varied significantly (Fig. 4). Nonetheless, all the forest types had the rainy season with the highest Fsoil rate.
The Q10 values for the forest type ranged from 1.6 (FT5) to 11.8 (FT3), with the average Q10 value being 4.09. The sub-tropical pine (FT3: Q10 11.8) and alpine scrub (FT9: Q10 9.08) forest types had exceptionally high-temperature sensitivity, while all the remaining forest types ranged between 1.60 and 2.67 (Supplementary Table S1).
Fsoil rates under dominant/major tree species in different forest types and seasons
Temperate Pinus wallichiana A.B.Jacks. (12.6 μmol m−2 s−1) and tropical S. robusta (4.47 μmol m−2 s−1) had the highest Fsoil rates among tree species in rainy and winter seasons, respectively (Supplementary Table S3, Fig. 5). Alpine dwarf shrub R. anthopogon had the least Fsoil rate for both the seasons among the species (Fig. 5).
Almost all the tree species investigated showed a significant difference in the means for both seasons in the Fsoil rates (Fig. 5). The highest Fsoil increment from winter to rainy season was 548% in R. anthopogon (FT9), followed by 328% in Rhododendron arboreum (FT3), and 320% in P. wallichiana (FT6). The average increment for all the species taken together was 190%.
This research recorded the highest Q10 value for tree species in the sub-tropical pine forest (Supplementary Table S3) viz., R. arboreum (12.25) > P. roxburghii (11.94) > Alnus nepalensis (6.11). Interestingly, R. arboreum showed the highest (FT3, Q10 12.25) and lowest (FT5, Q10 1.77) temperature sensitivity values in different forest types under different elevations.
Impact of surface litter on Fsoil and distance from tree base (spatial variation)
The Fsoil measurements with surface litter yielded discordant results among the forest types (Fig. 6, Supplementary Table S4). Irrespective of statistical significance, only the tropical (FT1) and sub-tropical (FT3) forest types showed higher Fsoil rates (around 1.2 times higher) with surface litter. In contrast, the temperate sites (FT4, FT5, FT6, and FT7) showed the opposite. Statistically, FT3, FT4, and FT7 showed no significant differences in Fsoil rates with and without surface litter as determined through paired sample t-tests (Fig. 6).
Only two tree species, i.e., T. grandis (FT1) (Fig. 7a) and Aesculus indica (FT5) (Fig. 7b), showed a statistically significant difference between the distances for Fsoil rates. Generally, most species’ Fsoil rate was the highest closest to the tree base (Supplementary Table S5). The average Fsoil for all the species combined (Fig. 7c) in terms of spatial variation showed a significant difference (F(2, 36): 9.299; p = 0.0006). The average Fsoil rate near the tree base (0 cm) was 1.24 and 1.31 times greater than the Fsoil rates at 100 and 250 cm distances from the tree base. Non-parametric Spearman’s correlation between the distance from tree base and Fsoil rates showed a weak negative statistically significant correlation (ρ = − 0.301; p < 0.0001) (Fig. 7d).
Impact of distance from the tree base on soil CO2 efflux rates. a for Tectona grandis (FT1), b for Aesculus indica (FT5), c average for all the species combined, and d Spearman’s correlation between Fsoil rates and distance from the tree base. Bars represent the mean, while the error bars represent the standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001, and ns: p > 0.05
Effect of tree age (stem girth) and elevation on tree species Fsoil rates
We tested one dominant/major tree species from the 8 tree-bearing forest types (FT1–FT8) for the impact of stem girth on Fsoil rates using linear and non-linear regression models. Only A. nepalensis (FT5) showed a significant positive linear effect (R2 = 0.7771; p = 0.048) on Fsoil (2.25–4.58 μmol m−2 s−1) with increase in tree stem girth (103–300 cm) (Fig. 8).
Simple linear regression between the tree’s circumference and the corresponding Fsoil rate for Alnus nepalensis (site 5). The regression line is indicated in blue, the observations are indicated by blue dots, the red-dotted lines mark the extent of the confidence interval (95%), and the area under it is in a blue shade
To observe the impact of elevation on Fsoil, we selected R. arboreum due to its widest occurrence range (1710 m at FT3 to 2981 m at FT7). Under both seasons, the species showed a difference in Fsoil rates at different elevations (Fig. 9) (Rainy season: F(3, 18), 14.087; p < 0.0001 and winter season: Welch’s F(3, 7.5), 33.120; p = 0.004). The post hoc tests showed R. arboreum to have the highest Fsoil rate in mid-temperate elevations, i.e., FT4 (2109 m) and FT5 (2210 m) under rainy and winter seasons, respectively (Fig. 9).
Statistical data analyses
The Fsoil rates for both seasons positively correlated with the average annual maximum and minimum air temperature, water vapor pressure, and atmospheric pressure. The Fsoil showed a strong negative correlation with elevation (Supplementary Fig. S4). Interestingly, all the positively correlating variables mentioned above showed a strong negative correlation with elevation. Annual average precipitation, solar radiation, PAR, slope, and slope aspect did not show a significant association with Fsoil for either season (Supplementary Fig. S4).
The Fsoil for the rainy season did not significantly correlate with tree species diversity, richness, and biomass parameters. In contrast, the Fsoil for the winter season showed a moderate to strong positive correlation with species richness (r = 0.81, p = 0.015) and species diversity (r = 0.77, p = 0.024) (Supplementary Figure S2). Furthermore, the winter season Fsoil rates did not correlate with any soil parameter. In contrast, the rainy season Fsoil rate showed a strong negative correlation with soil moisture (r = − 0.85, p = 0.007), SOC (r = − 0.73, p = 0.039), TKN (r = − 0.76, p = 0.029), and DHA activity (r = − 0.75, p = 0.03). The soil pH positively correlated with the rainy reason Fsoil rate (r = 0.78, p = 0.023) (Supplementary Figure S3).
The dimension reduction PCA for both seasons had the first four principal components (PCs) with eigenvalues > 1. The first four PCs explained 91.12% and 89.37% of the total variance for rainy and winter season data, respectively (Supplementary Table S6). The PCA biplot (Fig. 10) is illustrated for PC1 and PC2 since these two PCs explained the highest variance under both seasons.
PCA biplot for both seasons (Fig. 10) indicated a clear ordination of sites along PC1. The tropical and sub-tropical forests were in the positive space of PC1, while the temperate–alpine forest types in the negative space. For PC2, the forest types FT1, FT4, FT5, FT6, and FT7 were present in the positive space for the rainy season, while only FT1, FT5, and FT6 were for the winter season. A three-dimensional (3D) representation of the site score plot (Supplementary Fig. S5) under both seasons and the loading correlations (Supplementary Table S7) was used to visualize the ordination of sites on the principal axes.
During the rainy season, soil temperature, atmospheric temperature variables, water vapor pressure, and atmospheric pressure positively influence the variable loadings on PC1. In contrast, elevation and soil properties show a negative influence. This trend is also similar in the winter season. The PC2, however, had different trends in both seasons. In the rainy season, precipitation, soil electrical conductivity (EC), and total biomass density (TBD) were major influencing variables. In the winter season, the influencing variables were primarily tree species richness, species diversity, TBD, and EC. The 3D scatter plot (Supplementary Fig. S5) also indicates that FT1 and FT2 ordinate on the right corners, while FT7 and FT8 are on the left. The remaining sites were grouped roughly in the center of the PCA plot.
Discussion
Variation in Fsoil rates under different forest types
The vegetation composition and density influenced the Fsoil rates of the forest types, as indicated by the positive correlation of Fsoil with Margalef’s richness index (MR), Shannon–Wiener diversity index (H′), and the total biomass density (TBD) (Supplementary Table S7). The selected forest types had different vegetational compositions (Kaushal et al., 2021). The vegetation impacts the Fsoil rates through root respiration, soil microclimate, quality, and the quantity of litter input (detritus) (Raich & Tufekciogul, 2000). Hence, we believe the vegetation contributed differentially to these aspects, causing variable Fsoil rates under different forest types.
The reduced density and diversity of herbs and shrubs during the winter months, with only a few selective evergreen perennials surviving the harsh cold and drier winters, could explain the positive association of MR and H′ with Fsoil in this season. The preponderance of herbaceous vegetation during the rainy season could have masked the impact of tree layer on Fsoil rates. Seasonal pattern of herbaceous density is evident in the Himalayas (Joshi et al., 2012; Shameem et al., 2010) and was visually recognizable in the major forest types studied (Fig. 2e, f, h, i, k, and l). So, in addition to the tree species composition and diversity, the forest floor vegetation also impacts the Fsoil rates in these major forest types of Central Himalayas on a seasonal basis.
The coniferous- and broadleaf dominant forest types, as well as the tropical, sub-tropical, temperate, sub-alpine, and alpine forest types, did not exhibit any Fsoil patterns in common (Fig. 4). Although these trends are prevalent globally (Raich & Tufekciogul, 2000; Yan et al., 2014) and regionally (Joshi, 1994; Kumar et al., 2023; Tewary et al., 1982), the upper temperate to alpine forest types (FT7–FT9) had comparatively lower Fsoil rates than the other forest types (Supplementary Table S1). As the forest types are along an elevational gradient (Table 1), they have differential climate and edaphic structures, which could have masked variation due to the forest types. Earlier studies in the region (Joshi et al., 1991; Tewary et al., 1982) were conducted with smaller elevational gradients or a single climate zone, therefore, could elucidate forest-type differences. Given this, Raich and Potter (1995) could also not ascertain the global differential impact of vegetation types on Fsoil.
Temporal variation (seasonal) of Fsoil rates among different forest types and tree species
Despite having higher Fsoil rates during the rainy season, three tree species, viz., Adina cordifolia (Roxb.) Brandis (FT1), Syzygium cumini (L.) Skeels (FT1), and Quercus leucotrichophora (FT5), did not exhibit significant seasonal variation (Fig. 5) owing to considerable within-season data variance, which masked the between-season variation. Our Fsoil rates were marginally higher than those reported for similar forest types or tree species (Table 2). Interestingly, our results were more comparable to the studies which used the IRGA-based technique, while those using the alkali-absorption or soda-lime method had noticeably lower values. The IRGA approach is the most sensitive for instantaneous in situ Fsoil measurements and yields higher results than the soda lime method (Pongracic et al., 1997).
The alkali absorption method is known to over or under-estimate the Fsoil rates for spatially heterogeneous environments (King & Harrison, 2002). The IRGA-based systems are sensitive in capturing the seasonal Fsoil variation in forest ecosystems (Ferreira et al., 2018). Thus, a comparison with previous estimates (Table 2) deploying chemical absorption systems would be irrational.
Since the objectives of this study were to capture the variations in Fsoil on account of spatial, temporal, and vegetational features, the use of a chamber-based system was the most appropriate. However, one of the issues of spatial heterogeneity faced by us for the forest-type soil CO2 efflux comparisons could be overcome with the eddy covariance technique. Although, a major drawback of this method is that there should be a complete absence of any CO2 source or sink element between the soil and eddy flux sensors (Vermue et al., 2008). If this assumption is not met, then the night-time flux estimates will reflect ecosystem level respiration (soil and autotrophic components), as during the daytime, there is a complex interplay of soil respiration, autotrophic respiration, and photosynthesis (Renchon et al., 2021). Regionally, studies incorporating the eddy covariance technique in forest ecosystems used an IRGA-based chamber for determining the rates of soil respiration (Sarma et al., 2022; Singh et al., 2019). In other studies, computing the net ecosystem exchange (NEE) in forest ecosystems determined the total ecosystem respiration and did not represent its constituent elements, i.e., soil respiration and autotrophic respiration (Gnanamoorthy et al., 2020; Rodda et al., 2021). Therefore, for NEE studies, we compared our results only with those which used IRGA-based soil CO2 flux chambers for Fsoil determination.
Temperature and soil moisture (regulated through precipitation) are key regulators of the global Fsoil rates (Phillips & Nickerson, 2015; Raich & Potter, 1995; Singh & Gupta, 1977). Microbial activity and organic matter decomposition are positively influenced by adequate soil temperature and moisture, which increases the heterotrophic component of respiration and, thereby, the Fsoil rates (Singh & Gupta, 1977; Wildung et al., 1975). We found evidence to support this notion in our results wherein under both seasons, soil moisture was positively correlated with soil dehydrogenase enzyme (DHA) activity (Supplementary Fig. S3). The summer and winter seasons had near similar Fsoil rates with the maxima during the rainy season (Fig. 4). Rainy season had 2.8 to 2.2 times greater soil moisture than the summer and winter seasons, respectively. Soil temperature in the rainy season varied insignificantly from the summer, although it had a 2.4 times higher value than the winter. Therefore, maximum Fsoil during the rainy season is due to the elevated soil moisture and warmer temperature. If temperature acted alone, the summer and rainy seasons would have similar Fsoil results.
Rainy season Fsoil maxima could also be due to the increased density of fine root biomass. Regionally, previous studies have established the prominence of fine root biomass and necromass in Q. leucotrichophora–, A. nepalensis–, and P. roxburghii–dominant forest types during the rainy and least during the winter season (Joshi & Garkoti, 2021; Usman et al., 1999). Strong positive statistically significant association between root respiration and fine root biomass is well established globally and regionally (Dilustro et al., 2005; Joshi, 1994; Lee & Jose, 2003). Therefore, the enhanced root biomass and herbaceous growth during the rainy season explain the biotic component behind the rainy season Fsoil maxima.
Other factors associated with the rainy season, such as the prominence of earthworms and soil macrofauna, could also contribute to the higher Fsoil rates (Schulze, 1967). Rainy season Fsoil maxima also indicate that forest types do not suffer from deficient soil O2 diffusion due to waterlogging (Davidson et al., 1998). Birch effect (Jarvis et al., 2007) also did not attribute to the Fsoil maxima since the rainy season in the Central Himalayas gradually deposits 65–75% of the total annual rainfall, making it a warm and wet period (Singh & Singh, 1987). Temperature and moisture, combined with several seasonally regulated biotic and abiotic environmental factors, therefore, regulate the temporal variation in the Fsoil rates for these major forest types of Central Himalayas.
Impact of surface litter, distance from tree base, and age class of trees on Fsoil rates
We conducted these analyses in the winter season to primarily collect tree-specific (i.e., autotrophic component of soil CO2 efflux) respiration and minimize the disproportionate impact of the heterotrophic component. During winters, the microbial activity is subdued due to temperature and moisture limitations. Moreover, the roots (autotrophic component) contribute an estimated 50% or more to the total Fsoil in Central Himalayas (Joshi, 1994; Joshi et al., 1991; Singh & Gupta, 1977).
Surface litter inclusion only had a positive effect on tropical (FT1) and sub-tropical (FT3) forest types (Supplementary Table S4), with the surface litter making up about 19% of the total Fsoil rate (Fig. 6). Our findings were similar to the sub-tropical Himalayan pine forest, where the litter layer contributed 22% to the total soil CO2 efflux (Joshi et al., 1991). However, the temperate sites (FT4–FT7) had a reverse trend with no contribution (Fig. 6) from surface litter inclusion. Negative surface litter impact was also observed in Central European temperate deciduous forests where low temperature and high precipitation hindered the decomposition activity, thereby reducing litter-based CO2 efflux (Fekete et al., 2014). Therefore, poor litter decomposition due to subdued microbial activity in the winter season resulted in a negative trend in our temperate elevation forest types. Higher winter temperatures (12–14 °C) in the lower elevations than the temperate sites (4–7 °C) led to greater microbial activity and subsequent surface litter impact. Our findings are consistent with the Central Himalayan pattern, where Singh and Singh (1987) showed that tropical sal forest (like FT1) exhibited faster litter decomposition rates than the sub-tropical pine forest (like FT3) due to warmer temperatures and intense microarthropod activity.
Even with poor litter respiration, the with-litter Fsoil for temperate forests should resemble the without-litter layer and not show lower results comparatively (Fig. 6). We hypothesize that slowly decomposing litter creates an accumulation layer on the soil surface that maintains the soil microclimate, reduces aeration, and serves as a diffusion barrier for soil gases (Walkiewicz et al., 2021). Therefore, removing the litter fraction altered these conditions, increasing the without-litter Fsoil rates. The anthropogenic removal of forest floor litter could thus have a detrimental impact in the form of increased Fsoil rates in temperate regions. Lower litter respiration in the temperate regions could also be due to smaller measurement time by the IRGA method compared to the 24-h alkali-trap incubation method by the previous studies (Joshi et al., 1991).
For spatial variability (Fig. 7c), only 15% of the tree species investigated showed statistical significance (Supplementary Table S5; Fig. 7). The non-significance for most species was due to larger within-group variation, which masked the variation between the groups (distances from the tree base). Reduction in Fsoil rates with increasing distance from the tree base was seen elsewhere, with root biomass as the primary cause for this trend (Lee, 2018; Schwendenmann & Macinnis-Ng, 2016). Globally, the top 40 cm of the soil surface has 75% of the root biomass (Jackson et al., 1996) and up to 90% in the Central Himalayas (Usman et al., 1999). Since our study sites had a significant slope gradient (Table 1), increasing the distance from the tree base might increase the soil surface and horizontal root zone depth, thereby reducing Fsoil. Moreover, greater nutrient concentrations caused by stemflow and throughfall (up to 46% in the Himalayas) localize near the stem base which could have enhanced the Fsoil in this zone (Singh & Singh, 1987).
Only A. nepalensis, out of the nine tree species investigated, showed a significant positive linear effect of stem diameter (the corollary of age class) on Fsoil rates (Fig. 8). Different studies have found varying effects of age on Fsoil rates, including positive (Ewel et al., 1987), seasonal (Schurman & Thomas, 2021), and no influence (Wang et al., 2011). The increased root biomass and associated litter inputs, which enhance the autotrophic and heterotrophic components of respiration, are primarily responsible for the positive effect (Bréchet et al., 2011; Ewel et al., 1987; Karki et al., 2021). Since our forest types are natural primary forests with adequate species diversity (Kaushal et al., 2021), we could not capture the spatial variation in Fsoil. The random presence of trees in different girth classes increased the topographic heterogeneity of surrounding vegetation, unlike a plantation with even-aged trees, where the spatial impact is more evident (Ewel et al., 1987). Variable girth classes also impact the edaphic properties and cause microclimatic differences, thereby increasing the background noise and diluting the impact of stem girth on Fsoil rates. The lateral spread of roots and associated CO2 efflux for large trees would also not show directly near the base. Further complexities, like the xylary CO2 efflux of root respiration from the above-ground parts (Bloemen et al., 2014), reduce the rhizosphere component’s impact on Fsoil. Therefore, further research into this aspect is needed to elucidate the driving mechanisms behind the influence of tree size in the Himalayan forests.
Impact of elevation, climatic, and edaphic properties on Fsoil rates along with temperature sensitivity of Fsoil
Mean annual maximum and minimum temperature, water vapor pressure, atmospheric pressure, soil temperature, soil moisture, soil organic carbon content, soil Kjeldahl nitrogen, and soil dehydrogenase enzyme activity strongly correlated with elevation (Supplementary figures S3 and S4). Moreover, Fsoil is also strongly correlated with elevation; hence, this creates the situation of multicollinearity among the variables, due to which elevation is subduing the true impact of climatic and edaphic variables on Fsoil. In the event of multicollinearity, the true relative contribution of the independent variables is difficult to assess (Blalock, 1963). Therefore, comparing our trends with global or regional patterns would be inappropriate.
Smaller vector angles between elevation and soil parameters in the PCA biplot confirm the positive association, and the opposite direction of elevation and climate-associated vectors illustrate their negative association (Fig. 10). Higher elevations being richer in organic carbon, nitrogen, active microbial activity, and soil moisture have reduced Fsoil rates primarily due to lower soil temperature. Similar trends were observed in the Italian Alps, where the Fsoil rates were regulated by temperature (Badraghi et al., 2021). All the forest types were ordinated along PC1 for both seasons in the order of their elevational gradient (Fig. 10). Soil and the atmospheric temperature had one of the highest positive loading correlations with PC1 in both seasons (Supplementary Table S7). Temperature and elevation are, therefore, the chief regulators for Fsoil for major forest types of Central Himalayas along elevational gradients. Soil moisture, edaphic, and other climatic factors also influence Fsoil; however, temperature is the limiting factor. The alpine regions being rich in soil nutrients, active microbial activity, and soil moisture, should have optimum Fsoil, but are limited by lower soil temperature.
With an increase in elevation, the atmospheric pressure decreases. Moreover, higher elevations being colder result in more condensation and a reduction of water vapor pressure (Korner, 2007). Therefore, high-elevation forest types had lower atmospheric and water vapor pressure than tropical forest types. Greater pressure is associated with reduced Fsoil rates (Davidson et al., 2002; Lund et al., 1999); however, the high atmospheric pressure associated with tropical forests had the highest Fsoil rates while the lowest in the low-pressure alpine region. In our opinion, the impact of temperature and moisture is far greater along the elevation in regulating the Fsoil rates. Therefore, the atmospheric pressure is not causing any significant influence. Moreover, the multicollinearity also hinders the impact in deciphering the associations as discussed earlier. For determining the true impact of climatic constrains such as atmospheric and water vapor pressure within the site, analyses must be conducted modulating the pressure conditions.
We also selected light-associated climatic variables, viz., solar radiation and PAR, since these components are known to regulate the local microclimate, particularly soil temperature and moisture (Chen et al., 1993), which have been discussed to be critical in influencing the Fsoil rates. Our results corresponded with those of the temperate deciduous forest of the UK where Fenn et al. (2010) found total Fsoil to have no association with solar radiations. Since the studied forest types have medium to high canopy density, other climatic parameters such as temperature were more strongly influencing Fsoil. Being a mountainous region, even the slope aspects also influence the intensity of solar radiations (Singh & Singh, 1987), which could have led to uneven solar radiations influence. Fenn et al. (2010) also attributed the masking influence of soil temperature to the impact of solar radiation on Fsoil. Like solar radiation, PAR did not associate with Fsoil in our study. Although, Mitra et al. (2019) showed PAR as the chief regulator of diurnal variations in Rs by regulating photosynthesis and rhizosphere activity, however, they reported a 1-to-3-h delay in the response of PAR and Fsoil in their continuous diurnal study. Since our estimates were instantaneous therefore, we did not capture any trend.
Due to its widespread prevalence along the elevational gradient, R. arboreum was chosen to study the specie-specific elevation effect on Fsoil rates (Fig. 9). Lower Fsoil rates in the highest and lowest elevations for this species were due to lower soil and atmospheric temperatures in upper temperate FT7 and reduced canopy density in sub-tropical FT3 (Kaushal et al., 2021). Reduced canopy density increases the influx of solar radiations and wind, creating a localized microclimate of increased soil temperature but limiting soil moisture (Badraghi et al., 2021), causing Fsoil reduction.
The absence of any significant association between edaphic properties and Fsoil rates during winter reflects the independence of autotrophic Rs components towards seasonal soil properties. Maximum Fsoil contribution during winter is due to the autotrophic component (Joshi et al., 1991). Roots also supplement exudates to the microorganisms associated with the rhizosphere region (rhizosphere priming) (Canarini et al., 2019), thereby reducing the impact of bulk soil properties on Fsoil. Among soil properties, for all the forest types, our pH range (4.2–6.8) was above the threshold (pH < 3) to cause pH-associated Fsoil reduction (Luo & Zhou, 2006). The Italian Alps (Badraghi et al., 2021) and the Central Himalayan forests (Joshi, 1994; Joshi et al., 1991; Rawat et al., 2021) showed a positive association of Fsoil with soil pH. The SOC and nitrogen serve as energy and protein sources for the soil microorganisms (Tewary et al., 1982), and positive interaction between these elements and Fsoil is therefore posited (Bargali et al., 1992). However, we obtained a negative correlation due to the multicollinearity of edaphic properties and Fsoil with elevation (Supplementary Fig. S3a). The upper temperate and sub-alpine forest types although having higher concentration of organic carbon and nitrogen show lower Fsoil rates on account of lower temperature which acts as a limiting factor. (Supplementary Fig. S3a). Previous investigations have demonstrated a positive association of soil phosphorous and potassium with Fsoil rates (Bargali et al., 1992). Our study did not observe this association indicating that phosphorous and potassium levels varied along the elevational gradient in these major forest types.
After removing the outliers, the average Q10 for forest types and tree species was approximately 2.30, which aligns with the global median of 2.40 (Raich & Schlesinger, 1992). The Q10 for Fsoil is generally around two as it is a biochemical process (Singh & Gupta, 1977). In our investigation, the Fsoil Q10 values for the sub-tropical pine–dominant forest (FT3, Q10 11.80) and the alpine scrub forest (FT9, Q10 9.08) were exorbitantly high. This is primarily due to the reduced vertical forest profile. Alpine meadow (FT9) has no trees or large shrubs; only the prostrate to decumbent shrubs R. anthopogon and Rhododendron lepidotum Wall. Ex G.Don defines the microclimate (Rai et al., 2012). Sub-tropical FT3 had the lowest stem density among all the sites with tree growth from (sites 1–8) (Kaushal et al., 2021). Therefore, greater daytime solar radiations and surface warming, cooler night-time surface temperatures due to radiative loss (less canopy blanketing), and even stronger wind force (influencing moisture) all lead to larger variations in the temperature and moisture patterns (Rambo & North, 2009). The Q10 also showed a negative correlation (non-significant) with stand density (r, − 0.65). Furthermore, at FT9, the Q10 outside the under-canopy zone of R. anthopogon shrubs was 9.08, while that under the shrubs was 4.67. Similarly, the Q10 for pine-dominant (FT3) forest was 11.80, which was higher than A. nepalensis (Q10, 6.11) but nearly identical to P. roxburghii (Q10, 11.94) and R. arboreum (Q10, 12.25). This is because A. nepalensis forms a much denser canopy and dense understory vegetation growth, while P. roxburghii has an open sparse canopy, and R. arboreum was a scarce understory tree species. Italian Alps (Badraghi et al., 2021) also showed a similar trend where tree height and closeness of canopy modulated the Q10. Though comparing the Q10 values with global studies seems direct, however, due to the use of different models in the Q10 computation, the comparison of the Q10 values becomes irrational (Fang & Moncrieff, 2001).
Conclusion
The major forest types chosen demonstrated varying Fsoil rates along the elevational range, which were directly influenced by the species richness and diversity of these forest types. In addition, the stand density of the tree species was also critical since it influences the Fsoil through microclimate regulation. Although no specific pattern existed between the forest types studied, the high-elevation sub-alpine and alpine scrub types had an overall reduced Fsoil rate primarily because of lower soil and atmospheric temperatures. The temporal variation had a uniform impact along the elevational gradient, where the rainy season showed higher Fsoil rates among the forest types and under individual tree species. This trend was primarily regulated by soil moisture and temperature, and neither of these abiotic components alone can lead to higher Fsoil rates. Apart from the abiotic climatic influence, the biotic influence of increased herbaceous/ground vegetation during the rainy season was a probable factor behind this rainy season’s Fsoil maxima. The presence of surface litter significantly impacts total Fsoil rates. It enhances the total soil CO2 efflux through the contribution from litter decomposition and acts as a barrier layer in cold temperate regions, reducing the overall soil CO2 efflux.
Spatially, maximum Fsoil rates were observed near the tree base, which reduces with increasing distance from the base. The age of the trees did not show a clear pattern across the elevational range. Therefore, for tree-associated spatial Fsoil rates further, validation is required. We believe that segregation of the two Rs components and the corrections for xylary CO2 efflux would help decipher the spatial heterogeneity. Soil properties were either negatively associated or did not show any association with the Fsoil rates. This is primarily because elevation influenced (multicollinearity) soil properties through the abiotic climate variables (temperature and moisture). Very high Q10 values for the alpine scrub and less dense sub-tropical pine forest indicate the temperature sensitivity of these forests. Therefore, these regions will suffer the strongest impact from climate change-associated warming and can be selected as indicators of climate change impact.
Although the forest types are legally present in protected areas, anthropogenic disturbance is evident (Kaushal et al., 2021). Afforestation of forest types having poor stem density is recommended; such an initiative will enhance the biodiversity and carbon stocks, modulate the region’s microclimate, and regulate the Fsoil rates. Afforestation plans must preserve the original vegetational composition of the forest types and not alter it significantly. Exotic or commercially important species (e.g., teak, eucalyptus, pine.) must be avoided to prevent a detrimental impact on Fsoil rates. Clearing the forest floor litter for anthropogenic use or through human-induced forest fires is disadvantageous and must be checked. It not only disrupts the nutrient dynamics but also could lead to higher total CO2 efflux, as shown by our study. Due to their highest temperature sensitivity, the alpine regions must be stringently monitored for disturbance events such as overgrazing or extraction of alpine shrubs. The conservation of these alpine shrubs is crucial since they are the only major vertical component regulating the microclimate of this region.
Through our research, we were able to identify certain trends and patterns. Subsequent research, particularly which segregates the components of Fsoil and then investigates the impact of vegetation, edaphic, climatic, and other environmental factors, will help to elucidate the mechanisms that control Fsoil in the Himalayas.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Allen, S. E., Grimshaw, H. M., Parkinson, J. A., & Quarmby, C. (1974). Chemical analysis of ecological materials. Blackwell Scientific Publications.
Anderson, J. M., & Ingram, J. S. I. (1993). Tropical soil biology and fertility: A handbook of methods. C. A. B International, Wallingford, Oxfordshire.
Badraghi, A., Ventura, M., Polo, A., Borruso, L., Giammarchi, F., & Montagnani, L. (2021). Soil respiration variation along an altitudinal gradient in the Italian Alps: Disentangling forest structure and temperature effects. PLoS ONE, 16(8), e0247893. https://doi.org/10.1371/journal.pone.0247893
Bargali, S. S., Joshi, M., & Bargali, K. (1992). Seasonal pattern of total soil respiration in an age series of Eucalypt plantation and mixed broad-leaved forest in Tarai belt of Kumaun Himalaya. Oecologia Montana, 1(2), 7–11.
Bastida, F., Zsolnay, A., Hernández, T., & García, C. (2008). Past, present and future of soil quality indices: A biological perspective. Geoderma, 147(3–4), 159–171. https://doi.org/10.1016/j.geoderma.2008.08.007
Becker, A., & Bugmann, H. (2001). Global change and mountain regions — An IGBP initiative for collaborative research. In G. Visconti, M. Beniston, & E. D. Iannorelli (Eds.), Global change and protected areas. Advances in Global Change Research (pp. 3–9). Springer, Dordrecht. https://doi.org/10.1007/0-306-48051-4_1
Bhuyan, S. I., Laskar, I., Tripathi, O. P., & Khan, M. L. (2014). Effect of different land use patterns on soil carbon-dioxide emission in Eastern Himalaya. International Journal of Innovative Science, Engineering and Technology, 1(9), 476–480.
Blalock, H. M., Jr. (1963). Correlated independent variables: The problem of multicollinearity. Social Forces, 42(2), 233–237. https://doi.org/10.1093/sf/42.2.233
Bloemen, J., Agneessens, L., Meulebroek, L. V., Aubrey, D. P., McGuire, M. A., Teskey, R. O., & Steppe, K. (2014). Stem girdling affects the quantity of CO2 transported in xylem as well as CO2 efflux from soil. New Phytologist, 201(3), 897–907. https://doi.org/10.1111/nph.12568
Bréchet, L., Ponton, S., Alméras, T., Bonal, D., & Epron, D. (2011). Does spatial distribution of tree size account for spatial variation in soil respiration in a tropical forest? Plant and Soil, 347(1), 293–303. https://doi.org/10.1007/s11104-011-0848-1
Byanjankar, S., Dhamala, M. K., Maharjan, S. R., & Kayastha, S. P. (2020). Soil respiration and its temperature sensitivity to different ecosystems in Annapurna Conservation Area. Nepal. Nepal Journal of Environmental Science, 8, 69–81. https://doi.org/10.3126/njes.v8i1.34471
Canarini, A., Kaiser, C., Merchant, A., Richter, A., & Wanek, W. (2019). Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Frontiers in Plant Science, 10, 157. https://doi.org/10.3389/fpls.2019.00157
Cardoso, E. J. B. N., Vasconcellos, R. L. F., Bini, D., Miyauchi, M. Y. H., Santos, C. A. D., Alves, P. R. L., Paula, A. M. D., Nakatani, A. S., Pereira, J. D. M., & Nogueira, M. A. (2013). Soil health: Looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Scientia Agricola, 70, 274–289. https://doi.org/10.1590/S0103-90162013000400009
Casida, L. E., Jr. (1977). Microbial metabolic activity in soil as measured by dehydrogenase determinations. Applied and Environmental Microbiology, 34(6), 630–636. https://doi.org/10.1128/aem.34.6.630-636.1977
Chakraborty, J. S., Singh, S., Singh, N., & Jeeva, V. (2021). Methane and carbon dioxide flux heterogeneity mediated by termite mounds in moist tropical forest soils of Himalayan foothills, India. Ecosystems, 1–16. https://doi.org/10.1007/s10021-021-00630-y
Champion, H. G., & Seth, S. K. (1968). A revised survey of forest types of India. Natraj Publishers.
Chen, J., Franklin, J. F., & Spies, T. A. (1993). Contrasting microclimates among clearcut, edge, and interior of old-growth Douglas-fir forest. Agricultural and Forest Meteorology, 63(3–4), 219–237. https://doi.org/10.1016/0168-1923(93)90061-l
Dar, J. A., Ganie, K. A., & Sundarapandian, S. (2015). Soil CO2 efflux among four coniferous forest types of Kashmir Himalaya. India. Environmental Monitoring and Assessment, 187(11), 1–13. https://doi.org/10.1007/s10661-015-4927-2
Davidson, E. A., Belk, E., & Boone, R. D. (1998). Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Global Change Biology, 4(2), 217–227. https://doi.org/10.1046/j.1365-2486.1998.00128.x
Davidson, E. A., Savage, K. V. L. V., Verchot, L. V., & Navarro, R. (2002). Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agricultural and Forest Meteorology, 113(1–4), 21–37. https://doi.org/10.1016/S0168-1923(02)00100-4
Dhital, D., Manandhar, R., Manandhar, P., & Maharjan, S. R. (2022). Soil CO2 efflux dynamics and its relationship with the environmental variables in a sub-tropical mixed forest. Open Journal of Forestry, 12(3), 312–336. https://doi.org/10.4236/ojf.2022.123017
Dilustro, J. J., Collins, B., Duncan, L., & Crawford, C. (2005). Moisture and soil texture effects on soil CO2 efflux components in southeastern mixed pine forests. Forest Ecology and Management, 204(1), 87–97. https://doi.org/10.1016/j.foreco.2004.09.001
Epron, D., Farque, L., Lucot, É., & Badot, P. M. (1999). Soil CO2 efflux in a beech forest: Dependence on soil temperature and soil water content. Annals of Forest Science, 56(3), 221–226. https://doi.org/10.1051/forest:19990304
Ewel, K. C., Cropper Jr, W. P., & Gholz, H. L. (1987). Soil CO2 evolution in Florida slash pine plantations. I. Changes through time. Canadian Journal of Forest Research, 17(4), 325–329. https://doi.org/10.1139/x87-054
Fang, C., & Moncrieff, J. B. (2001). The dependence of soil CO2 efflux on temperature. Soil Biology and Biochemistry, 33(2), 155–165. https://doi.org/10.1016/S0038-0717(00)00125-5
FAO. (2007). Digital soil map of the world. FAO-UN Land and Water Division.
Fekete, I., Kotroczó, Z., Varga, C., Nagy, P. T., Várbíró, G., Bowden, R. D., Tóth, J. A., & Lajtha, K. (2014). Alterations in forest detritus inputs influence soil carbon concentration and soil respiration in a Central-European deciduous forest. Soil Biology and Biochemistry, 74, 106–114. https://doi.org/10.1016/j.soilbio.2014.03.006
Fenn, K. H., Malhi, Y., & Morecroft, M. D. (2010). Soil CO2 efflux in a temperate deciduous forest: Environmental drivers and component contributions. Soil Biology & Biochemistry, 42(10), 1685–1693. https://doi.org/10.1016/j.soilbio.2010.05.028
Ferreira, C. R. P. C., Antonino, A. C. D., Sampaio, E. V. D. S. B., Correia, K. G., Lima, J. R. D. S., Soares, W. D. A., & Menezes, R. S. C. (2018). Soil CO2 efflux measurements by alkali absorption and infrared gas analyzer in the Brazilian semiarid region. Revista Brasileria Ciência Do Solo, 42. https://doi.org/10.1590/18069657rbcs20160563
Fick, S. E., & Hijmans, R. J. (2017). WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. International Journal of Climatology, 37(12), 4302–4315. https://doi.org/10.1002/joc.5086
Friedlingstein, P., O’sullivan, M., Jones, M. W., Andrew, R. M., Hauck, J., Olsen, A., & Zaehle, S. (2020). Global carbon budget 2020. Earth System Science Data, 12(4), 3269-3340. https://doi.org/10.5194/essd-12-3269-2020
FSI (2019). India state of forest report 2019. Forest Survey of India, Ministry of Environment, Forest and Climate Change. Government of India, Dehradun.
Giardina, C. P., Litton, C. M., Crow, S. E., & Asner, G. P. (2014). Warming-related increases in soil CO2 efflux are explained by increased below-ground carbon flux. Nature Climate Change, 4, 822–827. https://doi.org/10.1038/nclimate2322
Gnanamoorthy, P., Selvam, V., Burman, P. K. D., Chakraborty, S., Karipot, A., Nagarajan, R., Ramasubramanian, R., Song, Q., Zhang, Y., & Grace, J. (2020). Seasonal variations of net ecosystem (CO2) exchange in the Indian tropical mangrove forest of Pichavaram. Estuarine, Coastal and Shelf Science, 243, 106828. https://doi.org/10.1016/j.ecss.2020.106828
Grand, S., Rubin, A., Verrecchia, E. P., & Vittoz, P. (2016). Variation in soil respiration across soil and vegetation types in an alpine valley. PLOS ONE, 11(9), e0163968. https://doi.org/10.1371/journal.pone.0163968
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 9.
Hanson, P. J., Wullschleger, S. D., Bohlman, S. A., & Todd, D. E. (1993). Seasonal and topographic patterns of forest floor CO2 efflux from an upland oak forest. Tree Physiology, 13(1), 1–15. https://doi.org/10.1093/treephys/13.1.1
Hari, M., & Tyagi, B. (2022). Terrestrial carbon cycle: A tipping edge of climate change between atmosphere and biosphere ecosystems. Environmental Science: Atmospheres. https://doi.org/10.1039/D1EA00102G
Jackson, R. B., Canadell, J., Ehleringer, J. R., Mooney, H. A., Sala, O. E., & Schulze, E. D. (1996). A global analysis of root distributions for terrestrial biomes. Oecologia, 108(3), 389–411. https://doi.org/10.1007/BF00333714
Jain, A. K. (2020). Geological evolution of the Himalayan mountains. In N. Gupta, S. Tandon (Eds.), Geodynamics of the Indian Plate (pp. 363 – 393). Springer Geology, Springer, Cham. https://doi.org/10.1007/978-3-030-15989-4_10
Jarvis, P., Rey, A., Petsikos, C., Wingate, L., Rayment, M., Pereira, J., Banza, J., David, J., Miglietta, F., Borghetti, M., & Manca, G. (2007). Drying and wetting of Mediterranean soils stimulates decomposition and carbon dioxide emission: The “Birch effect.” Tree Physiology, 27(7), 929–940. https://doi.org/10.1093/treephys/27.7.929
Jenkins, M. E., & Adams, M. A. (2011). Respiratory quotients and Q10 of soil respiration in sub-alpine Australia reflect influences of vegetation types. Soil Biology and Biochemistry, 43(6), 1266–1274. https://doi.org/10.1016/j.soilbio.2011.02.017
Jina, B. S., Bohra, C. P. S., Rawat, Y. S., & Bhatt, M. D. (2008). Seasonal changes in soil respiration of degraded and non-degraded sites in oak and pine forests of Central Himalaya. Scientific World, 6(6), 89–93. https://doi.org/10.3126/sw.v6i6.2641
Joshi, M. (1994). Patterns of forest floor respiration in broadleaf and conifer forest ecosystems in parts of central Himalaya. Proceedings of the Indian National Science Academy Part B, 60, 67–74.
Joshi, M., Mer, G. S., Singh, S. P., & Rawat, Y. S. (1991). Seasonal pattern of total soil respiration in undisturbed and disturbed ecosystems of Central Himalaya. Biology and Fertility of Soils, 11(4), 267–272. https://doi.org/10.1007/BF00335846
Joshi, M., Rawat, Y. S., & Ram, J. (2012). Seasonal variations in species diversity, dry matter and net primary productivity of herb layer of Quercus leucotrichophora-Pinus roxburghii mixed forest in Kumaun Himalaya. India. Journal of Forestry Research, 23(2), 223–228. https://doi.org/10.1007/s11676-011-0214-4
Joshi, R. K., & Garkoti, S. C. (2020). Litter dynamics, leaf area index and forest floor respiration as indicators for understanding the role of Nepalese alder in white oak forests in central Himalaya, India. Ecological Indicators, 111, 106065. https://doi.org/10.1016/j.ecolind.2020.106065
Joshi, R. K., & Garkoti, S. C. (2021). Influence of Nepalese alder on soil physico-chemical properties and fine root dynamics in white oak forests in the central Himalaya, India. Catena, 200, 105140. https://doi.org/10.1016/j.catena.2020.105140
Karhu, K., Auffret, M. D., Dungait, J. A. J., Hopkins, D. W., Prosser, J. I., Singh, B. K., Subke, J. A., Wookey, P. A., Ågren, G. I., Sebastià, M. T., Gouriveau, F., Bergkvist, G., Meir, P., Nottingham, A. T., Salinas, N., & Hartley, I. P. (2014). Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature, 513(7516), 81–84. https://doi.org/10.1038/nature13604
Karki, H., Bargali, K., & Bargali, S. S. (2021). Spatial and seasonal pattern of fine root biomass and turnover rate in different land use systems in Central Himalaya. India. Russian Journal of Ecology, 52(1), 36–48. https://doi.org/10.1134/S1067413621010070
Kaushal, S., & Baishya, R. (2021). Stand structure and species diversity regulate biomass carbon stock under major Central Himalayan forest types of India. Ecological Processes, 10, 14. https://doi.org/10.1186/s13717-021-00283-8
Kaushal, S., Siwach, A., & Baishya, R. (2021). Diversity, regeneration, and anthropogenic disturbance in major Indian Central Himalayan forest types: Implications for conservation. Biodiversity and Conservation, 30, 2451–2480. https://doi.org/10.1007/s10531-021-02203-w
King, J. A., & Harrison, R. (2002). Measuring soil respiration in the field: An automated closed chamber system compared with portable IRGA and alkali absorption methods. Communications in Soil Science and Plant Analysis, 33(3–4), 403–423. https://doi.org/10.1081/CSS-120002753
Körner, C. (2007). The use of ‘altitude’ in ecological research. Trends in Ecology & Evolution, 22(11), 569–574. https://doi.org/10.1016/j.tree.2007.09.006
Kumar, A. D., & Ramakrishnan, P. S. (1985). Litter dynamics in khasi pine (Pinus kesiya Royle ex Gordon) of north-eastern India. Forest Ecology and Management., 10(1–2), 135–153. https://doi.org/10.1016/0378-1127(85)90018-0
Kumar, P., Singh, R., Singh, H., Chand, T., & Bala, N. (2020). Assessment of soil carbon dioxide efflux and its controlling factors in moist temperate forest of West Himalayas. Current Science, 119(4), 661.
Kumar, S., Kumar, M., Verma, A. K., Joshi, R. K., Hansada, P., Geise, A., & Garkoti, S. K. (2023). Seasonal dynamics of soil and microbial respiration in the banj oak and chir pine forest of the central Himalaya, India. Applied Soil Ecology, 182, 104740. https://doi.org/10.1016/j.apsoil.2022.104740
Kuzyakov, Y. (2006). Sources of CO2 efflux from soil and review of partitioning methods. Soil Biology and Biochemistry, 38(3), 425–448. https://doi.org/10.1016/j.soilbio.2005.08.020
Laishram, J., Saxena, K. G., Maikhuri, R. K., & Rao, K. S. (2012). Soil quality and soil health: A review. International Journal of Ecology and Environmental Sciences, 38(1), 19–37.
Lal, R. (2008). Carbon sequestration. Philosophical Transactions of the Royal Society B: Biological Science, 363(1492), 815–830
Lal, R., Monger, C., Nave, L., & Smith, P. (2021). The role of soil in regulation of climate. Philosophical Transactions of the Royal Society B, 376(1834), 20210084. https://doi.org/10.1098/rstb.2021.0084
Lashof, D., & Ahuja, D. (1990). Relative contributions of greenhouse gas emissions to global warming. Nature, 344, 529–531. https://doi.org/10.1038/344529a0
Lee, J. S. (2018). Relationship of root biomass and soil respiration in a stand of deciduous broadleaved trees—A case study in a maple tree. Journal of Ecology and Environment, 42(1), 1–8. https://doi.org/10.1186/s41610-018-0078-z
Lee, K. H., & Jose, S. (2003). Soil respiration, fine root production, and microbial biomass in cottonwood and loblolly pine plantations along a nitrogen fertilization gradient. Forest Ecology and Management, 185(3), 263–273. https://doi.org/10.1016/S0378-1127(03)00164-6
Lei, J., Guo, X., Zeng, Y., Zhou, J., Gao, Q., & Yang, Y. (2021). Temporal changes in global soil respiration since 1987. Nature Communications, 12(1), 403. https://doi.org/10.1038/s41467-020-20616-z
Lorenz, K., & Lal, R. (2010). The importance of carbon sequestration in forest ecosystems. In K. Lorenz & R. Lal Carbon sequestration in forest ecosystems (1st ed., pp. 1 – 21). Springer, Dordrecht. https://doi.org/10.1007/978-90-481-3266-9_6
Lovenduski, N. S., & Bonan, G. B. (2017). Reducing uncertainty in projections of terrestrial carbon uptake. Environmental Research Letters, 12(4), 044020. https://doi.org/10.1088/1748-9326/aa66b8
Lund, C. P., Riley, W. J., Pierce, L. L., & Field, C. B. (1999). The effects of chamber pressurization on soil-surface CO2 flux and the implications for NEE measurements under elevated CO2. Global Change Biology, 5(3), 269–281.
Luo, Y., & Zhou, X. (2006). Soil respiration and the environment. Academic Press.
Maier, M., Schack-Kirchner, H., Hildebrand, E. E., & Schindler, D. (2011). Soil CO2 efflux vs. soil respiration: Implications for flux models. Agricultural and Forest Meteorology, 151(12), 1723–1730. https://doi.org/10.1016/j.agrformet.2011.07.006
Mariappan, S., Hartley, I. P., Cressey, E. L., Dungait, J. A., & Quine, T. A. (2022). Soil burial reduces decomposition and offsets erosion-induced soil carbon losses in the Indian Himalaya. Global Change Biology, 28(4), 1643–1658. https://doi.org/10.1111/gcb.15987
Metcalfe, D. B., Fisher, R. A., & Wardle, D. A. (2011). Plant communities as drivers of soil respiration: Pathways, mechanisms, and significance for global change. Biogeosciences, 8(8), 2047–2061. https://doi.org/10.5194/bg-8-2047-2011
Mishra, S., Chaudhary, L. B., Jain, M. K., Kumar, V., & Behera, S. K. (2020). Interaction of abiotic factor on soil CO2 efflux in three forest communities in tropical deciduous forest from India. Environmental Monitoring and Assessment, 191(Suppl 3), 796. https://doi.org/10.1007/s10661-019-7689-4
Mitra, B., Miao, G., Minick, K., McNulty, S. G., Sun, G., Gavazzi, M., King, J. S., & Noormets, A. (2019). Disentangling the effects of temperature, moisture, and substrate availability on soil CO2 efflux. Journal of Geophysical Research: Biogeosciences, 124, 2060–2075. https://doi.org/10.1029/2019JG005148
Mundim, K. C., Baraldi, S., Machado, H. G., & Vieira, F. M. (2020). Temperature coefficient (Q10) and its applications in biological systems: Beyond the Arrhenius theory. Ecological Modelling, 431, 109127. https://doi.org/10.1016/j.ecolmodel.2020.109127
Nirola, M. P., Wangdi, N., Mayer, M., Schindlbacher, A., & Jandl, R. (2016). Drivers of soil respiration in two mountain forests in Bhutan. Masters of Science in Mountain Forestry, University of Natural Resources and Life Sciences.
Noh, N. J., Son, Y., Lee, S. K., Yoon, T. K., Seo, K. W., Kim, C., Lee, W. K., Bae, S. W., & Hwang, J. (2010). Influence of stand density on soil CO2 efflux for a Pinus densiflora forest in Korea. Journal of Plant Research, 123, 411–419. https://doi.org/10.1007/s10265-010-0331-8
Pandey, R., Rawat, M., Singh, R., & Bala, N. (2023). Large scale spatial assessment, modelling and identification of drivers of soil respiration in the Western Himalayan temperate forest. Ecological Indicators, 146, 109927. https://doi.org/10.1016/j.ecolind.2023.109927
Phillips, C. L., & Nickerson, N. (2015). Soil respiration. Reference Module in Earth Systems and Environmental Sciences, Elsevier. https://doi.org/10.1016/B978-0-12-409548-9.09442-2
Ponder, F., Jr. (2005). Effect of soil compaction and biomass removal on soil CO2 efflux in a Missouri forest. Communications in Soil Science and Plant Analysis, 36(9–10), 1301–1311. https://doi.org/10.1081/CSS-200056935
Pongracic, S., Kirschbaum, M. U. F., & Raison, R. J. (1997). Comparison of soda lime and infrared gas analysis techniques for in situ measurement of forest soil respiration. Canadian Journal of Forest Research, 27(11), 1890–1895. https://doi.org/10.1139/x97-139
Rai, I. D., Adhikari, B. S., & Rawat, G. S. (2012). Floral diversity along sub-alpine and alpine ecosystems in Tungnath area of Kedarnath Wildlife Sanctuary. Uttarakhand. Indian Forester, 138(10), 927.
Raich, J. W., & Potter, C. S. (1995). Global patterns of carbon dioxide emissions from soils. Global Biogeochemical Cycles, 9(1), 23–36. https://doi.org/10.1029/94GB02723
Raich, J. W., & Schlesinger, W. H. (1992). The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus b: Chemical and Physical Meteorology, 44(2), 81–99. https://doi.org/10.3402/tellusb.v44i2.15428
Raich, J. W., & Tufekciogul, A. (2000). Vegetation and soil respiration: Correlations and controls. Biogeochemistry, 48(1), 71–90. https://doi.org/10.1023/A:1006112000616
Rambo, T. R., & North, M. P. (2009). Canopy microclimate response to pattern and density of thinning in a Sierra Nevada forest. Forest Ecology and Management, 257(2), 435–442. https://doi.org/10.1016/j.foreco.2008.09.029
Ramesh, T., Manjaiah, K. M., Tomar, J. M. S., & Ngachan, S. V. (2013). Effect of multipurpose tree species on soil fertility and CO2 efflux under hilly ecosystems of Northeast India. Agroforestry Systems, 87(6), 1377–1388. https://doi.org/10.1007/s10457-013-9645-6
Rawat, M., Arunachalam, K., & Arunachalam, A. (2021). Tree species influence soil respiration in a temperate forest of Uttrakhand Himalaya. India. Journal of Sustainable Forestry, 40(8), 820–830. https://doi.org/10.1080/10549811.2020.1822873
Renchon, A. A., Drake, J. E., Macdonald, C. A., Sihi, D., Hinko-Najera, N., Tjoelker, M. G., & Pendall, E. (2021). Concurrent measurements of soil and ecosystem respiration in a mature eucalypt woodland: advantages, lessons, and questions. Journal of Geophysical Research: Biogeosciences, 126(3), e2020JG006221. https://doi.org/10.1029/2020JG006221
Rodda, S. R., Thumaty, K. C., Praveen, M. S. S., Jha, C. S., & Dadhwal, V. K. (2021). Multi-year eddy covariance measurements of net ecosystem exchange in tropical dry deciduous forest of India. Agricultural and Forest Meteorology, 301, 108351. https://doi.org/10.1016/j.agrformet.2021.108351
Sánchez-Cañete, E. P., Barron-Gafford, G. A., & Chorover, J. (2018). A considerable fraction of soil-respired CO2 is not emitted directly to the atmosphere. Scientific Reports, 8(1), 1–10. https://doi.org/10.1038/s41598-018-29803-x
Sanders, N. J., & Rahbek, C. (2012). The patterns and causes of elevational diversity gradients. Ecography, 35(1), 1–3. https://doi.org/10.1111/j.1600-0587.2011.07338.x
Sarma, D., Burman, P. K. D., Chakraborty, S., Gogoi, N., Bora, A., Metya, A., Datye, A., Murkute, C., & Karipot, A. (2022). Quantifying the net ecosystem exchange at a semi-deciduous forest in northeast India from intra-seasonal to the seasonal time scale. Agricultural and Forest Meteorology, 314, 108786. https://doi.org/10.1016/j.agrformet.2021.108786
Schickhoff, U., Singh, R. B., & Mal, S. (2016). Climate change and dynamics of glaciers and vegetation in the Himalaya: An overview. In R. Singh, U. Schickhoff, & S. Mal (Eds.), Climate change, glacier response, and vegetation dynamics in the Himalaya (1st ed., pp. 1 – 26). Springer, Cham. https://doi.org/10.1007/978-3-319-28977-9_1
Schulze, E. D. (1967). Soil respiration of tropical vegetation types. Ecology, 48(4), 652–653. https://doi.org/10.2307/1936509
Schurman, J. S., & Thomas, S. C. (2021). Linking soil CO2 efflux to individual trees: Size-dependent variation and the importance of the Birch effect. Soil Systems, 5(1), 7. https://doi.org/10.3390/soilsystems5010007
Schwendenmann, L., & Macinnis-Ng, C. (2016). Soil CO2 efflux in an old-growth southern conifer forest (Agathis australis)–magnitude, components and controls. The Soil, 2(3), 403–419. https://doi.org/10.5194/soil-2-403-2016
Shameem, S. A., Soni, P., & Bhat, G. A. (2010). Comparative study of herb layer diversity in lower Dachigam National Park, Kashmir Himalaya, India. International Journal of Biodiversity and Conservation, 2(10), 308–315.
Singh, D., Sharma, P., Kumar, U., Daverey, A., & Arunachalam, K. (2021). Effect of forest fire on soil microbial biomass and enzymatic activity in oak and pine forests of Uttarakhand Himalaya. India. Ecological Processes, 10(1), 29. https://doi.org/10.1186/s13717-021-00293-6
Singh, J. S., & Gupta, S. R. (1977). Plant decomposition and soil respiration in terrestrial ecosystems. The Botanical Review, 43(4), 449–528. https://doi.org/10.1007/BF02860844
Singh, J. S., & Singh, S. P. (1987). Forest vegetation of the Himalaya. The Botanical Review, 53(1), 80–192. https://doi.org/10.1007/BF02858183
Singh, N., & Parida, B. R. (2019). Environmental factors associated with seasonal variations of night-time plant canopy and soil respiration fluxes in deciduous conifer forest, Western Himalaya. India. Trees, 33(2), 599–613. https://doi.org/10.1007/s00468-018-1804-y
Singh, N., Parida, B. R., Charakborty, J. S., & Patel, N. R. (2019). Net ecosystem exchange of CO2 in deciduous pine forest of lower Western Himalaya. India. Resources, 8(2), 98. https://doi.org/10.3390/resources8020098
Sivaranjani, S., & Panwar, V. P. (2021). Environmental controls and influences of Pinus roxburghii Sarg. (Chir pine) plantation on temporal variation in soil carbon dioxide emission and soil organic carbon stock under humid subtropical region. Environmental Monitoring and Assessment, 193, 1–14. https://doi.org/10.1007/s10661-021-09419-x
Sundarapandian, S., & Dar, J. (2013). Variation in soil CO2 efflux in Pinus wallichiana and Abies pindrow temperate forests of Western Himalayas. India. Forest Research, 3(116), 2. https://doi.org/10.4172/2168-9776.1000116
Tewary, C. K., Pandey, U., & Singh, J. S. (1982). Soil and litter respiration rates in different microhabitats of a mixed oak-conifer forest and their control by edaphic conditions and substrate quality. Plant and Soil, 65(2), 233–238. https://doi.org/10.1007/BF02374653
Tiwari, P., Bhattacharya, P., Rawat, G. S., Rai, I. D., & Talukdar, G. (2021). Experimental warming increases ecosystem respiration by increasing above-ground respiration in alpine meadows of Western Himalaya. Scientific Reports, 11(1), 2640. https://doi.org/10.1038/s41598-021-82065-y
Trumbore, S. (2006). Carbon respired by terrestrial ecosystems–recent progress and challenges. Global Change Biology, 12(2), 141–153. https://doi.org/10.1111/j.1365-2486.2006.01067.x
Usman, S., Singh, S. P., & Rawat, Y. S. (1999). Fine root productivity and turnover in two evergreen central Himalayan forests. Annals of Botany, 84(1), 87–94. https://doi.org/10.1006/anbo.1999.0894
Vermue, E., Elbers, J., & Hoosbeek, M. (2008). A comparative field study of four soil respiration systems. Accessed on 18 March 2023 https://edepot.wur.nl/120659
Vikram, K., Chaudhary, H., Notup, T., Dinakaran, J., & Rao, K. S. (2022). Soil respiration under different land use systems in Kumaon region of Central Himalaya, India. International Journal of Ecology and Environmental Sciences, 48(5), 649–661. https://doi.org/10.55863/ijees.2022.0649
Walkiewicz, A., Rafalska, A., Bulak, P., Bieganowski, A., & Osborne, B. (2021). How can litter modify the fluxes of CO2 and CH4 from forest soils? A Mini-Review. Forests, 12(9), 1276. https://doi.org/10.3390/f12091276
Wang, B., Jiang, Y., Wei, X., Zhao, G., Guo, H., & Bai, X. (2011). Effects of forest type, stand age, and altitude on soil respiration in subtropical forests of China. Scandinavian Journal of Forest Research, 26(1), 40–47. https://doi.org/10.1080/02827581.2010.538082
Wangdi, N., Mayer, M., Nirola, M. P., Zangmo, N., Orong, K., Ahmed, I. U., Darabant, A., Jandl, R., Gratzer, G., & Schindlbacher, A. (2017). Soil CO2 efflux from two mountain forests in the eastern Himalayas, Bhutan: Components and controls. Biogeosciences, 14(1), 99–110. https://doi.org/10.5194/bg-14-99-2017
Wani, O. A., Kumar, S., Hussain, N., Wani, A. I. A., Subhash, B., Parvej, A., Rashid, M., Popescu, S. M., & Mansoor, S. (2022). Multi-scale processes influencing global carbon storage and land-carbon-climate nexus: A critical review. Pedosphere. https://doi.org/10.1016/j.pedsph.2022.07.002
Wildung, R. E., Garland, T. R., & Buschbom, R. L. (1975). The interdependent effects of soil temperature and water content on soil respiration rate and plant root decomposition in arid grassland soils. Soil Biology and Biochemistry, 7(6), 373–378. https://doi.org/10.1016/0038-0717(75)90052-8
Yan, W. D., Xu, W. M., Tian, D. L., Peng, Y. Y., Zhen, W., Zhang, C., & Xu, J. (2014). Soil CO2 flux in different types of forests under a subtropical microclimatic environment. Pedosphere, 24(2), 243–250. https://doi.org/10.1016/S1002-0160(14)60010-2
Zhao, J. F., Liao, Z. Y., Yang, L. Y., Shi, J. K., & Tan, Z. H. (2021). Characteristics of soil respiration and its components of a mixed dipterocarp forest in China. Forests, 12(9), 1159. https://doi.org/10.3390/f12091159
Zuur, A. F., Ieno, E. N., & Smith, G. M. (2007). Analysing ecological data. Springer.
Acknowledgements
The authors express their gratitude to the Principal Chief Conservator of Forests (PCCF) & Chief Wildlife Warden (CWLW), Uttarakhand Forest Department, Uttarakhand, along with the director of Rajaji National Park, and the Divisional Forest Officers (DFOs) for Dehradun, Chakrata, and Kedarnath Wildlife Sanctuary for providing the required field permissions and assistance during the field surveys. We thank Mr. Aakash Goswami for his assistance during the field visits. All authors thank the three anonymous reviewers for their constructive comments and suggestions which considerably improved the manuscript and its readability.
Funding
This study is funded by Science and Engineering Research Board (SERB) through research project no. EEQ/2016/000164. The first author is thankful to the University Grants Commission (UGC), New Delhi, for CSIR-UGC senior research fellowship. The corresponding author acknowledges the Institution of Eminence (IoE), University of Delhi for providing additional funds for the study through Faculty Research Programme (FRP) grant (IoE/2021/12/FRP).
Author information
Authors and Affiliations
Contributions
S. K. and R. B conceptualized this study. S. K. collected the in situ soil CO2 efflux data and soil sampling, conducted laboratory experiments, performed statistical data analyses and wrote the original manuscript draft. K. S. R., P. L. U., and R. B. reviewed and edited the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaushal, S., Rao, K.S., Uniyal, P.L. et al. Patterns and determinants of soil CO2 efflux in major forest types of Central Himalayas, India. Environ Monit Assess 195, 876 (2023). https://doi.org/10.1007/s10661-023-11470-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11470-9