Abstract
The immune system affects all phases of tumor growth from initiation to progression and dissemination. Tumor-infiltrating lymphocytes (TILs) are mononuclear immune cells that infiltrate tumor tissue. Several retrospective studies have suggested the potential of TILs as a prognostic as well as predictive factor of chemotherapy in some breast cancers. On the other hand, programmed cell death protein-1 (PD-1) and programmed cell death-ligand 1 (PD-L1) eliminate T cell activation in various types of cancers. Prospective trials to evaluate the efficacy of antibody agents to PD-1 and PD-L1 are ongoing in patients with breast cancer. The findings of these studies appear to support the potential of immune checkpoint inhibitors targeting the PD-1/PD-L1 axis in triple negative breast cancer. Further studies are needed in order to confirm previous findings on TILs and promote the development of new immune therapy approaches for breast cancer patients. Furthermore, the search for TILs will soon be introduced into actual clinical practice, for which the standardization of evaluation methods and establishment of a simple evaluation method are expected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous lymphocytes have been detected in cancer tissue for more than 100 years [1], and these immune-related cells including various types of lymphocytes have been referred to as tumor-infiltrating lymphocytes (TILs) [2]. TILs are assumed to be closely related to the proliferation and elimination of cancer cells, and lymphocytes within cancer tissue have been suggested to transfer specific information between cancer cells and lymphocytes [3]. Furthermore, the state of expression of TILs in situ has been identified as a useful factor for predicting prognoses and responses to drug therapies, and has been attracting attention as a new biological marker in various types of cancers [4].
One theory on the presence of immune cells in TILs that co-exist with cancer cells and do not attack them by binding programmed cell death protein-1 (PD-1) on lymphocytes and programmed cell death-ligand 1 (PD-L1) on cancer cells has recently been recognized [5]. In addition, therapeutic antibody agents possessing the ability to bind to PD-1 and PD-L1 proteins, which inhibit the co-existence of lymphocytes and cancer cells, have been developed [6,7,8].
In the present review, we focus on the value of TILs evaluations for predicting prognoses and drug responses in patients with breast cancer, and the utility of examining PD-L1 expression in cancer cells.
Mechanism of cancer immunity
The involvement of immunological mechanisms in all processes from cancer development to metastasis has been clarified in immunological studies. Dendritic cells, a type of immune cell, incorporate specific antigens possessed by cancer cells in cancer tissue, and activate T cells with antigen specificity after they move to the lymph nodes. These T cells attack cancer cells as cytotoxic (killer) T cells, and cancer cells release various cytokines to inhibit their immune function and evade this attack. Cancer cells also inhibit the immune system in order to promote their own proliferation by inducing regulatory T (T-reg) cells [9], which inhibit an excessive immune response by the host [10].
Histopathology of lymphocyte infiltration in breast cancer tissue
Various grades of tumor-associated lymphocytes infiltration are recognized in breast cancer tissues. Mohammed et al. previously reported that 93.6% of breast cancer cases showed low- to high-grade inflammatory cell infiltration [11]. This finding has recently been referred to as TILs, and many in vitro and in vivo studies have been conducted on the functions of TILs since the 1970s [2]. These TILs were immunohistochemically found to have some types of immunoglobulins, such as IgG and IgM [12].
On the other hand, medullary carcinoma, a special type of invasive breast cancer, shows prominent and diffuse lymphoplasmacytic infiltration between syncytial cancer nests (Fig. 1), which is considered to be one of the characteristics of medullary carcinoma. In addition, the prognosis of medullary carcinoma is excellent despite the high nuclear grade [13].
Stanton et al. defined lymphocyte predominant breast cancer (LPBC) as a state in which more than 50% of tumor tissue is occupied by lymphocytes. They reported that the median incidence of LPBC was 20% in triple negative (TN) tumors, 6% in hormone receptor (HR)-positive tumors, and 16% in HER2-positive tumors (Table 1), and also indicated the high incidence of TILs in TN as well as HER2-positive breast cancer [14].
Functions of TILs in breast cancer
TILs are mononuclear immune cells that accumulate in cancer tissue and are considered to perform an important immune system-related function. The functions of TILs were previously analyzed and evaluated in cell culture systems; however, the usefulness of a histopathological method to evaluate the state of expression of TILs in situ has recently been demonstrated. In 2006, Galon et al. [4] reported a strong relationship between the state of expression of TILs in situ and outcomes in colorectal and breast cancer patients. A number of retrospective studies then showed the usefulness of identifying the state of expression of TILs in order to predict the outcomes and effects of drug therapy in breast cancer patients [15]. On the other hand, differences in the immune system due to variations in the characteristics of TILs-constituting immune cells in each cancer tissue were identified. These characteristics are closely related to the prognosis and prediction of responses to drug therapy. Therefore, the importance of evaluating TILs in actual clinical practice is increasing.
Evaluation method of TILs in breast cancer
In situ TILs evaluation methods and cut-off values have varied among previous clinical studies on TILs, and, thus, have not yet been standardized. In 2014, the International Working Group prepared guidelines on TILs evaluation methods (Table 2) [16]. TILs are evaluated in 4- to 5-μm-thick hematoxylin and eosin (H&E)-stained histological sections via light microscopy. TILs have been classified based on their location into stromal-TILs (str-TILs), which are present in the stromal tissue of cancer, and intratumoral-TILs (i-TILs), which are present between cancer cells. Mononuclear immune cells localized in stromal tissue and present between the nests of cancer cells are evaluated as str-TILs. The evaluation of str-TILs is relatively easy for pathology experts, and the concordance rate among observers is reportedly high. However, i-TILs represent mononuclear immune cells adjacent to cancer cells within each cancer cell nest. The evaluation of i-TILs in H&E-stained preparations is difficult, even for experts, and the concordance rate among observers is low [17]. The International Working Group has suggested that the evaluation of i-TILs requires an immunohistochemical analysis of lymphocyte-specific markers such as CD3 and CD8. The guidelines established by the International Working Group state that although the evaluation of TILs using a needle biopsy specimen is possible, it needs to be performed after identifying the distribution and state of expression of TILs in all fields because of the heterogeneity of TILs in tissue. Moreover, although several studies [18, 19] reported that the evaluation of TILs in residual tumors after preoperative drug therapy is useful, cancer cells may diffusely remain when the pathological therapeutic effect is high, and difficulties are associated with deciding on the range to be evaluated. In the guidelines of the International Working Group, the evaluation of TILs using tissue microarrays is not currently recommended because of the issue of heterogeneity. Although the quantitation of TILs and classification of cells constituting TILs based on immunohistological characteristics may be important, evidence is still insufficient and, thus, it is not recommended. The guidelines also do not currently recommend TILs measurements using analytical software. In a number of previous studies, the grade of TILs was evaluated by employing a method based on the quantity of immune cells presented with the proportion rate (%); however, difficulties have been associated with setting a distinct cut-off value, such as the Ki67 labeling index [20, 21]. Thus, in the evaluation criteria established by the International Working Group, the str-TILs expression level has been classified into three grades: low (0–10%), medium (10–40%), and high (40–90%) (Fig. 2). The standardization of TILs evaluation methods has just begun, and may be corrected in the future.
Histopathological findings of low-, medium-, and high-grade TILs (HE stain). a Low-grade TILs. A few lymphocytes are present in the surrounding tissues of cancer nests. b Medium-grade TILs. Many lymphocytes are scattered in the surrounding connective tissue of cancer nests. c High-grade TILs. Numerous lymphocytes are distributed in the adjacent areas of cancer nests
Usefulness of TILs as a prognostic factor in breast cancer
The usefulness of TILs as a prognostic factor has recently been clarified by translational research performed by a number of large-scale prospective clinical studies (Table 3). In 2013, Loi et al. [22] reported that the expression of TILs was a prognostic factor for ER-negative breast cancer (particularly TN breast cancer) in the Breast International Group (BIG) 2-98 study. In 2013, Adams et al. [23] showed that the expression of TILs was a very strong prognostic factor for TN breast cancer in the Eastern Cooperative Oncology Group (ECOG) 2197 and ECOG 1199. In addition, Loi et al. [24] reported at the 2015 San Antonio Breast Cancer Symposium that they performed a meta-analysis of these studies and large-scale studies, such as the Finland Herceptin Trial (FinHER), for the usefulness of the expression of TILs as a prognostic factor involving TN breast cancer patients registered in these studies, and found that it was a potent prognostic factor for TN breast cancer.
Although translational research on HER2-positive breast cancer has been performed by the Neo ALTTO [25] and FinHER trials [26], TN breast cancer has been examined in more detail. Hida et al. [27] suggested that the prognostic and predictive impacts of TILs differ between TN breast cancer and HER2-positive breast cancers treated with standard systemic therapies. Further investigations are needed in order to identify the usefulness of TILs as a prognostic factor for this cancer type.
Reliable studies have not yet been conducted on the usefulness of TILs as a prognostic factor for ER-positive breast cancer, and this may be due to the low frequency of the strong expression of TILs.
Usefulness of TILs as a predictor of drug effects in breast cancer
Several clinical studies on postoperative chemotherapy clarified that TILs are a predictor of the effects of anticancer drugs. The BIG 2-98 study [22] suggested a relationship between the expression of TILs and effects of anthracycline on HER2-positive breast cancer, and a relationship was also observed between epirubicin sensitivity and the expression of TILs in the National Epirubicin Adjuvant Trial (NEAT)/BR9601 study [28].
In several studies on preoperative drug therapy, the relationship between the state of expression of TILs and therapeutic effects was investigated (Table 4). Denkert et al. [29] reported a relationship between the pathological response rate and expression of TILs in the GeparDuo and GeparTrio studies, in which the pathological complete response (pCR) rate in the group with the strong expression of TILs was significantly higher than that in the group with the weak expression of TILs. These findings demonstrated the potential of the state of expression of TILs as a predictor of the effects of preoperative chemotherapy. However, its usefulness in preoperative trastuzumab-combined chemotherapy for HER2-positive breast cancer and preoperative endocrine therapy for ER-positive breast cancer remains controversial. We previously revealed that the status of TILs in pre-treatment tumors predicted responses to preoperative chemotherapy concomitant with trastuzumab in HER2-positive breast cancer [30].
The state of expression of TILs in residual tumors after preoperative drug therapy has been suggested as an important factor for evaluating the drug sensitivity of cancer. In 2014, Dieci et al. [31] compared the expression of TILs between before and after preoperative chemotherapy for TN breast cancer, and observed that, among the non-pCR group, outcomes were significantly more favorable with the strong rather than the weak expression of TILs in residual tumors. Miyashita et al. [18] also reported in 2015 that outcomes became more favorable with an increase in the CD8/FOXP3 ratio in TILs after preoperative chemotherapy in TN breast cancer.
Our previous findings [19] suggested that the expression of TILs in residual tumors following preoperative trastuzumab-combined chemotherapy was stronger than that in pre-treatment tumors, and patients with high residual-TILs showed better prognoses than those with low residual-TILs among non-pCR patients with HER2-positive breast cancer. Accordingly, although the prognosis of patients is generally considered to be poorer in the non-pCR group than in the pCR group after preoperative chemotherapy, it may be possible to extract cases with favorable prognoses from the non-pCR group by evaluating the expression of TILs in residual tumors. However, as described above, difficulties are associated with evaluating TILs in residual tumors after preoperative drug therapy, and, thus, further investigations are needed.
Significance of immunohistochemical evaluations of TILs
In order to effectively use molecular targeted drugs involved in this novel tumor immunity, the characteristics of immune cells constituting TILs may need to be examined in more detail, in addition to an evaluation of their expression. Killer T cells, which attack cancer cells, are positive for CD8, and T-reg cells, which inhibit immune responses to cancer cells, are positive for CD4, CD25, and FOXP3. In 2016, Asano et al. [32] reported that the degree of involvement of TILs in antitumor effects was based on the ratios of the expression levels of these markers in TILs.
Mechanism of the PD-1 and PD-L1 checkpoint pathway
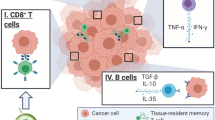
PD-1 is a one of the receptors of T-reg cells and is expressed on the surface of T cells. PD-L1 is a ligand of PD-1 and is localized on cancer cells and lymphocytes. PD-1 and PD-L1 are immune checkpoint proteins, and their direct binding inhibits the killer T cell attack against cancer cells, resulting in the co-existence of cancer cells and killer T cells. PD-1 and PD-L1 binding modulates decreases in cytokine production by T cells and inhibitory signals are transmitted to T cells by dendritic cells. PD-1 and PD-L1 inhibitors promote killer T cell-induced tumor immune responses by inhibiting these immune checkpoints, thereby exerting antitumor effects [33] (Fig. 3). PD-L1 expressed in cancer cells is considered to act as an immune-resistant factor. PD-L1 is detected in cancer cells, but not in normal epithelial cells [30, 33]. The existence of PD-1 has been confirmed in lymphocytes and PD-L1 in breast cancer cells, and the immune check point mechanism may also be accommodated to breast cancer. In addition, a correlation was found between the behavior of lymphocytes around cancer cells and the expression of PD-L1 on these cells (Fig. 4). Moreover, PD-L1 was immunohistochemically detected not only in cancer cells, but also in lymphocytes around cancer cells, and PD-L1 inhibitors are considered to bind to PD-L1-positive cancer cells as well as lymphocytes and inhibit the binding of PD-1-positive lymphocytes to PD-L1 positive cells. However, differences in and the significance of PD-L1 expression between tumor cells and lymphocytes currently remain unclear. Further research is required in order to clarify the mechanisms underlying PD-L1 expression between tumor cells and lymphocytes.
Actions of PD-1 and PD-L1 on immune checkpoints. PD-1 and PD-L1 are proteins expressed on the cell membranes of T lymphocytes and cancer cell surfaces, respectively. When PD-1 on the surface of T cells is bound by PD-L1 on the cancer cell surface, T lymphocytes become unable to attack the target cancer cells
Molecular targeted agents against PD-1 and PD-L1
Based on the mechanism of the PD-1/PD-L1 pathway, many antibody agents have been developed that target the PD-1/PD-L1 immune checkpoint. A number of clinical studies recently demonstrated that immuno-targeting therapy using PD-1 and PD-L1 inhibitors was very effective against tumors, such as melanoma [34], non-small cell lung cancer [35], and renal cell carcinoma [36], and several molecular targeted agents, including nivolumab and pembrolizumab, have been approved as therapies for these malignant tumors. A worldwide-scale clinical study on breast cancer is currently ongoing, and efficacy for TN (ER-negative, PgR-negative, and HER2-negative) breast cancer is expected [37]. The presence of immune cells controlled by PD-1 and PD-L1 in TILs may be a precondition for the effects of these immune checkpoint inhibitors [38].
Examination method of PD-L1 expression
An evaluation of PD-1 and PD-L1 expression levels is also reportedly useful for assessing the efficacy of immune checkpoint inhibitors. However, antibodies that accurately evaluate the expression of PD-L1 by immunohistochemistry have yet to be developed. Furthermore, a suitable cut-off value for the expression of PD-L1 evaluated by immunohistochemistry has not been established [39]. However, PD-L1 using the antibody 22C3 has been used in a companion diagnosis for deciding on the indication of pembrolizumab for non-small lung cancer, and a cut-off value of ≥1% is used for second-line treatments and ≥50% for first-line treatments [40, 41]. On the other hand, PD-L1 using the antibody 28-8 has been used in a complementary diagnosis for deciding on the indication of nivolumab for non-small lung cancer, and a cut-off value of ≥1% is used for second-line treatments [41, 42]. Sun et al. [43] previously suggested that the expression of PD-L1 in TN breast cancer cells was not the same between the different clones of antibodies. They revealed that the positive rates of PD-1 markedly differed between the antibodies used, such as 28-8 and E1L3N. Further validation studies are needed in order to identify appropriate antibodies for companion and complementary diagnoses.
Mechanism of the antitumor agent trastuzumab-TDB
The molecular targeted drug, trastuzumab not only directly inhibits HER2 proliferation signals, but also induces the antitumor effects of natural killer (NK) cells using antibody-dependent cellular cytotoxicity (ADCC) activity [44, 45]. On the other hand, the potentiation of TILs expression in breast cancer tissue by trastuzumab has been reported, suggesting that trastuzumab exerts another effect on tumor immunity separate from ADCC activity. On the other hand, we revealed that the status of TILs in pre-treatment tumors predicted responses to neoadjuvant chemotherapy concomitant with trastuzumab in HER2-positive breast cancer. In addition, anti-HER2 immunotherapy research is currently focused on anti-HER2 vaccines and new immunotherapy using HER2 antigen-specific T lymphocytes, and dendritic cells are considered to play an important role in tumor immune escape and tolerance. A previous study showed that HER2-specific T lymphocytes generated from dendritic cells killed HER2-positive breast cancer cell lines, and immunotherapy has potential as a treatment option for HER2-positive breast cancer. Furthermore, a new HER2-targeting agent possessing a CD3 binding site is expected to induce a stronger immune response by binding T lymphocytes. Trastuzumab-T cell-dependent bispecific antibody (TDB) is a new drug that utilizes the immune responses induced by trastuzumab and is a bispecific antibody [46]. Trastuzumab-TDB has 2 arms, similar to trastuzumab, and induces strong immune responses in HER2-positive breast tumors by binding to HER2 on breast cancer cells through one arm and CD3 on T cells through the other, thereby exerting antitumor effects (Fig. 5). A clinical study on the usefulness of this drug for HER2-positive breast cancer is currently ongoing.
Conclusion
The state of the in situ expression of TILs has been identified as a useful factor for predicting patient prognoses and responses to drug therapies not only in breast cancer, but also in cancers arising in various organs, such as the colon, ovary, and head and neck. However, advances have been achieved in research on TILs, particularly in the field of breast cancer, and the significance of evaluating TILs has been emphasized by subgroup analyses because breast cancer may be divided into several subtypes based on the status of ER, PgR, and HER2 expression. Furthermore, neoadjuvant chemotherapy, particularly that developed for breast cancer, and the relationship between responses to chemotherapy and the expression of TILs need to be analyzed in the short term.
On the other hand, recent studies on PD-L1 have been conducted on the tumors of many organs, and the focus of research has shifted from TILs to PD-L1 expression in tumor cells because effective PD-1 and PD-L1 inhibitors have been produced, examined in clinical studies, and used in clinical practice.
A number of studies have demonstrated the usefulness of TILs as a prognostic factor and predictor of the effects of drugs; however, these were retrospective studies on breast cancer. Therefore, a prospective clinical study to clarify the true usefulness of TILs is warranted. The search for TILs will soon be introduced into actual clinical practice, for which the standardization of evaluation methods and establishment of a simple evaluation method are desired.
References
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357:539–545
Klein E, Becker S, Svedmyr E, Jondal M, Vanky F (1976) Tumor infiltrating lymphocytes. Ann N Y Acad Sci 276:207–216
Galon J, Angell HK, Bedognetti D, Marincola FM (2013) The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 39:11–26
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci 99:12293–12297
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704
Nishimura H, Agata Y, Kawasaki A, Sato M, Imamura S, Minato N, Yagita H, Nakano T, Honjo T (1996) Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4−CD8−) thymocytes. Int Immunol 8:773–780
Chen DS, Mellman I (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39:1–10
Coussens LM, Zitvogel L, Palucka AK (2013) Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science 339:286–291
Mohammed ZMA, Going JJ, Edwards B, Doughty JC, McMillan DC (2012) The relationship between components of tumor inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br J Cancer 107:864–873
Richters A, Kaspersky CL (1975) Surface immunoglobulin positive lymphocytes in human breast cancer tissue and homolateral axillary lymph nodes. Cancer 35:129–133
WHO (2012) Carcinomas with medullary features. In: Lakhani SR, Ellis IO (eds) WHO classification of tumours of the breast. WHO Press, Geneva, pp 46–47
Stanton AE, Adams S, Disis ML (2016) Variation in incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA oncol 2:1354–1360
Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, Loi S (2016) Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol 13:228–241
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S, International TILs Working Group 2014 (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26:259–271
Buisseret L, Desmedt C, Garaud S, Fornili M, Wang X, Van den Eyden G, de Wind A, Duquenne S, Boisson A, Naveaux C, Rothé F, Rorive S, Decaestecker C, Larsimont D, Piccart-Gebhart M, Biganzoli E, Sotiriou C, Willard-Gallo K (2017) Reliability of tumor-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod Pathol. doi:10.1038/modpathol.2017.43
Miyashita M, Sasano H, Tamaki K, Hirakawa H, Takahashi Y, Nakagawa S, Watanabe G, Tada H, Suzuki A, Ohuchi N, Ishida T (2015) Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res 17:124
Kurozumi S, Inoue K, Matsumoto H, Hayashi Y, Tozuka K, Kubo K, Komatsu K, Takai K, Nagai SE, Oba H, Horiguchi J, Takeyoshi I, Kurosumi M (2015) Prognostic value of tumor-infiltrating lymphocytes in residual tumors after neoadjuvant chemotherapy concomitant with trastuzumab for HER2-positive breast cancer. Cancer Res 76(suppl):abstr: P4-14-17
Nishimura R, Osako T, Okumura Y, Hayashi M, Arima N (2010) Clinical significance of Ki-67 in neoadjuvant chemotherapy for primary breast cancer as a predictor for chemosensitivity and for prognosis. Breast Cancer 17:269–275
Honma N, Horii R, Iwase T, Saji S, Younes M, Ito Y, Akiyama F (2015) Ki-67 evaluation at the hottest spot predicts clinical outcome of patients with hormone receptor-positive/HER2-negative breast cancer treated with adjuvant tamoxifen monotherapy. Breast Cancer 22:71–78
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 31:860–867
Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, Wolff AC, Wood WC, Davidson NE, Sledge GW, Sparano JA, Badve SS (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32:2959–2966
Loi S, Drubay D, Adams S, Francis PA, Joensuu H, Dieci MV, Badve S, Demaria S, Gray R, Piccart MJ, Lehtinen PLK, Andre F, Denkert C, Salgado R, Michiels S (2016) Pooled individual patient data analysis of stromal tumor infiltrating lymphocytes in primary triple negative breast cancer treated with anthracycline-based chemotherapy. Cancer Res 76(suppl):abstr S1–03
Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, de Azambuja E, Eidtmann H, Ellis CE, Baselga J, Piccart-Gebhart MJ, Michiels S, Bradbury I, Sotiriou C, Loi S (2015) Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO Trial. JAMA Oncol 1:448–454
Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C, Piccart MJ, Loibl S, Denkert C, Smyth MJ, Joensuu H, Sotiriou C (2014) Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 25:1544–1550
Hida AI, Sagara Y, Yotsumoto D, Kanemitsu S, Kawano J, Baba S, Rai Y, Oshiro Y, Aogi K, Sagara Y, Ohi Y (2016) Prognostic and predictive impacts of tumor-infiltrating lymphocytes differ between Triple-negative and HER2-positive breast cancers treated with standard systemic therapies. Breast Cancer Res Treat 158:1–9
Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, Earl HM, Poole CJ, Hiller L, Dunn JA, Bowden SJ, Twelves C, Bartlett JM, Mahmoud SM, Rakha E, Ellis IO, Liu S, Gao D, Nielsen TO, Pharoah PD, Caldas C (2014) Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol 25:1536–1543
Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Törne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28:105–113
Kurozumi S, Inoue K, Kurosumi M, Matsumoto H, Hayashi Y, Tozuka K, Kubo K, Komatsu K, Takai K, Nagai SE, Oba H, Horiguchi J (2016) Values of tumor-infiltrating lymphocytes (TILs), CD8+ TILs, and PDL-1 for predicting pathological complete response and prognosis in HER2-positive breast cancer receiving neoadjuvant chemotherapy with trastuzumab. J Clin Oncol 34(suppl):abstr 589
Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, Ficarra G, Mathieu MC, Delaloge S, Curigliano G, Andre F (2015) Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol 26:1518
Asano Y, Kashiwagi S, Goto W, Kurata K, Noda S, Takashima T, Onoda N, Tanaka S, Ohsawa M, Hirakawa K (2016) Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br J Surg 103:845–854
Topalian SL, Taube JM, Anders RA, Pardoll DM (2016) Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 16:275–287
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23–34
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135
Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, Margolin KA, Plimack ER, Lambert AM, Waxman IM, Hammers HJ (2015) Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 33:1430–1437
Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L (2016) Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol 34:2460–2467
Gibney GT, Weiner LM, Atkins MB (2016) Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 17:e542–e551
Gandini S, Massi D, Mandalà M (2016) PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: a systematic review and meta-analysis. Crit Rev Oncol Hematol 100:88–98
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, KEYNOTE-001 Investigators (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018–2028
Mino-Kenudson Mari (2016) Programmed cell death ligand-1 (PD-L1) expression by immunohistochemistry: could it be predictive and/or prognostic in non-small cell lung cancer? Cancer Biol Med 13:157–170
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639
Sun WY, Lee YK, Koo JS (2016) Expression of PD-L1 in triple-negative breast cancer based on different immunohistochemical antibodies. J Transl Med 14:173
Hurvitz SA, Betting DJ, Stern HM, Quinaux E, Stinson J, Seshagiri S, Zhao Y, Buyse M, Mackey J, Driga A (2012) Analysis of Fcgamma receptor IIIa and IIa polymorphisms: lack of correlation with outcome in trastuzumab-treated breast cancer patients. Clin Cancer Res 18:3478–3486
Sliwkowski MX, Mellman I (2013) Antibody therapeutics in cancer. Science 341:1192–1198
Junttila TT, Li J, Johnston J, Hristopoulos M, Clark R, Ellerman D, Wang BE, Li Y, Mathieu M, Li G, Young J, Luis E, Lewis Phillips G, Stefanich E, Spiess C, Polson A, Irving B, Scheer JM, Junttila MR, Dennis MS, Kelley R, Totpal K, Ebens A (2014) Antitumor efficacy of a bispecific antibody that targets HER2 and activates T cells. Cancer Res 74:5561–5571
Issa-Nummer Y, Darb-Esfahani S, Loibl S, Kunz G, Nekljudova V, Schrader I, Sinn BV, Ulmer HU, Kronenwett R, Just M, Kühn T, Diebold K, Untch M, Holms F, Blohmer JU, Habeck JO, Dietel M, Overkamp F, Krabisch P, von Minckwitz G, Denkert C (2013) Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer–a substudy of the neoadjuvant GeparQuinto trial. PLoS One 8:e79775
Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD, Schem C, Fisch K, Darb-Esfahani S, Mehta K, Sotiriou C, Wienert S, Klare P, André F, Klauschen F, Blohmer JU, Krappmann K, Schmidt M, Tesch H, Kümmel S, Sinn P, Jackisch C, Dietel M, Reimer T, Untch M, Loibl S (2015) Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 33:983–991
West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH (2011) Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res 13:R126
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurozumi, S., Fujii, T., Matsumoto, H. et al. Significance of evaluating tumor-infiltrating lymphocytes (TILs) and programmed cell death-ligand 1 (PD-L1) expression in breast cancer. Med Mol Morphol 50, 185–194 (2017). https://doi.org/10.1007/s00795-017-0170-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-017-0170-y