Abstract
Increased levels of total tumor-infiltrating lymphocytes (TILs) are generally associated with good prognosis in several breast cancer subtypes. Subtypes of TILs impact both tumor cells and immune cells in a variety of different ways, leading to either a pro-tumor or antitumor effect. Tumor-infiltrating CD8+ T cells and natural killer (NK) cells perform as effector cells against tumor cells and are associated with better clinical outcome. Immunotherapy approaches that improve the antitumor activity and proliferation of CD8+ T and NK cells include PD-1/PD-L1 blockade, CAR T cell therapy, or ex vivo-stimulated NK cells. A subset of CD8+ T cells, tissue-resident memory T cells, has also recently been associated with good prognosis in breast cancer patients, and has potential to serve as a predictive biomarker and therapeutic target. Tumor-infiltrating B cells also secrete apoptosis-inducing IgG antibodies and can act as antigen-presenting cells to prime CD4+ and CD8+ T cells. On the other hand, regulatory T and regulatory B cells modulate the immune response from CD8+ T cells and NK cells by secreting immunosuppressive cytokines and inhibiting maturation of antigen-presenting cells (APCs). These regulatory cells are typically associated with poor prognosis, therefore rendering suppression of their regulatory function a key immunotherapeutic strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Breast cancer is the most common type of cancer for women globally and is one of the leading causes of cancer-related deaths in women in the USA, second only to lung cancer [1, 2]. One in 8 women in the USA is expected to develop breast cancer during their lifetime, with the risk of breast cancer development increasing with age [3]. Over 275,000 cases and 40,000 deaths are estimated to occur due to breast cancer in the USA in 2020, representing approximately 15.4% and 7.0% of all estimated new cancer cases and cancer-related deaths in 2020. Five-year survival rates for breast cancer patients decrease from 99% to just 27% with the transition from local stage 0 or 1A cancer to metastatic stage IV cancer, highlighting a need for systemic treatment that can eradicate microscopic as well as macroscopic metastasis.
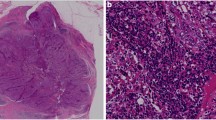
There are several systemic treatment options for patients with metastatic breast cancer: chemotherapy, hormonal therapy, targeted therapy, and immunotherapy. With each of these therapies, patients with higher levels of total tumor-infiltrating lymphocytes (TILs) tend to exhibit better treatment outcomes [4,5,6,7]. TILs are comprised of T cells, B cells, and natural killer (NK) cells (Fig. 1), which represent about 75%, 20%, and 5% of TILs in breast tumors, respectively [8, 9]. The subtypes of immune filtrate also play a role in predicting prognosis. Regulatory T and B cells, which modulate the immune response rather than augment it, are both negatively associated with breast cancer prognosis in all breast cancer subtypes [10, 11]. Thus, immunotherapy, which seeks to improve the level and composition of TILs, has become an exciting avenue for the treatment of breast cancer patients. This review aims to identify the role of different TIL subtypes in breast cancer and discuss different immunotherapeutic strategies that target these cells.
Tumor-infiltrating lymphocyte subsets in the tumor microenvironment (TME). (I) Naïve CD8+ T cells become activated upon binding to antigen-presenting dendritic cells (DCs) in the lymph nodes. Once activated, CD8+ effector T cells recognize and bind to tumor cells, inducing apoptosis via granzyme release. (II) Regulatory T cells inhibit the antitumor immune response by secreting immunosuppressive cytokines and restricting the activity of DCs by the binding of CTLA-4 to CD80/86 on DCs. (III) NK cells recognize tumor cells as “non-self” and bind to induce apoptosis by releasing granzymes into the cell, as well as secrete immunostimulatory cytokines that recruit CD8+ effector T cells into the TME. (IV) B cells secrete tumor antigen-specific IgG antibodies that lead to apoptosis upon binding to the tumor, but B cells can also secrete immunosuppressive cytokines like TGF-β, IL-10, and IL-35 that promote tumor growth. Figure not drawn to scale. Created with BioRender.com
2 CD8+ T cells
To differentiate into CD8+ effector T cells that recognize and attack tumor cells, naïve CD8+ T cells must first become stimulated by dendritic cells (DCs) in lymphoid organs [12]. DCs will uptake and process tumor-associated antigens, presenting the MHC-peptide complexes on their surface. T cell antigen receptors (TCR) on the surface of CD8+ T cells recognize the MHC-peptide complex on the DCs, bind, and are activated in an antigen-specific manner (Fig. 1). At this stage, these early effector CD8+ T cells can further differentiate into memory precursor cells, which have the capacity to survive long-term as central memory T cells (TCM) and effector memory T cells (TEM) [13]. Co-stimulatory molecules on the surface of DCs also have the ability to prompt T cells to undergo clonal expansion, forming a large pool of CD8+ effector T cells [12]. During early human development, DCs are also responsible for inducing self-tolerant T cells by presenting self-antigens to naïve T cells in the thymus, ensuring that the only T cells that enter circulation are those with no or low affinity to self-antigens [12, 14]. Upon recognition of the target cell via surface antigens, effector CD8+ T cells release lytic granules containing perforin, granzymes, and serine proteases (Fig. 1) [15]. Perforin polymerizes to form pores in the target cell membrane, allowing granzymes and serine proteases to enter the target cell. Upon entry, the granzymes activate an enzyme cascade that leads to DNA degradation of the target cell, triggering the cell to undergo apoptosis.
Triple-negative breast cancer (TNBC) and HER2+ patients with higher levels of infiltrating CD8+ T cells are more likely to achieve an objective response rate (ORR) with immune checkpoint inhibitor (ICI) therapy [16]. In addition to CD8+ effector T cells, this cell population can also consist of CD8+ TCM, TEM, T stem cell memory (TSCM), and naïve CD8+ T cells [17]. ER+ breast cancer patients who respond to ICI immunotherapy are more likely to have exhausted T cell infiltration (CTLA-4+/PD-1+ CD8+ T cells), as these cells are the target cell population of ICIs [18]. The association between response rate and TIL levels was seen in TNBC tumors, but had not been shown in ER+ tumors until recently [19]. Spatial localization of CD8+ T cells within breast tumors also contributes to their prognostic ability. CD8+ TILs found within cancer islands (i.e., tumor parenchyma) of breast tumors have a stronger association with relapse-free survival (RFS) in TNBC patients than CD8+ TILs found within the stroma [20]. Similar findings were also found in ER− (TNBC and HER2+) breast cancer patients [21]. Additionally, high HLA-I expression levels on primary HER2+ breast tumor cells in patients are positively associated with a reduced relapse rate, likely due to the contribution of CD8+ T cells [22].
Although CD8+ T cells are positively correlated with better clinical outcome in breast cancer patients, breast cancers employ several methods of developing resistance to CD8+ T cell antitumor activity, thus reducing their clinical benefit. The tumor microenvironment (TME) secretes immunosuppressive cytokines, such as IL-6, IL-17, or TGF-β, which are associated with poor clinical outcomes [23, 24]. By releasing immunosuppressive cytokines, the TME also elevates levels of tumor-associated macrophages (TAMs), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs) that restrict CD8+ T cell infiltration, proliferation, and activity within the tumor [23, 25,26,27,28]. Human breast cancer cells can also induce an immunosuppressive environment by upregulating PD-L1 expression, which induces T cell suppression and inhibits T cell activity upon binding of tumor PD-L1 to the PD-1 or B7-1 receptors located on T and B cells [29]. Further, TCR signaling, a marker of T cell functionality, is decreased in peripheral blood of HR+ metastatic breast cancer patients compared to healthy donors, particularly in PD-1+ T cells [30].
As the presence of CD8+ T cells in tumors is strongly associated with improved RFS and objective response, several treatment strategies target CD8+ T cells directly. For example, antibodies that target the PD-1/PD-L1 axis have become increasingly popular for treating breast cancer, particularly in TNBC patients. In a systematic analysis of ICIs in clinical trials, anti-PD-L1 immunotherapy demonstrated an ORR of 28% compared to anti-PD-1 (16%) and anti-CTLA-4 (no significant response) [16]. Indeed, atezolizumab, an anti-PD-L1 antibody, and pembrolizumab, an anti-PD-1 antibody, are the only ICIs currently approved by the FDA for treatment of TNBC [31, 32]. Chimeric antigen receptor (CAR) T cell therapy involves adapting the CD8+ T cell receptor to target tumor antigens, expanding the cells ex vivo, and transferring the expanded CD8+ CAR T cells to the patient by infusion [33]. This strategy is successful in a variety of hematologic malignancies [33,34,35], but has recently shown potential in solid tumors as well. Particularly, CAR T cells directed towards the human MUC1 cleavage product, a protein expressed in 95% of breast cancers, have demonstrated success in vivo and are currently in a phase I clinical trial for the treatment of metastatic breast cancer (NCT04020575) [36, 37]. Another method of stimulating CD8+ T cells for breast cancer treatment is by the use of a cancer vaccine. For example, DC-based vaccines stimulate DCs in vitro with various molecules (IFN-γ, LPS, IL-4, GM-CSF, etc.) and human tumor-associated antigens like p53 and HER2 to improve antigen presentation to CD8+ T cells [38,39,40]. These DC-based vaccines directed towards p53 and HER2 lead to increases in tumor-specific CD8+ effector T cells, NK cells, and Th1 cytokine secretion, which resulted in an improvement of the 3-year progression-free survival (PFS) rate from 31.0 to 76.9% in a clinical trial with stage II/IIIA PR−/ER− breast cancer patients [39, 41, 42].
3 CD4+ regulatory T cells (Tregs)
CD4+CD25+FoxP3+ human regulatory T cells (Tregs) play an important role in regulating the immune system to prevent autoimmunity, allergy responses, and to induce tolerance to organ grafts [43,44,45,46]. FoxP3 (forkhead box P3) contributes in several ways to Treg differentiation: it upregulates CD25, differentiates α/β TCR-positive T cells to Tregs in the thymus, and induces suppressive activity even in non-Tregs when expressed at high levels [47, 48]. Intratumoral Tregs in breast cancer primarily develop from tumor-infiltrating naïve human CD4+ T cells in a CCL18-dependent manner [49]. CCL18 is a chemokine secreted by TAMs that recruits naïve CD4+ T cells to the tumor by binding to the PITPNM3 receptor on CD4+ cells. Accordingly, breast cancer patients exhibit upregulation of CCL18 in peripheral blood compared to healthy volunteers, and high CCL18 expression is associated with poor prognosis and cancer progression [50, 51]. Knockdown of the PITPNM3 receptor in vivo with CD4-aptamer-siRNA chimeras in a mouse model (humanized NSG mice injected with isolated human CD4+CD25+CD127− Tregs throughout the study) of human MDA-MB-231 breast cancer led to a reduction in intratumoral Tregs that was associated with inhibition of tumor progression [49].
Once activated, Tregs have the ability to induce an immunosuppressive TME in several ways. Antigen-specific Tregs can inhibit maturation of antigen-presenting cells (APCs) that are essential for the development of cytotoxic CD8+ T cells via binding of CTLA-4 expressed by Tregs to CD80/86 expressed by APCs (Fig. 1) [52, 53]. The TCR repertoire from tumor-infiltrating Tregs specifically react against autologous tumors and mutated neoantigens, suggesting that these Tregs are activated and undergo clonal expansion within the TME [54]. In addition to modulating the immune response in an antigen-specific manner, activated Tregs also function in a nonspecific manner. Tregs consume IL-2 through their high affinity IL-2 receptor, which would otherwise mediate and stimulate cytotoxic activity of CD8+ effector T cells [52, 55]. Additionally, Tregs secrete immunosuppressive cytokines such as IL-10, TGF-β, and IL-35 (Fig. 1), which inhibit CD8+ T cell-mediated immunity and promote tumor growth and metastasis [52, 56,57,58]. Lastly, degradation of ATP by human Tregs into adenosine by CD39 and CD73 leads to suppression of effector CD8+ T cells by engagement of the A2a receptor present on the surface of CD8+ T cells [59]. Although Tregs can act nonspecifically, Treg activation and expansion typically still requires TCR engagement [60, 61].
CD4+CD25+FoxP3+ Tregs, as well as the Treg subset T follicular regulatory (Tfr) cells, are present at increased levels in peripheral blood and breast tissue of breast cancer patients than in healthy volunteers for all breast cancer subtypes [62,63,64]. Increased levels of Tregs are strongly associated with increased risk of relapse, lower RFS, and overall survival, and can identify patients with higher risks of relapse after 5 years [64]. Additionally, Tfr cells in human breast tumors have increased FoxP3+ levels and IL-10 production, suggesting that they have an increased capacity for immunosuppression [62]. FoxP3+ lymphocytes combined with cytoplasmic FoxP3+ in tumor cells lead to worse overall survival in breast cancer patients than either phenotype alone [11]. Moreover, the proportion of FoxP3+ Tregs increases significantly with progression of normal breast tissue to ductal carcinoma in situ (DCIS), and DCIS to invasive ductal carcinoma (IDC) [65]. Interestingly, CD4+ Tregs are also able to inhibit pre-invasive breast cancer from developing into invasive breast cancer by suppressing pro-tumorigenic Th2 responses [66]. As Tregs can co-infiltrate tumors along with CD8+ T cells and CD20+ B cells, higher levels of Tregs within TNBC tumors have also been correlated with better prognosis [67], highlighting a need to normalize the data through representation of the Treg level as a ratio of CD8/Treg as performed in other studies [68, 69].
Tregs also possess the ability to suppress immunostimulation induced by immunotherapeutic approaches. For example, DC vaccines induce antitumor immunity, but this immunity does not always lead to tumor regression due to Treg expansion after DC infusion [70]. An in vivo study in mice resolved this phenomenon by combining a DC vaccine with a synthetic peptide known to inhibit Foxp3, resulting in improved therapeutic efficacy of the DC vaccine and reduced IL-10 secretion by Foxp3+ murine breast cancer cells in vitro [71]. IL-2, an NK-cell stimulatory cytokine, administered along with trastuzumab resulted in no objective responses in HER2+ metastatic breast cancer patients along with no NK cell expansion [72]. This outcome is possibly due to a concurrent activation and expansion of Tregs as previously demonstrated in melanoma and renal cancer upon IL-2 administration [72, 73]. Strategies that aim to improve IL-2 therapy focus on engineering the IL-2 cytokine to selectively stimulate CD8+ or NK cells rather than Tregs, such as the IL-2 “superkine” or PEGylated IL-2 [74, 75]. PEG blocks the IL-2Rα subunit binding region, which typically activates Tregs, whereas the IL-2Rβ subunit binding region, which activates CD8+ T cells, is not blocked [75]. Additionally, radiotherapy induces higher proportions of Tregs compared to effector T cells due to their higher radioresistance levels [76].
Although Treg levels can be associated with better prognosis [67], they are more often associated with a worse outcome and the ability to reduce therapeutic benefit of NK and CD8+ T cell-directed therapies; thus, strategies have developed to modulate Treg activity. CTLA-4, a molecule commonly upregulated on activated T cells and constitutively expressed on Tregs, acts to inhibit CD8+ effector T cell function through activation of Tregs and by blocking the B7-1 and B7-2 ligands on APCs that would otherwise bind and activate CD8+ naïve T cells [77, 78]. CTLA-4 blockade is known to suppress Treg activity and activate CD8+ effector T cells [77], and its use in breast cancer demonstrates clinical benefit [79, 80]. In a phase I clinical trial, stable disease was achieved in 42% of HR+ breast cancer patients receiving tremelimumab (anti-CTLA-4 mAb) and exemestane (aromatase inhibitor) therapy, and 36% of these responding patients had previously failed exemestane therapy [80]. In an ongoing phase II trial, anti-CTLA-4 therapy also has promising results when combined with anti-PD-1, achieving an ORR of 12% and a median overall survival of 12 months in patients with metaplastic breast cancer [79]. Another method involves using anti-CD25 mAb to deplete CD25+FoxP3+ Tregs, resulting in a long-lasting depletion of circulating Tregs and a priming and boosting of effector T cell response when given concurrently with HLA-A2-binding peptide vaccination in metastatic breast cancer patients [81]. Although more indirect, standard neoadjuvant chemotherapy treatment regimen of carboplatin, docetaxel, and trastuzumab also results in significantly decreased Treg levels in the peripheral blood, particularly in HER2+ breast cancer patients who also had better clinical outcomes [82]. Overall, the CD8/Treg ratio increased in HER2+ breast cancer patients, indicating that the chemotherapy did not negatively affect CD8+ T cells significantly. However, the method of reducing Treg proliferation is also critical because apoptotic human Tregs can induce oxidative stress in the TME by conversion of ATP to adenosine via CD39 and CD73, suppressing T cell activation at levels similar to or greater than live Tregs [83].
4 Tissue-resident memory T cells
Tissue-resident memory T cells (TRM) are memory T cells that permanently localize within peripheral tissues rather than recirculating throughout the body [84, 85]. TRM cells exhibit key differences from TEM cells, including chromatin landscape [86] and TCR repertoire [87]. Human TRM cells characteristically express the CD103 (αEβ7) integrin and the C-type lectin CD69 [88], while also displaying a downregulation of Kruppel-like factor 2 (KLF2) and sphingosine 1-phosphate type 1 (S1PR1) genes, resulting in TRM retention within tissues [89]. CD103 is a transmembrane receptor on the surface of TRM cells that binds to the E-cadherin ligand expressed on epithelial cells, favoring retention of these cells within epithelial tissues [90]. In fact, CD103 binding to E-cadherin facilitates antigen recognition on epithelial tumor cells [91]. CD69 plays a role in limiting TRM recirculation as well by downregulating S1PR1 [92], which promotes egress of naïve T cells from lymph nodes [93], and can be used as a phenotypic marker to distinguish TRM cells from TEM cells [94]. However, not all TRM cells express CD69 and CD103 [95], indicating that additional biomarkers need to be discovered to better characterize these cells.
To upregulate CD103, one of the specific markers of residency, CD8+ T cells typically require both antigen stimulation and TGF-β signaling [96, 97]; although some TRM cells can be induced through antigen-independent means [98]. In mice, TRM cells were observed to develop from memory precursor cells resulting from downregulated or absent KLRG1 expression in CD8+ effector cells [99]. Within epithelial tissue, CD8+ TRM cells bind to E-cadherin located on the epithelial tumor cells via the CD103 integrin [91]. This binding, along with TCR engagement, results in polarization of cytolytic granules at the immune synapse. Human TRM cells express high levels of mRNA encoding for cytotoxic molecules such as granzyme B, perforin, and IFN-γ [87], suggesting that TRM cells may have antitumor effects through direct cytotoxic ability and/or recruitment of cells via IFN-γ to the TME (Fig. 1) [99]. In vitro, human TRM cells (CD103+) display increased levels of apoptotic activity compared to CD8+ effector cells (CD103−) after incubation with autologous tumor cells [88].
Using gene expression data from the METABRIC consortium, Savas et al. found that a TRM gene signature is associated with an improved RFS and overall survival (OS) in TNBC patients after chemotherapy [87]. Patients with high CD8+ signature and a high TRM signature have better prognosis than patients with only a high CD8+ signature. A separate study found a similar correlation between a TRM gene signature and progression-free and overall survival in TNBC patients after treatment with anti-PD-1 antibody [100]. Intraepithelial CD8+CD103+ TIL in basal-like breast tumors also have a positive association with RFS and OS [101]. CD103+CD69+ TRM cells make up 40% of CD8+ TILs in human breast tumors on average [102]. Patients with a poor prognosis (defined as having a relapse less than 3 years after diagnosis) have TRM cells make up just 20% of CD8+ TILs. Alternatively, patients with a good prognosis (defined as having a relapse in more than 5 years after diagnosis) have TRM cells make up 60% of CD8+ TILs. Additionally, an in vivo study in mice that activates DCs with dectin-1 observed an induction of CD8+CD103+ T cells after treatment that resulted in an antitumor response to breast cancer [103]. In melanoma, adoptive cell transfer of glycoprotein B-expressing B16 variant cells into immunized mice elicits a TRM cell-dependent antitumor effect [104]. These recent studies suggest that the TRM signature may serve as a predictive biomarker and potential therapeutic target in the future with further elucidation of its antitumor effect.
5 Natural killer (NK) cells
Natural killer (NK) cells are innate lymphocytes that express a range of inhibitor receptors that are activated when NK cells encounter infected or transformed cells [105, 106]. Some of these inhibitory receptors respond to MHC class I molecules, but others can recognize non-MHC class I molecules [107]. Through these receptors, NK cells are able to differentiate between normal and altered self-cells and provide the first-line immune defense against foreign cells [108]. Human NK cells are further characterized by their level of CD56 expression: CD56dim cells are recognized as functionally mature cells and make up approximately 90% of NK cells, whereas CD56bright cells are considered to be functionally immature, directing their focus towards cytokine production rather than cytotoxicity [109,110,111].
NK cells exhibit antitumor potential in two primary ways: by secretion of key cytokines such as IFN-γ, TNF-α, and GM-CSF, and by directly binding to tumor cells via their activating receptors to induce apoptosis (Fig. 1) [112, 113]. These cytokines lead to tumor site recruitment and functioning of other hematopoietic cells and enhance antigen-specific T cell responses [114]. Tumor cells are first recognized by NK cells as “non-self” due to their reduced amount of surface MHC-I molecules, which are expressed on almost all healthy cells of the body [115]. Upon binding of the NK cell to the target cell, perforin inserts itself into the cell membrane and creates a pore, allowing for entry of granzymes into the target cell cytoplasm to trigger apoptosis of the cell [116, 117].
One strategy that human breast cancer cells use to neutralize the NK cell response is the accumulation of actin filaments within the cell upon binding of the NK cell, known as an “actin response” [118]. This buildup of actin filaments leads to a significant decrease in granzyme B levels and is also associated with modifications of the NK cell receptor ligands at the synapse between the cells. Thus, breast cancer cells that undergo the actin response are also significantly more likely to evade apoptosis induced by NK cells. Additionally, tumor secretion of IL-18 was also demonstrated to contribute to NK cell immunosuppression by regulation of NK cell differentiation [119]. Exposure of human NK cells to IL-18 leads to a higher proportion of CD56dimCD16−/dim NK cells, which display lower levels of activating receptors and cytolytic molecules [120], compared to CD56dimCD16+ NK cells [119], which have strong antitumor activity [121]. Additionally, chronic exposure (i.e., with a persistent tumor) of the target cells to NK cell receptors may cause hyporesponsiveness, reducing the cytotoxic ability of NK cells [122].
In patients with primary HER2+ breast cancer, baseline levels of tumor-infiltrating NK cells are prognostic for achieving pathological complete response (pCR) with anti-HER2 mAb and standard chemotherapy treatment [22]. Decreased HLA-I expression is associated with increased pCR rates, likely due to higher rates of recognition of low HLA-I expressing tumor cells by NK cells. However, HLA-Ihigh tumors have the highest rates of pCR, likely because they are targeted by cytotoxic CD8+ T cells rather than NK cells. Further, tumor-infiltrating NK cells combined with high m-CD155 expression are able to predict improved patient survival across all breast cancer subtypes, likely due to the recruitment of NK cells by m-CD155 present on the surface of breast cancer cells [123, 124]. Higher levels of circulating CD57+ NK-cell numbers in HER2+ breast cancer patients are inversely correlated with achieving pCR with early treatment of anti-HER2 mAbs, indicating that CD57+ NK cell levels could be used as a biomarker for primary resistance to anti-HER2 mAb treatment [125]. Characteristics of CD57+ NK cells that could be leading to this observation include lower expression levels of surface CXCR3 (which is involved in NK-cell tumor homing), lower expression of activating receptors, and a lower proliferative capacity compared to CD57− NK cells [126].
Methods of utilizing NK cells during treatment of breast cancer include treating patients with therapeutic drugs/cytokines that stimulate patients’ NK cells and administering ex vivo-stimulated NK cells into the patient. Several cytokines have been used to stimulate NK cell proliferation and activity in patients, including IL-2 [127, 128], IL-15 [129, 130], and IL-12 [131, 132]. Trastuzumab, a humanized mAb targeting HER2, utilizes antibody-dependent cellular cytotoxicity (ADCC) by recruiting NK cells to trastuzumab-bound HER2+ tumor cells via the FcγIII receptor on NK cells [133, 134]. Indeed, NK activity was correlated with both early (6 months post-treatment) and long-term response (12 months post-treatment) of HER2+ breast cancer patients receiving trastuzumab therapy [133]. On the other hand, ADCC function was only associated with early response (6 months post-treatment). Apart from stimulating NK cells within the patient, different therapeutic strategies are employed that utilize adoptively transferred NK cells to induce antitumor activity. In phase I clinical trial, NK cells stimulated ex vivo with IL-2 were given to ovarian and breast cancer patients that had undergone a lymphodepleting regimen of cyclophosphamide and fludarabine, along with 200 cGy in some patients [135]. Of the 20 patients, 20% achieved a partial response and 60% achieved stable disease with treatment. However, success of treatment was limited by poor NK cell expansion in the patient after NK cell infusion, possibly due to a highly immunosuppressed environment and rejection of the infused NK cells by effector T cells. Recent studies have shown success in vitro and in vivo by taking a similar approach to CAR-T therapy and directing NK cells towards a tumor-associated antigen through the addition of a chimeric antigen receptor (CAR) [136, 137]. Potential advantages of CAR-NK therapy over CAR-T therapy include lower negative side effects by reducing the risk of inducing graft-versus-host disease [138, 139], promotion of DC migration into the tumor [140], and broader clinical applications from a single construct [141]. A similar approach that targets HER2+ cells by conjugating trastuzumab onto NK cells, rather than adapting its surface receptor to redirect it as CAR-NK cells do, is currently in clinical trials (NCT04319757) [142].
6 B cells
As opposed to the cell-mediated immunity induced by T cells, B cells play a key role in the development of humoral immunity through the germinal center (GC) reaction [143]. CCR7-stimulated DCs migrate to secondary lymphoid organs and present antigens via MHCII to naïve T cells, promoting their differentiation into T-follicular helper (Tfh) cells [143, 144]. The interaction between Tfh cells, follicular DCs, and B cells leads to the activation and maturation of B cells into memory B cells and long-living plasma cells [145], as indicated by the induction of Ig antibody production after Tfh and B cell interaction [62]. Specifically, during maturation, B cells can undergo B cell receptor (BCR) selection, class switch recombination, and clonal expansion in the GC reaction [146]. Plasma cells, which are responsible for secreting the antibodies that induce adaptive humoral immunity, develop from the pool of memory B cells formed from the GC reaction [147,148,149]. Affinity maturation of B cells can occur intratumorally in breast tumors, leading to production of high-affinity antibodies against tumor antigens [150]. In breast cancer, human B cells and plasma cells tend to aggregate around the neoplasia and fibrotic areas that result from CD8+ effector T cell function [151]. This suggests that the T cell immune response acts first, followed by the infiltration of B lymphocytes.
B cells impact breast cancer growth and metastasis in a variety of opposing ways. Tumor antigen-specific immunoglobulin G antibodies (IgG) secreted by activated B cells can induce lysis of tumors cells by apoptosis (Fig. 1) [152]. Additionally, adoptively transferred activated B cells can stimulate antitumor T cell immunity by the host. Levels of MUC1-specific IgG antibodies are significantly associated with better overall survival in breast cancer patients after endocrine therapy or chemotherapy with or without trastuzumab [153]. IgG antibodies can also promote proliferation of CD4+ and CD8+ T cells by facilitating the internalization of tumor antigens by DCs that are then presented to activate T cells, demonstrated in mice [154]. However, the antigen that the antibody is directed towards is important for prognosis. For example, HSPA4 membrane protein-specific IgG antibodies promote tumor metastasis upon binding to the HSPA4 antigen via the NF-κB pathway, and consequently are associated with poor prognosis of breast cancer patients [155]. B cells can also act as APCs by presenting antigens to CD4+ and CD8+ T cells [156]. In vitro, murine antigen-presenting B cells activate either effector T cells or Tregs depending on if they are in an activated or exhausted state. B cells can also differentiate to regulatory B cells (Bregs) by CD40 or Toll-like receptor (TLR) engagement, or by other pro-inflammatory cytokines [157, 158]. An in vivo study in mice showed that tumor-evoked Bregs reserve the ability to induce TGF-β-dependent conversion of naïve CD4+ T cells to immunosuppressive Tregs, which plays a role in the metastasis of breast cancer to the lungs [159]. Similar to Tregs, Bregs secrete IL-10, TGF-β, and IL-35 immunomodulating cytokines that restrict CD8+ effector T cell activation and proliferation (Fig. 1) [160,161,162]. Thus, it is essential to consider the impact of treatment on separate B cell subsets when designing therapies that target B cells directly.
B cells are found to infiltrate tumors at high levels in about 20% of breast cancer patients and can represent up to 40% of all TILs [9, 163, 164]. The prognostic value of B cells has not always been clear; several earlier studies were contradictory in defining the relationship between tumor-infiltrating B cells and prognosis [165,166,167,168]. In TNBC patients, plasma cell infiltration, along with Ig gene expression, is positively associated with disease-free survival (DFS), demonstrating the importance of humoral immunity on treatment response [169]. Infiltrating CD20+ (B cells) and PD-L1+ TILs are significantly associated with improved survival and pCR after neoadjuvant chemotherapy in inflammatory breast cancer patients, suggesting that a combination of PD-1/PD-L1 immunotherapy and an immunomodulatory therapy that stimulates B cell responses may improve prognosis for these patients [170]. Recent clinical studies that demonstrate a correlation between B cell infiltration and poor prognosis focus on the Breg subset [10, 171]. Bregs with a CD19+CD24hiCD38hi phenotype are increased in the peripheral blood of IDC breast cancer patients compared to healthy controls and correlate with levels of circulating Tregs [171]. A separate study of breast cancer patients also showed this correlation between Bregs and Tregs, and also found that the coexistence of these cells within TIL aggregates was associated with a shorter metastasis-free survival (MFS) [10]. Similar to the relationship between regulatory T cells and effector T cells, B cells can produce antitumor or pro-tumor effects depending on their subtype and IgG specificity. However, more research needs to be done to better characterize B cell subsets both functionally and phenotypically.
Immunotherapies that target B cells directly are not nearly as established as those that target T cells, possibly because of their less understood phenotypic characteristics and mechanisms of action [172]. In an in vivo mouse model of breast cancer, ex vivo LPS and CD40-stimulated B cells were able to restrict lung metastasis upon adoptive transfer, particularly when combined with adoptively transferred T cells [152]. Additionally, treatment of mice with anti-CD20 antibody results in increased metastasis if treatment started after tumor establishment, whereas mice treated with anti-CD20 before tumor challenge have reduced metastasis, indicating that the timing of B cell-targeted therapy plays a key role in treatment efficacy [173]. This phenomenon is likely to due to anti-CD20-mediated enrichment of CD20lo Bregs after depletion of the CD20hi B cells that have immunostimulatory properties. Some therapies are designed to specifically inhibit Breg cell activity, such as the use of CXCR5-targeted CpG-ODN [173], Stat3-inactivating resveratrol [174], or IL-10 depletion [175]. This strategy leads to the inhibition of Breg-dependent Treg conversion and improves the efficacy of adoptively transferred effector B cells. While these strategies show in vivo efficacy in mice, the lack of clinical trials using B cell-targeted therapy in breast cancer indicates that more work needs to be done in determining the role that B cells play in breast cancer and methods of stimulating B effector cells while inhibiting Breg activity.
7 TIL composition across breast cancer subtypes
The TNBC subtype, followed by HER2+, displays the highest levels of total tumor-infiltrating lymphocytes, whereas hormone-receptor (HR) positive breast cancers have much lower infiltration levels [176]. In fact, hormone receptor expression is negatively associated with TIL level, Treg/Th2 ratio, and CD8+ effector T cells and Tregs present at the tumor edge [177]. Although higher levels of total TILs are generally associated with better clinical outcomes, TNBC and HER2+ breast cancer patients exhibit lower survival rates compared to HR+ patients [178], indicating other factors (e.g., TIL subtypes) are also involved. For example, both TNBC and HER2+ tumors have a lower intratumoral CD8/Treg ratio compared to HR+/HER2− tumors, indicating higher levels of immunosuppression in the TME [176, 177]. Additionally, the high genomic instability of TNBC and HER2+ tumor cells that allow for better recognition of foreign antigens by the immune system simultaneously increases the chances of developing abnormal signaling pathways, like EGFR, MET, and PI3K, that would otherwise control their proliferation and survival [176]. Although breast cancer subtypes can trend toward higher or lower TIL levels, TIL levels can vary greatly within each subtype [179, 180]. Lymphocyte infiltration levels are associated with better clinical outcome in several types of breast cancer, including TNBC [19, 179], HER2+ [19], and ER− breast cancer [180]. Additionally, incremental increases in TIL levels in both the tumor and the surrounding stroma area in TNBC patients lead to corresponding increases in chemotherapy response and overall survival [19, 181]. In luminal breast cancer, a greater tumor burden is associated with increased intratumoral CD8+ effector cells, Tregs, and TIL level [177]. Similarly, stage I breast cancer patients tend to have lower levels of these parameters compared to stage II+ patients.
Classifying cancer into six distinct subtypes (wound healing, IFN-γ dominant, inflammatory, lymphocyte depleted, immunologically quiet, and TGF-β dominant) can help provide a clearer picture of patients’ TIL level and composition [182]. These immune subtypes are based on cluster analysis of immune gene expression signatures across 33 types of cancer using data from The Cancer Genome Atlas (TCGA). As reviewed by Gatti-Mays et al., the IFN-γ dominant subtype, followed by wound healing and inflammatory, represents the most common subtype in breast cancer, comprising 60% of basal-like breast cancers and just under half of HER2+ and luminal B breast cancers [23]. A higher lymphocyte signature leads to a better prognosis in IFN-γ dominant and wound healing subtypes, whereas it leads to a worse outcome in the inflammatory subtype, possibly indicative of an already balanced immune response [182]. The IFN-γ dominant subtype is characterized by strong CD8+ levels, TCR diversity, and a high M1/M2 macrophage ratio, which is associated with improved OS. However, the IFN-γ dominant subtype also has the least favorable prognosis, indicating either that the immune response could not keep up with tumor growth or that tumor cells were able to escape immune recognition. Although breast cancer was traditionally considered immunologically quiescent, no breast cancers were identified as immunologically quiet in this analysis [23, 182]. With further research of these immune subtypes in breast cancer, characterizing patients by immune subtype may serve as an important tool for better predicting patients’ outcome and identifying ideal treatment regimens.
8 Impact of therapeutics on TIL levels and composition
Many breast cancer treatments achieve clinical benefit in patients through reversal of the immunosuppressive TME to an immune-permissive environment, either by directly targeting immune cells or by indirectly affecting these cells (Table 1). With HER2-targeted therapies, such as trastuzumab and T-DM1 (trastuzumab conjugated to the chemotherapeutic agent emtansine), an increase in TILs is observed upon treatment, possibly reflecting some HER2-dependent immunosuppression prior to therapy [190, 191]. Additionally, in HER2+ patients treated with docetaxel, trastuzumab, and pertuzumab, another HER2-targeting antibody, every 10% increase in stromal TILs is associated with a longer overall survival [6]. HER2+ breast cancer patients that respond to trastuzumab therapy are also more likely to have higher levels of NK cells and ADCC activity [133]. Radiotherapy can have both immune-suppressing and immune-stimulating effects via a higher proportion of radioresistant immunomodulatory cells like Tregs after therapy and induction of immunogenic cell death that leads to maturation of APCs and activation of antitumor CD8+ effector T cells [192, 193]. Additionally, a HER2-derived peptide administered along with GM-CSF designed to enhance CD8+ T cell response demonstrates an increased ability to elicit cytotoxic T lymphocytes in vaccinated patients [189].
PD-L1 is expressed in up to 60% of breast cancer patients and its expression is positively correlated with a high level of TILs [194, 195]. PD-L1 is also expressed in HR− and TNBC patients at greater levels, indicating a large subset of patients that may benefit more from PD-1/PD-L1 blockade therapy [194]. Indeed, both anti-PD-1 and anti-PD-L1 antibodies have demonstrated success when used as an immunotherapy in TNBC patients [183, 185, 187]. Anti-PD-L1 therapy can also induce greater levels of plasma cytokines and CD8+ T cell proliferation compared to baseline levels [196]. Further, clinical benefit is augmented in patients with PD-L1+ tumors when compared to patients with PD-L1− tumors, reflecting a biomarker that may be useful in determining potential therapeutic benefit [183, 185]. PD-1/PD-L1 blockade is also effective in other breast cancer subsets as well, such as one study that observed a 4% overall response rate and 19% clinical benefit (ORR + stable disease > 24 weeks) rate in ER+ patients after treatment with vorinostat (HDAC inhibitor), tamoxifen (ER modulator), and pembrolizumab (anti-PD-1 mAb) [18]. Currently, atezolizumab (anti-PD-L1 mAb) plus nab-paclitaxel and pembrolizumab (anti-PD-1 mAb) plus nab-paclitaxel are the only approved regimens with immunotherapy for breast cancer, and are currently only approved for TNBC patients.
CTLA-4 is expressed on activated T cells and its inhibition is hypothesized to increase counts of CD8+ effector T cells through the inactivation of FoxP3+CD4+ Tregs [77, 197]. Breast cancer patients typically have higher levels of CTLA-4 expression compared to healthy volunteers, indicating enhanced immunosuppressive function in these patients [198]. Early clinical trials testing anti-CTLA-4 mAb in breast cancer patients observed an increase in overall counts and percentages of ICOS+ CD4+ and CD8+ T cells along with a decreased level of FoxP3+ CD4+ Tregs in some patients after treatment [80, 199]. Although none of the breast cancer patients achieved an objective response in these trials, some patients did exhibit stable disease and the toxicity profile was shown to be tolerable and consistent with previous studies using anti-CTLA-4 mAb in other indications. Based on the success of combined nivolumab (anti-PD-1 mAb) and ipilimumab (anti-CTLA-4 mAb) treatment compared to single-agent therapy in melanoma [200], recent studies are also testing the safety and efficacy of the combination of anti-PD-1 and anti-CTLA-4 mAbs in breast cancer patients [79, 201].
9 Conclusions
The presence of TILs within breast tumors can play a key role in determining clinical outcome. Here, we have discussed different subsets of TILs (T cells, NK cells, B cells) and how they interact with breast cancer and impact prognosis. Lymphocytes can have antitumor or pro-tumor effects, depending on their differentiation and activation status, and can significantly impact a patient’s outcome. Current immunotherapeutic approaches seek to stimulate or inhibit lymphocyte activity based on their observed interaction with breast cancer. Several factors contribute to a patient’s response to immunotherapy, including TIL level and composition, PD-L1 expression, and receptor expression (HER2, ER, PR), but there remains a need to identify additional predictive biomarkers aside from PD-L1 [202]. It remains essential to continue developing our knowledge in how infiltrating lymphocytes interact with breast cancer to be able to discover new therapeutic targets and strategies.
References
The, L. (2018). GLOBOCAN 2018: Counting the toll of cancer. Lancet (London, England), 392(10152), 985.
Howlader, N., Noone, A., Krapcho, M. e., Miller, D., Brest, A., Yu, M., et al. (2019). SEER cancer statistics review, 1975–2016 (pp. 1423–1437). National Cancer Institute.
Society, A. C. (2020). Cancer facts and figures, 2020.
Asano, Y., Kashiwagi, S., Goto, W., Takada, K., Takahashi, K., Shibutani, M., et al. (2020). Predicting therapeutic efficacy of endocrine therapy for stage IV breast cancer by tumor-infiltrating lymphocytes. Molecular and clinical oncology, 13(2), 195–202. https://doi.org/10.3892/mco.2020.2063.
Denkert, C., Loibl, S., Noske, A., Roller, M., Muller, B., Komor, M., et al. (2010). Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol, 28(1), 105–113.
Luen, S. J., Salgado, R., Fox, S., Savas, P., Eng-Wong, J., Clark, E., et al. (2017). Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: A retrospective analysis of the CLEOPATRA study. The Lancet. Oncology, 18(1), 52–62. https://doi.org/10.1016/S1470-2045(16)30631-3.
Emens, L. A., Cruz, C., Eder, J. P., Braiteh, F., Chung, C., Tolaney, S. M., et al. (2019). Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: A phase 1 study. JAMA Oncology, 5(1), 74–82. https://doi.org/10.1001/jamaoncol.2018.4224.
Zgura, A., Galesa, L., Bratila, E., & Anghel, R. (2018). Relationship between tumor infiltrating lymphocytes and progression in breast cancer. Maedica, 13(4), 317–320. https://doi.org/10.26574/maedica.2018.13.4.317.
Chin, Y., Janseens, J., Vandepitte, J., Vandenbrande, J., Opdebeek, L., & Raus, J. (1992). Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res, 12(5), 1463–1466.
Ishigami, E., Sakakibara, M., Sakakibara, J., Masuda, T., Fujimoto, H., Hayama, S., et al. (2019). Coexistence of regulatory B cells and regulatory T cells in tumor-infiltrating lymphocyte aggregates is a prognostic factor in patients with breast cancer. Breast Cancer, 26(2), 180–189. https://doi.org/10.1007/s12282-018-0910-4.
Takenaka, M., Seki, N., Toh, U., Hattori, S., Kawahara, A., Yamaguchi, T., et al. (2013). FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Molecular and clinical oncology, 1(4), 625–632. https://doi.org/10.3892/mco.2013.107.
Banchereau, J., & Steinman, R. M. (1998). Dendritic cells and the control of immunity. Nature, 392(6673), 245–252. https://doi.org/10.1038/32588.
Obar, J. J., & Lefrançois, L. (2010). Memory CD8+ T cell differentiation. Annals of the New York Academy of Sciences, 1183, 251–266. https://doi.org/10.1111/j.1749-6632.2009.05126.x.
Kumar, B. V., Connors, T. J., & Farber, D. L. (2018). Human T cell development, localization, and function throughout life. Immunity, 48(2), 202–213. https://doi.org/10.1016/j.immuni.2018.01.007.
Charles, A., Janeway, J., Travers, P., Walport, M., & Shlomchik, M. J. (2001). Immunobiology: The immune system in health and disease (5ed.). New York: Garland Science.
Zou, Y., Zou, X., Zheng, S., Tang, H., Zhang, L., Liu, P., et al. (2020). Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: A systematic review and meta-analysis. Ther Adv Med Oncol, 12, 1758835920940928. https://doi.org/10.1177/1758835920940928.
Vahidi, Y., Bagheri, M., Ghaderi, A., & Faghih, Z. (2020). CD8-positive memory T cells in tumor-draining lymph nodes of patients with breast cancer. BMC Cancer, 20(1), 257. https://doi.org/10.1186/s12885-020-6714-x.
Terranova-Barberio, M., Pawlowska, N., Dhawan, M., Moasser, M., Chien, A. J., Melisko, M. E., et al. (2020). Exhausted T cell signature predicts immunotherapy response in ER-positive breast cancer. Nat Commun, 11(1), 3584. https://doi.org/10.1038/s41467-020-17414-y.
Loi, S., Sirtaine, N., Piette, F., Salgado, R., Viale, G., Van Eenoo, F., et al. (2013). Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol, 31(7), 860–867. https://doi.org/10.1200/JCO.2011.41.0902.
Egelston, C. A., Avalos, C., Tu, T. Y., Rosario, A., Wang, R., Solomon, S., et al. (2019). Resident memory CD8+ T cells within cancer islands mediate survival in breast cancer patients. JCI Insight, 4(19). https://doi.org/10.1172/jci.insight.130000.
Ali, H. R., Provenzano, E., Dawson, S. J., Blows, F. M., Liu, B., Shah, M., et al. (2014). Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol, 25(8), 1536–1543. https://doi.org/10.1093/annonc/mdu191.
Muntasell, A., Rojo, F., Servitja, S., Rubio-Perez, C., Cabo, M., Tamborero, D., et al. (2019). NK cell infiltrates and HLA class I expression in primary HER2(+) breast cancer predict and uncouple pathological response and disease-free survival. Clin Cancer Res, 25(5), 1535–1545. https://doi.org/10.1158/1078-0432.CCR-18-2365.
Gatti-Mays, M. E., Balko, J. M., Gameiro, S. R., Bear, H. D., Prabhakaran, S., Fukui, J., et al. (2019). If we build it they will come: Targeting the immune response to breast cancer. NPJ Breast Cancer, 5, 37. https://doi.org/10.1038/s41523-019-0133-7.
Kawaguchi, K., Sakurai, M., Yamamoto, Y., Suzuki, E., Tsuda, M., Kataoka, T. R., et al. (2019). Alteration of specific cytokine expression patterns in patients with breast cancer. Sci Rep, 9(1), 2924. https://doi.org/10.1038/s41598-019-39476-9.
Doedens, A. L., Stockmann, C., Rubinstein, M. P., Liao, D., Zhang, N., DeNardo, D. G., et al. (2010). Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res, 70(19), 7465–7475. https://doi.org/10.1158/0008-5472.CAN-10-1439.
Ruffell, B., Chang-Strachan, D., Chan, V., Rosenbusch, A., Ho, C. M., Pryer, N., et al. (2014). Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell, 26(5), 623–637. https://doi.org/10.1016/j.ccell.2014.09.006.
Almand, B., Clark, J. I., Nikitina, E., van Beynen, J., English, N. R., Knight, S. C., et al. (2001). Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol, 166(1), 678–689. https://doi.org/10.4049/jimmunol.166.1.678.
Ngamcherdtrakul, W., & Yantasee, W. (2019). siRNA therapeutics for breast cancer: recent efforts in targeting metastasis, drug resistance, and immune evasion. Translational Research, 214, 105–120. https://doi.org/10.1016/j.trsl.2019.08.005.
Chen, L., & Han, X. (2015). Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future. The Journal of clinical investigation, 125(9), 3384–3391. https://doi.org/10.1172/JCI80011.
Formenti, S. C., Hawtin, R. E., Dixit, N., Evensen, E., Lee, P., Goldberg, J. D., et al. (2019). Baseline T cell dysfunction by single cell network profiling in metastatic breast cancer patients. J Immunother Cancer, 7(1), 177. https://doi.org/10.1186/s40425-019-0633-x.
Narayan, P., Wahby, S., Gao, J. J., Amiri-Kordestani, L., Ibrahim, A., Bloomquist, E., et al. (2020). FDA approval summary: Atezolizumab plus paclitaxel protein-bound for the treatment of patients with advanced or metastatic TNBC whose tumors express PD-L1. Clinical cancer research, 26(10), 2284–2289.
FDA (2020). FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple#:~:text=Approvals%20and%20Databases-,FDA%20grants%20accelerated%20approval%20to%20pembrolizumab%20for%20locally%20recurrent,metastatic%20triple%20negative%20breast%20cancer&text=On%20November%2013%2C%202020%2C%20the,KEYTRUDA%2C%20Merck%20%26%20Co.). Accessed 11/29/2020.
Brentjens, R. J., Davila, M. L., Riviere, I., Park, J., Wang, X., Cowell, L. G., et al. (2013). CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med, 5(177), 177ra138. https://doi.org/10.1126/scitranslmed.3005930.
Kalos, M., Levine, B. L., Porter, D. L., Katz, S., Grupp, S. A., Bagg, A., et al. (2011). T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med, 3(95), 95ra73. https://doi.org/10.1126/scitranslmed.3002842.
Grupp, S. A., Kalos, M., Barrett, D., Aplenc, R., Porter, D. L., Rheingold, S. R., et al. (2013). Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med, 368(16), 1509–1518. https://doi.org/10.1056/NEJMoa1215134.
Bamdad, C. C., Stewart, A. K., Huang, P., Smagghe, B. J., Moe, S. T., Swanson, T. E., et al. (2020). Abstract P3-11-11: First-in-human CAR T for solid tumors targets the MUC1 transmembrane cleavage product. Cancer Research, 80(4 Supplement), P3-11-11-P13-11-11. https://doi.org/10.1158/1538-7445.Sabcs19-p3-11-11.
Bajgain, P., Tawinwung, S., D'Elia, L., Sukumaran, S., Watanabe, N., Hoyos, V., et al. (2018). CAR T cell therapy for breast cancer: harnessing the tumor milieu to drive T cell activation. J Immunother Cancer, 6(1), 34. https://doi.org/10.1186/s40425-018-0347-5.
Svane, I. M., Pedersen, A. E., Johansen, J. S., Johnsen, H. E., Nielsen, D., Kamby, C., et al. (2007). Vaccination with p53 peptide-pulsed dendritic cells is associated with disease stabilization in patients with p53 expressing advanced breast cancer; monitoring of serum YKL-40 and IL-6 as response biomarkers. Cancer Immunology, Immunotherapy, 56(9), 1485–1499. https://doi.org/10.1007/s00262-007-0293-4.
Czerniecki, B. J., Koski, G. K., Koldovsky, U., Xu, S., Cohen, P. A., Mick, R., et al. (2007). Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res, 67(4), 1842–1852. https://doi.org/10.1158/0008-5472.CAN-06-4038.
Lowenfeld, L., Zaheer, S., Oechsle, C., Fracol, M., Datta, J., Xu, S., et al. (2016). Addition of anti-estrogen therapy to anti-HER2 dendritic cell vaccination improves regional nodal immune response and pathologic complete response rate in patients with ER(pos)/HER2(pos) early breast cancer. Oncoimmunology, 6(9), e1207032–e1207032. https://doi.org/10.1080/2162402X.2016.1207032.
Svane, I. M., Pedersen, A. E., Johnsen, H. E., Nielsen, D., Kamby, C., Gaarsdal, E., et al. (2004). Vaccination with p53-peptide-pulsed dendritic cells, of patients with advanced breast cancer: Report from a phase I study. Cancer Immunol Immunother, 53(7), 633–641. https://doi.org/10.1007/s00262-003-0493-5.
Qi, C.-J., Ning, Y.-L., Han, Y.-S., Min, H.-Y., Ye, H., Zhu, Y.-L., et al. (2012). Autologous dendritic cell vaccine for estrogen receptor (ER)/progestin receptor (PR) double-negative breast cancer. Cancer Immunology, Immunotherapy, 61(9), 1415–1424. https://doi.org/10.1007/s00262-011-1192-2.
Brunkow, M. E., Jeffery, E. W., Hjerrild, K. A., Paeper, B., Clark, L. B., Yasayko, S.-A., et al. (2001). Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nature Genetics, 27(1), 68–73. https://doi.org/10.1038/83784.
Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M., & Toda, M. (1995). Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. The Journal of Immunology, 155(3), 1151–1164.
Hall, B. M., Pearce, N. W., Gurley, K. E., & Dorsch, S. E. (1990). Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporinte. III. Further characterization of the CD4+ suppressor cell and its mechanisms of action. [Article]. Journal of Experimental Medicine, 171(1), 141–157. https://doi.org/10.1084/jem.171.1.141.
Akdis, M., Verhagen, J., Taylor, A., Karamloo, F., Karagiannidis, C., Crameri, R., et al. (2004). Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. The Journal of experimental medicine, 199(11), 1567–1575. https://doi.org/10.1084/jem.20032058.
Fontenot, J. D., Gavin, M. A., & Rudensky, A. Y. (2003). Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunology, 4(4), 330–336. https://doi.org/10.1038/ni904.
Sakaguchi, S., Yamaguchi, T., Nomura, T., & Ono, M. (2008). Regulatory T cells and immune tolerance. Cell, 133(5), 775–787. https://doi.org/10.1016/j.cell.2008.05.009.
Su, S., Liao, J., Liu, J., Huang, D., He, C., Chen, F., et al. (2017). Blocking the recruitment of naive CD4+ T cells reverses immunosuppression in breast cancer. Cell Research, 27(4), 461–482. https://doi.org/10.1038/cr.2017.34.
Nariţa, D., Seclaman, E., Ursoniu, S., Ilina, R., Cireap, N., & Anghel, A. (2011). Expression of CCL18 and interleukin-6 in the plasma of breast cancer patients as compared with benign tumor patients and healthy controls. Rom J Morphol Embryol, 52(4), 1261–1267.
Sun, J. H., Fan, N., & Zhang, Y. (2016). Correlation between serum level of chemokine (C-C motif) ligand 18 and poor prognosis in breast cancer. Genet Mol Res, 15(3). https://doi.org/10.4238/gmr.15038632.
Ohue, Y., & Nishikawa, H. (2019). Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer science, 110(7), 2080–2089. https://doi.org/10.1111/cas.14069.
Collins, A. V., Brodie, D. W., Gilbert, R. J. C., Iaboni, A., Manso-Sancho, R., Walse, B., et al. (2002). The interaction properties of costimulatory molecules revisited. Immunity, 17(2), 201–210. https://doi.org/10.1016/S1074-7613(02)00362-X.
Ahmadzadeh, M., Pasetto, A., Jia, L., Deniger, D. C., Stevanović, S., Robbins, P. F., et al. (2019). Tumor-infiltrating human CD4(+) regulatory T cells display a distinct TCR repertoire and exhibit tumor and neoantigen reactivity. Science immunology, 4(31), eaao4310. https://doi.org/10.1126/sciimmunol.aao4310.
Pipkin, M. E., Sacks, J. A., Cruz-Guilloty, F., Lichtenheld, M. G., Bevan, M. J., & Rao, A. (2010). Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity, 32(1), 79–90. https://doi.org/10.1016/j.immuni.2009.11.012.
Halak, B. K., Maguire, H. C., & Lattime, E. C. (1999). Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor site. Cancer Research, 59(4), 911–917.
Xu, C., Wang, Z., Cui, R., He, H., Lin, X., Sheng, Y., et al. (2015). Co-expression of parathyroid hormone related protein and TGF-beta in breast cancer predicts poor survival outcome. BMC Cancer, 15, 925. https://doi.org/10.1186/s12885-015-1873-x.
Collison, L. W., Workman, C. J., Kuo, T. T., Boyd, K., Wang, Y., Vignali, K. M., et al. (2007). The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature, 450(7169), 566–569. https://doi.org/10.1038/nature06306.
Mandapathil, M., Hilldorfer, B., Szczepanski, M. J., Czystowska, M., Szajnik, M., Ren, J., et al. (2010). Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. The Journal of biological chemistry, 285(10), 7176–7186. https://doi.org/10.1074/jbc.M109.047423.
Takahashi, T., Kuniyasu, Y., Toda, M., Sakaguchi, N., Itoh, M., Iwata, M., et al. (1998). Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: Induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol, 10(12), 1969–1980. https://doi.org/10.1093/intimm/10.12.1969.
Thornton, A. M., & Shevach, E. M. (1998). CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med, 188(2), 287–296. https://doi.org/10.1084/jem.188.2.287.
Song, H., Liu, A., Liu, G., Wu, F., & Li, Z. (2019). T follicular regulatory cells suppress Tfh-mediated B cell help and synergistically increase IL-10-producing B cells in breast carcinoma. Immunologic Research, 67(4), 416–423. https://doi.org/10.1007/s12026-019-09090-y.
Sarvenaz, K., Hamid, A., Ramesh, O., Habibollah, M., Fahimeh, J.-A., Farzaneh Tofighi, Z., et al. (2019). The anti-tumoral effect of β-D-mannuronic acid (M2000) as a novel NSAID on Treg cells frequency and MMP-2, MMP-9, CCL22 and TGFβ1 gene expression in pre-surgical breast cancer patients. Iranian Journal of Allergy, Asthma and Immunology, 18(1). https://doi.org/10.18502/ijaai.v18i1.633.
Bates, G. J., Fox, S. B., Han, C., Leek, R. D., Garcia, J. F., Harris, A. L., et al. (2006). Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. Journal of Clinical Oncology, 24(34), 5373–5380.
Lal, A., Chan, L., DeVries, S., Chin, K., Scott, G. K., Benz, C. C., et al. (2013). FOXP3-positive regulatory T lymphocytes and epithelial FOXP3 expression in synchronous normal, ductal carcinoma in situ, and invasive cancer of the breast. Breast Cancer Research and Treatment, 139(2), 381–390. https://doi.org/10.1007/s10549-013-2556-4.
Martinez, L. M., Robila, V., Clark, N. M., Du, W., Idowu, M. O., Rutkowski, M. R., et al. (2019). Regulatory T cells control the switch from in situ to invasive breast cancer. Front Immunol, 10, 1942. https://doi.org/10.3389/fimmu.2019.01942.
Yeong, J., Thike, A. A., Lim, J. C. T., Lee, B., Li, H., Wong, S.-C., et al. (2017). Higher densities of Foxp3+ regulatory T cells are associated with better prognosis in triple-negative breast cancer. Breast Cancer Research and Treatment, 163(1), 21–35. https://doi.org/10.1007/s10549-017-4161-4.
Peng, G.-L., Li, L., Guo, Y.-W., Yu, P., Yin, X.-J., Wang, S., et al. (2019). CD8(+) cytotoxic and FoxP3(+) regulatory T lymphocytes serve as prognostic factors in breast cancer. American journal of translational research, 11(8), 5039–5053.
Solis-Castillo, L. A., Garcia-Romo, G. S., Diaz-Rodriguez, A., Reyes-Hernandez, D., Tellez-Rivera, E., Rosales-Garcia, V. H., et al. (2020). Tumor-infiltrating regulatory T cells, CD8/Treg ratio, and cancer stem cells are correlated with lymph node metastasis in patients with early breast cancer. Breast Cancer, 27(5), 837–849. https://doi.org/10.1007/s12282-020-01079-y.
Vasir, B., Wu, Z., Crawford, K., Rosenblatt, J., Zarwan, C., Bissonnette, A., et al. (2008). Fusions of dendritic cells with breast carcinoma stimulate the expansion of regulatory T cells while concomitant exposure to IL-12, CpG oligodeoxynucleotides, and anti-CD3/CD28 promotes the expansion of activated tumor reactive cells. J Immunol, 181(1), 808–821. https://doi.org/10.4049/jimmunol.181.1.808.
Moreno Ayala, M. A., Gottardo, M. F., Imsen, M., Asad, A. S., & Bal de Kier Joffé, E., Casares, N., et al. (2017). Therapeutic blockade of Foxp3 in experimental breast cancer models. Breast Cancer Research and Treatment, 166(2), 393–405. https://doi.org/10.1007/s10549-017-4414-2.
Mani, A., Roda, J., Young, D., Caligiuri, M. A., Fleming, G. F., Kaufman, P., et al. (2009). A phase II trial of trastuzumab in combination with low-dose interleukin-2 (IL-2) in patients (PTS) with metastatic breast cancer (MBC) who have previously failed trastuzumab. Breast Cancer Research and Treatment, 117(1), 83–89. https://doi.org/10.1007/s10549-008-0251-7.
Ahmadzadeh, M., & Rosenberg, S. A. (2006). IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood, 107(6), 2409–2414. https://doi.org/10.1182/blood-2005-06-2399.
Levin, A. M., Bates, D. L., Ring, A. M., Krieg, C., Lin, J. T., Su, L., et al. (2012). Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkineʼ. Nature, 484(7395), 529–533. https://doi.org/10.1038/nature10975.
Charych, D. H., Hoch, U., Langowski, J. L., Lee, S. R., Addepalli, M. K., Kirk, P. B., et al. (2016). NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin Cancer Res, 22(3), 680–690. https://doi.org/10.1158/1078-0432.Ccr-15-1631.
Qu, Y., Zhang, B., Liu, S., Zhang, A., Wu, T., & Zhao, Y. (2010). 2-Gy whole-body irradiation significantly alters the balance of CD4+ CD25- T effector cells and CD4+ CD25+ Foxp3+ T regulatory cells in mice. Cellular & molecular immunology, 7(6), 419–427. https://doi.org/10.1038/cmi.2010.45.
Selby, M. J., Engelhardt, J. J., Quigley, M., Henning, K. A., Chen, T., Srinivasan, M., et al. (2013). Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res, 1(1), 32–42. https://doi.org/10.1158/2326-6066.Cir-13-0013.
Buchbinder, E., & Hodi, F. S. (2015). Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. The Journal of clinical investigation, 125(9), 3377–3383. https://doi.org/10.1172/JCI80012.
Adams, S., Othus, M., Patel, S. P., Chae, Y. K., Miller, K., Chugh, R., et al. (2020). Dual anti-CTLA-4 and anti-PD-1 blockade in metaplastic carcinoma of the breast: Dart (SWOG S1609, Cohort 36). Journal of Clinical Oncology, 38(15_suppl), 1073–1073. https://doi.org/10.1200/JCO.2020.38.15_suppl.1073.
Vonderheide, R. H., LoRusso, P. M., Khalil, M., Gartner, E. M., Khaira, D., Soulieres, D., et al. (2010). Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res, 16(13), 3485–3494. https://doi.org/10.1158/1078-0432.Ccr-10-0505.
Rech, A. J., Mick, R., Martin, S., Recio, A., Aqui, N. A., Powell Jr., D. J., et al. (2012). CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Science translational medicine, 4(134), 134ra162–134ra162. https://doi.org/10.1126/scitranslmed.3003330.
Sánchez-Margalet, V., Barco-Sánchez, A., Vilariño-García, T., Jiménez-Cortegana, C., Pérez-Pérez, A., Henao-Carrasco, F., et al. (2019). Circulating regulatory T cells from breast cancer patients in response to neoadjuvant chemotherapy. Translational Cancer Research, 8(1), 59–65.
Maj, T., Wang, W., Crespo, J., Zhang, H., Wang, W., Wei, S., et al. (2017). Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nature Immunology, 18(12), 1332–1341. https://doi.org/10.1038/ni.3868.
Masopust, D., Vezys, V., Marzo, A. L., & Lefrancois, L. (2001). Preferential localization of effector memory cells in nonlymphoid tissue. Science, 291(5512), 2413–2417. https://doi.org/10.1126/science.1058867.
Hogan, R. J., Zhong, W., Usherwood, E. J., Cookenham, T., Roberts, A. D., & Woodland, D. L. (2001). Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med, 193(8), 981–986. https://doi.org/10.1084/jem.193.8.981.
Milner, J. J., Toma, C., Yu, B., Zhang, K., Omilusik, K., Phan, A. T., et al. (2017). Runx3 programs CD8(+) T cell residency in non-lymphoid tissues and tumours. Nature, 552(7684), 253–257. https://doi.org/10.1038/nature24993.
Savas, P., Virassamy, B., Ye, C., Salim, A., Mintoff, C. P., Caramia, F., et al. (2018). Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med, 24(7), 986–993. https://doi.org/10.1038/s41591-018-0078-7.
Djenidi, F., Adam, J., Goubar, A., Durgeau, A., Meurice, G., de Montpreville, V., et al. (2015). CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol, 194(7), 3475–3486. https://doi.org/10.4049/jimmunol.1402711.
Skon, C. N., Lee, J. Y., Anderson, K. G., Masopust, D., Hogquist, K. A., & Jameson, S. C. (2013). Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol, 14(12), 1285–1293. https://doi.org/10.1038/ni.2745.
Cepek, K. L., Shaw, S. K., Parker, C. M., Russell, G. J., Morrow, J. S., Rimm, D. L., et al. (1994). Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature, 372(6502), 190–193. https://doi.org/10.1038/372190a0.
Le Floc'h, A., Jalil, A., Franciszkiewicz, K., Validire, P., Vergnon, I., & Mami-Chouaib, F. (2011). Minimal engagement of CD103 on cytotoxic T lymphocytes with an E-cadherin-Fc molecule triggers lytic granule polarization via a phospholipase Cgamma-dependent pathway. Cancer Res, 71(2), 328–338. https://doi.org/10.1158/0008-5472.Can-10-2457.
Bankovich, A. J., Shiow, L. R., & Cyster, J. G. (2010). CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem, 285(29), 22328–22337. https://doi.org/10.1074/jbc.M110.123299.
Wei, S. H., Rosen, H., Matheu, M. P., Sanna, M. G., Wang, S. K., Jo, E., et al. (2005). Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol, 6(12), 1228–1235. https://doi.org/10.1038/ni1269.
Kumar, B. V., Ma, W., Miron, M., Granot, T., Guyer, R. S., Carpenter, D. J., et al. (2017). Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell reports, 20(12), 2921–2934. https://doi.org/10.1016/j.celrep.2017.08.078.
Steinert, E. M., Schenkel, J. M., Fraser, K. A., Beura, L. K., Manlove, L. S., Igyártó, B. Z., et al. (2015). Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell, 161(4), 737–749. https://doi.org/10.1016/j.cell.2015.03.031.
Wang, D., Yuan, R., Feng, Y., El-Asady, R., Farber, D. L., Gress, R. E., et al. (2004). Regulation of CD103 expression by CD8+ T cells responding to renal allografts. J Immunol, 172(1), 214–221. https://doi.org/10.4049/jimmunol.172.1.214.
Lee, Y. T., Suarez-Ramirez, J. E., Wu, T., Redman, J. M., Bouchard, K., Hadley, G. A., et al. (2011). Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol, 85(9), 4085–4094. https://doi.org/10.1128/JVI.02493-10.
Casey, K. A., Fraser, K. A., Schenkel, J. M., Moran, A., Abt, M. C., Beura, L. K., et al. (2012). Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol, 188(10), 4866–4875. https://doi.org/10.4049/jimmunol.1200402.
Byrne, A., Savas, P., Sant, S., Li, R., Virassamy, B., Luen, S. J., et al. (2020). Tissue-resident memory T cells in breast cancer control and immunotherapy responses. Nature Reviews Clinical Oncology, 17(6), 341–348. https://doi.org/10.1038/s41571-020-0333-y.
Loi, S., Schmid, P., Cortés, J., Cescon, D. W., Winer, E. P., Toppmeyer, D., et al. (2019). Abstract LB-225: RNA molecular signatures as predictive biomarkers of response to monotherapy pembrolizumab in patients with metastatic triple-negative breast cancer: KEYNOTE-086. Cancer Research, 79(13 Supplement), LB-225–LB-225. https://doi.org/10.1158/1538-7445.Am2019-lb-225.
Wang, Z. Q., Milne, K., Derocher, H., Webb, J. R., Nelson, B. H., & Watson, P. H. (2016). CD103 and intratumoral immune response in breast cancer. Clin Cancer Res, 22(24), 6290–6297. https://doi.org/10.1158/1078-0432.CCR-16-0732.
Egelston, C., Srinivasan, G., Avalos, C., Huang, Y., Rosario, A., Wang, R., et al. (2017). CD8+ tissue resident memory T cells are associated with good prognosis in breast cancer patients. The Journal of Immunology, 198(1 Supplement), 196.111–196.111.
Wu, T.-C., Xu, K., Banchereau, R., Marches, F., Yu, C. I., Martinek, J., et al. (2014). Reprogramming tumor-infiltrating dendritic cells for CD103+ CD8+ mucosal T-cell differentiation and breast cancer rejection. Cancer immunology research, 2(5), 487–500. https://doi.org/10.1158/2326-6066.CIR-13-0217.
Park, S. L., Buzzai, A., Rautela, J., Hor, J. L., Hochheiser, K., Effern, M., et al. (2019). Tissue-resident memory CD8+ T cells promote melanoma–immune equilibrium in skin. Nature, 565(7739), 366–371. https://doi.org/10.1038/s41586-018-0812-9.
Kiessling, R., Klein, E., Pross, H., & Wigzell, H. (1975). “Natural” killer cells in the mouse II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol, 5(2), 117–121. https://doi.org/10.1002/eji.1830050209.
Kiessling, R., Klein, E., & Wigzell, H. (1975). “Natural” killer cells in the mouse I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol, 5(2), 112–117. https://doi.org/10.1002/eji.1830050208.
Lanier, L. L. (2008). Up on the tightrope: Natural killer cell activation and inhibition. Nat Immunol, 9(5), 495–502. https://doi.org/10.1038/ni1581.
Dunn, P. L., & North, R. J. (1991). Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun, 59(9), 2892–2900. https://doi.org/10.1128/IAI.59.9.2892-2900.1991.
Cooper, M. A., Fehniger, T. A., & Caligiuri, M. A. (2001). The biology of human natural killer-cell subsets. Trends Immunol, 22(11), 633–640. https://doi.org/10.1016/s1471-4906(01)02060-9.
Cooper, M. A., Fehniger, T. A., Turner, S. C., Chen, K. S., Ghaheri, B. A., Ghayur, T., et al. (2001). Human natural killer cells: A unique innate immunoregulatory role for the CD56(bright) subset. Blood, 97(10), 3146–3151. https://doi.org/10.1182/blood.v97.10.3146.
De Maria, A., Bozzano, F., Cantoni, C., & Moretta, L. (2011). Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc Natl Acad Sci U S A, 108(2), 728–732. https://doi.org/10.1073/pnas.1012356108.
Fauriat, C., Long, E. O., Ljunggren, H. G., & Bryceson, Y. T. (2010). Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood, 115(11), 2167–2176. https://doi.org/10.1182/blood-2009-08-238469.
Krzewski, K., & Coligan, J. E. (2012). Human NK cell lytic granules and regulation of their exocytosis. Front Immunol, 3, 335. https://doi.org/10.3389/fimmu.2012.00335.
Waggoner, S. N., Cornberg, M., Selin, L. K., & Welsh, R. M. (2011). Natural killer cells act as rheostats modulating antiviral T cells. Nature, 481(7381), 394–398. https://doi.org/10.1038/nature10624.
Ljunggren, H. G., & Karre, K. (1985). Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med, 162(6), 1745–1759. https://doi.org/10.1084/jem.162.6.1745.
Praper, T., Sonnen, A., Viero, G., Kladnik, A., Froelich, C. J., Anderluh, G., et al. (2011). Human perforin employs different avenues to damage membranes. J Biol Chem, 286(4), 2946–2955. https://doi.org/10.1074/jbc.M110.169417.
Keefe, D., Shi, L., Feske, S., Massol, R., Navarro, F., Kirchhausen, T., et al. (2005). Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity, 23(3), 249–262. https://doi.org/10.1016/j.immuni.2005.08.001.
Al Absi, A., Wurzer, H., Guerin, C., Hoffmann, C., Moreau, F., Mao, X., et al. (2018). Actin cytoskeleton remodeling drives breast cancer cell escape from natural killer-mediated cytotoxicity. Cancer Res, 78(19), 5631–5643. https://doi.org/10.1158/0008-5472.CAN-18-0441.
Park, I. H., Yang, H. N., Lee, K. J., Kim, T. S., Lee, E. S., Jung, S. Y., et al. (2017). Tumor-derived IL-18 induces PD-1 expression on immunosuppressive NK cells in triple-negative breast cancer. Oncotarget, 8(20), 32722–32730. https://doi.org/10.18632/oncotarget.16281.
Mamessier, E., Pradel, L. C., Thibult, M. L., Drevet, C., Zouine, A., Jacquemier, J., et al. (2013). Peripheral blood NK cells from breast cancer patients are tumor-induced composite subsets. J Immunol, 190(5), 2424–2436. https://doi.org/10.4049/jimmunol.1200140.
Beziat, V., Duffy, D., Quoc, S. N., Le Garff-Tavernier, M., Decocq, J., Combadiere, B., et al. (2011). CD56brightCD16+ NK cells: A functional intermediate stage of NK cell differentiation. J Immunol, 186(12), 6753–6761. https://doi.org/10.4049/jimmunol.1100330.
Joncker, N. T., Shifrin, N., Delebecque, F., & Raulet, D. H. (2010). Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med, 207(10), 2065–2072. https://doi.org/10.1084/jem.20100570.
Triki, H., Charfi, S., Bouzidi, L., Ben Kridis, W., Daoud, J., Chaabane, K., et al. (2019). CD155 expression in human breast cancer: Clinical significance and relevance to natural killer cell infiltration. Life Sci, 231, 116543. https://doi.org/10.1016/j.lfs.2019.116543.
Chan, C. J., Andrews, D. M., McLaughlin, N. M., Yagita, H., Gilfillan, S., Colonna, M., et al. (2010). DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J Immunol, 184(2), 902–911. https://doi.org/10.4049/jimmunol.0903225.
Muntasell, A., Servitja, S., Cabo, M., Bermejo, B., Perez-Buira, S., Rojo, F., et al. (2019). High numbers of circulating CD57(+) NK cells associate with resistance to HER2-specific therapeutic antibodies in HER2(+) primary breast cancer. Cancer Immunol Res, 7(8), 1280–1292. https://doi.org/10.1158/2326-6066.CIR-18-0896.
Bjorkstrom, N. K., Riese, P., Heuts, F., Andersson, S., Fauriat, C., Ivarsson, M. A., et al. (2010). Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood, 116(19), 3853–3864. https://doi.org/10.1182/blood-2010-04-281675.
Marlind, J., Kaspar, M., Trachsel, E., Sommavilla, R., Hindle, S., Bacci, C., et al. (2008). Antibody-mediated delivery of interleukin-2 to the stroma of breast cancer strongly enhances the potency of chemotherapy. Clin Cancer Res, 14(20), 6515–6524. https://doi.org/10.1158/1078-0432.CCR-07-5041.
Tonini, G., Nunziata, C., Prete, S. P., Pepponi, R., Turriziani, M., Masci, G., et al. (1998). Adjuvant treatment of breast cancer: A pilot immunochemotherapy study with CMF, interleukin-2 and interferon alpha. Cancer Immunol Immunother, 47(3), 157–166. https://doi.org/10.1007/s002620050516.
Gillgrass, A. E., Chew, M. V., Krneta, T., & Ashkar, A. A. (2015). Overexpression of IL-15 promotes tumor destruction via NK1.1+ cells in a spontaneous breast cancer model. BMC Cancer, 15, 293. https://doi.org/10.1186/s12885-015-1264-3.
Knudson, K. M., Hicks, K. C., Alter, S., Schlom, J., & Gameiro, S. R. (2019). Mechanisms involved in IL-15 superagonist enhancement of anti-PD-L1 therapy. J Immunother Cancer, 7(1), 82. https://doi.org/10.1186/s40425-019-0551-y.
Bekaii-Saab, T. S., Roda, J. M., Guenterberg, K. D., Ramaswamy, B., Young, D. C., Ferketich, A. K., et al. (2009). A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. Mol Cancer Ther, 8(11), 2983–2991. https://doi.org/10.1158/1535-7163.MCT-09-0820.
Parihar, R., Nadella, P., Lewis, A., Jensen, R., De Hoff, C., Dierksheide, J. E., et al. (2004). A phase I study of interleukin 12 with trastuzumab in patients with human epidermal growth factor receptor-2-overexpressing malignancies: analysis of sustained interferon gamma production in a subset of patients. Clin Cancer Res, 10(15), 5027–5037. https://doi.org/10.1158/1078-0432.CCR-04-0265.
Beano, A., Signorino, E., Evangelista, A., Brusa, D., Mistrangelo, M., Polimeni, M. A., et al. (2008). Correlation between NK function and response to trastuzumab in metastatic breast cancer patients. J Transl Med, 6, 25. https://doi.org/10.1186/1479-5876-6-25.
Carson, W. E., Parihar, R., Lindemann, M. J., Personeni, N., Dierksheide, J., Meropol, N. J., et al. (2001). Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2 / neu-positive breast cancer cells. European Journal of Immunology, 31(10), 3016–3025. https://doi.org/10.1002/1521-4141(2001010)31:10<3016::AID-IMMU3016>3.0.CO;2-J.
Geller, M. A., Cooley, S., Judson, P. L., Ghebre, R., Carson, L. F., Argenta, P. A., et al. (2011). A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy, 13(1), 98–107. https://doi.org/10.3109/14653249.2010.515582.
Hu, Z. (2020). Tissue factor as a new target for CAR-NK cell immunotherapy of triple-negative breast cancer. Sci Rep, 10(1), 2815. https://doi.org/10.1038/s41598-020-59736-3.
Liu, Y., Zhou, Y., Huang, K. H., Fang, X., Li, Y., Wang, F., et al. (2020). Targeting epidermal growth factor-overexpressing triple-negative breast cancer by natural killer cells expressing a specific chimeric antigen receptor. Cell Prolif, 53(8), e12858. https://doi.org/10.1111/cpr.12858.
Miller, J. S., Soignier, Y., Panoskaltsis-Mortari, A., McNearney, S. A., Yun, G. H., Fautsch, S. K., et al. (2005). Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood, 105(8), 3051–3057. https://doi.org/10.1182/blood-2004-07-2974.
Hu, Y., Wang, J., Wei, G., Yu, J., Luo, Y., Shi, J., et al. (2019). A retrospective comparison of allogenic and autologous chimeric antigen receptor T cell therapy targeting CD19 in patients with relapsed/refractory acute lymphoblastic leukemia. Bone Marrow Transplantation, 54(8), 1208–1217. https://doi.org/10.1038/s41409-018-0403-2.
Barry, K. C., Hsu, J., Broz, M. L., Cueto, F. J., Binnewies, M., Combes, A. J., et al. (2018). A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med, 24(8), 1178–1191. https://doi.org/10.1038/s41591-018-0085-8.
Li, Y., Hermanson, D. L., Moriarity, B. S., & Kaufman, D. S. (2018). Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell, 23(2), 181–192 e185. https://doi.org/10.1016/j.stem.2018.06.002.
Li, H.-K. H., Wu, T.-S. E., Hsiao, C.-W. S., Yang, S.-H. S., Lee, C.-Y. S., Lin, Y.-L. J., et al. (2020). Abstract 2169: ACE1702: A potent and off-the-shelf oNK cell therapy product. Cancer Research, 80(16 Supplement), 2169–2169. https://doi.org/10.1158/1538-7445.Am2020-2169.
Goenka, R., Barnett, L. G., Silver, J. S., O'Neill, P. J., Hunter, C. A., Cancro, M. P., et al. (2011). Cutting edge: Dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J Immunol, 187(3), 1091–1095. https://doi.org/10.4049/jimmunol.1100853.
Ohl, L., Mohaupt, M., Czeloth, N., Hintzen, G., Kiafard, Z., Zwirner, J., et al. (2004). CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity, 21(2), 279–288. https://doi.org/10.1016/j.immuni.2004.06.014.
Tokunaga, R., Naseem, M., Lo, J. H., Battaglin, F., Soni, S., Puccini, A., et al. (2019). B cell and B cell-related pathways for novel cancer treatments. Cancer treatment reviews, 73, 10–19. https://doi.org/10.1016/j.ctrv.2018.12.001.
LeBien, T. W., & Tedder, T. F. (2008). B lymphocytes: How they develop and function. Blood, 112(5), 1570–1580. https://doi.org/10.1182/blood-2008-02-078071.
Fagraeus, A. (1948). Antibody production in relation to the development of plasma cells. In vivo and in vitro experiments. Acta Medica Scandinavica, 130(Suppl. 204).
Bernasconi, N. L., Traggiai, E., & Lanzavecchia, A. (2002). Maintenance of serological memory by polyclonal activation of human memory B cells. Science, 298(5601), 2199–2202.
Radbruch, A., Muehlinghaus, G., Luger, E. O., Inamine, A., Smith, K. G. C., Dörner, T., et al. (2006). Competence and competition: The challenge of becoming a long-lived plasma cell. Nature Reviews Immunology, 6(10), 741–750. https://doi.org/10.1038/nri1886.
Coronella, J. A., Spier, C., Welch, M., Trevor, K. T., Stopeck, A. T., Villar, H., et al. (2002). Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. The Journal of Immunology, 169(4), 1829–1836.
Roncati, L., Barbolini, G., Piacentini, F., Piscioli, F., Pusiol, T., & Maiorana, A. (2016). Prognostic factors for breast cancer: An immunomorphological update. Pathology & Oncology Research, 22(3), 449–452. https://doi.org/10.1007/s12253-015-0024-7.
Li, Q., Lao, X., Pan, Q., Ning, N., Yet, J., Xu, Y., et al. (2011). Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clinical cancer research, 17(15), 4987–4995.
Fremd, C., Stefanovic, S., Beckhove, P., Pritsch, M., Lim, H., Wallwiener, M., et al. (2015). Mucin 1-specific B cell immune responses and their impact on overall survival in breast cancer patients. Oncoimmunology, 5(1), e1057387–e1057387. https://doi.org/10.1080/2162402X.2015.1057387.
Carmi, Y., Spitzer, M. H., Linde, I. L., Burt, B. M., Prestwood, T. R., Perlman, N., et al. (2015). Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature, 521(7550), 99–104. https://doi.org/10.1038/nature14424.
Gu, Y., Liu, Y., Fu, L., Zhai, L., Zhu, J., Han, Y., et al. (2019). Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nature Medicine, 25(2), 312–322. https://doi.org/10.1038/s41591-018-0309-y.
Bruno, T. C., Ebner, P. J., Moore, B. L., Squalls, O. G., Waugh, K. A., Eruslanov, E. B., et al. (2017). Antigen-presenting intratumoral B cells affect CD4(+) TIL phenotypes in non-small cell lung cancer patients. Cancer immunology research, 5(10), 898–907. https://doi.org/10.1158/2326-6066.CIR-17-0075.
Mauri, C., Gray, D., Mushtaq, N., & Londei, M. (2003). Prevention of arthritis by interleukin 10–producing B cells. Journal of Experimental Medicine, 197(4), 489–501. https://doi.org/10.1084/jem.20021293.
Lampropoulou, V., Hoehlig, K., Roch, T., Neves, P., Gómez, E. C., Sweenie, C. H., et al. (2008). TLR-activated B cells suppress T cell-mediated autoimmunity. The Journal of Immunology, 180(7), 4763–4773.
Olkhanud, P. B., Damdinsuren, B., Bodogai, M., Gress, R. E., Sen, R., Wejksza, K., et al. (2011). Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Research, 71(10), 3505–3515. https://doi.org/10.1158/0008-5472.CAN-10-4316.
Parekh, V. V., Prasad, D. V., Banerjee, P. P., Joshi, B. N., Kumar, A., & Mishra, G. C. (2003). B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-β1. The Journal of Immunology, 170(12), 5897–5911.
Carter, N. A., Rosser, E. C., & Mauri, C. (2012). Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Research & Therapy, 14(1), R32. https://doi.org/10.1186/ar3736.
Shen, P., Roch, T., Lampropoulou, V., O'Connor, R. A., Stervbo, U., Hilgenberg, E., et al. (2014). IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature, 507(7492), 366–370. https://doi.org/10.1038/nature12979.
An, T., Sood, U., Pietruk, T., Cummings, G., Hashimoto, K., & Crissman, J. D. (1987). In situ quantitation of inflammatory mononuclear cells in ductal infiltrating breast carcinoma. Relation to prognostic parameters. Am J Pathol, 128(1), 52–60.
Disis, M. L., Pupa, S. M., Gralow, J. R., Dittadi, R., Menard, S., & Cheever, M. A. (1997). High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol, 15(11), 3363–3367. https://doi.org/10.1200/jco.1997.15.11.3363.
Scholl, S., Bièche, I., Pallud, C., Champème, M. H., Beuvon, F., Hacene, K., et al. (1996). Relevance of multiple biological parameters in breast cancer prognosis. The Breast, 5(1), 21–30. https://doi.org/10.1016/S0960-9776(96)90045-4.
Rody, A., Holtrich, U., Pusztai, L., Liedtke, C., Gaetje, R., Ruckhaeberle, E., et al. (2009). T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Research, 11(2), R15. https://doi.org/10.1186/bcr2234.
Schmidt, M., Böhm, D., von Törne, C., Steiner, E., Puhl, A., Pilch, H., et al. (2008). The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Research, 68(13), 5405–5413. https://doi.org/10.1158/0008-5472.Can-07-5206.
Mahmoud, S. M., Lee, A. H., Paish, E. C., Macmillan, R. D., Ellis, I. O., & Green, A. R. (2012). The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat, 132(2), 545–553. https://doi.org/10.1007/s10549-011-1620-1.
Yeong, J., Lim, J. C. T., Lee, B., Li, H., Chia, N., Ong, C. C. H., et al. (2018). High densities of tumor-associated plasma cells predict improved prognosis in triple negative breast cancer. Frontiers in immunology, 9, 1209–1209. https://doi.org/10.3389/fimmu.2018.01209.
Arias-Pulido, H., Cimino-Mathews, A., Chaher, N., Qualls, C., Joste, N., Colpaert, C., et al. (2018). The combined presence of CD20 + B cells and PD-L1 + tumor-infiltrating lymphocytes in inflammatory breast cancer is prognostic of improved patient outcome. Breast Cancer Research and Treatment, 171(2), 273–282. https://doi.org/10.1007/s10549-018-4834-7.
Gheybi, M. K., Farrokhi, S., Ravanbod, M. R., Ostovar, A., Mehrzad, V., & Nematollahi, P. (2017). The correlation of CD19 + CD24 + CD38 + B cells and other clinicopathological variables with the proportion of circulating Tregs in breast cancer patients. Breast Cancer, 24(6), 756–764. https://doi.org/10.1007/s12282-017-0775-y.
Garaud, S., Buisseret, L., Solinas, C., Gu-Trantien, C., de Wind, A., Van den Eynden, G., et al. (2019). Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight, 5(18), e129641. https://doi.org/10.1172/jci.insight.129641.
Bodogai, M., Lee Chang, C., Wejksza, K., Lai, J., Merino, M., Wersto, R. P., et al. (2013). Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Research, 73(7), 2127–2138. https://doi.org/10.1158/0008-5472.CAN-12-4184.
Lee-Chang, C., Bodogai, M., Martin-Montalvo, A., Wejksza, K., Sanghvi, M., Moaddel, R., et al. (2013). Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. Journal of immunology (Baltimore, Md. : 1950), 191(8), 4141–4151. https://doi.org/10.4049/jimmunol.1300606.
Tao, H., Lu, L., Xia, Y., Dai, F., Wang, Y., Bao, Y., et al. (2015). Antitumor effector B cells directly kill tumor cells via the Fas/FasL pathway and are regulated by IL-10. Eur J Immunol, 45(4), 999–1009. https://doi.org/10.1002/eji.201444625.
Stanton, S. E., Adams, S., & Disis, M. L. (2016). Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: A systematic review. JAMA Oncology, 2(10), 1354–1360. https://doi.org/10.1001/jamaoncol.2016.1061.
Glajcar, A., Szpor, J., Hodorowicz-Zaniewska, D., Tyrak, K. E., & Okoń, K. (2019). The composition of T cell infiltrates varies in primary invasive breast cancer of different molecular subtypes as well as according to tumor size and nodal status. Virchows Archiv : an international journal of pathology, 475(1), 13–23. https://doi.org/10.1007/s00428-019-02568-y.
Fallahpour, S., Navaneelan, T., De, P., & Borgo, A. (2017). Breast cancer survival by molecular subtype: A population-based analysis of cancer registry data. CMAJ open, 5(3), E734–E739. https://doi.org/10.9778/cmajo.20170030.
Gao, G., Wang, Z., Qu, X., & Zhang, Z. (2020). Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: A systematic review and meta-analysis. BMC Cancer, 20(1), 179–179. https://doi.org/10.1186/s12885-020-6668-z.
Kurozumi, S., Matsumoto, H., Kurosumi, M., Inoue, K., Fujii, T., Horiguchi, J., et al. (2019). Prognostic significance of tumour-infiltrating lymphocytes for oestrogen receptor-negative breast cancer without lymph node metastasis. Oncology letters, 17(3), 2647–2656. https://doi.org/10.3892/ol.2019.9938.
Adams, S., Gray, R. J., Demaria, S., Goldstein, L., Perez, E. A., Shulman, L. N., et al. (2014). Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 32(27), 2959–2966. https://doi.org/10.1200/JCO.2013.55.0491.
Thorsson, V., Gibbs, D. L., Brown, S. D., Wolf, D., Bortone, D. S., Ou Yang, T.-H., et al. (2018). The immune landscape of cancer. Immunity, 48(4), 812–830.e814. https://doi.org/10.1016/j.immuni.2018.03.023.
Schmid, P., Adams, S., Rugo, H. S., Schneeweiss, A., Barrios, C. H., Iwata, H., et al. (2018). Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. New England Journal of Medicine, 379(22), 2108–2121. https://doi.org/10.1056/NEJMoa1809615.
Conte, P. F., Dieci, M. V., Bisagni, G., De Laurentiis, M., Tondini, C. A., Schmid, P., et al. (2020). Phase III randomized study of adjuvant treatment with the ANTI-PD-L1 antibody avelumab for high-risk triple negative breast cancer patients: The A-BRAVE trial. Journal of Clinical Oncology, 38(15_suppl), TPS598–TPS598. https://doi.org/10.1200/JCO.2020.38.15_suppl.TPS598.
Dirix, L. Y., Takacs, I., Jerusalem, G., Nikolinakos, P., Arkenau, H.-T., Forero-Torres, A., et al. (2018). Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Research and Treatment, 167(3), 671–686. https://doi.org/10.1007/s10549-017-4537-5.
Nanda, R., Chow, L. Q. M., Dees, E. C., Berger, R., Gupta, S., Geva, R., et al. (2016). Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 34(21), 2460–2467. https://doi.org/10.1200/JCO.2015.64.8931.
Cortés, J., Lipatov, O., Im, S. A., Gonçalves, A., Lee, K. S., Schmid, P., et al. (2019). LBA21 - KEYNOTE-119: phase III study of pembrolizumab (pembro) versus single-agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC). Annals of Oncology, 30, v859–v860. https://doi.org/10.1093/annonc/mdz394.010.
Cortes, J., Cescon, D. W., Rugo, H. S., Nowecki, Z., Im, S.-A., Yusof, M. M., et al. (2020). KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. Journal of Clinical Oncology, 38(15_suppl), 1000–1000. https://doi.org/10.1200/JCO.2020.38.15_suppl.1000.
Mittendorf, E. A., Ardavanis, A., Litton, J. K., Shumway, N. M., Hale, D. F., Murray, J. L., et al. (2016). Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget, 7(40), 66192–66201. https://doi.org/10.18632/oncotarget.11751.
Gennari, R., Menard, S., Fagnoni, F., Ponchio, L., Scelsi, M., Tagliabue, E., et al. (2004). Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clinical cancer research, 10(17), 5650–5655.
Müller, P., Kreuzaler, M., Khan, T., Thommen, D. S., Martin, K., Glatz, K., et al. (2015). Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Science translational medicine, 7(315), 315ra188–315ra188.
Kachikwu, E. L., Iwamoto, K. S., Liao, Y.-P., DeMarco, J. J., Agazaryan, N., Economou, J. S., et al. (2011). Radiation enhances regulatory T cell representation. International Journal of Radiation Oncology*Biology*Physics, 81(4), 1128–1135. https://doi.org/10.1016/j.ijrobp.2010.09.034.
Apetoh, L., Ghiringhelli, F., Tesniere, A., Obeid, M., Ortiz, C., Criollo, A., et al. (2007). Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med, 13(9), 1050–1059. https://doi.org/10.1038/nm1622.
Wimberly, H., Brown, J. R., Schalper, K., Haack, H., Silver, M. R., Nixon, C., et al. (2015). PD-L1 Expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer immunology research, 3(4), 326–332. https://doi.org/10.1158/2326-6066.CIR-14-0133.
Schalper, K. A., Velcheti, V., Carvajal, D., Wimberly, H., Brown, J., Pusztai, L., et al. (2014). <em>In Situ</em> Tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clinical cancer research, 20(10), 2773–2782. https://doi.org/10.1158/1078-0432.Ccr-13-2702.
Emens, L. A., Braiteh, F. S., Cassier, P., Delord, J.-P., Eder, J. P., Fasso, M., et al. (2015). Abstract 2859: Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC). Cancer Research, 75(15 Supplement), 2859–2859. https://doi.org/10.1158/1538-7445.Am2015-2859.
Linsley, P. S., Bradshaw, J., Greene, J., Peach, R., Bennett, K. L., & Mittler, R. S. (1996). Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity, 4(6), 535–543. https://doi.org/10.1016/S1074-7613(00)80480-X.
Khalife, E., Khodadadi, A., Talaeizadeh, A., Rahimian, L., Nemati, M., & Jafarzadeh, A. (2018). Overexpression of regulatory T cell-related markers (FOXP3, CTLA-4 and GITR) by peripheral blood mononuclear cells from patients with breast cancer. Asian Pacific journal of cancer prevention : APJCP, 19(11), 3019–3025. https://doi.org/10.31557/APJCP.2018.19.11.3019.
Zhou, J., Bashey, A., Zhong, R., Corringham, S., Messer, K., Pu, M., et al. (2011). CTLA-4 blockade following relapse of malignancy after allogeneic stem cell transplantation is associated with T cell activation but not with increased levels of T regulatory cells. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation, 17(5), 682–692. https://doi.org/10.1016/j.bbmt.2010.08.005.
Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob, J. J., Cowey, C. L., Lao, C. D., et al. (2015). Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. The New England journal of medicine, 373(1), 23–34. https://doi.org/10.1056/NEJMoa1504030.
Barroso-Sousa, R., Trippa, L., Lange, P., Andrews, C., McArthur, H. L., Haley, B. B., et al. (2019). Nimbus: A phase II study of nivolumab plus ipilimumab in metastatic hypermutated HER2-negative breast cancer. Journal of Clinical Oncology, 37(15_suppl), TPS1115–TPS1115. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS1115.
Adams, S., & Mittendorf, E. A. (2019). Lack of robust prognostic biomarkers for immunotherapy in breast cancer—Adverse events—In Reply. JAMA Oncology, 5(11), 1640–1640. https://doi.org/10.1001/jamaoncol.2019.3605.
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health under Award Number R44CA217534, the Wayne D. Kuni & Joan E. Kuni Foundation, and OHSU Center for Women’s Health Circle of Giving Award. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH and US government.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.N. and W.N., literature search, M.N., writing—original draft preparation, M.N.; writing—review and editing, M.N., W.N., S.L., W.Y.; funding acquisition, W.Y., W.N. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
OHSU and W.Y. have a significant financial interest in PDX Pharmaceuticals, Inc. This potential personal and institutional conflict of interest has been reviewed and managed by OHSU.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nelson, M.A., Ngamcherdtrakul, W., Luoh, SW. et al. Prognostic and therapeutic role of tumor-infiltrating lymphocyte subtypes in breast cancer. Cancer Metastasis Rev 40, 519–536 (2021). https://doi.org/10.1007/s10555-021-09968-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-021-09968-0