Abstract
The adaptive immune system is a crucial component of cancer immunoediting and has emerged, in recent years, as a key foundation on which the success of novel cancer therapeutics have been built, namely the checkpoint inhibitors. Early observational studies have highlighted the importance of tumor-infiltrating lymphocytes (TILs) in patient outcomes across multiple solid tumor types, reflecting its role in cancer immunosurveillance. High TILs in triple-negative breast cancer (TNBC), a poor prognostic subgroup, has been shown to be associated with improved survival. When evaluated in functional TIL subsets, a positive correlation has also been observed with cytotoxic CD8+ TILs but results were conflicting for FOXP3 regulatory T cells. Overexpression of immune response genes corresponded to increased lymphocytic infiltration and improved outcomes in TNBC. Understanding the functional biology of TILs and its interaction with cancer in the immunoediting processes will help guide immunotherapeutic approaches in TNBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancer, defined by a lack of expression of estrogen receptor (ER), progesterone receptor (PR), and Her2, portends the poorest prognosis amongst breast cancer subtypes. These patients have higher relapse rates within 3 years from diagnosis and are more likely to develop visceral metastases [1]. Further molecular and phenotypic subclasses have been identified in this heterogeneous group of breast cancer in an effort to better classify these patients based on tumor biology and their associated clinical outcomes [2–8]. Gene expression profiling to molecularly classify triple-negative breast cancers (TNBCs) have found basal-like tumors to comprise the majority of TNBCs (70–80 %). All of the other intrinsic molecular subtypes were also noted to be present in TNBCs with Her2-enriched being the next most common (8–17 %), followed by the luminal subtype (6–11 %) and lastly the normal breast-like subtype (1–7 %) [5, 6]. Another subtype, the Claudine-low tumors which appears enriched for mesenchymal and stem cell features, has recently been identified in 30 % of TNBCs [5]. Based on these molecular classifications, the basal-like, Her2-enriched, and Claudine-low subtypes were found to have correspondingly poorer prognosis compared to the luminal A subtypes [5]. More recently, Lehmann et al. analyzed the gene expression profiles of almost 600 TNBCs and identified 6 subtypes based on cluster analysis: basal-like 1, basal-like 2, immunomodulatory, mesenchymal, mesenchymal stem-like, and luminal androgen receptor subtypes [7]. However, the correlation between these 6 molecular subtypes and patient outcomes have yet to be determined. Phenotypic subclassification by immunohistochemistry have also identified two subgroups of TNBCs distinguished by the expression of basal markers, cytokeratin 5/6 and/or epidermal growth factor receptor (EGFR) [8]. A significantly poorer prognosis was noted in the group positive for basal markers. In the recent years, newer prognostic models based on immune responses within the tumor microenvironment have garnered increasing interests.
Recent successes with immunotherapy, namely the use of immune checkpoint inhibitors in advanced melanomas and non-small cell lung cancer [9–11], have revived interests in understanding the complex interactions between cancer and the immune system, termed immunoediting [12, 13]. The cancer immunoediting process is comprised of three phases, the first of which, cancer elimination, was conceived by Paul Ehrlich in 1909. He hypothesized that the immune system was able to repress neoplastic processes, but it was not until almost a century later that the idea of a functional cancer immunosurveillance process was proven in murine models [14]. Genetically modified lymphocyte-deficient mice grew sarcomas more rapidly and in greater frequency after subcutaneous injection of chemical carcinogens compared to their wild-type controls.
Cancer immunosurveillance has indirectly been suggested by many early observational studies showing that increased immune infiltrates in tumors correlated with survival outcomes in many solid tumors, breast cancer notwithstanding [15–20]. When evaluated as a composite group, many studies have failed to show a correlation between survival outcomes and lymphocytic infiltration in breast cancer [21–26]. Conversely, when segregated into their phenotypic subtypes, a significant association between tumor-infiltrating lymphocytes (TILs) and patient outcomes was seen in the TNBC cohort. In this paper, we will review and consolidate the recent evidence linking TILs and its subsets to breast cancer outcomes in TNBC.
Immune Evaluation in Breast Cancer
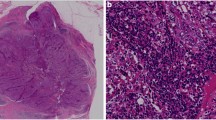
The evaluation of TILs in breast cancer across studies has been heterogeneous. Multiple varying parameters, including the type of tissue samples utilized, inflammatory cell population studied, site, and quantification of the immune cells, have made direct comparisons between the various studies difficult. Recent efforts by the International TILs Working Group have resulted in a consensus guideline on the histopathological evaluation of TILs in breast cancer to facilitate standardization of assessments and interpretation of data across studies [27]. The guidelines were based upon the methodology initially described by Denkert et al. [28] The study included all mononuclear cells (with the exclusion of polymorphonuclear leucocytes) in either the tumor cell nests or stromal compartment on full hematoxylin and eosin-stained sections. Intratumoral TILs were defined as lymphocytes with direct cell-to-cell contact with carcinoma cells, while stromal TILs were found in the intervening stroma in the absence of direct contact with carcinoma cells. TILs were measured as a percentage of each compartment and assessed as a continuous variable for statistical analyses. The guidelines also defined a group of breast cancers with particularly high TIL infiltrate, the lymphocyte predominant breast cancer (LPBC), where there were more lymphocytes than tumor cells, using an arbitrary cutoff of 50–60 % (Fig. 1).
International TILs Working Group Consensus guidelines [27]
The majority of TNBCs have been reported to be basal-like breast cancers by gene expression profiling, ranging from 70 to 90 % [4, 5, 29]. To facilitate the identification of this intrinsic molecular subgroup in clinical practice, Nielsen et al. identified a surrogate panel of immunohistochemistry markers that could accurately discriminate basal-like tumors from the other intrinsic molecular subtypes with a sensitivity of 76 % and specificity of 100 % [30]. In this review, core basal breast cancers, as described in the following sections, are defined accordingly as cytokeratin 5/6 and/or epidermal growth factor receptor-1 positivity in addition to the triple-negative phenotype described by Nielsen et al. [22, 24, 31–35].
Prognostic Value of TILs in TNBC
Stromal Mononuclear Cells Are Strong Independent Prognostic Markers for Breast Cancer Outcomes
Four large retrospective studies evaluating the role of TILs based on the International TILs Working Group guidelines have been published. These studies included more than 4000 patient samples, of which 1070 were from TNBC patients, enrolled in prospective randomized controlled trials involving adjuvant chemotherapy as detailed in Table 1 [25, 26, 36, 37]. In all but one study, the patient cohorts received adjuvant chemotherapy.
The stroma appeared to be more highly infiltrated by TILs (median 10 %) as compared to the intratumoral compartment (median 0–5 %). TILs were most consistently associated with high-grade tumors and ER negativity. The distribution of TILs was heterogeneous across the different breast cancer subtypes. Stromal TILs were higher in density in the ER-negative/Her2-negative (median 15–25 %) and Her2-positive breast cancer subgroups (median 15–20 %) when compared to the ER-positive subgroup (median 7.5–10 %) [25, 26, 36]. The median proportion of lymphocyte predominant breast cancer (LPBC) in the TNBC subgroup ranged between 4.4 and 11.6 % [26, 36-38].
The studies failed to show any prognostic significance of TILs in the composite breast cancer cohort. When evaluated in distinct phenotypic subgroups, TILs however were associated with improved outcomes in the triple-negative group. This prognostic value appeared to be most consistent when TILs within the stromal component were evaluated. Stromal TILs evaluated as a continuous variable (per 10 % increment) was found to significantly correlate with better outcomes [25, 26, 36, 37]. Interestingly, the adjusted point estimate reduction for relapse or death was similar across the studies, ranging from 16 to 18 % for every 10 % increase in stromal TIL infiltration. In addition, a 15 to 21 % reduction in overall mortality was also seen [25, 26, 36, 37].
When evaluated as a binary variable, patients with LPBC were found to have an even more significant improvement in relapse rates and survival (DFS hazard ratio (HR) 0.30, 95 % confidence interval (95 %CI) 0.011–0.81, adjusted p = .018; OS HR 0.29, 95 %CI 0.091–0.92, adjusted p = .036) [26]. A 21 % improvement in 5- and 10-year overall survival in the LPBC cohort was noted by Loi et al. and Dieci et al., respectively, although it did not reach statistical significance in the latter [26, 36].
Cytotoxic T Cells Are Associated with Improved Breast Cancer Outcomes
Cytotoxic CD8+ T cells play a crucial role in tumor-specific cellular adaptive immunity. Following tumor antigen interaction via the major histocompatibility (MHC) class 1 molecule, CD8+ T cells exert anti-tumor activity by mediating and facilitating type 1 immune responses within the tumor microenvironment [39, 40].
Studies evaluating the role of infiltrating cytotoxic T lymphocytes utilized a mixture of tissue samples including microarray constructs [21, 22, 31, 32] and full-faced H&E sections [33, 41]. CD8+ TILs were evaluated in a non-parametric manner with a variety of cutoff values used across the studies. Despite the heterogeneity of methodologies, CD8+ TILs tend to be found in higher density within the stroma compared to the intratumoral compartment [21, 22, 31, 33, 41]. High CD8+ TILs were shown to correlate with higher grade breast cancers, ER and PR negativity [21, 22, 31, 33], and notably, with the core basal phenotype [22, 31].
The prognostic significance of CD8+ TILs was not consistent across six more recent publications [21, 22, 31–33, 41]. In the ER negative cohort, CD8+ TILs correlated positively with outcomes in all but one study [33]. In the majority of studies, however, a higher frequency of CD8+ TIL infiltration, either total, intratumoral, or stromal, was associated with an improvement in breast cancer-specific survival (BCSS) in the ER-negative population.
Four of the six studies with subset analysis of more than 2000 TNBC patients have shown significant differences in breast cancer outcomes as detailed in Table 2 [22, 31, 32, 41]. Increased CD8+ TILs were associated with improved survival outcomes although the site, intratumoral or stromal, varied across the studies [22, 41]. Even after adjusting for potential confounders, increased intratumoral TILs were a significant independent prognostic factor for breast cancer-specific survival (HR 0.48, 95 %CI 0.34–0.67; p < .001) [22]. Intriguingly, within the triple-negative group, CD8+ TILs were associated with a better prognosis in the core basal phenotype [22, 31, 32] but this finding was not replicated in the five negative phenotype [22, 32], which is known to have a better prognosis.
Regulatory T Cells Have Conflicting Correlation with Breast Cancer Outcomes
Regulatory T cells (Tregs), characterized by the expression of CD4, CD25, and FOXP3, function to suppress inappropriate immune responses and are responsible for immunological tolerance [42]. In cancer, Tregs appear to have the ability to abrogate tumor-specific CD8+ T cell responses and hence postulated to be deleterious in anti-tumor immunity [43]. Tumor tissue may also secrete cytokines to traffic Tregs into the tumor microenvironment and promote the conversion of naïve T cells into FOXP3+ Treg, thereby impairing the effector immune responses [43]. FOXP3, localized to nucleus, was used to identify Tregs in the following studies discussed, as it is by far the most specific marker [44, 45].
A high density of FOXP3+ TILs was most consistently associated with high tumor grade, estrogen receptor negativity [24, 33, 34, 43, 46–49], and specifically, the core basal phenotype [24, 33, 34, 47]. The prognostic significance of FOXP3+ TILs in breast cancer is controversial. Similar to the studies on cytotoxic CD8+ TILs, the methodologies used to evaluate FOXP3+ Tregs in breast cancer have not been standardized. In addition, the discordance in findings across the studies also appears to be related to the different molecular subtypes of breast cancer and possible associations with CD8+ TILs. Within the ER-negative breast cancers, FOXP3+ TILs were not found to be prognostic for survival in three retrospective cohort studies [24, 46, 47]. Conversely, in the TNBC group, West et al. reported a longer relapse-free survival with higher densities of FOXP3+ TILs [48]. A significant improvement in BCSS with a median difference of 13 % at 15 years was also noted in the group with core basal phenotype [24]. Yan et al., however, reported a significant correlation with poorer survival in core basal breast cancers [34]. A higher proportion of concurrent staining with CXCR4 in FOXP3+ Tregs was also seen in the basal-like cancers compared to the luminal cancers in a small subset of this study. CXCR4 is a cognate receptor of CXCL12, a chemokine secreted by tumors to recruit Tregs [50]. Hence, the immune evasion process of core basal breast cancers could be facilitated by the recruitment of Tregs into the tumor microenvironment [34].
The correlation of outcomes with FOXP3+ TILs has been postulated to be related to the interplay with CD8+ T cell infiltration. Core basal tumors were found to have a lower ratio of stromal CD8+/FOXP3+ T cells, which corresponded to a poorer survival, as compared to luminal breast cancer subtypes [33]. Stromal CD8+/FOXP3+ ratio was reported to be an independent prognostic factor for survival. Low levels of CD8+ T cells relative to Tregs in the lymphoid aggregates suggest greater inhibition of the cytotoxic T lymphocytes and therefore a poorer tumor cell kill. In another study of ER-negative breast cancers, a strong correlation between FOXP3+ TILs and total CD8+ TILs was noted [48]. FOXP3+ TILs were significantly more abundant (>3-fold on average) in the high CD8+ TIL group than in the low CD8+ TIL group. High density of FOXP3+ TILs was not significantly associated with relapse-free survival in tumors that had low CD8+ TIL infiltration, suggesting that the prognostic value of FOXP3 TILs was dependent on the presence of cytotoxic T lymphocytes. Interestingly, Liu et al. had also shown that the prognostic effect of FOXP3+ intratumoral TILs was lost in the core basal breast cancer subgroup when CD8+ TILs was taken into account [24].
Other TIL Subsets
Another component of the adaptive immune system that has not been well characterized in the tumor microenvironment is humoral immunity. Matured B cells, identified by CD20 positivity, may mediate their effects on tumors through antibody production, direct cytotoxicity, tumor antigen presentation, and regulation of other immune cells [51]. B cells have been reported to be present in about 40 to 55 % of breast cancer full-face tissue sections [35, 52, 53]. Mahmoud et al. investigated the prognostic significance of infiltrating B lymphocytes in 1470 breast tumors and found a positive correlation between higher numbers of total CD20+ B cells and higher grade tumors, hormone receptor negativity, and basal phenotype breast cancer. Total CD20+ B cell count was associated with better prognosis in ER-negative tumors, independent of tumor size and nodal status in multivariate analysis (HR 0.61, 95 %CI 0.42–0.88, p = .008). This finding was replicated in the cohort of 249 breast cancer patients with core basal phenotype tumors (HR 0.46, 95 %CI 0.30–0.69, p < .001) [35].
Programmed death-1 (PD-1) is a member of the CD28/CTLA-4 family of co-receptors expressed on activated T cells. Upon interaction with its ligands after antigen recognition, PD-1 acts as a negative immune regulator that functions to dampen effector T cell activity [54]. The expression of PD-1 on TILs was found in 16 to 60 % of breast cancers and, when present, identifiable in up to 70 % of TILs [55, 56]. Of the TILs expressing PD-1, majority were cytotoxic CD8+ lymphocytes [56]. A significant correlation with high tumor grade was reported, but its correlation with ER status was conflicting in three studies which utilized different tissue materials [55–57]. In TNBC cohorts, the presence of PD-1+ TILs was significantly more likely and was found in at least 70 % of the tumors [55, 57]. In core basal tumors, over a quarter were infiltrated by PD-1+ TILs compared to the luminal A subgroup (~5 %) and, when present, was found to be a negative prognostic marker that correlated with a significant reduction in overall survival (unadjusted HR 3.140, 95 %CI 1.886–5.230, p < .0001) [55].
TILs and Immune-Related Gene Signatures
Gene expression profiling has been used in the clinical setting to aid in the prediction of clinical outcomes in patients with breast cancer. However, its utility has mainly been limited to ER-positive tumors to identify luminal A tumors at low risk of recurrence [58, 59]. These gene signatures, commonly associated with cell cycle progression and proliferation, tend to classify basal-like breast cancers as high risk and are hence less informative in further risk stratification of this subgroup. Within the molecularly heterogeneous triple-negative breast cancer cohort whereby approximately 80 % are molecularly defined basal-like breast cancers [3, 5, 60], there has been a lack of discriminating genomic tests that can help to predict relapse and survival to assist in therapeutic decision-making in the clinics.
Several recent studies have found immune gene signatures to be associated with breast cancer outcomes in ER-negative and TNBC using hierarchical clustering techniques and/or outcome correlation studies [60–67]. In general, gene expression signatures related to T cell responses, particularly of T helper 1 immune responses and/or B cell responses, have been found to correlate with favorable outcomes in basal-like breast cancers. An interesting study that was recently published attempted to correlate identified immune gene signatures from various research groups in 107 TNBC patients [60]. The study clustered patients into three distinct molecular groups and found a significant difference in event-free survival amongst them. Robust functional annotation of the clusters using various gene expression signatures showed that high immune response was a hallmark of the cluster with significantly better outcomes.
Overexpression of these immune-related genes is thought to reflect histopathological findings of the abundance of TILs in breast cancer, but correlation between the two entities has not been clear. Teschendorff et al. reported that overexpression of immune response genes correlating with improved prognosis appear to be independent of the degree of lymphocytic infiltration [61]. Conversely, in a study of 71 TNBCs, higher expression of interferon-regulated and immunoglobulin genes correlated with a significantly larger amount of TILs, suggesting an association between immune response gene upregulation and lymphocytic infiltration [62]. Another two studies further demonstrated the correlation between immune gene expression and TILs based on immunohistochemistry [64, 65]. Interestingly, aside from pro-immune response genes, immunosuppressive markers such as LAG3, IDO1, CTLA-4, TIGIT, BTLA, and FOXP3 were also found to correlate positively with increased lymphocytic infiltration [65]. These feedback immunosuppressive pathways appear to be part of a continuum process in cancer immunoediting, from tumor elimination to evasion.
Discussion
The “immunogenicity” of cancers, which is the tumor’s ability to induce host adaptive immunity, appears to play an important role in cancer immunosurveillance [68]. The first step involves the presentation of tumor antigens and its recognition by T lymphocytes [69]. Highly immunogenic cancers, such as melanomas and non-small cell lung cancers, have high mutational load [70] and hence more likely to generate neoantigens that may be recognized by cytotoxic T lymphocytes [71]. Breast cancers, on the other hand, are not typically immunogenic as they have lower frequencies of somatic mutation [70]. However, amongst the breast cancer subtypes, molecularly defined basal-like breast cancer has been shown to have mutational rates twice that of luminal A breast cancers [72], suggesting a possible differential neoepitope landscape and thus immunogenicity. In conjunction with this hypothesis, the studies discussed have demonstrated higher lymphocytic infiltration in triple-negative as compared to ER-positive breast cancers, likely due to increased T cell recognition of tumor neoantigens, resulting in increased T cell trafficking and clonal expansion in the tumor microenvironment. These tumor-antigen-specific lymphocytes appear to confer immunity against cancer cells and thus correlate with improved survival outcomes.
The identification of prognostic biomarkers in early TNBCs is crucial for many reasons. Firstly, TNBCs are comprised of a group of biologically and genomically diverse tumors with potentially varying outcomes [3, 73]. Comparative studies have shown that approximately 80 % of all triple-negative tumors are identified molecularly as basal-like [3, 5]. However, it appears that even within basal-like breast cancers, further distinct subclasses exist with significantly different survival outcomes [60]. Further refinement of this population of breast cancers using easily incorporable and reproducible prognostic factors may help to accurately distinguish a group of low-risk patients that may not require adjuvant chemotherapy. Secondly, these biomarkers may help us better understand the underlying pathogenic mechanisms and, in turn, may aid in identifying potential therapeutic targets.
As highlighted in our review, mononuclear TILs, defined by the International TILs Working Group guidelines, were associated with favorable outcomes in TNBC patients receiving adjuvant chemotherapy [25, 26, 36, 37]. Amongst the four large studies, only one included patients from two randomized controlled trials who had no adjuvant chemotherapy [36]. There was no significant heterogeneity in the prognostic effect of TILs according to whether chemotherapy was administered and vice versa. Although the population of patients who did not receive adjuvant chemotherapy in this study was small, it suggests that the prognostic value of TILs may be independent of adjuvant treatment. This remains to be validated in larger studies. Future prospective studies may consider incorporating TIL assessments and use it to stratify patients in treatment arms to help determine not only its prognostic but also predictive value.
Given the consistency of mononuclear TILs in predicting outcomes across the studies, it would appear that the specific composition and function of TILs did not seem to matter. However, when TILs were evaluated according to their functional subsets, cytotoxic CD8+ TILs appeared to correlate with prognosis but the findings were equivocal for FOXP3+ Tregs.
One potential explanation is the heterogeneity in study methodologies. Aside from its nuclear expression in T cells, FOXP3 expression has also been reported on normal and carcinoma breast tissue [74]. The localisation of FOXP3 in breast tumor cells (i.e., nuclear or cytoplasmic) appears to have prognostic implications [49, 75]. In light of these findings, caution needs to be taken when interpreting immunohistochemistry and gene expression data of FOXP3 on breast cancer tissue samples. Another possible reason for the discordance in the prognostic value of FOXP3 Tregs is the complex interplay between various cellular components of the innate and adaptive immunity, and the tumor [76]. The effect of each functional immune component may be modulated by other immune cell populations. Exemplifying this dynamic relationship were three studies that demonstrated an interaction between suppressor FOXP3 TILs and effector CD8+ TILs in ER-negative and core basal breast cancers whereby the prognostic value of Tregs appeared to be dependent on the presence of cytotoxic T lymphocytes [24, 33, 48]. Hence, a comprehensive analysis of the various immune components in the tumor microenvironment is needed to provide a clearer picture of their functional role in cancer immunoediting.
There exists a continuum between tumor elimination and evasion in the process of cancer immunoediting. Effector CD8+ TILs were interestingly found to correlate positively with suppressor Tregs infiltration [48], upregulation of negative immune checkpoint PD-1 [56], and overexpression of immunosuppressive genes [65]. To further illustrate this feedback immunosuppressive pathways, a recent publication in melanomas suggested that CD8+ T cells may orchestrate an immunosuppressive tumor microenvironment through production of interferon-γ, which drives upregulation of PD-L1 and indoleamine-2,3-dioxygenase (IDO), and chemokine CCL22, which mediates recruitment of Tregs [77]. Additionally, tumor-evoked B regulatory cells were found to induce TGF-β-dependent conversion from resting CD4+ T cells to FOXP3+ Tregs, a process that allows breast cancer cells to metastasize to the lungs in murine models [78]. Understanding the mechanisms of immune evasion in cancer has led to the development of drugs that inhibit negative regulatory checkpoints, thereby reversing immunotolerance. Although these drugs are currently only being studied in patients with metastatic TNBC, its utility in the adjuvant setting to re-ignite effective cancer immunosurveillance is a highly attractive proposition and is eagerly awaited.
Accumulating evidence on the prognostic utility of TILs in TNBCs have brought about the first steps in harmonization of the methodology used by pathologists when evaluating TILs. Current guidelines evaluate tumor-infiltrating T and B lymphocytes and plasma cells as a composite entity and recommend the use of full-face sections. Future efforts to refine these guidelines to include the various immune cell subsets and their relationship with one another may unveil further prognostic biomarkers and contribute to better the understanding of the biological immune processes associated with patient outcomes. Likewise, correlative studies of TILs with genomic profiling may offer insights into the tumor microenvironment and may potentially improve outcome prediction models in TNBC. On a practical note, it may be worthwhile to determine concordance between tissue microarrays and full-face sections in relation to the prognostic value of TILs, given potential problems of intratumoral heterogeneity. This will help inform adequacy of using tissue microarrays, an otherwise very practical and effective tool for high-throughput analysis and experimental standardization, in assessing TILs in TNBC.
Conclusion
We now have robust evidence that TILs, a reflection of functional cancer immunosurveillance, are an important prognostic marker in TNBC in the adjuvant setting. Apart from current methods used for prognostication, TILs may further refine outcome prediction. However, its utility in the clinical setting to aid in decisions on adjuvant chemotherapy has not been established. Further efforts to understand the functional biology of TIL subsets and its interactions in the tumor microenvironment are essential in developing effective immunotherapeutic approaches in TNBCs. Correlation of TILs on histopathology with emerging molecular subtypes in TNBC, which include not only biological processes from the tumor but also the tumor microenvironment, may allow the development of an even more powerful prognostic marker in this very heterogenous cohort of patients.
References
Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115(2):423–8.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52.
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48.
Tischkowitz M, Brunet JS, Begin LR, Huntsman DG, Cheang MC, Akslen LA, et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007;7:134.
Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5(1):5–23.
Prat A, Adamo B, Cheang MC, Anders CK, Carey LA, Perou CM. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist. 2013;18(2):123–33.
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67.
Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14(5):1368–76.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23.
Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–17.
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–8.
Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60.
Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–11.
Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28A(4-5):859–64.
Clark Jr WH, Elder DE, Guerry D, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81(24):1893–904.
Jass JR. Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol. 1986;39(6):585–9.
Lipponen PK, Eskelinen MJ, Jauhiainen K, Harju E, Terho R. Tumour infiltrating lymphocytes as an independent prognostic factor in transitional cell bladder cancer. Eur J Cancer. 1992;29A(1):69–75.
Nacopoulou L, Azaris P, Papacharalampous N, Davaris P. Prognostic significance of histologic host response in cancer of the large bowel. Cancer. 1981;47(5):930–6.
Rilke F, Colnaghi MI, Cascinelli N, Andreola S, Baldini MT, Bufalino R, et al. Prognostic significance of HER-2/neu expression in breast cancer and its relationship to other prognostic factors. Int J Cancer. 1991;49(1):44–9.
Baker K, Lachapelle J, Zlobec I, Bismar TA, Terracciano L, Foulkes WD. Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology. 2011;58(7):1107–16.
Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14(2):R48.
Torhorst J, Bucher C, Kononen J, Haas P, Zuber M, Kochli OR, et al. Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol. 2001;159(6):2249–56.
Liu S, Foulkes WD, Leung S, Gao D, Lau S, Kos Z, et al. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res. 2014;16(5):432.
Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–50.
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–7.
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs working group 2014. Ann Oncol. 2015;26(2):259–71.
Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–13.
Bertucci F, Finetti P, Cervera N, Esterni B, Hermitte F, Viens P, et al. How basal are triple-negative breast cancers? Int J Cancer. 2008;123(1):236–40.
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367–74.
Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2010;29(15):1949–55.
Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25(8):1536–43.
Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, et al. CD8(+) cytotoxic T cell and FOXP3(+) regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130(2):645–55.
Yan M, Jene N, Byrne D, Millar EK, O’Toole SA, McNeil CM, et al. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res. 2011;13(2):R47.
Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat. 2012;132(2):545–53.
Dieci MV, Mathieu MC, Guarneri V, Conte P, Delaloge S, Andre F, and Goubar A. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015
Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–66.
Loi S. Host antitumor immunity plays a role in the survival of patients with newly diagnosed triple-negative breast cancer. J Clin Oncol. 2014;32(27):2935–7.
Dobrzanski MJ, Reome JB, Hylind JC, Rewers-Felkins KA. CD8-mediated type 1 antitumor responses selectively modulate endogenous differentiated and nondifferentiated T cell localization, activation, and function in progressive breast cancer. J Immunol. 2006;177(11):8191–201.
Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50.
Chen Z, Chen X, Zhou E, Chen G, Qian K, Wu X, et al. Intratumoral CD8(+) cytotoxic lymphocyte is a favorable prognostic marker in node-negative breast cancer. PLoS One. 2014;9(4), e95475.
Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87.
Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69(5):2000–9.
Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–41.
Qin FX. Dynamic behavior and function of Foxp3+ regulatory T cells in tumor bearing host. Cell Mol Immunol. 2009;6(1):3–13.
Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–80.
Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Lee AH, Ellis IO, et al. An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Res Treat. 2011;127(1):99–108.
West NR, Kost SE, Martin SD, Milne K, Deleeuw RJ, Nelson BH, et al. Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer. 2013;108(1):155–62.
Takenaka M, Seki N, Toh U, Hattori S, Kawahara A, Yamaguchi T, et al. FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Mol Clin Oncol. 2013;1(4):625–32.
Wei S, Kryczek I, Zou W. Regulatory T-cell compartmentalization and trafficking. Blood. 2006;108(2):426–31.
Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185(9):4977–82.
Marsigliante S, Biscozzo L, Marra A, Nicolardi G, Leo G, Lobreglio GB, et al. Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett. 1999;139(1):33–41.
Helal TE, Ibrahim EA, Alloub AI. Immunohistochemical analysis of tumor-infiltrating lymphocytes in breast carcinoma: relation to prognostic variables. Indian J Pathol Microbiol. 2013;56(2):89–93.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139(3):667–76.
Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: implication for immunotherapy. BMC Cancer. 2008;8:57.
Sun S, Fei X, Mao Y, Wang X, Garfield DH, Huang O, et al. PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol Immunother. 2014;63(4):395–406.
Sotiriou C, Piccart MJ. Taking gene-expression profiling to the clinic: when will molecular signatures become relevant to patient care? Nat Rev Cancer. 2007;7(7):545–53.
Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360(8):790–800.
Jezequel P, Loussouarn D, Guerin-Charbonnel C, Campion L, Vanier A, Gouraud W, et al. Gene-expression molecular subtyping of triple-negative breast cancer tumours: importance of immune response. Breast Cancer Res. 2015;17:43.
Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 2007;8(8):R157.
Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, et al. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9(5):R65.
Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D, Bontempi G, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14(16):5158–65.
Rody A, Holtrich U, Pusztai L, Liedtke C, Gaetje R, Ruckhaeberle E, et al. T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res. 2009;11(2):R15.
Lee HJ, Lee JJ, Song IH, Park IA, Kang J, Yu JH, et al. Prognostic and predictive value of NanoString-based immune-related gene signatures in a neoadjuvant setting of triple-negative breast cancer: relationship to tumor-infiltrating lymphocytes. Breast Cancer Res Treat. 2015;151(3):619–27.
Sabatier R, Finetti P, Cervera N, Lambaudie E, Esterni B, Mamessier E, et al. A gene expression signature identifies two prognostic subgroups of basal breast cancer. Breast Cancer Res Treat. 2011;126(2):407–20.
Nagalla S, Chou JW, Willingham MC, Ruiz J, Vaughn JP, Dubey P, et al. Interactions between immunity, proliferation and molecular subtype in breast cancer prognosis. Genome Biol. 2013;14(4):R34.
DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482(7385):405–9.
Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–65.
Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–8.
Schumacher TN, and Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74.
Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61-70.
Weigelt B, Baehner FL, Reis-Filho JS. The contribution of gene expression profiling to breast cancer classification, prognostication and prediction: a retrospective of the last decade. J Pathol. 2010;220(2):263–80.
Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, et al. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007;129(7):1275–86.
Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Menard S, et al. FOXP3 expression and overall survival in breast cancer. J Clin Oncol. 2009;27(11):1746–52.
Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306.
Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T (regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5(200):200ra116.
Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011;71(10):3505–15.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Koo Si-Lin, Loh Kiley, Sulastri Kamis, Jabed Iqbal, Rebecca Dent, and Yap Yoon Sim declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Biomarkers
Rights and permissions
About this article
Cite this article
Si-Lin, K., Kiley, L., Kamis, S. et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer. Curr Breast Cancer Rep 7, 232–241 (2015). https://doi.org/10.1007/s12609-015-0196-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-015-0196-x