Abstract
Aims
We investigated the effects of volatile organic compounds (VOCs) emitted by pine litter, specifically terpenes, on soil microbial biomass carbon and nitrogen and heterotrophic soil respiration under different microclimatic scenarios of water availability and temperature.
Methods
Soil in glass jars (0.6 L headspace) was exposed to pine needle litter, avoiding any physical contact between soils and litter. Treatments were subjected to two moisture levels, control and drought (20 % and 10 % gravimetric soil water content respectively) and to different temperatures (temperature response curve from 5 °C to 45 °C).
Results
In control soils, exposure to litter was associated with a significant decrease in microbial biomass carbon and ninhydrin extractable organic nitrogen, and with a significant increase in heterotrophic respiration (up to 46 %) under optimum temperature (25 °C). Drought, on the other hand, restricted the effects of litter exposure on heterotrophic respiration but exposure to litter was associated with a significant increase in microbial biomass nitrogen. We did not detect significant overall microbial consumption of terpenes in this study.
Conclusions
These results suggest either that other VOCs not measured in the study were being consumed and/or that VOCs emissions were triggering strong changes in the composition and functioning of soil microbial communities. More studies under field conditions are needed to assess the magnitude of litter VOCs effects on carbon and nitrogen cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The emissions of volatile organic compounds (VOCs) from litter are the result of enzymatic activities of decomposer communities (mainly bacteria and fungi) in litter over time (Insam and Seewald 2010; Stahl and Parkin 1996) and other non-enzymatic (abiotic) thermochemical reactions (Warneke et al. 1999). Significant litter VOCs emissions have been reported in different forest ecosystems from spring to autumn (Aaltonen et al. 2011; Isidorov and Jdanova 2002; Isidorov et al. 2010; Schade and Goldstein 2001) although, in general, litter VOCs emissions represent a small fraction of the total litter C pool (Asensio et al. 2007a).
Biogenically produced VOCs include a wide range of chemical species: alcohols, aldehydes, aromatics, esters, ethers, hydrocarbons, ketones, nitriles, terpenes, sulfides (Fall 1999; Fuentes et al. 2000). Among the VOCs produced naturally in plants, the terpene group accounts for a large portion of VOCs emissions (Peñuelas and Llusià 2003). Terpenes are present in plant tissues in significant amounts, especially in coniferous species, and can be released to the soil by root and leaf litter and plant root exudates (Asensio et al. 2008a; Hayward et al. 2001; Janson 1993; Lin et al. 2007). Within the group of terpenes, monoterpenes (C10H16) play an important role on the interactions between organisms (Peñuelas and Llusià 2004; Peñuelas and Staudt 2010), including soil organisms (reviewed by Wenke et al. 2010). Monoterpenes can have inhibitory effects on microbial growth (Smolander et al. 2006; Uusitalo et al. 2008; Vokou et al. 2002) and have been shown to inhibit net mineralization of nitrogen and net nitrification (Amaral and Knowles 1997; Paavolainen et al. 1998; Smolander et al. 2006; Uusitalo et al. 2008; White 1988). This suggests that the occurrence of plant monoterpenes in soils can affect microbial population demography and the nutritional status of soils and, therefore, it may affect nitrogen (N) cycle.
Soil microorganisms can both produce and consume VOCs. Several authors have described the ability of soil microorganisms to use VOCs as a carbon source (reviewed by Insam and Seewald 2010). In fact, previous studies of soil VOCs exchanges in field conditions reported the potential of soils to act as a sink of VOCs (Asensio et al. 2007a). As a result of microbial consumption of VOCs, soil carbon dioxide (CO2) efflux has been shown to increase (Amaral and Knowles 1998; Owen et al. 2007; Ramirez et al. 2009; Smolander et al. 2006; Vokou et al. 2002). For instance, Ramirez et al. (2009) showed that soils exposed to litter VOCs absorbed 80 % of the VOCs emitted by litter increasing soil respiration rates by 15 % under controlled laboratory conditions. Thus, litter VOCs can be a rapidly mineralizable carbon (C) source for soil microorganisms, increasing soil CO2 efflux and potentially affecting C cycle. Therefore, terpenes (and other VOCs) can serve as carbon source for some microbes, and at the same time, they can show inhibitory effects for other species growth. Given that changes on soil microbial activities may have significant effects on soil CO2 emissions (Curiel Yuste et al. 2011), it is important to understand the overall effect of VOCs on structure and functioning of soil microbial communities, which are responsible of at least half of the soil CO2 efflux.
Furthermore, there is little information on how different environmental (biotic and abiotic) factors and their interactions may affect the microbial use of VOCs. For example, although the diffusion of VOCs through the soil matrix is enhanced under dry conditions (Moldrup et al. 2000), microbial activity decreases sharply with drought below a threshold (e.g. Curiel Yuste et al. 2007) and therefore microbes cannot benefit from increasing VOCs emissions under higher temperatures (Asensio et al. 2008b).
In the present study we aimed to investigate the effects of litter VOCs on decomposition process driven by microbes and on microbial biomass C and N contents of soil under different microclimatic scenarios of water availability and temperature. Given that volatile terpenes can play an important role on soil ecology and biogeochemistry, we have focused only on terpene emissions from pine litter. More specifically our aims were to answer these three questions: 1) Given that literature reports contradictory (inhibitory and stimulatory) effects of terpenes over soil microorganisms activity, can we observe evidence of negative or positive effects of terpenes on heterotrophic respiration and microbial biomass C and N in our experimental system?, 2) Could temperature increase enhance litter production and microbial consumption of terpenes?, and 3) Could microbes take profit of increasing VOCs diffusivity under moderate water stress?.
Material and methods
Study site, soil and litter sampling and experiment set up
Soil and litter samples were collected in a pure stand of Pinus halepensis Mill. at Collserola National Park (Barcelona) during the first week of January 2010. The Collserola National Park is a maquis located at 350 m altitude in central Catalonia, north-east Spain, 41°27′N, 2°7.7′E. The dominant species are 1–3 m tall Arbutus unedo L., Bupleurum fruticosum L., Cistus albidus L., Erica arborea L., Pinus halepensis Mill.., Quercus coccifera L. and Quercus ilex L. The climate is typical Mediterranean, with cool winters and warm, dry summers with a mean annual temperature of 14.5 °C and a mean annual precipitation of 610 mm.
Five pine trees of similar age were selected randomly within the study area (30 × 30 m). No understory vegetation existed in the study site. The soil type was Haploxerept calcic (Soil Taxonomy, USDA). Litter pine needles and two soil cores (6 cm diameter; 12 cm depth) from the top 10 cm soil (including organic and mineral horizons) were collected at 1.5–2 m distance from each pine tree. Soil sub-samples were used to calculate water content of each soil core after drying soil at 60 °C for 48 h. Soil water content at the field site ranged from 6 % to 21 %. Fresh soil was bulked from ten soil cores to make one composite sample and sieved (2 mm mesh size) to remove larger roots, woody fragments and stones. Physical and chemical properties of the soil were characterized (Table 1). Organic carbon content (oxidable carbon) was determined by wet oxidation with 0.4N K2Cr2O7 and by subsequent titration with 0.2N (NH4)2Fe(SO4)2·6H2O, and results were used to estimate total soil organic matter (Nelson and Sommers 1982). Total N was determined by the Kjeldahl method (Bremner and Mulvaney 1982). The electrical conductivity and soil pH were measured at soil water ratios of 1:5 and 1:2.5 respectively. The remaining sieved soil was dried in an oven at 60 °C, mixed and stored at 4 °C until the start of the incubation experiments (within 1 week). Litter samples were stored at room temperature and allowed to equilibrate with ambient room moisture until measurements.
Four days before measurement, water was added to the soil samples to reach 20 % water content for the control soil treatment (CS) and 10 % for the drought soil treatment (DS). Hermetically sealed glass jars with one septa port were filled with 100 g of hydrated soil + 5 g of litter, 100 g of soil alone or 5 g of litter alone. The amount of litter used was based on estimates of litter inputs for pine stands at Collserola National Park. Additional empty jars were used as blanks. A total of 4 replicates were prepared for each of the following treatments: CS: control soil, DS: drought soil, CSL: control soil-litter; DSL: drought soil-litter; L: litter and blanks (empty jars). In soil-litter treatments, a 0.2 mm metallic mesh was used to avoid physical contact between needle litter and soil, holding the needles 2–3 cm above the soil during the entire experiment. All treatments were held at 20 °C until measurements.
To assess the effects of temperature on CO2, VOCs (terpenes) emitted by soil and litter and soil microbial biomass C and N, the different treatments (CS, DS, CSL, DSL, L and blanks) were placed in a thermal bath and subjected to temperature response curves following a similar procedure to Curiel Yuste et al. (2007).
CO2 and terpene sampling and analysis
CO2 efflux from each jar was measured at 5 temperature steps: 5, 15, 25, 35 and 45 °C. Jars were incubated for 24 h at each temperature, and CO2 measurements were conducted after each temperature application. Thirty minutes prior to measurement, jars were opened to equilibrate the air CO2 concentrations of the jar and the room. Then we used a modified soil chamber connected to an EGM-4 portable system (PP-systems, UK) to measure soil CO2 efflux. A commercial soil chamber of PP-systems, the SRC-1, was enlarged with a 1 l.13 cm long PVC tube of the same diameter as the SRC-1 chamber and with a rubber rim at the end. Glass jars containing the incubated soils were then introduced into the enlarged chamber, sealing the inner volume as a closed system. The modified chamber produced CO2 readings similar to those obtained with the commercial SRC-1. Three respiration measurements were made on each jar and the average was used as the final value. Each respiration measurement took 2–3 min approximately.
Terpenes were sampled at time 0 (T0 = 0) and after the 24-hour incubation period (TF = 24 h) at each temperature, and prior to open the jar for soil CO2 equilibration. Terpene analyses were performed by a GC-MS system (Agilent Technologies, GC: 7890A, MS: 5975C inert MSD with Triple-Axis Detector, Palo Alto, CA, USA). Terpenes were sampled from the glass jars heads space using a 10 mL gastight syringe (#1010, Hamilton CO., Reno, Nevada) and injected into 20 mL head space vials. The vials were previously evacuated using a vacuum pump and cleaned with N2 5.0 in order to avoid contamination from laboratory air. Helium 5.0 was used to clean the syringe. After injecting the 10 mL, the over pressure generated in the 20 mL head space vials was balanced with atmospheric pressure using a needle syringe through the septa. Vials were then introduced in a Head Space incubator (CTC Analytics, MH 01-00B, Zwingen, Switzerland) at 50 °C during 1 min to avoid condensation inside the vials, and later injected into the column with an automatic sample processor (Combi PAL, CTC Analytics, MXY 02-01B, Zwingen, Switzerland) using a Head Space 2.5 mL syringe (CTC Analytics, MSH 02-00B, Zwingen, Switzerland). Samples were injected into a 30 m × 0.25 mm × 0.25 μm film thickness capillary column (HP-5MS, Agilent Technologies). Helium flow was 0.5 ml min−1. Total GC run time was 30 min and the solvent delay was 4 min. After the sample injection, the initial temperature of 70 °C was held for 2 min, then increased at 20 °C min−1 to 230 °C, and thereafter at 20 °C min−1 up to 240 °C. This temperature was maintained for 1 min, and thereafter increased at 20 °C min−1 up to 270 °C, and maintained at that temperature for another 5 min.
The identification of terpenes was conducted by comparing the retention times with liquid standards from Fluka (Buchs, Switzerland) volatilized in separated vials, and comparing the fractionation mass spectra with standards spectra and Nist05a and wiley7n mass spectra libraries. Terpene concentrations were determined from calibration curves. The calibration curves for common monoterpenes, α-pinene, Δ3-carene, β-pinene, β-myrcene, p-cymene, limonene and sabinene, and common sesquiterpenes such as α-humulene were determined once every seven analyses using four different terpene concentrations. Terpene calibration curves were always highly significant (r 2 > 0.99) in the relationship between signal and terpene emission rates. The quantification of the peaks was conducted using the fractionation product with mass 93. Detection limit for our analytical method was about 5 ng. The accumulation of terpenes in the jar head space (actual terpenes concentration) was calculated as the difference between TF and T0 terpene concentrations.
Carbon and nitrogen analyses and metabolic quotient calculation
Microbial biomass carbon (MBC) in soil (soil alone and soil-litter treatments) was estimated at the end of the incubations following the fumigation extraction method: two portions of moist soil (20 g oven-dry soil) were weighed, the first (not fumigated) was immediately extracted with 80 ml of 0.5 M K2SO4 for 30 min by shaking, and then filtered (Whatman no. 42); the second one was fumigated for 24 h at 25 °C with ethanol-free CHCl3 and then extracted as described above. Extractable organic C was determined after oxidation with 0.4 M K2Cr2O7 at 150 °C for 30 min and back titration with 0.1 N ammonium-iron(II)sulphate-6-hydrate (Vance et al. 1987). MBC was calculated as follows:
where EC is the difference between organic C extracted from fumigated soil and organic C extracted from non-fumigated soil and 2.22 is the conversion factor from EC into microbial biomass (Wu et al. 1990).
Microbial biomass nitrogen (MBN) was estimated on fumigated and non fumigated samples following Joergensen and Brookes (1990). Ninhydrin reagent solution (Sigma N7285) was used to measure ninhydrin-reactive nitrogen in non-fumigated and fumigated 0.5 M K2SO4 soil extracts in order to determine the N content of K2SO4 extractable soil organic matter (SOM). Ninhydrin reagent solution (0.5 ml) was slowly added to 1 ml of non-fumigated and fumigated 0.5 M K2SO4 soil extracts and the mixture was thoroughly mixed and heated in a vigorously boiling water bath for 25 min. The solution was then cooled to room temperature, 5 ml of ethanol-water (1:1 v:v of 95 % ethanol:water) was added, the solution was thoroughly mixed and the absorbance was read at 570 nm against the control within 60 min, when the color was stable. A standard curve was prepared by measuring the absorbance of six different concentrations of L-leucine-N (from 0 to 15 μg N ml−1) dissolved in 0.5 M K2SO4. MBN was calculated as follows:
where EN is the difference between N extracted from fumigated soils and N extracted from non-fumigated soils and 2.2 is the conversion factor from EN into microbial biomass (Jenkinson 1988; Joergensen and Brookes 1990).
The metabolic quotient (qCO2) was calculated as the cumulative amount of CO2 produced on each treatment divided by the microbial biomass C at the end of the experiment (Anderson and Domsch 1993).
Statistical analyses
Statistical analyses were performed with Statistica 6.0 (StatSoft, Inc., 1984–2001, Tulsa, OK, USA). Normality of variables was tested by the Kolmogorov-Smirnov test and variables were logarithmically transformed to accomplish normal distribution and homogeneity of variance.
For the four different treatments containing soil (CS, DS, CSL and DSL) the effects of litter (presence/absence) and water availability (control/drought) on the dependent variables CO2 efflux and total monoterpene concentrations were tested with a repeated-measures Analysis of Variance (ANOVA) using temperature as the within-factor (repeated measurement). Repeated-measures ANOVA were also used separately in control and drought soils to test the effect of litter presence and temperature on CO2 efflux. Similarly, repeated-measures ANOVA were used separately in control and drought soils to test the “non-additive” effect of litter presence (priming) and temperature on CO2 efflux. With litter alone treatments (L), repeated-measures ANOVA analyses were performed to test the effects of temperature on litter CO2 production and monoterpene concentrations.
For the other soil variables measured at the end of the experiment (MBC, MBN, metabolic quotient and C/N ratios) a two-way ANOVA was performed to test the effects of litter presence and water availability on those variables.
For all the analyses performed, differences between treatments within the same temperature were tested using post-hoc test comparisons (Fisher’s test, P ≤ 0.05).
Results
Extractable organic carbon and nitrogen and soil microbial biomass C and N
Litter and drought treatments did not affect Extractable Organic Carbon (EOC) in soil (Table 2). However, litter and drought treatments significantly decreased ninhydrin Extractable Organic Nitrogen (EON) (Table 2).
Litter VOCs significantly reduced soil MBC (Fig. 1a). Contrarily, litter VOCs increased soil MBN (Fig. 1b), especially in drought soils (post-hoc test P < 0.05; Fig. 1b). On average (control and drought data together) pine litter VOCs decreased microbial biomass C/N ratio by 70 % (Table 2).
a Soil microbial biomass carbon (MBC), b soil microbial biomass nitrogen (MBN) and c metabolic quotient (qCO2) in control soil (CS), control soil-litter (CSL), drought soil (DS) and drought soil-litter (DSL) treatments measured at the end of the experiment (1 week). Significant effects from the two-way ANOVA (factors: litter and water) are indicated inside the panel. Significant differences between treatments (P < 0.05, Fisher’s post-hoc test) are indicated with different letters. Error bars are standard error of the mean, n = 4
Drought did not affect MBC (Fig. 1a) but enhanced the effect of litter increasing MBN (post hoc test DSL > DS, P < 0.01, Fig. 1b). However, the interaction between litter and drought narrowly failed significance for MBN (two way ANOVA, interaction litter x drought P < 0.1, Fig. 1b).
Soil and litter CO2 emissions
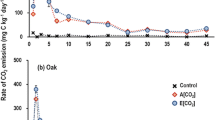
Soil-litter treatments showed significantly higher CO2 emissions than soil alone treatments independently of water availability (ANOVA litter P < 0.05 both control and drought treatments, Fig. 2). CO2 emissions in litter alone treatments (L) were always lower than in soil treatments (Fig. 2). The “non-additive” increase on CO2 induced by litter VOCs (CO2 not explained by the addition of soil alone + litter alone respiration) was significantly affected by temperature in control soils (Fig. 2) being maximum at 25 °C (46 % non-additive significant increase in CO2, Fig. 2). Non-additive effects in drought soils were not significant, although maximum differences between DSL and DS + L were found also at 25 °C (Fig. 2). The metabolic quotient (qCO2) in soil treatments was significantly higher when litter was present (Fig.1c), especially in the control soil-litter treatment (post-hoc tests P < 0.05; Fig. 1c).
CO2 emissions in control and drought soil-litter (non-additive), soil alone, litter alone and soil + litter (additive) treatments. Differences between non-additive and additive CO2 emissions (non-additive effect) are tested by repeated measurements ANOVA on control and drought treatments separately. Significant differences between non-additive and additive CO2 emissions within the same temperature are indicated with one asterisk (P < 0.05, Fisher’s post-hoc test). Error bars are standard errors of the mean, n = 4
Temperature significantly affected soil CO2 production in control and drought soils (Fig. 2). Drought significantly reduced soil CO2 emissions (35 % on average), in both soil alone and soil-litter treatments (ANOVA water P < 0.01; Fig. 2). The maximum effect of drought independently of litter presence, was obtained at 25 °C (50 % decrease relative to control in soil-litter and soil alone treatments, Fig. 2). Control soils (both CS and CSL soils) showed minimum CO2 emissions at the lowest and the highest temperatures, with maximum CO2 emissions at 25 °C. Contrarily, CO2 in drought soils (both DS and DSL) decreased from 5 °C to 45 °C (Fig. 2).
Soil and litter terpene concentrations in the head space
Terpenes measured in treatments containing litter (L, CSL and DSL) consisted of the monoterpenes α-pinene (80 %) and Δ3-carene (20 %). The sesquiterpene caryophyllene was also detected in treatments containing litter, but the concentrations were almost insignificant. Terpenes measured in soil alone treatments (CS and DS) consisted also of α-pinene and Δ3-carene, although in different proportions (60 % and 40 % α-pinene and Δ3-carene respectively).
At the lower temperatures, no significant differences between treatments were found in total monoterpene concentrations. However, at 35 °C total monoterpenes in litter alone treatments were significantly higher than in treatments containing soil, independently of soil water availability (L vs. CSL, CS, DSL and DS; post hoc P < 0.01 and P < 0.05, Fig. 3). At 45 °C monoterpene concentrations in CSL and DSL increased massively and reached levels similar to those measured in L treatments (Fig. 3). Temperature significantly affected monoterpene concentrations in soil-litter treatments (ANOVA temperature and temperature × litter effects P < 0.0001) while it did not affect soil alone treatments (Fig. 3). No significant differences were found in total monoterpene concentrations between control and drought soil treatments at any temperature (Fig. 3).
Total monoterpene concentrations in the head space in control soil-litter (CSL), control soil (CS), drought soil-litter (DSL), drought soil (DS) and in litter alone (L) treatments. Significant differences between treatments within the same temperature are indicated with different letters (P < 0.05, Fisher’s post-hoc test). Error bars are standard errors of the mean, n = 4
Discussion
Litter effects on soil microbial biomass C and N and heterotrophic respiration
The observed changes in MBC and MBN and depletion of soil ninhydrin EON on soils exposed to litter avoiding any physical contact (Fig. 1a and b and Table 2) suggest that VOCs emitted by litter are triggering important changes in the soil and microbial stoichiometry, and probably in the taxonomic composition of soil microbial communities. The fact that the experiment was a short-term study may explain part of these results, since microbes did not have much time to adapt to the new conditions imposed by treatments. Drying soils at 60 °C, and posterior rewetting and incubation at 20 °C for 4 days before the assay, might have changed the microbial community composition as compared to the composition in its natural environment. However, it was expected that any flush of CO2 due to drying at 60 °C would have been essentially completed after incubating the moistened soils for 4 days at room temperature. In any case, recolonization by both native and non-native microbes, as well as microbial activity levels, should have been consistently homogeneous between all treatments at the beginning of the incubation.
Changes in soil heterotrophic respiration associated to litter exposure have been already observed in a recent publication (Ramirez et al. 2009). We have different plausible interpretations to our observations. Monoterpenes present in essential oils are known to possess antimicrobial activity against certain bacteria and fungi (Cox et al. 2000; Flamini et al. 1999), and have been shown to decrease soil microbial biomass carbon (Smolander et al. 2006; Uusitalo et al. 2008) and to reduce net mineralization of N, possibly through N immobilization by microbes (Bremner and McCarty 1988; White 1991, 1994). Therefore, the observed decrease in C/N ratio of microbes may be the consequence of a combined effect of microbial growth inhibition and net N immobilization in the surviving living microbes triggered by VOCs emissions. This may also explain the observed depletion of ninhydrin EON in soils exposed to litter (Table 2).
Besides the observed change in microbial C/N ratio, results showed that under optimum temperature and moisture conditions, CO2 efflux of soils exposed to litter was about 46 % higher than in soils not exposed to litter (25 °C, control soil; Fig. 2). This increase was not explained by the sum of respirations of soil- and litter-alone treatments (non-additive effect, Fig. 2). The 46 % “extra” carbon released in soils exposed to pine litter at 25 °C could, however, not be explained by an overall microbial consumption of terpenes, since no significant decrease in overall terpenes concentrations was observed in soil-litter treatments relative to litter alone at this temperature (Fig. 3). It also might be that the toxic effects of monoterpenes could have further generated labile C necromass, favoring the establishment of a fast-growing microbial community, which typically have very low carbon-use efficiency i.e., the amount of C incorporated to the microbial biomass per amount of C respired (Stickland and Rousk 2010). Accordingly, the metabolic quotient qCO2 (the microbial respiration per unit microbial biomass) was significantly higher in control soil-litter treatments (Fig. 1c). Thus, a shift towards microbial communities dominated by fast-growing species could partly explain the high CO2 efflux concurrently with the low MBC in soil-litter treatments as compared to soil alone. A change from fungal to bacterial-dominated soil community may have occurred since bacterial decomposition pathways support high turnover rates of easily available substrates (Wardle 1992; 1998) and can adapt their metabolism by decreasing their C-use efficiency. However, there also exist fast-growing fungal groups that can respond quickly to the addition of labile C sources (Chiginevaa et al. 2009; de Graaff et al. 2010). We did not explicitly test for fungi: bacteria ratios in our study, therefore the categorization of soil organisms between r-strategists (non specialist, fast growth) versus k-strategists (complex substrates, slow growth) proposed by Fontaine et al. (2003) may describe better the potential shift observed in our soil microbial communities.
On the other hand, pine litter emits other volatile compounds besides terpenes, methanol (CH3OH) being one of the dominant compounds (Bäck et al. 2010; Ewen et al. 2004). Methanol emissions can represent from 30 % to 95 % of the total pine litter VOC emissions, depending on the pine species litter type (Gray et al. 2010; Ramirez et al. 2009). Microbial consumption of methanol and other VOCs in soils exposed to red maple litter VOCs has been reported by Ramirez et al. (2009). These authors found that the amount of non-additive CO2 produced by soil-litter treatments was similar in quantity to the carbon produced as total VOCs from the litter alone treatments. Thus, it is possible that methanol and other VOC types not measured in this experiment were metabolized by microbes, therefore increasing soil respiration in our soil-litter treatments.
On average, the amount of C lost as terpenes represented 0.01 % of the C emitted as CO2 in this experiment. There is not much information yet on the quantitative role of soil-and-litter VOCs emissions in the carbon cycle in different ecosystems, however, some authors have found the contribution of soil VOCs fluxes to ecosystem carbon cycling may be very low (Aaltonen et al. 2011, Asensio et al. 2007b). Indeed, our results show once more that C lost as terpenes is very low as compared to C lost as CO2.
Whatever the mechanisms, our results show that litter VOCs can affect strongly microbial biomass and its metabolic activity when water is not limiting. Therefore, our results indicate that litter VOCs may play an important role in the formation, functioning and stoichiometry of the microbial communities responsible of SOM decomposition.
Temperature and water availability effects on litter terpenes emissions and microbial activity
Both soil and litter were a source of terpenes, although the soil source was very small. Temperature increased litter terpene emissions, but no significant overall consumption of litter terpenes was detected in this study. No overall consumption of terpenes was observed at peak decomposition rates, obtained at 25 °C. Only at 35 °C, terpene concentrations decreased significantly in soils exposed to litter with respect to litter alone treatments (Fig. 3). However, we did not find a concomitant increase in CO2 efflux in soil-litter treatments at this temperature (i.e. release of non-additive CO2, Fig. 2) and therefore the microbial use of terpenes as carbon source was not clear, but not impossible. Our methods cannot distinguish microbial consumption of terpenes separately from soil terpene emissions. There can be concurrent emissions of terpenes from the soil and consumption rate of terpenes by soil microbes, resulting in an overall small emission of terpenes from the soil (Fig. 4). Overall, our results indicate no overall uptake of terpenes in the soil, although terpene consumption by microbes might have been masked by larger soil emissions. Other studies have demonstrated the catabolism of selected terpenes (d-limonene, α-pinene, γ-terpinene, terpinolene and geraniol) by forest soil microbial cultures and soil samples (Misra et al. 1996; Owen et al. 2007), our results indicate that microbial consumption of litter terpenes may not exceed emissions of terpenes in all soils and could depend on the substrate.
Moisture limitations, on the other hand, restricted the overall effect of litter VOCs on heterotrophic soil respiration (Fig. 2). These results provide evidence that microbes probably do not benefit from increasing VOCs diffusivity in drought soils, or from any other VOC-mediated effect on microbial communities (e. g. the use of labile C in necromass generated by terpenes toxicity) because they were experiencing a direct physiological stress that limited their metabolic activity. Drought limits the diffusion of nutrients and extracellular enzymes in the soil pore space (Sardans and Peñuelas 2005) as well as affect the physiological performance of microbes (Harris 1981). Indeed, CO2 efflux decreased in drought treatments soils as compared to control soils (Fig. 2).
Interestingly, drought significantly increased MBN in the presence of litter (Fig. 1b). This drought x litter interaction effect on MBN might be consequence of a shift in resources allocation from growth pathways to protective molecules like osmolytes, which are needed to survive under water stress conditions. Bacteria can accumulate amino compounds such as proline, glutamine, and glycine betaine, to reduce their internal water potential to avoid dehydrating and dying when soils dry and water potential declines (Schimel et al. 2007). Thus, increased MBN levels might be a consequence of increasing N-rich osmolytes inside microbial cells under the water stress imposed in drought soil-litter treatments. Given that accumulating osmolytes requires energy, litter VOCs may have provided advantageous carbon resources as compared to drought soil alone treatments, where no changes in MBN were observed (Fig. 1b). We, therefore, hypothesize that under this stressed conditions microbes could have used the C provided by litter VOCs to synthesize N-rich protective molecules.
Conclusions and final remarks
Results showed that litter VOCs can affect microbial biomass C and N and can influence heterotrophic respiration. Whatever the mechanisms behind the observed changes, our results suggest that the interaction litter-microbes could induce profound changes in soil nutrient dynamics and microbial community functioning. More studies are warranted to address the impact of terpenes and other litter VOCs on shaping microbial community composition.
We have found evidence for both positive and negative effects of VOCs over soil microbial activity; our observations likely meant more than one effect. The decrease in MBC observed in soil-litter treatments suggests once more that terpenes can have inhibitory effects on soil microorganisms. It was not possible to distinguish microbial consumption of terpenes from the overall observed flux of terpenes from the soil with the methods we used. The inhibitory effects of litter terpenes on soil microorganism activity might have dominated over the positive/stimulatory effects, in this specific microbial community. We further observed that, although microbial metabolic activity was reduced under drought stress, microbes might have used litter VOCs to synthesize N-rich protective compounds. This suggests that litter VOCs might play a role on changes in microbial biomass C and N under drought stress conditions.
References
Aaltonen H, Pumpanen J, Pihlatie M, Hakola H, Hellén H, Kulmala L, Vesala T, Bäck J (2011) Boreal pine forest floor biogenic volatile organic compound emissions peak in early summer and autumn. Agr Forest Meteorol 151:682–691
Amaral JA, Knowles R (1997) Inhibition of methane consumption in forest soils and pure cultures of methanotrophs by aqueous forest soil extracts. Soil Biol Biochem 29:1713–1720
Amaral JA, Knowles R (1998) Inhibition of methane consumption in forest soils by monoterpenes. J Chem Ecol 24:723–734
Anderson TH, Domsch KH (1993) The metabolic quotient for CO2 (QCO2) as specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol Biochem 25:393–395
Asensio D, Peñuelas J, Llusià J, Ogaya R, Filella I (2007a) Interannual and interseasonal soil CO2 efflux and VOC exchange rates in a Mediterranean holm oak forest in response to experimental drought. Soil Biol Biochem 39:2471–2484
Asensio D, Peñuelas J, Ogaya R, Llusià J (2007b) Seasonal soil VOC exchange rates in a Mediterranean holm oak forest and their responses to drought conditions. Atmos Environ 41:2456–2466
Asensio D, Owen S, Llusià J, Peñuelas J (2008a) The distribution of volatile isoprenoids in the soil horizons around Pinus halepensis trees. Soil Biol Biochem 40:2937–2947
Asensio D, Peñuelas J, Prieto P, Estiarte M, Filella I, Llusià J (2008b) Interannual and seasonal changes in the soil exchange rates of monoterpenes and other VOCs in a Mediterranean shrubland. Eur J Soil Sci 59:878–891
Bäck J, Aaltonen H, Hellén H, Kajos MK, Patokoski J, Taipale R, Pumpanen J, Heinonsalo J (2010) Variable emissions of microbial volatile organic compounds (MVOCs) from root-associated fungi isolated from Scots pine. Atmos Environ 44:3651–3659
Bremner JM, McCarty GW (1988) Effects of terpenoids on nitrification in soil. Soil Sci Soc Am J 52:1630–1633
Bremner JM, Mulvaney CS (1982) Nitrogen-total. In: Page AL et al (eds) Methods of soil analysis part 2—chemical and microbiological proprieties, 2nd edn. Am Soc Agron, Madison, pp 595–624
Chiginevaa NI, Aleksandrovab AV, Tiunovc AV (2009) The addition of labile carbon alters residue fungal communities and decreases residue decomposition rates. Appl Soil Ecol 42:264–270
Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmington JR, Wyllie SG (2000) The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol 88:170–175
Curiel Yuste J, Baldocchi DD, Gershenson A, Goldstein A, Misson L, Wong S (2007) Microbial soil respiration and its dependency on carbon inputs, soil temperature and moisture. Glob Chang Biol 13:2018–2035
Curiel Yuste J, Peñuelas J, Estiarte M, Garcia-Mas J, Mattana S, Ogaya R, Pujol M, Sardans J (2011) Drought-resistant fungi control soil organic matter decomposition and its response to temperature. Glob Chang Biol 17:1475–1486
de Graaff M-A, Classen AT, Castro HF, Schadt CW (2010) Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytol 188:1055–1064
Ewen RJ, Jones PRH, Ratcliffe NM, Spencer-Phillips PTN (2004) Identification by gas chromatography–mass spectrometry of the volatile organic compounds emitted from the wood-rotting fungi Serpula lacrymans and Coniophora puteana, and from Pinus sylvestris timber. Mycol Res 108:806–814
Fall R (1999) Biogenic emission of volatile organic compound from higher plants. In: Hewitt CN (ed) Reactive hydrocarbon in the atmosphere. Academic, San Diego, pp 41–96
Flamini G, Cioni PL, Puleio R, Morelli I, Panizzi L (1999) Antimicrobial activity of the essential oil of Calamintha nepeta and its constituent pulegone against bacteria and fungi. Phytother Res 13:349–351
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–843
Fuentes JD, Lerdau M, Atkinson R, Baldocchi D, Bottenheim JW, Ciccioli P, Lamb B, Geron C, Gu L, Guenther A et al (2000) Biogenic hydrocarbons in the atmospheric boundary layer: a review. Bull Am Meteorol Soc 81:1537–1575
Gray CM, Monson RK, Fierer N (2010) Emissions of volatile organic compounds during the decomposition of plant litter. J Geophys Res 115:G03015. doi:10.1029/2010JG001291
Harris RF (1981) Effect of water potential on microbial growth and activity. In: Parr JF (ed) Water potential relations in soil microbiology. ASA, Madison, pp 23–95
Hayward S, Muncey RJ, James AE, Halsall CJ, Hewitt CN (2001) Monoterpene emissions from soil in a Sitka spruce forest. Atmos Environ 35:4081–4087
Insam H, Seewald MSA (2010) Volatile organic compounds (VOCs) in soils. Biol Fertil Soils 46:199–213
Isidorov V, Jdanova M (2002) Volatile organic compounds from leaves litter. Chemosphere 48:975–979
Isidorov VA, Smolewska M, Purzyńska-Pugacewicz A, Tyszkiewicz Z (2010) Chemical composition of volatile and extractive compounds of pine and spruce leaf litter in the initial stages of decomposition. Biogeosci Discuss 7:1727–1750
Janson R (1993) Monoterpene emissions from Scots pine and Norwegian spruce. J Geophys Res 98:2839–2850
Jenkinson DS (1988) The determination of microbial biomass carbon and nitrogen in soil. In: Wilson JR (ed) Advances in nitrogen cycling in agricultural ecosystems. Commonwealth Agricultural Bureau International, Wallingford, pp 368–386
Joergensen RG, Brookes PC (1990) Ninhydrin-reactive nitrogen measurements of microbial biomass in 0.5.-M K2SO4 soil extracts. Soil Biol Biochem 22:1023–1027
Lin C, Owen SM, Peñuelas J (2007) Volatile organic compounds in the roots and rhizosphere of Pinus spp. Soil Biol Biochem 39:951–960
Misra G, Pavlostathis SG, Perdue EM, Araujo R (1996) Aerobic biodegradation of selected monoterpenes. Appl Microbiol Biotechnol 45:831–838
Moldrup P, Olesen T, Schjonning P, Yamaguchi T, Rolston DE (2000) Predicting the gas diffusion coefficient in undisturbed soil from soil water characteristics. Soil Sci Soc Am J 64:94–100
Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter. In: Page AL (ed) Methods of soil analysis part 2—chemical and microbiological proprieties, 2nd edn. Am Soc Agron, Madison, pp 539–579
Owen SM, Clark S, Pompe M, Semple KT (2007) Biogenic volatile organic compounds as potential carbon sources for microbial communities in soil from the rhizosphere of Populus tremula. FEMS Microbiol Lett 268:34–39
Paavolainen L, Kitunen V, Smolander A (1998) Inhibition of nitrification in forest soil by monoterpenes. Plant Soil 205:147–154
Peñuelas J, Llusià J (2003) BVOCs: plant defense against climate warming? Trends Plant Sci 8:105–109
Peñuelas J, Llusià J (2004) Plant VOC emissions: making use of the unavoidable. Trends Ecol Evol 19:402–404
Peñuelas J, Staudt M (2010) BVOCs and global change. Trends Plant Sci 15:133–144
Ramirez KS, Lauber CL, Fierer N (2009) Microbial consumption and production of volatile organic compounds at the soil-litter interface. Biogeochemistry 99:97–107
Sardans J, Peñuelas J (2005) Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol Biochem 37:455–461
Schade GW, Goldstein AH (2001) Fluxes of oxygenated volatile organic compounds from a ponderosa pine plantation. J Geophys Res 106:3111–3123
Schimel J, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386–1394
Smolander A, Ketola RA, Kotiaho T, Kanerva S, Suominen K, Kitunen V (2006) Volatile monoterpenes in soil atmosphere under birch and conifers: effects on soil N transformations. Soil Biol Biochem 38:3436–3442
Stahl PD, Parkin TB (1996) Microbial production of volatile organic compounds in soil microcosms. Soil Sci Soc Am J 60:821–828
Stickland MS, Rousk J (2010) Considering fungal:bacterial dominance in soils—methods, controls, and ecosystem implications. Soil Biol Biochem 42:1385–1395
Uusitalo M, Kitunen V, Smolander A (2008) Response of C and N transformations in birch soil to coniferous resin volatiles. Soil Biol Biochem 40:2643–2649
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19:703–707
Vokou D, Chalkos D, Karamanlidou G, Yiangou M (2002) Activation of soil respiration and shift of the microbial population balance in soil as a response to Lavandula stoechas essential oil. J Chem Ecol 28:755–768
Wardle DA (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol Rev Camb Philos 67:321–358
Wardle DA (1998) Controls of temporal variability of the soil microbial biomass: a global scale synthesis. Soil Biol Biochem 30:1627–1637
Warneke C, Karl T, Judmaier H, Hansel A, Jordan A, Lindinger W, Crutzen PJ (1999) Acetone, methanol, and other partially oxidized volatile organic emissions from dead plant matter by abiological processes: significance for atmospheric HOx chemistry. Glob Biogeochem Cycles 13:9–17
Wenke K, Kai M, Birgit P (2010) Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 231:499–506
White CS (1988) Nitrification inhibition by monoterpenoids—theoretical-mode of action. Ecology 69:1631–1633
White CS (1991) The role of monoterpenes in soil-nitrogen cycling processes in ponderosa pine. Results from laboratory bioassays and field studies. Biogeochemistry 12:43–68
White CS (1994) Monoterpenes—their effects on ecosystem nutrient cycling. J Chem Ecol 20:1381–1406
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction-an automated procedure. Soil Biol Biochem 22:1167–1169
Acknowledgements
We are very grateful to Dr. S.M. Owen for reviewing the manuscript and Dr. Josep Mª Alcañiz for his help on soil type identification. We are indebted to the Spanish government projects CONSOLIDER INGENIO MONTES (CSD2008-00040), CGL2006-04025, CGL2010-17172 and SECASOL (CGL2009-08101) and the Catalan government project SGR2009-458. D. Asensio acknowledges the postdoctoral fellowship from the Spanish government, Ministerio de Educación.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Paul Bodelier.
Rights and permissions
About this article
Cite this article
Asensio, D., Yuste, J.C., Mattana, S. et al. Litter VOCs induce changes in soil microbial biomass C and N and largely increase soil CO2 efflux. Plant Soil 360, 163–174 (2012). https://doi.org/10.1007/s11104-012-1220-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1220-9