Abstract
The prognostic significance of tumor-infiltrating lymphocytes and immune signals has been described previously in triple-negative breast cancer (TNBC). Furthermore, recent studies have shown that immunologic parameters are relevant for the response to neoadjuvant chemotherapy (NAC) in breast cancer as well as for outcomes after adjuvant chemotherapy. However, immune signals are variable, and which signals are important is largely unknown. We, therefore, evaluated the expression of immune-related genes in TNBC treated with NAC. We retrospectively evaluated biopsy tissue from 55 patients with primary TNBC treated with NAC (anthracycline, cyclophosphamide, and docetaxel) against the NanoString nCounter GX Human Immunology Panel (579 immune-related genes). Higher expression of cytotoxic molecules, T cell receptor signaling pathway components, cytokines related to T helper cell type 1 (Th1), and B cell markers was associated with a pathologic complete response (pCR). Higher expression of NFKB1, MAPK1, TRAF1, CXCL13, GZMK, and IL7R was significantly associated with pCR, higher Miller-Payne grade, and lower residual cancer burden class. Expression of NFKB1, TRAF1, and CXCL13genes, in particular, was significantly correlated with a longer disease-free survival rate. Conversely, patients those who failed to achieve a pCR showed increased expression of genes related to neutrophils. Higher expression of cytotoxic molecules, T cell receptor signaling pathway components, Th1-related cytokines, and B cell markers is correlated with pCR and survival in TNBC patients treated with NAC. Our results suggest that the activation status of neutrophils may provide additional predictive information for TNBC patients treated with NAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancer (TNBC) is defined by negativity of estrogen receptor (ER) and progesterone receptor (PR) and the lack of human epidermal growth factor receptor 2 (HER2) overexpression [1]. These cancers account for 10–15 % of all breast cancers, and they tend to show visceral metastasis and aggressive clinical behavior [2]. Patients with this breast cancer subtype receive no benefit from molecularly targeted treatments such as endocrine therapy or trastuzumab [3]. In the case of operable TNBC, only systematic chemotherapy has been shown to be effective in an adjuvant or neoadjuvant setting; however, a growing body of evidence suggests that immunotherapy may show great potential for combating the disease [4].
Patients showing a pathologic complete response (pCR) to neoadjuvant chemotherapy (NAC) may experience prolonged disease-free survival [5, 6]. Therefore, investigating the predictive factors associated with response to chemotherapy in patients with TNBC is important. Predictive factors for NAC success have been reported by several investigators, and some factors such as tumor grade and biology-based tumor type (based on gene expression profiles) are candidates [7, 8].
Several studies have shown that the presence of tumor-infiltrating lymphocytes (TILs) in cancer tissue is associated with an improved outcome [9, 10] and that the immune system participates in elimination of tumor cells and control of tumor growth [11, 12]. In addition, some investigators have reported that immunologic parameters are relevant for response to NAC in breast cancer [13–16] as well as for outcomes after adjuvant chemotherapy [17–19]. The predictive significance of TILs was proven by histological evaluation by hematoxylin and eosin (H&E) and immunohistochemical staining [14, 16]. Furthermore, Denkert et al. showed at the molecular level that the expression of T cell-related markers CD3D and CXCL9 was significantly associated with a pCR [13]. Another study also revealed that T cell-related genes (CD3D, LCK, CD48, SELL, GZMB, and PRF1) were correlated with a pCR in breast cancer patients with anthracycline-based NAC [15]. These immune signals are particularly strong in HER2-positive breast cancer and TNBCs [20, 21]. However, not all breast cancer patients with a high level of TILs or cytotoxic lymphocytes showed a pCR. Factors affecting response to NAC in these patients are largely unknown.
In this study, we aimed to identify the role of immune-related genes in TNBC treated with NAC. We used the NanoString nCounter GX Human Immunology Panel, which includes 579 immune-related genes. First, we investigated correlations between immune-related genes and the TIL level. Second, we evaluated the prognostic and predictive significance of immune-related genes in TNBC treated with NAC.
Materials and methods
Patients
One hundred eight TNBC patients diagnosed and treated with anthracycline- and taxane-based NAC and surgery between 2010 and 2012 at the Asan Medical Center were retrospectively reviewed. We included in this study 55 of the 108 cases that had available formalin-fixed, paraffin-embedded tissue samples. All patients received anthracycline and taxane-based regimens that included four cycles of 60 mg/m2 adriamycin and 600 mg/m2 cyclophosphamide followed by four cycles of 75 mg/m2 docetaxel. Surgery was performed approximately 3–4 weeks after the last chemotherapy cycle. The occurrence of a pCR was defined as the absence of residual invasive carcinoma in the breast and regional lymph nodes (ypT0/Tis, N0). Approval of the present study was obtained from the Institutional Review Board of the Asan Medical Center.
The patients’ medical records were reviewed for clinical information, and histological parameters were evaluated based on H&E-stained slides and pathology reports. The clinicopathological parameters evaluated in each case included the patient’s age at diagnosis, gender, tumor size, histological subtype, histological grade, pathological (p) T stage, lymphovascular invasion, lymph node metastasis, overall tumor stage, presence or absence of recurrence, most recent follow-up date, and survival status. Expression of ER, PR, and HER2 was evaluated in full sections at the time of diagnosis. ER and PR levels were regarded as positive if there were at least 1 % positive tumor nuclei present in the sections [22]. HER2-overexpressing tumors were defined as those with scores of 3+ or 2+ after fluorescence in situ hybridization (FISH) or silver in situ hybridization amplification [23].
Histological evaluation
The entire tumor bed was submitted for pathologic evaluation. Pre-NAC biopsy and post-NAC surgery samples of the 55 tumors were stained with H&E, and then histopathologically analyzed to determine the TILs (defined as the mean percentage of the stroma of invasive carcinoma infiltrated by lymphocytes and plasma cells in 10 % increments; if <10 % of stroma was infiltrated by TILs, 1 or 5 % criteria were used; all available full sections were evaluated) [24], histologic subtype and grade, tumor size, ypT stage, and ypN stage. Histologic type was defined based on the 2012 WHO classification criteria, and histologic grade was assessed using the modified Bloom-Richardson classification [25]. Miller–Payne grade and residual cancer burden (RCB) were also assessed [26].
Gene expression analysis using NanoString nCounter system
A total of 579 immunology-related human genes and 15 internal reference genes were implemented in the digital transcript counting (nCounter GX Human Immunology V2 kit assay NanoString, Seattle, WA) [27]. Total RNA (100 ng) was assayed on a nCounter Digital Analyzer (NanoString) according to the manufacturer’s instructions. Data were normalized by scaling with the geometric mean of the built-in control gene probes for each sample. Then, a log transformation (base 2) was performed. The heat map of gene expression for differentially expressed genes between pCR and non-pCR tumors was plotted and analyzed using the GENE-E software, version 3.0.230 (Broad Institute, Cambridge, MA) [28].
Immunohistochemical evaluation
Formalin-fixed, paraffin-embedded whole tissue sections were stained with an automatic immunohistochemical staining device (Benchmark XT; Ventana Medical Systems, Tucson, AZ). An antibody to myeloperoxydase (MPO, 1:2000, Dako, Glostrup, Denmark) was used. MPO-positive neutrophils were counted in five high-power fields (×400) of a hot spot.
Statistical analysis
Mann–Whitney U tests, Fisher’s exact tests, Spearman’s correlations, and Cox proportional hazard analyses were used as appropriate. Statistical significance was set at 5 %, and all statistical analyses were performed with SPSS statistical software (version 18; SPSS Inc., Chicago, IL).
Results
Clinicopathological characteristics of the cases
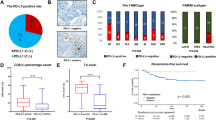
Patient ages ranged from 23 to 68 years (mean, 41.4 years). All patients were women. Of the cases analyzed, 4 were cT1 tumors, 31 were cT2, 18 were cT3, and 2 were cT4. The nodal statuses included 1 cN0 tumor, 27 cN1 tumors, 11 cN2 tumors, and 16 cN3 tumors. Twenty-one tumors were clinical stage II, and thirty-four were stage III. The overall rate of pCR was 30.9 % (17 of 55 tumors). The median patient follow-up was 38 months (range, 12–60 months). Patients with pCR and non-pCR showed no significant difference in age, clinical T or N stage, histologic type, or grade (Table 1).
Genes significantly correlated with TIL level
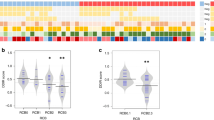
We analyzed the correlation of gene expression with TILs evaluated based on the H&E staining (Table 2). Genes associated with T cell surface molecules (CD96, CD2, IL2RG, IL2RB, CD3D, CD8A, CD3E, CD28, and IL7R), T cell receptor and cytokine signaling pathway components (STAT4, TRAF1, JAK2, and NFKB1), cytotoxic molecules (GZMB, GNLY, GZMA, PRF1, and KLRD1), T helper 1 (Th1) molecules (IFNG, CXCL10, TBX21, CXCL9, CXCL11, and CCL4), immunosuppressive markers (LAG3, IDO1, CTLA-4, TIGIT, BTLA, and FOXP3), B cell markers (BLNK and CD79A), and pattern recognition receptors (GBP5, CXCR6, IL18RAP, IL18, TLR4, and TLR7) were significantly correlated with the TIL level.
Genes differentially expressed between pCR and non-pCR tumors
We compared the gene expression of pCR and non-pCR tumors to discover genes that were differentially expressed between the two groups (Supplementary Table 1). Higher expression of cytotoxic molecules, T cell receptor signaling pathway components, cytokines related to Th1, and B cell markers [CR2, CD19, and MS4A1 (a gene related to CD20 protein)] was associated with a pCR (Fig. 1). Higher expression of NFKB1, MAPK1, TRAF1, CXCL13, GZMK, and IL7R was also significantly associated with a higher Miller–Payne grade and a lower RCB class. Refer to the supplementary file as Online Resource 1.
Genes associated with patient survival outcome
Patients with pCR experienced no disease recurrence during the follow-up period. In contrast, half of the patients without pCR (n = 38) experienced a disease recurrence (recurrence-free survival: 1.5–19.7 months). Many immune-related genes showed prognostic significance. Refer to the supplementary file as Online Resource 2. Among the genes significantly associated with a tumor’s response to NAC, expression of NFKB1 [hazard ratio (HR) = 0.177, 95 % confidence interval (95 % CI) 0.059–0.533, p = 0.002], TRAF1 (HR = 0.469, 95 % CI 0.241–0.912, p = 0.026), and CXCL13 (HR = 0.602, 95 % CI 0.419–0.864, p = 0.006) genes was independent better prognostic factors for disease-free survival.
Differentially expressed genes between pCR and non-pCR tumors with high-level CD8A expression
Some tumors had increased cytotoxic T cell infiltration, but not all of those tumors achieved a pCR (Fig. 1). To determine the significant genes affecting pCR status of these tumors, we first divided all tumors into two groups based on their median CD8A expression. Among the patients with a high level of CD8A expression (n = 28), a pCR was achieved in 11 patients (39.3 %). Tumors without pCR showed higher expression of several genes associated with neutrophils (DEFB1, DEFB103A, DEFB4A, and FCAR) than tumors with pCR (Fig. 2). Also, refer to the supplementary file as Online Resource 3. The correlation among the genes which show higher expression in the non-pCR group is summarized in supplementary file as Online Resource 4. Expression of other genes that show higher expression in the non-pCR group was positively correlated with neutrophil-related genes except six genes (TRAF5, MAPK1, CR2, PTK2, CASP3, and CCL18).
Neutrophil counts in tumors with high-level CD8A expression
To further define neutrophils’ role in NAC response of tumors with high-level CD8A expression, we counted neutrophils using MPO immunohistochemistry. The number of MPO-positive neutrophils was evaluated in five high-power fields of a hot spot. Neutrophil counts were not significantly different between pCR (31.2 ± 45.1) and non-pCR groups (21.8 ± 41.5, p = 0.378).
Discussion
This is the first study to investigate the correlation between expression of diverse immune-related genes and TIL level in breast cancer tissue samples. TILs evaluated by H&E staining were well correlated with T cell surface molecules (CD96, CD2, IL2RG, IL2RB, CD3D, CD8A, CD3E, CD28, and IL7R), T cell receptor and cytokine signaling pathway components (STAT4, TRAF1, JAK2, and NFKB1), cytotoxic molecules (GZMB, GNLY, GZMA, PRF1, and KLRD1), and Th1 molecules (IFNG, CXCL10, TBX21, CXCL9, CXCL11, and CCL4). Recently, the International TIL Working Group reported guidelines for the evaluation of TILs in breast cancer [29]. They recommended assessment of TILs with a microscopic examination of whole tissue sections on H&E staining for routine pathology practice. Using immunohistochemistry to assess the clinical importance of the lymphocyte sub-typing is not currently recommended. We evaluated expression of the immune-related genes and found several genes (NFKB1, TRAF1, and TCXCL13), which have a predictive value for NAC response and prognostic significance in TNBC patients.
While most studies define TILs as CD3+ lymphocytes, some reports have tried to identify the lymphocytic subsets that comprise TILs. Denkert et al. showed that T cell-related markers CD3D and CXCL9 expression were significantly associated with a pCR [13]. A pCR to NAC was shown to be associated with an immunologic profile combining the absence of immunosuppressive FOXP3+ regulatory T cells and the presence of a high number of CD8+ T cells and cytotoxic TIA1- and granzyme B-positive cells [30]. Follicular helper T cell (Tfh) and Th1 gene expression signatures defined by Gu-Trantien et al. have also been shown to predict a pCR after NAC in breast cancer [19]. Similar to previous studies, our study showed that higher expression of cytotoxic molecules, T cell receptor signaling pathway components, cytokines related to Th1 cell markers, and Tfh marker CXCL13 was associated with pCR.
Most TILs are T lymphocytes [19, 31, 32]. B lymphocytes are less numerous in lymphocytic infiltrates of malignant tumors [33]. Consequently, less is known about the role of B lymphocytes (CD20+) as TIL components. The expression of immunoglobulin κC that is produced by plasma cells has been associated with the prognosis of patients with breast cancer [17]. A recent study revealed that B cells/plasma B cells (B/P) and monocytes/dendritic cells (M/D) metagenes provide the most robust therapy-predictive performance among the immune metagenes [34]. The spatio-temporal dynamics of 28 different immune cell types infiltrating colorectal carcinomas were analyzed previously [35]. The study showed that markers of the Tfh cell, which has been known to help the generation of B cell-mediated immune responses, have a strong correlation with B cell markers [36]. Our study also showed that higher expression of B cell markers and CXCL13 was associated with a pCR. This result may be evidence for a significant role of B cells as TIL components. Further studies are necessary to prove the beneficial role of B cells in breast cancer.
In our study not only pro-immune markers (CCL5, CD45RO, CD80, CXCL9, and CXCL13), but also immunosuppressive markers such as LAG3, IDO1, CTLA-4, TIGIT, BTLA, and FOXP3 showed a positive correlation with increased TILs. Our results are concordant with a recent study by Denkert et al., who showed that increased mRNA expression of PD-1, PD-L1, CTLA-4, and FOXP3 was positively correlated with increased TILs as well as treatment response [37]. Additionally, the positive correlation of immunosuppressive markers with better outcome and improved response to various therapies has been described in recent studies [38–41]. These results suggest a feedback activation of immunosuppressive pathways as part of the immune reaction. Various immune checkpoint inhibitors (i.e., inhibitors of CTLA-4, PD-1, or PD-L1) have been developed and used in tumors including malignant melanoma, renal cell carcinoma, and non-small cell carcinoma [42]. Biomarker studies for these therapeutic agents are ongoing, and the expression level of PD-1 and PD-L1 themselves is suggested as predictive biomarkers for those agents.
We found that the expression of genes associated with pattern recognition receptors (GBP5, CXCR6, IL18RAP, IL18, TLR4, and TLR7) was significantly correlated with TIL level. Pattern recognition receptors such as toll-like receptors (TLRs) and NOD-like receptors (NLRs) recognize pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) and activate inflammasomes, which generate inflammatory cytokines such as IL-1 and IL-18 [43]. Various expression patterns of TLRs have been reported in breast cancer and breast cancer cell lines, but the exact role of pattern recognition receptors and their downstream signaling effect are not clear [44]. Therefore, further studies exploring the relationship between TILs, pattern recognition receptors, downstream signaling molecules, and their role in breast cancer prognosis and treatment response are warranted.
The peripheral blood absolute neutrophil count/absolute lymphocyte count ratio (ANC/ALC) or the neutrophil/lymphocyte ratio (NLR) at diagnosis is also a prognostic indicator in malignant tumors including breast cancer [45–48]. Furthermore, recent studies have revealed that NLR is a negative predictive factor of advanced esophageal cancer [49], stage III-IV gastric cancer [50], and breast cancer with ER/PR-positivity and HER2-negativity in the NAC setting [51]. However, the prognostic or predictive significance of neutrophils in tissues has not been extensively investigated. In patients with a high level of CD8A expression tumors that did not achieve a pCR showed higher expression of several genes directly or indirectly related to neutrophils. These genes encode cytotoxic and chemoattractant peptides made by neutrophils (DEFB1, DEFB103A, and DEFB4A) and a cell surface receptor molecule (FCAR). However, the number of neutrophils was not significantly different between tumors with pCR and non-pCR. These results propose the possibility of the activation status of cancer tissue neutrophils as a predictive factor of pCR in breast cancer treated with NAC. Further studies exploring the role of neutrophils in response to NAC in TNBC are warranted.
In conclusion, higher expression of cytotoxic molecules, T cell receptor signaling pathway components, cytokines related to Th1 and Tfh cells, and B cell markers is correlated with pCR and survival in TNBC patients treated with NAC. Our results suggest that the activation status of neutrophils may provide additional predictive information for TNBC patients treated with NAC.
References
Penault-Llorca F, Viale G (2012) Pathological and molecular diagnosis of triple-negative breast cancer: a clinical perspective. Ann Oncol 23(Suppl 6):vi19–vi22
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434
Dawson SJ, Provenzano E, Caldas C (2009) Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer 45(Suppl 1):27–40
Stagg J, Allard B (2013) Immunotherapeutic approaches in triple-negative breast cancer: latest research and clinical prospects. Ther Adv Med Oncol 5:169–181
Fisher CS, Ma CX, Gillanders WE, Aft RL, Eberlein TJ, Gao F, Margenthaler JA (2012) Neoadjuvant chemotherapy is associated with improved survival compared with adjuvant chemotherapy in patients with triple-negative breast cancer only after complete pathologic response. Ann Surg Oncol 19:253–258
Kong X, Moran MS, Zhang N, Haffty B, Yang Q (2011) Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer 47:2084–2090
Osako T, Horii R, Matsuura M, Domoto K, Ide Y, Miyagi Y, Takahashi S, Ito Y, Iwase T, Akiyama F (2010) High-grade breast cancers include both highly sensitive and highly resistant subsets to cytotoxic chemotherapy. J Cancer Res Clin Oncol 136:1431–1438
Darb-Esfahani S, Loibl S, Muller BM, Roller M, Denkert C, Komor M, Schluns K, Blohmer JU, Budczies J, Gerber B, Noske A, du Bois A, Weichert W, Jackisch C, Dietel M, Richter K, Kaufmann M, von Minckwitz G (2009) Identification of biology-based breast cancer types with distinct predictive and prognostic features: role of steroid hormone and HER2 receptor expression in patients treated with neoadjuvant anthracycline/taxane-based chemotherapy. Breast Cancer Res 11:R69
Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353:2654–2666
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213
Zitvogel L, Kroemer G (2008) The immune response against dying tumor cells: avoid disaster, achieve cure. Cell Death Differ 15:1–2
Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, Andre F, Tursz T, Kroemer G, Zitvogel L (2007) The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 220:47–59
Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Torne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28:105–113
Issa-Nummer Y, Darb-Esfahani S, Loibl S, Kunz G, Nekljudova V, Schrader I, Sinn BV, Ulmer HU, Kronenwett R, Just M, Kuhn T, Diebold K, Untch M, Holms F, Blohmer JU, Habeck JO, Dietel M, Overkamp F, Krabisch P, von Minckwitz G, Denkert C (2013) Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer–a substudy of the neoadjuvant GeparQuinto trial. PLoS One 8:e79775
West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH (2011) Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res 13:R126
Yamaguchi R, Tanaka M, Yano A, Tse GM, Yamaguchi M, Koura K, Kanomata N, Kawaguchi A, Akiba J, Naito Y, Ohshima K, Yano H (2012) Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol 43:1688–1694
Schmidt M, Hellwig B, Hammad S, Othman A, Lohr M, Chen Z, Boehm D et al (2012) A comprehensive analysis of human gene expression profiles identifies stromal immunoglobulin kappa C as a compatible prognostic marker in human solid tumors. Clin Cancer Res 18:2695–2703
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 31:860–867
Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, Veys I, Haibe-Kains B, Singhal SK, Michiels S, Rothe F, Salgado R, Duvillier H, Ignatiadis M, Desmedt C, Bron D, Larsimont D, Piccart M, Sotiriou C, Willard-Gallo K (2013) CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 123:2873–2892
Ignatiadis M, Singhal SK, Desmedt C, Haibe-Kains B, Criscitiello C, Andre F, Loi S, Piccart M, Michiels S, Sotiriou C (2012) Gene modules and response to neoadjuvant chemotherapy in breast cancer subtypes: a pooled analysis. J Clin Oncol 30:1996–2004
Bianchini G, Gianni L (2014) The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol 15:e58–e68
Spitale A, Mazzola P, Soldini D, Mazzucchelli L, Bordoni A (2009) Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol 20:628–635
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2014) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med 138:241–256
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S et al (2014) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group. Ann Oncol 26:259–271
Lakhani SREI, Schnitt SJ, Tan PH, van de Vijver MJ (eds) (2012) WHO classification of tumours of the breast. International Agency for Research on Cancer, Lyon
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, Buzdar AU, Singletary SE, Hortobagyi GN, Pusztai L (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25:4414–4422
Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K (2008) Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26:317–325
Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP (2006) GenePattern 2.0. Nat Genet 38:500–501
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26:259–271
Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, Fumoleau P, Ghiringhelli F (2008) Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res 14:2413–2420
Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM (2012) Leukocyte composition of human breast cancer. Proc Natl Acad Sci USA 109:2796–2801
Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, Perez S, Pasqual N, Faure C, Ray-Coquard I, Puisieux A, Caux C, Blay JY, Menetrier-Caux C (2009) Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res 69:2000–2009
Cimino-Mathews A, Ye X, Meeker A, Argani P, Emens LA (2013) Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: a pilot study. Hum Pathol 44:2055–2063
Alistar A, Chou JW, Nagalla S, Black MA, D’Agostino R Jr, Miller LD (2014) Dual roles for immune metagenes in breast cancer prognosis and therapy prediction. Genome Med 6:80
Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, Bruneval P, Fridman WH, Becker C, Pages F, Speicher MR, Trajanoski Z, Galon J (2013) Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39:782–795
King C, Tangye SG, Mackay CR (2008) T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol 26:741–766
Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD, Schem C, Fisch K, Darb-Esfahani S, Mehta K, Sotiriou C, Wienert S, Klare P, André F, Klauschen F, Blohmer JU, Krappmann K, Schmidt M, Tesch H, Kümmel S, Sinn P, Jackisch C, Dietel M, Reimer T, Untch M, Loibl S (2015) Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 33:983–991
West NR, Kost SE, Martin SD, Milne K, Deleeuw RJ, Nelson BH, Watson PH (2013) Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer 108:155–162
Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B (2009) Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 27:186–192
Jacquemier J, Bertucci F, Finetti P, Esterni B, Charafe-Jauffret E, Thibult ML, Houvenaeghel G, Van den Eynde B, Birnbaum D, Olive D, Xerri L (2012) High expression of indoleamine 2,3-dioxygenase in the tumour is associated with medullary features and favourable outcome in basal-like breast carcinoma. Int J Cancer 130:96–104
Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C, Piccart MJ, Loibl S, Denkert C, Smyth MJ, Joensuu H, Sotiriou C (2014) Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 25:1544–1550
Callahan MK, Postow MA, Wolchok JD (2014) CTLA-4 and PD-1 pathway blockade: combinations in the clinic. Front Oncol 4:385
Terlizzi M, Casolaro V, Pinto A, Sorrentino R (2014) Inflammasome: cancer’s friend or foe? Pharmacol Ther 143:24–33
Bhatelia K, Singh K, Singh R (2014) TLRs: linking inflammation and breast cancer. Cell Signal 26:2350–2357
Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, Widmann WD (2012) Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol 19:217–224
Forget P, Machiels JP, Coulie PG, Berliere M, Poncelet AJ, Tombal B, Stainier A, Legrand C, Canon JL, Kremer Y, De Kock M (2013) Neutrophil:lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol 20(Suppl 3):S650–S660
Noh H, Eomm M, Han A (2013) Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer 16:55–59
Dirican A, Kucukzeybek BB, Alacacioglu A, Kucukzeybek Y, Erten C, Varol U, Somali I, Demir L, Bayoglu IV, Yildiz Y, Akyol M, Koyuncu B, Coban E, Ulger E, Unay FC, Tarhan MO (2015) Do the derived neutrophil to lymphocyte ratio and the neutrophil to lymphocyte ratio predict prognosis in breast cancer? Int J Clin Oncol 20:70–81
Sato H, Tsubosa Y, Kawano T (2012) Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg 36:617–622
Jin H, Zhang G, Liu X, Chen C, Yu H, Huang X, Zhang Q, Yu J (2013) Blood neutrophil-lymphocyte ratio predicts survival for stages III–IV gastric cancer treated with neoadjuvant chemotherapy. World J Surg Oncol 11:112
Koh YW, Lee HJ, Ahn JH, Lee JW, Gong G (2014) Prognostic significance of the ratio of absolute neutrophil to lymphocyte counts for breast cancer patients with ER/PR-positivity and HER2-negativity in neoadjuvant setting. Tumour Biol 35:9823–9830
Acknowledgments
This study was supported by a grant (2013-0866) from the Asan Institute for Life Sciences, Seoul, Korea. We thank professor Chan Sik Park, Asan Medical Center for comments that greatly improved the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hee Jin Lee and Jeong-Ju Lee have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, H.J., Lee, JJ., Song, I.H. et al. Prognostic and predictive value of NanoString-based immune-related gene signatures in a neoadjuvant setting of triple-negative breast cancer: relationship to tumor-infiltrating lymphocytes. Breast Cancer Res Treat 151, 619–627 (2015). https://doi.org/10.1007/s10549-015-3438-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3438-8