Abstract
A dramatic reduction in mortality among people living with HIV (PLWH) has been achieved during the modern antiretroviral therapy (ART) era. However, ART does not restore gut barrier function even after long-term viral suppression, allowing microbial products to enter the systemic blood circulation and induce chronic immune activation. In PLWH, a chronic state of systemic inflammation exists and persists, which increases the risk of development of inflammation-associated non-AIDS comorbidities such as metabolic disorders, cardiovascular diseases, and cancer. Clostridium butyricum is a human butyrate-producing symbiont present in the gut microbiome. Convergent evidence has demonstrated favorable effects of C. butyricum for gastrointestinal health, including maintenance of the structural and functional integrity of the gut barrier, inhibition of pathogenic bacteria within the intestine, and reduction of microbial translocation. Moreover, C. butyricum supplementation has been observed to have a positive effect on various inflammation-related diseases such as diabetes, ulcerative colitis, and cancer, which are also recognized as non-AIDS comorbidities associated with epithelial gut damage. There is currently scant published research in the literature, focusing on the influence of C. butyricum in the gut of PLWH. In this hypothesis review, we speculate the use of C. butyricum as a probiotic oral supplementation may well emerge as a potential future synergistic adjunctive strategy in PLWH, in tandem with ART, to restore and consolidate intestinal barrier integrity, repair the leaky gut, prevent microbial translocation from the gut, and reduce both gut and systemic inflammation, with the ultimate objective of decreasing the risk for development of non-AIDS comorbidities in PLWH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cumulative evidence has demonstrated that compared to uninfected individuals, people living with HIV (PLWH) experience a significantly increased risk of non-AIDS comorbidities, even in the context of long-term, effective antiretroviral therapy (ART). One recent meta-analysis, which included 236,127 women living with HIV, has shown that this population had a dramatically higher overall pooled risk of cervical cancer, compared to uninfected individuals, with a risk ratio (RR) of 6.07 (95% CI 4.40–8.37) [1]. Chronic systemic inflammation in PLWH has been considered to be the major contributor that facilitates the development of these comorbidities, which include the metabolic syndromes, cardiovascular diseases, cancers, and HIV-associated neurocognitive disorder (HAND) [2,3,4,5]. One systematic review of 29 studies has indicated that higher levels of monocyte activation and inflammatory markers in cerebrospinal fluid are consistently associated with neurocognitive impairment in PLWH [2]. The fundamental underlying mechanisms linking chronic inflammation to non-AIDS comorbidities are complex, and may include immune exhaustion, premature immune senescence, and direct harm to organs through the release of pro-inflammatory cytokines [3].
The elevated level of microbial translocation associated with the leaky gut in HIV-infected individuals is recognized to be the major source of systemic inflammation in PLWH [6]. In addition to the uptake of nutrients and water, other critical functions of the intestinal barrier are to prevent toxic substances originating from the external environment from entering the portal and systemic circulation, and to preserve intestinal homeostasis. Once this barrier is disrupted, non-viable and viable intraluminal gut microbes and their pro-inflammatory products may translocate from the gut into the systemic circulation, a phenomenon referred to as microbial translocation. During HIV infection, the gastrointestinal tract is the major site of HIV replication, and is considered to be the primary location of the HIV viral reservoir as a result of greater expression of the C–C chemokine receptor (CCR)-5, a vital co-receptor for HIV to entry into the gut epithelial cells, and also the opportune localized presence of a large quantity of gut-associated lymphoid tissue (GALT) [7,8,9,10]. As such, among PLWH, rapid loss of gastrointestinal mucosal integrity and heightened gastrointestinal permeability are relatively common, leading to increased microbial translocation and chronic systemic inflammation [11]. Therefore, in addition to modern ART, strategies which ameliorate poor intestinal barrier function and which mitigate the chronic systemic inflammation present in PLWH have emerged as a research priority.
Clostridium butyricum is an anaerobic bacterium existing symbiotically in the human intestine, as well as in various environmental niches, such as soil and vegetables [12]. When used as an oral probiotic supplement, C. butyricum could produce an important metabolite short-chain fatty acid (SCFA), namely butyrate by fermentation, and influence other gut flora or the host health in various ways [13]. Encouragingly, C. butyricum, as well as butyrate, has been reported to restore and maintain the integrity of the gut barrier, inhibit the proliferation of pathogenic bacteria in the intestine, and reduce microbial translocation and systemic inflammation [14,15,16,17,18]. Moreover, C. butyricum supplementation has been shown to exert a beneficial effect on metabolic diseases, nonalcoholic fatty liver disease (NAFLD), ulcerative colitis (UC), tumorigenesis, and neurodegeneration, all of which have been recognized as relatively common comorbidities in PLWH [19,20,21,22,23,24,25]. However, there remain very few published research works which focus on the gastrointestinal and other effects of C. butyricum in PLWH. The present work is intended to discuss the influences of C. butyricum on the gastrointestinal system and systemically, and the implications of C. butyricum supplementation for gut health, systemic inflammation, and non-AIDS comorbidities in PLWH.
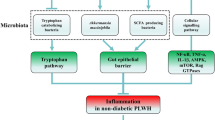
Based on the known favorable effects of C. butyricum on the human body, such as restoration and maintenance of the integrity of the gut barrier, inhibition of the growth of pathogenic bacteria in the intestine, reduction of microbial translocation, and the inherent characteristics of the clinical consequences of HIV infection present in PLWH, we speculate that oral supplementation of C. butyricum may serve as a potential mitigative strategy to ameliorate non-AIDS comorbidities in PLWH (Fig. 1).
(1) C. butyricum may alleviate inflammation through its interactions with immune cells (macrophages and T-helper cells). As such, C. butyricum is capable of inducing the secretion of anti-inflammatory cytokines such as IL-10 and TGF-β. In parallel, C. butyricum may drastically reduce the expression of pro-inflammatory cytokines. Within the gut of an HIV-infected individual, gut integrity is compromised, leading to a permanent leaky gut syndrome. (2) C. butyricum and its metabolites (butyric acid, butyrate) in addition to IL-10 increase the production of the mucin layer and protectin D1. The combination of anti-inflammatory cytokines, protectin D1, and the mucin layer participates in reducing contact between epithelial cells and pathogenic microbes and, thus, participates in protecting gut integrity. (3) Within the gut, C. butyricum has been shown to be capable of repressing the proliferation of pathogenic bacteria such as C. difficile, Helicobacter pylori, and Vibrio cholerae. Furthermore, evidence suggests that C. butyricum is important in promoting the growth of beneficial bacteria such as the butyrate-producing bacteria (particularly those carrying the buk and butyryl-CoA genes), Lactobacillus, and Bifidobacterium spp. In the context of HIV infection where it is known that pathogenic bacteria are augmented while beneficial bacteria are depleted, gut supplementation with C. butyricum may potentially help to regulate the overall microbial balance within the gut microbiome. Thus, we believe that C. butyricum may help to regulate the gut microbiome, alleviate inflammation, and significantly reduce the leaky gut syndrome and the consequences of the abnormal systemic condition observed in an HIV-infected individual. Indeed, several research teams [3, 26, 27] have demonstrated that the during HIV infection, chronic inflammation, the leaky gut syndrome, and the proliferation of pathogenic bacteria may be responsible for the evolution of non-AIDS conditions.
Profile of C. butyricum
Metabolism
C. butyricum is believed to exist symbiotically in the human gastro-intestine [28]. It consumes undigested dietary fiber, including indigestible carbohydrate, and generates SCFAs, particularly butyrate and acetate [13, 29]. Among them, butyrate outstands as one of the dominant metabolic end-products and the most multifunctional metabolite. Involved enzymes are quite critical in butyrate production process. C. butyricum mainly utilizes the butyrae kinase pathway to release butyrate in intestine. For other bacteria, such as Coprococcus eutactus, butyryl-coenzyme A (CoA) transferase pathway is the important metabolic pathway to produce butyrate [30]. There is a strong positive correlation between gene expression levels of butyrate kinase (buk), which codes buk and butyryl-CoA transferase respectively, and the concentrations of butyrate or the content of butyrate-producing bacteria within the intestine [31]. Apart from carbohydrate residues, protein residues such as amino acids may also be regarded as another butyrate source for C. butyricum in the human colon [32]. Specifically, lysine could be converted into butyrate and acetate, with aid from the microbial enzyme crotonyl CoA [32]. Butyrate not only plays as the preferred energy source supply for colonic epithelial cells, but also has a role in various metabolic processes, including reducing bile salt solubility by decreased pH, inhibiting ammonia absorption, lowering blood glucose by enhanced colonic glucagon-like peptide-1 (GLP-1) secretion, and regulating hepatic glucose or lipid homeostasis in a phosphoadenosine activated protein kinase dependent pathway [28, 29, 33, 34].
Efficacy in Alleviation of Inflammation
During the metabolic process, C. butyricum may influence specific behaviors of immune cells such as macrophages and T-lymphocytes, which may alter the release of inflammatory cytokines from these cells [35,36,37]. Increased levels of interleukin (IL)-10, an anti-inflammatory cytokine, plays an indispensable role in the protective effects of C. butyricum [38, 39]. Gao Q and colleagues have reported that C. butyricum may activate toll-like receptor (TLR)-2 within gut macrophages, enhance the release of IL-10, and inhibit cell apoptosis, thus further weakening inflammatory responses in vitro [35]. With the absence of IL-10 in colonic macrophages, the anti-inflammatory benefits of C. butyricum may be attenuated in mice with colitis [37]. Moreover, C. butyricum and butyrate may directly affect the behavior of helper T (Th) cells as well as their produced cytokines, such as TGF-β and IL-10. In asthmatic mice, C. butyricum has been shown to inhibit the secretion of Th2-related cytokines such as IL-4 and IL-13, increase the abundance of anti-inflammatory factors IL-10 and TGF-β, decrease bronchial reactivity, and relieve pulmonary inflammation [36]. Similarly, C. butyricum may reduce Th2 cytokines, including IL-5 and IL-13, and alleviate gastrointestinal inflammatory symptoms and diarrhea induced by food allergies in vivo [38]. Treatment with C. butyricum in animal models may decrease other pro-inflammatory cytokines, such as IL-17 in diarrhea, IL-23 in colitis, and interferon (IFN)-γ in NAFLD, resulting in improved clinical outcomes for these conditions [38, 40, 41]. In addition, representative metabolites SCFAs, such as butyric and valeric acid, were significantly reduced in HIV-positive patients, compared to normal people [42]. The supplementation of SCFAs contributed to a remarkable decrease in pro-inflammatory cytokines (such as IL-10) and an obvious depletion of effector T-lymphocytes in PLWH, leading to reduced inflammatory immune responses and improved life-quality assessment [43], suggesting that SCFA producer C. butyricum may also benefit PLWH. Thus, C. butyricum may exert profound anti-inflammatory effects both directly and indirectly.
There may be some possible mechanisms underlying these anti-inflammatory effects. The MyD88 and nuclear factor-kappa B (NF-κB) signaling pathway might be one possibility. MyD88 is a common regulatory element of innate inflammation, which may activate the NF-κB signaling, thus suppressing apoptosis and mediating inflammation [44]. C. butyricum has been reported to restrain the proliferation of colorectal cancer (CRC) cells, inhibit the mRNA and protein expression of MyD88 and NF-κB/p65, which is inversely proportional to multiplicities of infection (MOIs) of C. butyricum, and improve intestinal inflammation in both cell tests and mice [45]. Toll-like receptor 4 (TLR4)–dependent pathway may also be involved. TLR4, one TLR family member, could recognize lipopolysaccharide and bacterial endotoxins, trigger the production of varied pro-inflammatory factors, and herein mediate inflammatory responses [46]. C. butyricum could suppress intestinal inflammation and attenuate bacteria-induced gut damage, through downregulating the TLR4-dependent signal transduction pathways in Salmonella enteritidis–infected broiler chickens [18]. Moreover, regulation on the expression of some enzymes does matter during the anti-inflammatory response mentioned earlier. Matrix metalloprotein-9 (MMP-9) is a secretory member of the zinc metallopeptidase family, which can exacerbate airway inflammation by increasing inflammatory cells recruitment and inflammatory cytokines release [47]. In comparison to the negative controls, C. butyricum was effective in repressing MMP-9 expression, inhibiting degranulation of mast cells, and reducing respiratory inflammation, in mice with allergic airway inflammation [36].
Modulation of Immune Homeostasis
Along with regulating inflammation, C. butyricum may affect the release of inflammatory cell factors by regulating the balance among immune cells in the gut, as well as the homeostasis of the endogenous enteric immunity. To maintain the intestinal steady state, C. butyricum may regulate the functionality of various immune cells, typically dendritic cells (DCs) and regulatory T-cells (Tregs) [48, 49]. Compared with controls, C. butyricum intervention has been shown to inhibit the activation and proliferation of DCs, and alleviate Th1- or Th17-correlated inflammatory responses in the intestinal mucosa of mouse models with irritable bowel syndrome (IBS) [48]. Further data indicates that C. butyricum significantly suppresses the levels of secreted pro-inflammatory cytokines such as IL-1β and IL-6, and reduces the proportion of cells expressing T-cell immunoglobulin and mucin domain 3 (TIM3) in colonic DC clusters in both mice and humans with IBS [48, 50, 51]. C. butyricum has been shown to promote the induction of Tregs in the colon and the activity of anti-inflammatory Treg immune responses through enhancement of the secretion of TGF-β [39, 52]. Research data has further indicated that C. butyricum increases the expression of TGF-β in a TLR2-dependent manner within DCs, facilitates the differentiation of the Treg population, and establishes the intestinal immune tolerance seen in a murine colitis model [13, 53]. Several murine models have confirmed that C. butyricum contributes to the aggregation of Tregs in intestinal lymphatic organs and decreases inflammatory indices in injured organs, e.g., a reduction of the Th1 to Th17 cellular ratio [38, 49, 54]. When there is a lack of Treg cells, the anti-inflammatory effects of C. butyricum administration may be weakened in mice with colitis, suggesting that Treg cells may have a partially beneficial role in this process [37].
Reinforcement of the Gut Barrier
The intestinal epithelial barrier, composed of the mucous layer, the epithelial monolayer, and the lamina propria, maintains gut epithelial homeostasis by prevention of the transmigration of intestinal microorganisms, promotion of the absorption of nutrients, and by resisting the invasion of pathogens [55, 56]. In healthy mice, C. butyricum supplementation significantly thickens the first layer of the intestinal mucosa [57]. Butyrate, a metabolite of C. butyricum, has also been shown to increase the mucin proteins secreted by epithelial goblet cells, with up-regulation in the mucin gene and the involved mitogen-activated protein kinase (MAPK) pathway factors [58, 59], which is likely to be the underlying mechanism whereby C. butyricum affects the mucus layer. Similarly, in a murine model of colitis, C. butyricum has been seen to raise the production of mucin 2, exaggerate the protective properties of the mucous membrane, and mitigate colonic epithelial injury [60]. The second layer tight junction (TJ) complexes between epithelial cells, composed of claudin, occludin, adhesion junctions, and accessory proteins such as ZO-1, knits epithelial cells together [61]. Ingestion of C. butyricum upregulates the expression of different TJ proteins and anti-inflammatory cytokines, which may prevent the increase in intestinal tract permeability, prevent pathogen invasion, and reduce intestinal inflammation [60, 62]. In mouse models of diarrhea or pancreatitis, administration of C. butyricum has been seen to enhance the expression of TJ proteins including occludin and ZO-1, thus improving intestinal barrier function and reducing the risk of gut leakage [60, 63]. Similarly in mice with traumatic brain injury (TBI), C. butyricum promotes the recovery and expression of occludin in the colon, and protects the integrity of the intestinal mucosal barrier [25]. Moreover, reports have shown that C. butyricum may induce γδ T-cells to secrete higher levels of IL-17, which enhances the defensive role of the colonic lamina propria and maintains the expression levels of TJ proteins so as to protect gut barrier defensive functions in vivo [60, 64]. Ingestion of C. butyricum by mice with colitis may enrich a representative anti-inflammatory lipid metabolite, i.e., protectin D1 in the colon, thus enhancing the release of the anti-inflammatory factor IL-10, which attenuates gut inflammatory responses and strengthens the intestinal barrier [65].
Inhibition of Pathogenic Bacteria in the Intestine
C. butyricum has been reported to increase the proportion of several beneficial bacteria, such as Lactobacillus spp., and to inhibit the proliferation of some harmful bacterial species, thus preventing microbial translocation, improving gastrointestinal infections, and maintaining the dynamic microbial balance inherent to the gut microbiome [66]. Data has suggested that C. butyricum may reverse the dramatically decreased growth of butyrate-producing bacteria, and increase levels of SCFA metabolites, particularly butyrate, in the colon of diabetic mice [20]. Butyryl-CoA and buk are top two most commonly used genetic biomarkers for detection of butyrate-producing communities [67]. Normally, C. butyricum carries only the buk gene. However, researchers have observed that in diabetic mice, the orally administered C. butyricum strain may cause significant enrichment in levels of butyrate-producing bacteria carrying either the buk or the butyryl-CoA gene, which may be related to improvement of metabolic dysfunction and glucose homeostasis [20, 49]. Increases in levels of other beneficial anaerobic bacteria, such as Lactobacillus and Bifidobacterium spp., have also been observed after C. butyricum supplementation in mice [66]. Apart from that, in vitro studies have shown that after contact with C. butyricum, proliferation and cytotoxin production of Clostridium difficile, a typical pathogen linked to nosocomial diarrhea, are significantly inhibited [68]. Murine models of C. difficile infection (CDI) have demonstrated that C. butyricum can consume its nutrient source (succinic acid), produce SCFAs to inhibit growth, decrease release tumor necrosis factor (TNF)-α secretion, and reduce intestinal colonization of C. difficile, resulting in alleviation of intestinal inflammatory diarrhea [16]. It has been reported that C. butyricum and its butyrate product may limit the growth of Helicobacter pylori in vitro and in vivo [69]. This may be achieved via inhibition of the bacterial adhesion of H. pylori to gastric epithelial cells, thus easing clinical symptoms in patients having gastrointestinal H. pylori infection [70]. Similarly, when C. butyricum and enterohemorrhagic E. coli (EHEC) are co-cultured in vitro, the reproduction and enterotoxin production of EHEC are notably decreased [71]. Administration of C. butyricum has been observed to contribute to a 50% reduction in mortality rates of mice infected with EHEC [72]. Inhibitory effects of C. butyricum on growth of other enteric pathogens have also been noticed in vitro with respect to pathogens such as on Staphylococcus aureus, Vibrio cholerae, Shigella flexneri, and Salmonella species [18, 73, 74]. Possible underlying mechanisms for this effect include upregulation of levels of EGFR and anti-inflammatory elements such as IL-10 [73, 74]. This provides further evidence for amelioration of intestinal injury secondary to bacterial enteritis associated with C. butyricum treatment.
Effects of C. butyricum in the Context of Disease Spectrum
Regarding these beneficial characteristics of C. butyricum, it has been used in various ailments, such as metabolic disorders, cancer, gastrointestinal infection, neurological disorders, cerebrovascular diseases, and aging.
Effects of C. butyricum on Metabolic Disorders
C. butyricum inhabiting the gastrointestinal tract may enter the systemic circulatory system by transmigration through the gut barrier, and may interfere with host metabolic signals via endocrine modulation. The metabolite, butyrate, is postulated to be the trigger that enables these processes [40]. A negative correlation exists between enrichment of C. butyricum and fat accumulation, and the total number of this particular organism is reduced in murine models of type 2 diabetes [75]. Additional supplementation with C. butyricum is likely to benefit diabetic mice in the following metabolic ways: (1) Glucose. C. butyricum may increase insulin concentrations, promote glucose catabolism, and decrease levels of fructosamine. C. butyricum has also been shown to increase the abundance of peroxisomes and improves insulin sensitivity [19, 76]. (2) Lipids: Administration of C. butyricum upregulates the respiratory exchange ratio (RER), enhances mitochondrial function, stimulates ANGPTL4 production to prevent fat build-up, and eventually reduces weight gain [19, 76, 77]. (3) Metabolites: With C. butyricum supplementation, butyrate-producing gene expression is enhanced, and the reduction of butyric acid production is improved in obese mice with diabetes [76]. In the disease model of non-obese diabetic (NOD) mice, C. butyricum intake may induce intestinal Tregs production, inhibit the accumulation of pro-inflammatory cytokines such as IFN-γ in the pancreas, and decrease the degree of insulitis by reduction of pancreatic autoimmune injury, thus delaying the onset of diabetes [49]. Moreover, the levels of butyrate in feces are also increased with C. butyricum treatment [49].
Reports have revealed that in animal NAFLD models, besides the methods described above, treatment with C. butyricum may also reduce hepatic lipid accumulation at source and improve hepatic inflammatory indicators [40, 78]. Specifically, this therapy reduces the synthesis of triglycerides, promotes the conversion of cholesterol into bile acid, and boosts excretion of excessive lipids [78]. Also, the presence of C. butyricum has a protective effect on acute hepatic injury and raises the activity of anti-oxidant enzymes and their correlated factors, enhances anti-inflammatory reactions and anti-oxidant responses, and repairs the damaged liver to improve survival rates in mice [79]. Diabetic patients may also suffer from NAFLD, which ranges from hepatitis, fibrosis, cirrhosis, and eventually to hepatocellular carcinoma [13, 80]. The combination of C. butyricum and statin therapy can effectively improve the intestinal flora dysbiosis, lower blood lipid levels, reduce liver fibrosis and inflammation, and further alleviate liver function damage in patients with NAFLD [21]. One single-blind clinical trial with C. butyricum in elderly people revealed that intervention with C. butyricum could facilitate SCFA production, increase the probiotics abundance, and accelerate bile acid metabolism, eventually enhancing immunity and nutrition [81]. Dietary fiber could promote SCFA producers in greater abundance and more diversity, leading to increased GLP-1 production, improved hemoglobin A1c levels, and better prognosis in type 2 diabetes population [82].
Effects of C. butyricum on Cancer
Intestinal microorganisms, including the C. butyricum community, are essential for the maintenance of intra-epithelial homeostasis, while immune homeostasis imbalance, inflammation, and oncogenesis are inextricably linked [83,84,85].
CRC remains a major cause for cancer-related mortality worldwide [86]. Compared with the healthy population, butyric acid–producing genes in the gut microbiota of CRC patients showed a dramatic reduction, and also a reduction of the amount of butyric-producing bacteria (such as Lachnospiraceae) in their feces [87, 88]. Several CRC murine models have shown that C. butyricum restores weight loss, reduces tumor morbidity, lowers intestinal tumor burden, and improves tumor survival rate [24, 89, 90]. Evidence suggests that C. butyricum induces the up-regulation of the mir-200c cancer inhibitor at a molecular level, inhibiting colitis-related tumors [89]. It also enhances the expression of the p21 cell cycle inhibitor, suppresses the Wnt cell proliferation signal pathway, arrests the cell cycle, and thus inhibits proliferation of CRC cells [91, 92]. Researchers have also observed the enhancement of apoptosis in CRC cells, consistent with an increase of the Bax pro-apoptosis protein and a decreased level of the Bcl-2 anti-apoptosis protein [24]. C. butyricum and its metabolites are able to modify the tumor microenvironment in vitro and to restrain growth and reproduction of the intestinal enterotoxigenic Bacteroides fragilis carcinogenic bacterium [93]. In the murine CRC model, supplementation of C. butyricum triggers anti-inflammatory immuno-signals, which inhibits NF-κB signal transduction, and reduces the expression of the TLR4 and IL-22 pro-inflammatory elements, decreasing enteric leakage, and maintaining the integrity of the intestinal barrier [24, 90, 94]. Also, in a murine colon tumor model, a synergistic anti-tumor effect has been observed with the combination of C. butyricum and the targeting drug, lapatinib [95]. Clinical study suggests that higher fecal SCFA concentrations may indicate a longer progression-free survival and better efficacy when receiving the immune checkpoint blockade (ICB) therapy in solid cancer tumors [96].
Other than colon cancer, C. butyricum has also shown advantages for the treatment of other tumors, and may be used as an auxiliary anti-cancer strategy. C. butyricum strengthens the function of polymorphonuclear neutrophils, and may migrate into the bladder and release TNF-related apoptosis-inducing ligand (TRAIL) [97,98,99]. This may significantly inhibit the growth, and specifically induce apoptosis, of bladder cancer cells in a tumor-bearing murine model, and has been shown to be more effective than Bacillus Calmette-Guérin (BCG) therapy [97]. Cancer patients with higher amounts of blood butyrate seemed to exhibit a better chemotherapeutic response with oxaliplatin [100]. Compared with the group with no C. butyricum supplements, C. butyricum significantly improves the prognosis of non-small cell lung cancer (NSCLC) patients with early ICB treatment [101]. C. butyricum may raise overall survival rates, during which microbiota regulation and immunomodulation may be one potential mechanism of action [13, 101]. C. butyricum may also assist in neutralizing the more severe adverse effects of anti-cancer drugs used for pulmonary carcinoma, particularly diarrhea [102]. In patients with H. pylori infection, C. butyricum may prevent gastric cancer by eradicating H. pylori [103]. Oral supplement of C. butyricum in gastric cancer patients after gastrectomy can significantly reduce pro-inflammatory factors or cells, upregulate intestinal SCFAs, and increase the enrichment of beneficial bacteria such as Bacteroides, ultimately decreasing the incidence of postoperative complications [104].
Effects of C. butyricum on Gastrointestinal Infection
Gastrointestinal tract infection is caused by disruption of the balance of the originally existing intestinal microbiota by the invasion of different pathogens, and manifests as vomiting, intestinal spasm, diarrhea, and fever [105, 106]. Bacterial infections account for a significant portion of gastrointestinal infections [107]. A comparative study reported that the inflammatory bowel disease (IBD) susceptible population manifested lower fecal bacterial abundance, weaker fiber digestion efficacy, and insufficient SCFA-producing bacteria [108]. In the rat model of bacterial enteropathy, C. butyricum significantly relieves intestinal discomfort and reduces the frequency of diarrhea [109]. A number of animal models and clinical investigations have observed that probiotic supplementation may lower the risk of gastrointestinal tract infection and enhance host immunity [110,111,112]. Among the various probiotic bacterial species, C. butyricum is often used as a probiotic supplement to improve the symptoms of gut infections [112]. As has been previously mentioned, C. butyricum curbs the reproduction and harmful behavior of the deleterious bacterium, C. difficile [68]. The representative fermentation product butyrate exhibits potential antibacterial activities towards C. difficile, strengthens the intestinal barrier, and maintains host immune homeostasis [113, 114]. C. butyricum also downregulates the concentration of succinic acid in the intestines of mice, preventing the colonization of C. difficile, suppresses pro-inflammatory cytokines, such as TNF-α, induces macrophage aggregation in the intestinal lumen, and restores intestinal epithelial damage [112, 115, 116]. Additionally, C. butyricum also mobilizes the host immune system to function optimally. The butyrate metabolite has been reported to activate the GPR43/109a signal transduction pathway, induce the production of neutrophils, upregulate the release of pathogen-specific antibodies, and promote Th17 cells to participate in intestinal epithelial barrier reinforcement, which may ultimately weaken the pathogenicity of pathogens to a certain extent, just as for C. difficile [16, 112, 117]. Clinical data showed that C. butyricum may effectively suppress H. pylori to a certain extent in human [103]. When combined with antibiotics in H. pylori positive patients, it could help decrease variations in gut microbiota and alleviate the severity of treatment related diarrhea [118, 119]. C. butyricum can also be used as a probiotics to ameliorate stool consistency and frequency, and improve quality of life in IBS population [50]. In patients with UC, administration of C. butyricum can reduce the risk of postoperative pouchitis occurrence [22]. Therapy with C. butyricum to alleviate IBD is currently under study (NCT02614963). Therefore, C. butyricum is likely to prevent and treat gastrointestinal tract infection, and may be used as a probiotic for interventional therapeutic purposes.
Effects of C. butyricum on Neurological Disorders
Gastrointestinal microbiota may regulate CNS endocrine function, alter the signal transmission of neurotransmitters, and may even activate immune reactions within the nervous system via the microbiota-entero-brain axis that discourages and reduces harmful neuroinflammation [13, 120]. A meta-analysis showed that SCFA-producing bacteria were actually decreased in people with Parkinson’s disease [121]. In murine models, C. butyricum improves and treats neurodegenerative diseases by activating the Akt pathway and reducing cell apoptosis [25, 122]. C. butyricum has been shown to also alleviate or even reverse the hippocampal injury of mice caused by cerebral ischemia, mainly strengthening the spatial learning capacity and improving memory deficiency [122, 123]. H. Li and colleague observed that C. butyricum treatment also effectively enhances motor and sensory function, and reduces neurodegeneration in mice afflicted by TBI [25]. Nerve function benefits from increased enrichment of butyrate in the brain via anti-apoptotic mechanisms [124]. Research suggests that GLP-1 may protect the integrity of the blood–brain barrier by up-regulating levels of TJ proteins, and assumes a neuroprotective role in the murine brain injury model [125, 126]. Treatment with C. butyricum may increase the expression of the GLP-1 receptor in the intestine and brain, which may mediate intestinal and brain barrier function through GLP-1 signaling, and protecting the blood–brain barrier, alleviating brain edema, and addressing neuronal injury [25]. C. butyricum may also be used in the management of multiple sclerosis. Treatment with C. butyricum significantly reduces the myelin loss resulting from attack by the immune system and may mitigate neuropathological inflammation at lesional sites in mice [54].
Effects of C. butyricum on Cerebrovascular Diseases
Cerebral ischemia/reperfusion (I/R) can lead to severe brain damage [127]. Jing Sun and associates constructed a mice model of cerebral I/R, and observed that C. butyricum increases the content of butyrate in the brain, which enhances the activity of the superoxide dismutase (SOD) anti-oxidant, and significantly decreases protein expression of apoptosis-related Caspase-3 and Bax, thus improving neurological deficits present in these patients [124]. Diabetes may also aggravate cerebral injury induced by I/R, leading to an exacerbation of neural functional deficits [128, 129]. C. butyricum has been shown to be capable of reversing this situation in mice models. Apart from the mechanism already mentioned, C. butyricum may also reduce protein levels of phosphorylated Akt and inhibit the apoptosis of nerve cells. Thus, reconsidering its protective potential, C. butyricum may be used as an effective adjuvant therapeutic strategy for the treatment of cerebrovascular disease in diabetics [123]. Additionally, in murine models of vascular dementia or Alzheimer’s disease (AD), the therapeutic effects of C. butyricum include restoration of butyrate levels in the gut and brain, improvement of cognitive impairment, an increase in levels of Bcl-2, and a reduction of neuronal apoptosis [122, 130]. Clinical results showed that patients with acute ischemic stroke (AIS) usually present less SCFA-producing bacteria and lower fecal SCFAs concentrations, when compared to healthy controls [131]. Several clinical studies also found out that a negative correlation existed between SCFAs levels and stroke severity or disability risk in AIS patients [131,132,133].
Effects of C. butyricum on Aging
The lifespan of organisms may be influenced by C. butyricum. Subsequent to being fed with the C. butyricum probiotic strain, the lifespan of Caenorhabditis elegans (C. elegans) is significantly prolonged, locomotive efficiency is enhanced, and the resistance to pathogenic bacterial infection (such as Staphylococcus aureus) is enhanced when compared to the control group [134]. This may be attributable to regulation of Nrf2 transcription factor and IGF-1 signal transduction, which are two key factors associated with longevity [134,135,136].
Typical Non-AIDS Comorbidities in PLWH
Coincidentally, these above diseases are prevalent in PLWH, and are recognized as non-AIDS comorbidities. In developing countries, with the popularization and optimization of modern combined antiretroviral treatment (ART), survival rates of PLWH have been gradually improving over the past three decades [137]. However, with HIV virus replication controlled, non-AIDS comorbidities gradually emerge and increase, resulting in an elevated mortality rate in this population that may even exceed rates of mortality caused by HIV itself [138]. Based on many investigations, typically encountered non-AIDS comorbidities comprise various metabolic and neoplastic diseases, which will be elaborated on in the following sections.
For PLWH, the dysfunctional catabolic state caused by the uncontrollable HIV viremia and ART may induce variations in adipose tissue immunity, which may elevate prevailing obesity rates [139,140,141]. Excess adiposity may increase the risk of metabolic abnormalities, including diabetes and hepatic disease [142,143,144]. Compared to the HIV-negative population, obese or overweight PLWH have a higher risk of developing the preceding metabolic diseases [142, 144]. One all-male study observed that the incidence of diabetes in HIV-negative controls was over 4 times lower than that in PLWH [145]. Assuming identical weight gain, the increased risk of diabetes in PLWH is nearly twice as that of HIV-negative patients [144]. For PLWH, the lower the baseline CD4 + T-cell count and the older the protease inhibitors or nucleoside reverse transcriptase inhibitors used, the higher the risk is for the emergence of diabetes [145, 146]. Hepatic disease is the most common non-AIDS associated comorbidity in PLWH [147]. The prevalence of NAFLD in PLWH is higher than that in the general population, and the use of some specific antiretroviral drugs may be one reason for this [148,149,150]. PLWH are more prone to develop steatohepatitis or hepatic fibrosis than HIV-negative individuals, and this may be related to increased adipogenesis and decreased lipid clearance [151,152,153]. Lipodystrophy, attributed to the redistribution of human fatty tissue, is another common metabolic abnormality in PLWH, and considered an adverse effect of ART [154]. This could change the external appearance of individuals, affect their personality and life quality, and further increase the risk of diabetes and cardiovascular diseases through hyperlipidemia, insulin resistance, and impaired endothelial function [154,155,156]. Diabetes, NAFLD, and lipodystrophy constitute the common abnormal metabolic conditions seen in HIV.
Cancer accounts for 10–20% of mortality in PLWH [157, 158]. Compared to healthy individuals, PLWH also exhibit a heightened risk of malignancies, with a higher mortality rate and a poorer prognosis [159, 160]. For PLWH, malignant tumors are classified into two main categories, non-AIDS-defining cancers (NADCs) and traditional AIDS-defining cancers (ADCs); the latter includes Kaposi’s sarcoma, invasive cervical cancer, and non-Hodgkin’s lymphoma (NHL) [161, 162]. NADCs account for the majority (approximately two-thirds) of all cancers in PLWH [163, 164]. This is likely to be related to the prolongation of the average life expectancy of this population after ART treatment, and is expected to continue growing [165, 166]. Overall, in PLWH, the cumulative incidence rate of NADCs is approximately triple that of ordinary people [164, 167]. Among the NADCs, virus-associated NADCs such as hepatitis B virus–related hepatoma and EBV-related lymphoma have an even higher-than-expected incidence [164]. This may be explained by the uniquely low CD4 + T-cell counts found in PLWH [168]. Smoking may be a factor contributing towards a higher prevalence of ADCs (including laryngeal cancer and lung cancer) in PLWH, when compared to HIV-negative individuals [169, 170]. In people with Hodgkin lymphoma (HL), the 3-year survival rate for the HIV-positive cohort was observed to be statistically lower than that in the negative control cohort [171]. A similar trend was observed in those with cervical cancer and NHL [171, 172]. In fact, the implementation of ART has been observed to reduce the risk of most cancers among HIV-infected patients [173, 174]. This may be secondary to HIV viral suppression and the rejuvenation of cellular immunity among those PLWH using ART [175]. When ART is used in conjunction with anti-cancer therapeutic strategies, such as radiotherapy or chemotherapy, enhanced therapeutic effectiveness is observed for tumors in PLWH, compared to ART alone (albeit with an augmented adverse effect profile) [176, 177]. It is, therefore, particularly critical to weigh and consider the balance between therapeutic efficacy and toxicity when using multiple therapeutic modalities in tandem. Additionally, reduction of principal cancer risk factors such as smoking, alcohol consumption, and obesity should be promoted and implemented as adjunctive cancer prevention strategies in PLWH [162, 163].
Beyond this, the incident rate of a wide variety of disorders such as gastrointestinal infection, neurological disorders, cerebrovascular disease, and aging has also increased in PLWH, when compared with non-HIV-infected populations. The gastrointestinal tract is the principal site for HIV replication, due to high expression of CCR5 and abundant GALT [178]. HIV infection initially leads to disruption of gastrointestinal mucosal integrity, and subsequently to increased gut permeability and enhanced microbial translocation, and ultimately causes chronic intestinal inflammation [179], resulting in the increased incidence and severity of gastrointestinal infections such as enteritis. One primary manifestation of the neurological disorder spectrum in PLWH is the development of cognitive impairment [180]. When compared to healthy controls, approximately half of PLWH show impaired cognitive functioning, such as dementia [180, 181]. PLWH also experience a higher risk (ranging from 40 to 150%) for different cardiovascular diseases (CVDs), including acute myocardial infarction (AMI), heart failure, ischemic stroke, and coronary heart disease when compared to normal controls [182,183,184,185]. Also, CVD accounts for 20% of non-AIDS deaths in PLWH, and these patients exhibit a 70% higher mortality rate from CVD-related death than the non-HIV-infected population [186]. ART aims to extend the total lifespan of PLWH. However, in treated PLWH, with the associated prolongation of survival, the age of onset of senility-related complications or syndromes tends to be earlier, and the incident rate of these conditions is correspondingly increased [187]. Even with ART, systemic inflammation and immune disorders prevail, along with chronic persistent HIV infection attributable to the HIV reservoir, ultimately leading to an overall decline in life expectancy [188].
HIV Infection Promotes the Onset of Non-AIDS Comorbidities Through the Leaky Gut Syndrome
HIV-Associated Leaky Gut Syndrome
Gut microbiota strongly participates to gut homeostasis [189]. For example, evidences indicate that commensal bacteria can regulate the balance between pro-inflammatory and anti-inflammatory T-helper subsets (example Th17 and Tregs) involved in host responses [189]. In mice, Bacteroides fragilis can inhibit Th17 differentiation and Tregs proliferation. In humans, Th17 differentiation is also inhibited by the YCH46 strain of Bacteroides fragilis through the production of propionic acid [190]. In the context of HIV infection, it has been demonstrated that cell cultures of lamina propria with heat-killed Escherichia coli favor increased activation, proliferation, and HIV infection of CD4 + T-cells [191]. Concretely, compared to uninfected individuals, greater productions of TNF-α and IL-10 were noted in peripheral cells from HIV-infected patients when exposed to bacterial products of Prevotella spp., Bacteroides spp., and Erysipelotrichaceae [192]. Further ex vivo investigations have shown that altered gut microbiota contributes to enhance HIV replication ex vivo [193]. Altogether, studies in the HIV research field indicate that HIV infection profoundly perturbates the gut homeostasis through a particular feature referred to as HIV-associated gut dysbiosis syndrome. In turn, the gut dysbiosis strongly participates in the onset of the leaky gut syndrome. By definition, the leaky gut syndrome refers to an intestinal condition whereby the intestinal lining allows microorganisms (bacteria, fungi, etc.) and toxins to enter the bloodstream [194, 195]. In other words, during HIV infection, the leaky gut syndrome is a consequence of perturbations seen in the gut homeostasis, particularly changes in the immune system, inflammation, and dysbiosis [196]. This topic has been extensively investigated in previous research [27], and our group even believe that for this reason, the gut microbiota represents an important target to cure incomplete immune reconstitution in HIV infection [27, 197].
It has been shown that gut microbial communities differ significantly from that within the GI tracts of HIV-uninfected individuals [198, 199]. Most past studies [200,201,202] and one recent meta-analysis [203] uphold the preceding conclusion; however, some research groups have not observed any change in gut microbiota diversity during HIV infection, compared to HIV-negative individuals [192, 204]. It is, however, accepted that HIV infection provokes the onset of a change characterized by a reduction in symbiotic beneficial bacteria, and an elevation in levels of potentially pathogenic bacteria [205, 206], and this significantly impacts gut barrier integrity. Indeed, the diminution of beneficial bacteria (Akkermansia muciniphila, Bacteroides, Bacteroides vulvae, Diplococcus, and Arbuscular roseus) induces a significant reduction of their protective effects [digestion of carbohydrates into SCFA such as butyrate, acetate, and propionate] [207], which leaves the gut barrier relatively unprotected [208]. Furthermore, bacterial metabolites as butyrate can enhance Tregs differentiation [209], while Clostridium spp. are responsible for Tregs accumulation [52]. Conversely, the augmentation of levels of potentially pathogenic bacteria [Proteus, Enterococcus, Klebsiella, Shigella, and Streptococcus [201, 210]] and their toxins participate in the ultimate weakening and dismantling of the intestinal barrier. Consequently, microorganisms (bacteria, fungi, etc.) and toxins may readily translocate into the bloodstream. In short, gut microbiota and its related metabolites contribute (positively or negatively) in the gut homeostasis which is deteriorated by HIV infection.
In addition to microbial dysbiosis, HIV replication within intestinal cells directly instigates apoptosis and gut epithelial TJ disruption, which subsequently induces the leaky gut syndrome and promotes microbial translocation [211, 212]. Besides the apoptosis, it is also acknowledged that pyroptosis is responsible for the death of HIV-infected cells [213]. Interestingly, one category of HIV-positive individuals, referred to as elite controllers (ECs), do not possess the preceding gastrointestinal profile. It has been observed that HIV infection does not significantly affect the gut microbiome of ECs [214]. Thus, ECs tend to have a relatively analogous microbiomic composition to HIV-uninfected individuals [195, 214], and a conspicuous absence of the enduring leaky gut syndrome ubiquitously seen in HIV-infected individuals who are not ECs. Given that ECs (i) preserve their CD4 + T-cell counts in the gut [215], (ii) have lower levels of immune activation [216], and (iii) display smaller HIV reservoirs [216], it is therefore valid to further explore the association between the leaky gut syndrome and HIV reservoir persistence.
Furthermore, HIV infection induces the depletion of predominantly CD4 + T-cells [217, 218], and the exhaustion of immune cells in general (characterized by the loss of immune and/or secretory functions) [219,220,221]. However, it has been shown that T-helper 17 (Th17) cytokines, which are secreted by various cells including CD4 + T-cells, CD8 + T-cells, gamma delta T-cells, natural killer T-cells (NKT), and natural killer (NK) cells, are critical for gut barrier integrity. Indeed, Th17 cytokines promote mucosal barrier function through enhancement of the epithelial release of antimicrobial peptides, induction of mucus production, and promotion of wound healing [198, 222]. Thus, in an HIV infection context, both immune cell depletion and exhaustion contribute to the decrease of levels of Th17 cytokines and therefore promotes the leaky gut syndrome. The preceding mechanisms may be summarized as a modulation of the gut microbiome by HIV infection [198, 199, 223, 224], which progressively leads to (i) changes in microbial diversity [205, 206], (ii) damage to the intestinal barrier, (iii) impairment of mucosal immunological function, (iv) augmentation of microbial translocation, and (v) long-term immune activation. Therefore, during HIV infection, the gut microbiota and their metabolites are thought to be essential in contributing to the persistence of inflammation and immune activation, even in ART-treated individuals [225,226,227]. Nevertheless, it is worth mentioning that ART acts differently on the gut microbiota. Evidence suggests that PLWH receiving integrase inhibitors seem to have a gut microbiota more similar to healthy individuals [228]. In recent publications, Villoslada-Blanco et al. have successively indicated that the administration of integrase inhibitors alleviates both the leaky gut syndrome and the inflammation [229], and partially restores gut dysbiosis at the viral level [230]. These findings represent another area of investigation which may further explain the interesting role played by ART in the composition of gut microbiota. In the following section, we demonstrate how the leaky gut syndrome fosters the onset of non-AIDS comorbidities via inflammation and immune activation.
Leaky Gut Promotes Non-AIDS Comorbidities
Cumulative evidence suggests that the continuing state of inflammation seen in PLWH is primarily driven by the leaky gut syndrome during HIV infection, and is associated with an increased risk of development of non-AIDS comorbidities. Hence, translocated microbial products, which are recognized by the immune system as pathogen-associated molecular patterns (PAMPs), may induce inflammation and thus lead to development of non-AIDS comorbidities. Upon PAMP recognition, pattern recognition receptors (PRRs) present at the cell surface trigger pro-inflammatory and antimicrobial responses by activation of a multitude of intracellular signaling pathways, including adaptor molecules, kinases, and transcription factors [231]. In other words, microbial translocated products recognized by PRRs trigger PRR-mediated signaling and induction of an innate immune response (comprising gene expression and synthesis of a broad range of immune molecules, including cytokines, chemokines, cell adhesion molecules, and immunoreceptors [232], which ultimately sustains the prevailing inflammatory state). These processes, however, have several detrimental consequences as they may promote the onset of non-AIDS comorbidities.
The leaky gut facilitates the translocation of microbial products which in turn may activate monocytes and macrophages via interactions with TLRs (TLR4 for example). This activation is characterized by the increase circulating levels of IL-6, sCD14, and sCD163 [233]. In the context of HIV infection, the inflammation exhibited by monocytes underlies HIV pathology and is associated to age-related comorbidities. To illustrate this, Alzahrani et al. have indicated that monocytes activation is associated with all-risk of mortality in PLWH, particularly those on ART [27]. As such, the preceding immune cells increase glycolysis and lactate production, which ultimately favor immunosenescence and immune cell aging [234]. This context establishes an environment in which HAND, CVD, frailty, and osteoporosis can develop [235,236,237,238]. The preceding case represents pieces of evidence towards the major role played by the leaky in the development of non-AIDS comorbidities, and many studies have already reported findings corroborating this [6, 27, 212, 239].
In a past hypothesis review [212], our research team has explored the influence of the leaky gut syndrome on the onset of HBV infection. In that review, we have indicated that once in the portal vein, translocated microbes and their products are able to reach hepatocytes and activate the liver’s innate immune system. Consequently, hepatocytes are injured by PAMPs produced by intestinal microbes, and therefore become vulnerable to HBV. Beyond HBV infection, we believe that the leaky gut represents a widely opened gateway via which translocated microbial products may injure the liver and induce liver cancer. Based on published evidence, it has been shown that during HIV infection, gut microbiota enhances the degree of tryptophan catabolism to kynurenine [198]. In the context of the leaky gut syndrome, kynurenine may reach the liver and induce cancer by suppression of liver anti-tumor immunity (e.g., activation of the aryl hydrocarbon receptor and CD39 in tumor-associated immune cells which become dysfunctional). This phenomenon has been observed and reported by several research teams [240,241,242,243]. Additionally, secondary bile acids, which are known to be converted from primary bile acids (cholic acid and chenodeoxycholic acid) solely by gut microbial bile acid-inducible operon via the 7-α-dehydroxylation process [244, 245], have been shown to be capable of inducing liver cancer by promotion of the senescence-associated secretory phenotype (secretion of inflammatory cytokines, chemokines, proteases, and growth factors which promote liver cancer progression) in hepatic stellate cells [246]. A complete review of gut-liver axis mechanisms potentially involved in liver cancer development has been published by Yu and Schwabe [247], and subsequently by Ohtani and Hara [248].
Microbial translocation has also been linked to several pathological conditions and/or diseases. Indeed, Morris et al. [249] have observed that HIV-positive individuals displaying a leaky gut syndrome with higher circulating (1– > 3)-beta-D-glucan (BDG) levels had a higher frequency of cardiopulmonary abnormalities (including a reduced diffusion capacity for carbon monoxide), a higher pulmonary artery systolic pressure, and an increased tricuspid regurgitant jet velocity. Similarly, Isnard et al. [250] have observed an association between plasma BDG levels and subclinical coronary atherosclerotic plaque formation in ART-treated PLWH. Higher blood BDG levels have also been associated with (i) greater trunk and total body fat accumulation [251] and (ii) poorer cognitive functioning (elevated levels of BDG in cerebrospinal fluid are associated with worse Global Deficit Scores) [252] in PLWH. Furthermore, one cross-sectional study (including 11 years of follow-up after ART commencement) by Hoenigl et al. [253] has observed that soluble urokinase plasminogen activator receptor (a marker for monocyte, T-cell, and plasminogen activation) [254,255,256,257,258] is predictive of non-AIDS medical events, including myocardial infarction, stroke, non-AIDS malignancy, bacterial infection, and death from a non-AIDS-related event.
To date, several animal studies have observed the beneficial effects of the probiotic use of C. butyricum on gut microbes, intestinal health, and anti-inflammatory responses [15, 259,260,261,262,263]. In general, results indicate that dietary supplementation with C. butyricum may improve the preceding factors and also enhance mucosal barrier functioning. Administered to humans, C. butyricum is safe, and improves inflammation and immunity [81, 104, 264]. Intervention with C. butyricum administration in HIV-positive individuals may also help to mitigate the effects of HIV-associated gut dysbiosis syndrome. However, to our knowledge and in the particular context of HIV infection, specific clinical trials investigating the benefits of C. butyricum supplementation on gut health remain sparse in the literature. Future studies focusing on probiotic C. butyricum supplementation in humans will provide more definitive answers with regard to potential adverse effects and safety concerns.
Conclusion
Due to the enduring presence of chronic inflammation, HIV-infected individuals have an elevated incidence of a series of non-AIDS comorbidities, such as metabolic disorders, cancer, gastrointestinal infections, neurological disorders, and cerebrovascular diseases. The presence of C. butyricum in the gut has been shown to have multiple beneficial outcomes, such as amelioration of inflammation, regulation of immune homeostasis, maintenance of the structural and functional integrity of the intestinal barrier, and inhibition of intestinal pathogenic bacterial species. An increase in the abundance of C. butyricum in the gut helps to prevent and manage various inflammation-associated diseases, including metabolic syndromes, tumors, intestinal infections, and neurological diseases. Additionally, HIV infection may promote the emergence and development of the preceding non-AIDS complications via aggravation of a leaky gut, suggesting that C. butyricum may be a fundamental participant in relation to the non-AIDS complications associated with HIV infection. Therefore, it may be possible to inhibit the occurrence and slow down the progress of non-AIDS-related diseases by increasing the concentration of C. butyricum or its metabolites in the gut, thereby reducing the chronic inflammatory state and maintaining the dynamic equilibrium of the intestinal microecology. This may have clinical value for the monitoring of disease development and evaluation of the effects of treatment and of prognosis among HIV-infected individuals. However, further studies are warranted to confirm the clinical benefits of an enriched C. butyricum presence in HIV-positive patients, and to further explore the mechanisms whereby C. butyricum therapeutic utilization as a probiotic supplement exerts its effects on the maintenance and restoration of human intestinal microbiotic ecosystems in HIV-infected patients, and in the mitigation of non-AIDS complications in these individuals. Multidisciplinary research cooperation is necessary and inevitable for this quest, and future research priorities will require a focus on both the known and as yet undiscovered biological properties of C. butyricum, and to seamlessly and creatively combine the knowledge gleaned therefrom with the disciplines of metabonomics, microbiology, and oncology. This future research may well reveal whether C. butyricum is a potentially viable future therapeutic candidate for PLWH, and is likely to go a long way towards mitigating the persisting gastrointestinal inflammation present in HIV-infected individuals, decreasing non-HIV-related complications, and improving the overall quality of lives of PLWH.
References
Stelzle D et al (2021) Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health 9(2):e161–e169
Williams ME et al (2021) Cerebrospinal fluid immune markers and HIV-associated neurocognitive impairments: a systematic review. J Neuroimmunol 358:577649
Zicari S et al (2019) Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 11(3)
Savinelli S et al (2022) Obesity in HIV infection: host-pathogen interaction. AIDS 36(11):1477–1491
Yang Q et al (2020) Short-chain fatty acids: a soldier fighting against inflammation and protecting from tumorigenesis in people with diabetes. Front Immunol 11:590685
Routy JP, Royston L, Isnard S (2022) Aging with grace for people living with HIV: strategies to overcome leaky gut and cytomegalovirus coinfection. J Acquir Immune Defic Syndr 89(Suppl 1):S29-s33
Galperin C, Gershwin ME (1997) Immunopathogenesis of gastrointestinal and hepatobiliary diseases. JAMA 278(22):1946–1955
Lapenta C et al (1999) Human intestinal lamina propria lymphocytes are naturally permissive to HIV-1 infection. Eur J Immunol 29(4):1202–1208
Mehandru S et al (2005) The gastrointestinal tract is critical to the pathogenesis of acute HIV-1 infection. J Allergy Clin Immunol 116(2):419–422
Anton PA et al (2000) Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS 14(12):1761–1765
Neurath MF, Überla K, Ng SC (2021) Gut as viral reservoir: lessons from gut viromes, HIV and COVID-19. Gut 70(9):1605–1608
Mountzouris KC, McCartney AL, Gibson GR (2002) Intestinal microflora of human infants and current trends for its nutritional modulation. Br J Nutr 87(5):405–420
Stoeva MK et al (2021) Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 13(1):1–28
Wang Y et al (2018) Lactobacillus acidophilus and Clostridium butyricum ameliorate colitis in murine by strengthening the gut barrier function and decreasing inflammatory factors. Benef Microbes 9(5):775–787
Li W et al (2021) Effects of Clostridium butyricum on growth performance, gut microbiota and intestinal barrier function of broilers. Front Microbiol 12:777456
Hagihara M et al (2021) Clostridium butyricum enhances colonization resistance against Clostridioides difficile by metabolic and immune modulation. Sci Rep 11(1):15007
Wang K et al (2019) Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, intestinal structure, and inflammation in lipopolysaccharide-challenged weaned piglets. J Anim Sci 97(10):4140–4151
Zhao X et al (2020) Clostridium butyricum ameliorates Salmonella enteritis induced inflammation by enhancing and improving immunity of the intestinal epithelial barrier at the intestinal mucosal level. Front Microbiol 11:299
Shang H, Sun J, Chen YQ (2016) Clostridium butyricum CGMCC0313.1 modulates lipid profile, insulin resistance and colon homeostasis in obese mice. PLoS One 11(4):e0154373
Jia L et al (2017) Anti-diabetic effects of Clostridium butyricum CGMCC0313.1 through promoting the growth of gut butyrate-producing bacteria in type 2 diabetic mice. Sci Rep 7(1):7046
Zhu W et al (2022) Effects of Clostridium butyricum capsules combined with rosuvastatin on intestinal flora, lipid metabolism, liver function and inflammation in NAFLD patients. Cell Mol Biol (Noisy-le-grand) 68(2):64–69
Yasueda A et al (2016) The effect of Clostridium butyricum MIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis. Surg Today 46(8):939–949
Chen D et al (2020) Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett 469:456–467
Liu M et al (2020) Clostridium butyricum modulates gut microbiota and reduces colitis associated colon cancer in mice. Int Immunopharmacol 88:106862
Li H et al (2018) Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol Motil 30(5):e13260
Hsu DC, Sereti I (2016) Serious non-AIDS events: therapeutic targets of immune activation and chronic inflammation in HIV infection. Drugs 76(5):533–549
Alzahrani J et al (2019) Inflammatory and immunometabolic consequences of gut dysfunction in HIV: parallels with IBD and implications for reservoir persistence and non-AIDS comorbidities. EBioMedicine 46:522–531
Guo P et al (2020) Clostridium species as probiotics: potentials and challenges. J Anim Sci Biotechnol 11:24
Rivière A et al (2016) Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol 7:979
Sun H et al (2021) Increased production of short-chain fatty acids in microbacteria fermentation treated by fullerenols. J Nanosci Nanotechnol 21(10):5352–5362
Vital M et al (2013) A gene-targeted approach to investigate the intestinal butyrate-producing bacterial community. Microbiome 1(1):8
Bui TP et al (2015) Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal. Nat Commun 6:10062
Christiansen CB et al (2018) The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol 315(1):G53-g65
den Besten G et al (2015) Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 64(7):2398–2408
Gao Q et al (2012) Clostridium butyricum activates TLR2-mediated MyD88-independent signaling pathway in HT-29 cells. Mol Cell Biochem 361(1–2):31–37
Juan Z et al (2017) Oral administration of Clostridium butyricum CGMCC0313-1 reduces ovalbumin-induced allergic airway inflammation in mice. Respirology 22(5):898–904
Hayashi A et al (2013) A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe 13(6):711–722
Zhang J et al (2017) Oral administration of Clostridium butyricum CGMCC0313-1 inhibits β-lactoglobulin-induced intestinal anaphylaxis in a mouse model of food allergy. Gut Pathog 9:11
Atarashi K et al (2013) Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500(7461):232–236
Zhou D et al (2017) Clostridium butyricum B1 alleviates high-fat diet-induced steatohepatitis in mice via enterohepatic immunoregulation. J Gastroenterol Hepatol 32(9):1640–1648
Zhang HQ et al (2009) Therapeutic effects of Clostridium butyricum on experimental colitis induced by oxazolone in rats. World J Gastroenterol 15(15):1821–1828
Qing Y et al (2019) Gut microbiome, short-chain fatty acids, and mucosa injury in young adults with human immunodeficiency virus infection. Dig Dis Sci 64(7):1830–1843
Brauckmann V et al (2022) Influence of dietary supplementation of short-chain fatty acid sodium propionate in people living with HIV (PLHIV). J Eur Acad Dermatol Venereol 36(6):881–889
Ben-Neriah Y, Karin M (2011) Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol 12(8):715–723
Zhou M et al (2022) Clostridium butyricum inhibits the progression of colorectal cancer and alleviates intestinal inflammation via the myeloid differentiation factor 88 (MyD88)-nuclear factor-kappa B (NF-κB) signaling pathway. Ann Transl Med 10(8):478
Zhang Y et al (2022) Toll-like receptor 4 (TLR4) inhibitors: current research and prospective. Eur J Med Chem 235:114291
Piirilä P et al (2010) Matrix metalloproteinases-7, -8, -9 and TIMP-1 in the follow-up of diisocyanate-induced asthma. Allergy 65(1):61–68
Zhao Q et al (2019) Clostridium butyricum alleviates intestinal low-grade inflammation in TNBS-induced irritable bowel syndrome in mice by regulating functional status of lamina propria dendritic cells. World J Gastroenterol 25(36):5469–5482
Jia L et al (2017) Clostridium butyricum CGMCC0313.1 protects against autoimmune diabetes by modulating intestinal immune homeostasis and inducing pancreatic regulatory T cells. Front Immunol 8:1345
Sun YY et al (2018) The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Sci Rep 8(1):2964
Sisson G et al (2014) Randomised clinical trial: a liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome–a 12 week double-blind study. Aliment Pharmacol Ther 40(1):51–62
Atarashi K et al (2011) Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331(6015):337–341
Kashiwagi I et al (2015) Smad2 and Smad3 inversely regulate TGF-β autoinduction in Clostridium butyricum-activated dendritic cells. Immunity 43(1):65–79
Chen H et al (2019) Gut microbiota interventions with Clostridium butyricum and norfloxacin modulate immune response in experimental autoimmune encephalomyelitis mice. Front Immunol 10:1662
Ahmad R et al (2017) Gut permeability and mucosal inflammation: bad, good or context dependent. Mucosal Immunol 10(2):307–317
Paone P, Cani PD (2020) Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut 69(12):2232–2243
Long M et al (2018) Combined use of C. butyricum Sx-01 and L. salivarius C-1–3 improves intestinal health and reduces the amount of lipids in serum via modulation of gut microbiota in mice. Nutrients 10(7)
Jung TH et al (2015) Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr Res Pract 9(4):343–349
Burger-van Paassen N et al (2009) The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J 420(2):211–219
Hagihara M et al (2020) Clostridium butyricum modulates the microbiome to protect intestinal barrier function in mice with antibiotic-induced dysbiosis. iScience 23(1):100772
Peltonen S et al (2007) Tight junction components occludin, ZO-1, and claudin-1, -4 and -5 in active and healing psoriasis. Br J Dermatol 156(3):466–472
Pan LL et al (2019) Clostridium butyricum strains suppress experimental acute pancreatitis by maintaining intestinal homeostasis. Mol Nutr Food Res 63(13):e1801419
Pan LL et al (2019) Clostridium butyricum strains suppress experimental acute pancreatitis by maintaining intestinal homeostasis. Mol Nutr Food Res e1801419
Lee JS et al (2015) Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 43(4):727–738
Gobbetti T et al (2017) Protectin D1(n-3 DPA) and resolvin D5(n-3 DPA) are effectors of intestinal protection. Proc Natl Acad Sci U S A 114(15):3963–3968
Miao RX et al (2018) Effect of Clostridium butyricum supplementation on the development of intestinal flora and the immune system of neonatal mice. Exp Ther Med 15(1):1081–1086
Cooksley CM et al (2012) Targeted mutagenesis of the Clostridium acetobutylicum acetone-butanol-ethanol fermentation pathway. Metab Eng 14(6):630–641
Woo TDH et al (2011) Inhibition of the cytotoxic effect of Clostridium difficile in vitro by Clostridium butyricum MIYAIRI 588 strain. J Med Microbiol 60(Pt 11):1617–1625
Takahashi M et al (2000) Studies of the effect of Clostridium butyricum on Helicobacter pylori in several test models including gnotobiotic mice. J Med Microbiol 49(7):635–642
Erawijantari PP et al (2020) Influence of gastrectomy for gastric cancer treatment on faecal microbiome and metabolome profiles. Gut 69(8):1404–1415
Kunishima H et al (2019) The effect of gut microbiota and probiotic organisms on the properties of extended spectrum beta-lactamase producing and carbapenem resistant Enterobacteriaceae including growth, beta-lactamase activity and gene transmissibility. J Infect Chemother 25(11):894–900
Takahashi M et al (2004) The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice. FEMS Immunol Med Microbiol 41(3):219–226
Kuroiwa T, Kobari K, Iwanaga M (1990) Inhibition of enteropathogens by Clostridium butyricum MIYAIRI 588. Kansenshogaku Zasshi 64(3):257–263
Ma M et al (2021) Overexpression of pEGF improved the gut protective function of Clostridium butyricum partly through STAT3 signal pathway. Appl Microbiol Biotechnol 105(14–15):5973–5991
Obanda DN et al (2020) Abundance of the species Clostridium butyricum in the gut microbiota contributes to differences in obesity phenotype in outbred Sprague-Dawley CD rats. Nutrition 78:110893
Ji SK et al (2017) Preparing the gut with antibiotics enhances gut microbiota reprogramming efficiency by promoting xenomicrobiota colonization. Front Microbiol 8:1208
Zhao X et al (2014) Clostridium butyricum reduce lipogenesis through bacterial wall components and butyrate. Appl Microbiol Biotechnol 98(17):7549–7557
Seo M et al (2013) Clostridium butyricum MIYAIRI 588 improves high-fat diet-induced non-alcoholic fatty liver disease in rats. Dig Dis Sci 58(12):3534–3544
Liu J et al (2017) The hepatoprotective effect of the probiotic Clostridium butyricum against carbon tetrachloride-induced acute liver damage in mice. Food Funct 8(11):4042–4052
Diehl AM, Day C (2017) Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 377(21):2063–2072
Liu L et al (2022) Clostridium butyricum potentially improves immunity and nutrition through alteration of the microbiota and metabolism of elderly people with malnutrition in long-term care. Nutrients 14(17)
Zhao L et al (2018) Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359(6380):1151–1156
Belkaid Y, Harrison OJ (2017) Homeostatic immunity and the microbiota. Immunity 46(4):562–576
Wang JL et al (2014) Infection, antibiotic therapy and risk of colorectal cancer: a nationwide nested case-control study in patients with type 2 diabetes mellitus. Int J Cancer 135(4):956–967
Khandia R, Munjal A (2020) Interplay between inflammation and cancer. Adv Protein Chem Struct Biol 119:199–245
Zygulska AL, Pierzchalski P (2022) Novel diagnostic biomarkers in colorectal cancer. Int J Mol Sci 23(2)
Yang J, Yu J (2018) The association of diet, gut microbiota and colorectal cancer: what we eat may imply what we get. Protein Cell 9(5):474–487
Wang T et al (2012) Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. Isme j 6(2):320–329
Xiao Y et al (2017) Clostridium butyricum partially regulates the development of colitis-associated cancer through miR-200c. Cell Mol Biol (Noisy-le-grand) 63(4):59–66
Chen ZF et al (2015) Probiotics Clostridium butyricum and Bacillus subtilis ameliorate intestinal tumorigenesis. Future Microbiol 10(9):1433–1445
Gartel AL, Radhakrishnan SK (2005) Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res 65(10):3980–3985
Cheng X et al (2019) Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed Pharmacother 110:473–481
Shin DS, Rhee KJ, Eom YB (2020) Effect of probiotic Clostridium butyricum NCTC 7423 supernatant on biofilm formation and gene expression of Bacteroides fragilis. J Microbiol Biotechnol 30(3):368–377
Isono A et al (2007) Clostridium butyricum TO-A culture supernatant downregulates TLR4 in human colonic epithelial cells. Dig Dis Sci 52(11):2963–2971
Xin M et al (2019) Synergistic anti-tumour effects of Clostridium butyricum in combination with apatinib in CT26 colorectal tumour-bearing mice. Anticancer Drugs 30(10):991–997
Nomura M et al (2020) Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open 3(4):e202895
Shinnoh M et al (2013) Clostridium butyricum MIYAIRI 588 shows antitumor effects by enhancing the release of TRAIL from neutrophils through MMP-8. Int J Oncol 42(3):903–911
Kemp TJ et al (2005) Neutrophil stimulation with Mycobacterium bovis bacillus Calmette-Guerin (BCG) results in the release of functional soluble TRAIL/Apo-2L. Blood 106(10):3474–3482
Ludwig AT et al (2004) Tumor necrosis factor-related apoptosis-inducing ligand: a novel mechanism for Bacillus Calmette-Guérin-induced antitumor activity. Cancer Res 64(10):3386–3390
He Y et al (2021) Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab 33(5):988-1000.e7
Tomita Y et al (2020) Association of probiotic Clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol Res 8(10):1236–1242
Tian Y et al (2019) Effects of probiotics on chemotherapy in patients with lung cancer. Oncol Lett 17(3):2836–2848
Zhang J et al (2020) The efficacy and safety of Clostridium butyricum and Bacillus coagulans in Helicobacter pylori eradication treatment: an open-label, single-arm pilot study. Medicine (Baltimore) 99(45):e22976
Cao W et al (2022) Clostridium butyricum potentially improves inflammation and immunity through alteration of the microbiota and metabolism of gastric cancer patients after gastrectomy. Front Immunol 13:1076245
Lim SC, Knight DR, Riley TV (2020) Clostridium difficile and one health. Clin Microbiol Infect 26(7):857–863
Saberpour M, Bakhshi B, Najar-Peerayeh S (2020) Evaluation of the antimicrobial and antibiofilm effect of chitosan nanoparticles as carrier for supernatant of mesenchymal stem cells on multidrug-resistant Vibrio cholerae. Infect Drug Resist 13:2251–2260
Marder EP et al (2017) Incidence and trends of infections with pathogens transmitted commonly through food and the effect of increasing use of culture-independent diagnostic tests on surveillance - Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2013–2016. MMWR Morb Mortal Wkly Rep 66(15):397–403
De Filippo C et al (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 107(33):14691–14696
Oka K et al (2018) Establishment of an endogenous Clostridium difficile rat infection model and evaluation of the effects of Clostridium butyricum MIYAIRI 588 probiotic strain. Front Microbiol 9:1264
Gopalakrishnan V et al (2018) The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 33(4):570–580
Oka A, Sartor RB (2020) Microbial-based and microbial-targeted therapies for inflammatory bowel diseases. Dig Dis Sci 65(3):757–788
Ariyoshi T et al (2022) Effect of Clostridium butyricum on gastrointestinal infections. Biomedicines 10(2)
Chen MX et al (2019) Metabolome analysis for investigating host-gut microbiota interactions. J Formos Med Assoc 118(Suppl 1):S10-s22
Flint HJ et al (2012) The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 9(10):577–589
Hagihara M et al (2018) The impact of Clostridium butyricum MIYAIRI 588 on the murine gut microbiome and colonic tissue. Anaerobe 54:8–18
Hagihara M et al (2019) The impact of probiotic Clostridium butyricum MIYAIRI 588 on murine gut metabolic alterations. J Infect Chemother 25(8):571–577
Hayashi A et al (2021) The butyrate-producing bacterium Clostridium butyricum suppresses Clostridioides difficile infection via neutrophil- and antimicrobial cytokine-dependent but GPR43/109a-independent mechanisms. J Immunol 206(7):1576–1585
Imase K et al (2008) Efficacy of Clostridium butyricum preparation concomitantly with Helicobacter pylori eradication therapy in relation to changes in the intestinal microbiota. Microbiol Immunol 52(3):156–161
Shimbo I et al (2005) Effect of Clostridium butyricum on fecal flora in Helicobacter pylori eradication therapy. World J Gastroenterol 11(47):7520–7524
Carabotti M et al (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28(2):203–209
Hirayama M, Ohno K (2021) Parkinson’s disease and gut microbiota. Ann Nutr Metab 77(Suppl 2):28–35
Liu J et al (2015) Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed Res Int 2015:412946
Sun J et al (2016) Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res 1642:180–188
Sun J et al (2016) Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci Lett 613:30–35
Shan Y et al (2019) The glucagon-like peptide-1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke. J Neuroinflammation 16(1):242
Fukuda S et al (2016) Glucagon-like peptide-1 strengthens the barrier integrity in primary cultures of rat brain endothelial cells under basal and hyperglycemia conditions. J Mol Neurosci 59(2):211–219
Manzanero S, Santro T, Arumugam TV (2013) Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int 62(5):712–718
Murányi M, Lacza Z (2006) Influence of diabetes mellitus on cerebral ischemia and reperfusion injury. Orv Hetil 147(39):1885–1889
Chen R, Ovbiagele B, Feng W (2016) Diabetes and stroke: epidemiology, pathophysiology, pharmaceuticals and outcomes. Am J Med Sci 351(4):380–386
Sun J et al (2020) Effect of Clostridium butyricum against microglia-mediated neuroinflammation in Alzheimer’s disease via regulating gut microbiota and metabolites butyrate. Mol Nutr Food Res 64(2):e1900636
Tan C et al (2021) Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. JPEN J Parenter Enteral Nutr 45(3):518–529
Chou PS et al (2023) The prognostic biomarkers of plasma trimethylamine N-oxide and short-chain fatty acids for recanalization therapy in acute ischemic stroke. Int J Mol Sci 24(13)
Henry N et al (2021) Short chain fatty acids taken at time of thrombectomy in acute ischemic stroke patients are independent of stroke severity but associated with inflammatory markers and worse symptoms at discharge. Front Immunol 12:797302
Kato M et al (2018) Clostridium butyricum MIYAIRI 588 increases the lifespan and multiple-stress resistance of Caenorhabditis elegans. Nutrients 10(12)
Zhao Y et al (2018) Changes to social feeding behaviors are not sufficient for fitness gains of the Caenorhabditis elegans N2 reference strain. Elife 7
Samuelson AV, Carr CE, Ruvkun G (2007) Gene activities that mediate increased life span of C. elegans insulin-like signaling mutants. Genes Dev 21(22):2976–2994
Noubissi EC, Katte JC, Sobngwi E (2018) Diabetes and HIV. Curr Diab Rep 18(11):125
Escota GV et al (2018) Understanding mechanisms to promote successful aging in persons living with HIV. Int J Infect Dis 66:56–64
Tate T et al (2012) HIV infection and obesity: where did all the wasting go? Antivir Ther 17(7):1281–1289
Couturier J et al (2015) Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS 29(6):667–674
Koethe JR et al (2016) Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 32(1):50–58
Mohammed SS et al (2007) HIV-positive patients with nonalcoholic fatty liver disease have a lower body mass index and are more physically active than HIV-negative patients. J Acquir Immune Defic Syndr 45(4):432–438
McCutchan JA et al (2012) Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 78(7):485–492
Herrin M et al (2016) Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr 73(2):228–236
Brown TT et al (2005) Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 165(10):1179–1184
Butt AA et al (2009) HIV infection and the risk of diabetes mellitus. AIDS 23(10):1227–1234
Weber R et al (2006) Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A: D study. Arch Intern Med 166(15):1632–1641
Younossi ZM et al (2016) Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1):73–84
Perazzo H et al (2018) Predictive factors associated with liver fibrosis and steatosis by transient elastography in patients with HIV mono-infection under long-term combined antiretroviral therapy. J Int AIDS Soc 21(11):e25201
Maurice JB et al (2017) Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS 31(11):1621–1632
Vodkin I et al (2015) Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: a case-control study. Aliment Pharmacol Ther 41(4):368–378
Reeds DN et al (2003) Alterations in lipid kinetics in men with HIV-dyslipidemia. Am J Physiol Endocrinol Metab 285(3):E490–E497
Lui G et al (2016) Liver fibrosis and fatty liver in Asian HIV-infected patients. Aliment Pharmacol Ther 44(4):411–421
Haugaard SB et al (2007) Impaired proinsulin secretion before and during oral glucose stimulation in HIV-infected patients who display fat redistribution. Metabolism 56(7):939–946
Wierzbicki AS et al (2008) HIV lipodystrophy and its metabolic consequences: implications for clinical practice. Curr Med Res Opin 24(3):609–624
Likanonsakul S et al (2013) Polymorphisms in Fas gene is associated with HIV-related lipoatrophy in Thai patients. AIDS Res Hum Retroviruses 29(1):142–150
Goehringer F et al (2017) Causes of death in HIV-infected individuals with immunovirologic success in a national prospective survey. AIDS Res Hum Retroviruses 33(2):187–193
Engels EA et al (2017) Cancer-attributable mortality among people with treated human immunodeficiency virus infection in North America. Clin Infect Dis 65(4):636–643
Coghill AE et al (2015) Elevated cancer-specific mortality among HIV-infected patients in the United States. J Clin Oncol 33(21):2376–2383
Powles T et al (2009) Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol 27(6):884–890
Berretta M et al (2016) New treatment strategies for HIV-positive cancer patients undergoing antiblastic chemotherapy. Expert Opin Pharmacother 17(18):2391–2403
Shmakova A, Germini D, Vassetzky Y (2020) HIV-1, HAART and cancer: a complex relationship. Int J Cancer 146(10):2666–2679
Robbins HA et al (2015) Excess cancers among HIV-infected people in the United States. J Natl Cancer Inst 107(4)
Hernández-Ramírez RU et al (2017) Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV 4(11):e495–e504
Shiels MS et al (2018) Projected cancer incidence rates and burden of incident cancer cases in HIV-infected adults in the United States Through 2030. Ann Intern Med 168(12):866–873
Cobucci RN et al (2015) Assessing the impact of HAART on the incidence of defining and non-defining AIDS cancers among patients with HIV/AIDS: a systematic review. J Infect Public Health 8(1):1–10
Park LS et al (2016) Time trends in cancer incidence in persons living with HIV/AIDS in the antiretroviral therapy era: 1997–2012. AIDS 30(11):1795–1806
Kesselring A et al (2011) Immunodeficiency as a risk factor for non-AIDS-defining malignancies in HIV-1-infected patients receiving combination antiretroviral therapy. Clin Infect Dis 52(12):1458–1465
Reid E et al (2018) Cancer in people living with HIV, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 16(8):986–1017
Shiels MS et al (2010) Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J Acquir Immune Defic Syndr 55(4):510–515
Cingolani A et al (2017) Survival and predictors of death in people with HIV-associated lymphoma compared to those with a diagnosis of lymphoma in general population. PLoS ONE 12(10):e0186549
Dryden-Peterson S et al (2016) HIV infection and survival among women with cervical cancer. J Clin Oncol 34(31):3749–3757
Chiao EY et al (2008) Human immunodeficiency virus-associated squamous cell cancer of the anus: epidemiology and outcomes in the highly active antiretroviral therapy era. J Clin Oncol 26(3):474–479
Hleyhel M et al (2015) Trends in survival after cancer diagnosis among HIV-infected individuals between 1992 and 2009. Results from the FHDH-ANRS CO4 cohort. Int J Cancer 137(10):2443–53
Bruyand M et al (2015) Cancer risk and use of protease inhibitor or nonnucleoside reverse transcriptase inhibitor-based combination antiretroviral therapy: the D: A: D study. J Acquir Immune Defic Syndr 68(5):568–577
Leitch H, Trudeau M, Routy JP (2003) Effect of protease inhibitor-based highly active antiretroviral therapy on survival in HIV-associated advanced Kaposi’s sarcoma patients treated with chemotherapy. HIV Clin Trials 4(2):107–114
Castillo JJ, Echenique IA (2012) Rituximab in combination with chemotherapy versus chemotherapy alone in HIV-associated non-Hodgkin lymphoma: a pooled analysis of 15 prospective studies. Am J Hematol 87(3):330–333
Wong JK, Yukl SA (2016) Tissue reservoirs of HIV. Curr Opin HIV AIDS 11(4):362–370
Godfrey C et al (2019) Obesity and fat metabolism in human immunodeficiency virus-infected individuals: immunopathogenic mechanisms and clinical implications. J Infect Dis 220(3):420–431
Clifford DB, Ances BM (2013) HIV-associated neurocognitive disorder. Lancet Infect Dis 13(11):976–986
Tedaldi EM, Minniti NL, Fischer T (2015) HIV-associated neurocognitive disorders: the relationship of HIV infection with physical and social comorbidities. Biomed Res Int 2015:641913
Freiberg MS et al (2013) HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 173(8):614–622
Sico JJ et al (2015) HIV status and the risk of ischemic stroke among men. Neurology 84(19):1933–1940
Butt AA et al (2011) Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med 171(8):737–743
Freiberg MS et al (2011) The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes 4(4):425–432
Croxford S et al (2017) Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort. Lancet Public Health 2(1):e35–e46
Rodés B et al (2022) Ageing with HIV: challenges and biomarkers. EBioMedicine 77:103896
Masters MC et al (2022) Chronic HIV infection and aging: application of a geroscience-guided approach. J Acquir Immune Defic Syndr 89(Suppl 1):S34–S46
Bandera A et al (2018) Altered gut microbiome composition in HIV infection: causes, effects and potential intervention. Curr Opin HIV AIDS 13(1):73–80
Sun CY et al (2023) T helper 17 (Th17) cell responses to the gut microbiota in human diseases. Biomed Pharmacother 161:114483
Dillon SM et al (2012) HIV-1 infection of human intestinal lamina propria CD4+ T cells in vitro is enhanced by exposure to commensal Escherichia coli. J Immunol 189(2):885–896
Lozupone CA et al (2013) Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe 14(3):329–339
Dillon SM et al (2016) Enhancement of HIV-1 infection and intestinal CD4+ T cell depletion ex vivo by gut microbes altered during chronic HIV-1 infection. Retrovirology 13:5
Deeks SG, Tracy R, Douek DC (2013) Systemic effects of inflammation on health during chronic HIV infection. Immunity 39(4):633–645
Nowak P et al (2015) Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS 29(18):2409–2418
Pickard JM et al (2017) Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev 279(1):70–89
Zaongo SD, Chen Y (2023) Gut microbiota: a potential key player in boosting immune reconstitution of immunological nonresponders. Future Microbiol 18:83–85
Vujkovic-Cvijin I, Somsouk M (2019) HIV and the gut microbiota: composition, consequences, and avenues for amelioration. Curr HIV/AIDS Rep 16(3):204–213
Ouyang J et al (2020) The bacterium Akkermansia muciniphila: a sentinel for gut permeability and its relevance to HIV-related inflammation. Front Immunol 11:645
Lu J et al (2019) Changes in peripheral blood inflammatory factors (TNF-α and IL-6) and intestinal flora in AIDS and HIV-positive individuals. J Zhejiang Univ Sci B 20(10):793–802
Zhou Y et al (2018) Alterations in the gut microbiota of patients with acquired immune deficiency syndrome. J Cell Mol Med 22(4):2263–2271
Sun Y et al (2016) Fecal bacterial microbiome diversity in chronic HIV-infected patients in China. Emerg Microbes Infect 5(4):e31
Tuddenham SA et al (2020) The impact of human immunodeficiency virus infection on gut microbiota α-diversity: an individual-level meta-analysis. Clin Infect Dis 70(4):615–627
Ling Z et al (2016) Alterations in the fecal microbiota of patients with HIV-1 infection: an observational study in a Chinese population. Sci Rep 6:30673
Dillon SM, Frank DN, Wilson CC (2016) The gut microbiome and HIV-1 pathogenesis: a two-way street. AIDS 30(18):2737–2751
Deusch S et al (2018) Effects of HIV, antiretroviral therapy and prebiotics on the active fraction of the gut microbiota. AIDS 32(10):1229–1237
Roy CC et al (2006) Short-chain fatty acids: ready for prime time? Nutr Clin Pract 21(4):351–366
Zaongo SD et al (2023) Candida albicans can foster gut dysbiosis and systemic inflammation during HIV infection. Gut Microbes 15(1):2167171
Furusawa Y et al (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504(7480):446–450
Geng ST et al (2020) Regulation of gut microbiota on immune reconstitution in patients with acquired immunodeficiency syndrome. Front Microbiol 11:594820
Zaongo SD et al (2022) HIV Infection Predisposes to Increased Chances of HBV Infection: Current Understanding of the Mechanisms Favoring HBV Infection at Each Clinical Stage of HIV Infection. Front Immunol 13:853346
Ouyang J et al (2021) Microbiota-meditated immunity abnormalities facilitate hepatitis B virus co-infection in people living with HIV: a review. Front Immunol 12:755890
Steele AK et al (2014) Microbial exposure alters HIV-1-induced mucosal CD4+ T cell death pathways ex vivo. Retrovirology 11:14
Vesterbacka J et al (2017) Richer gut microbiota with distinct metabolic profile in HIV infected Elite Controllers. Sci Rep 7(1):6269
Ciccone EJ et al (2011) CD4+ T cells, including Th17 and cycling subsets, are intact in the gut mucosa of HIV-1-infected long-term nonprogressors. J Virol 85(12):5880–5888
Buckheit RW 3rd et al (2013) The implications of viral reservoirs on the elite control of HIV-1 infection. Cell Mol Life Sci 70(6):1009–1019
Vidya Vijayan KK et al (2017) Pathophysiology of CD4+ T-cell depletion in HIV-1 and HIV-2 infections. Front Immunol 8:580
Le Hingrat Q et al (2021) The Hitchhiker Guide to CD4(+) T-cell depletion in lentiviral infection. A critical review of the dynamics of the CD4(+) T cells in SIV and HIV infection. Front Immunol 12:695674
Baxter AE et al (2016) Single-Cell Characterization of Viral Translation-Competent Reservoirs in HIV-Infected Individuals. Cell Host Microbe 20(3):368–380
Nguyen S et al (2019) Elite control of HIV is associated with distinct functional and transcriptional signatures in lymphoid tissue CD8(+) T cells. Sci Transl Med 11(523)
Wang S et al (2020) An atlas of immune cell exhaustion in HIV-infected individuals revealed by single-cell transcriptomics. Emerg Microbes Infect 9(1):2333–2347
Eyerich K, Dimartino V, Cavani A (2017) IL-17 and IL-22 in immunity: driving protection and pathology. Eur J Immunol 47(4):607–614
Rocafort M et al (2019) Evolution of the gut microbiome following acute HIV-1 infection. Microbiome 7(1):73
Ceccarelli G et al (2019) Challenges in the management of HIV infection: update on the role of probiotic supplementation as a possible complementary therapeutic strategy for cART treated people living with HIV/AIDS. Expert Opin Biol Ther 19(9):949–965
Massanella M, Fromentin R, Chomont N (2016) Residual inflammation and viral reservoirs: alliance against an HIV cure. Curr Opin HIV AIDS 11(2):234–241
Zilberman-Schapira G et al (2016) The gut microbiome in human immunodeficiency virus infection. BMC Med 14(1):83
Zevin AS et al (2016) Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS 11(2):182–190
Villanueva-Millán MJ et al (2017) Differential effects of antiretrovirals on microbial translocation and gut microbiota composition of HIV-infected patients. J Int AIDS Soc 20(1):21526
Villoslada-Blanco P et al (2022) Integrase inhibitors partially restore bacterial translocation, inflammation and gut permeability induced by HIV infection: impact on gut microbiota. Infect Dis Ther 11(4):1541–1557
Villoslada-Blanco P et al (2022) Impact of HIV infection and integrase strand transfer inhibitors-based treatment on the gut virome. Sci Rep 12(1):21658
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4(7):499–511
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124(4):783–801
Anzinger JJ et al (2014) Monocytes as regulators of inflammation and HIV-related comorbidities during cART. J Immunol Res 2014:569819
Palmer CS et al (2016) Emerging role and characterization of immunometabolism: relevance to HIV pathogenesis, serious non-AIDS events, and a cure. J Immunol 196(11):4437–4444
Angelidou K et al (2018) Changes in Inflammation but not in T-cell activation precede non-AIDS-defining events in a case-control study of patients on long-term antiretroviral therapy. J Infect Dis 218(2):239–248
Fukui SM, Piggott DA, Erlandson KM (2018) Inflammation strikes again: frailty and HIV. Curr HIV/AIDS Rep 15(1):20–29
Shan Z et al (2018) Gut microbial-related choline metabolite trimethylamine-N-oxide is associated with progression of carotid artery atherosclerosis in HIV infection. J Infect Dis 218(9):1474–1479
Yeoh HL et al (2017) Immunometabolic and lipidomic markers associated with the frailty index and quality of life in aging HIV+ men on antiretroviral therapy. EBioMedicine 22:112–121
Isnard S et al (2021) Gut leakage of fungal-related products: turning up the heat for HIV infection. Front Immunol 12:656414
Mezrich JD et al (2010) An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 185(6):3190–3198
Liu Y et al (2018) Tumor-repopulating cells induce PD-1 expression in CD8(+) T cells by transferring kynurenine and AhR activation. Cancer Cell 33(3):480-494.e7
Greene LI et al (2019) A role for tryptophan-2,3-dioxygenase in CD8 T-cell suppression and evidence of Tryptophan catabolism in breast cancer patient plasma. Mol Cancer Res 17(1):131–139
Takenaka MC et al (2019) Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci 22(5):729–740
Feng HY, Chen YC (2016) Role of bile acids in carcinogenesis of pancreatic cancer: An old topic with new perspective. World J Gastroenterol 22(33):7463–7477
Parks DJ et al (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science 284(5418):1365–1368
Yoshimoto S et al (2013) Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499(7456):97–101
Yu LX, Schwabe RF (2017) The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol 14(9):527–539
Ohtani N, Hara E (2021) Gut-liver axis-mediated mechanism of liver cancer: a special focus on the role of gut microbiota. Cancer Sci 112(11):4433–4443
Morris A et al (2012) Serum (1→3)-β-D-glucan levels in HIV-infected individuals are associated with immunosuppression, inflammation, and cardiopulmonary function. J Acquir Immune Defic Syndr 61(4):462–468
Isnard S et al (2021) Circulating β-d-glucan as a marker of subclinical coronary plaque in antiretroviral therapy-treated people with human immunodeficiency virus. Open Forum Infect Dis 8(6):ofab109
Dirajlal-Fargo S et al (2019) Changes in the fungal marker β-D-glucan after antiretroviral therapy and association with adiposity. Open Forum Infect Dis 6(11):ofz434
Hoenigl M et al (2016) (1→3)-β-D-glucan levels correlate with neurocognitive functioning in hiv-infected persons on suppressive antiretroviral therapy: a cohort study. Medicine (Baltimore) 95(11):e3162
Hoenigl M et al (2019) Soluble urokinase plasminogen activator receptor is predictive of non-AIDS events during antiretroviral therapy-mediated viral suppression. Clin Infect Dis 69(4):676–686
Rasmussen LJ et al (2016) Soluble urokinase plasminogen activator receptor (suPAR) is a novel, independent predictive marker of myocardial infarction in HIV-1-infected patients: a nested case-control study. HIV Med 17(5):350–357
Hodges GW et al (2015) suPAR: a new biomarker for cardiovascular disease? Can J Cardiol 31(10):1293–1302
Hayek SS et al (2015) Soluble urokinase receptor and chronic kidney disease. N Engl J Med 373(20):1916–1925
Raggam RB et al (2014) Soluble urokinase plasminogen activator receptor predicts mortality in patients with systemic inflammatory response syndrome. J Intern Med 276(6):651–658
Hoenigl M et al (2013) Diagnostic accuracy of soluble urokinase plasminogen activator receptor (suPAR) for prediction of bacteremia in patients with systemic inflammatory response syndrome. Clin Biochem 46(3):225–229
Wang T et al (2022) Protease or Clostridium butyricum addition to a low-protein diet improves broiler growth performance. Appl Microbiol Biotechnol 106(23):7917–7931
Liu L et al (2022) Functional comparison of Clostridium butyricum and sodium butyrate supplementation on growth, intestinal health, and the anti-inflammatory response of broilers. Front Microbiol 13:914212
Zhang C et al (2022) Clostridium butyricum improves the intestinal health of goats by regulating the intestinal microbial community. Front Microbiol 13:991266
Liu M et al (2023) Regulatory effects of the probiotic Clostridium butyricum on gut microbes, intestinal health, and growth performance of chickens. J Poult Sci 60(2):2023011
Xu L et al (2021) Dietary supplementation with Clostridium butyricum improves growth performance of broilers by regulating intestinal microbiota and mucosal epithelial cells. Anim Nutr 7(4):1105–1114
Choi Y et al (2023) Effect of Clostridium butyricum on high-fat diet-induced intestinal inflammation and production of short-chain fatty acids. Dig Dis Sci 68(6):2427–2440
Funding
This study was funded by the Medical Research Projects (No. CSTB2022BSXM-JCX0081, No. CSTB2023TIAD-KPX0063-3, and No. CSTB2023NSCQ-MSX0392) of Chongqing Science & Technology Bureau, Joint Project of Chongqing Health Commission and Science and Technology Bureau (Nos. 2024ZDXM015 and 2024GDRC012), and the Chongqing Public Health Medical Center Research Initiation Fund (KYLW202321).
Author information
Authors and Affiliations
Contributions
QY and SDZ wrote the first draft edition of the manuscript. LZ and JY provided critical revisions of this manuscript. JY and JO conceived and designed the manuscript. QY and SDZ also prepared Fig. 1. All authors have reviewed the manuscript and approved the study for publication.
Corresponding authors
Ethics declarations
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Q., Zaongo, S.D., Zhu, L. et al. The Potential of Clostridium butyricum to Preserve Gut Health, and to Mitigate Non-AIDS Comorbidities in People Living with HIV. Probiotics & Antimicro. Prot. 16, 1465–1482 (2024). https://doi.org/10.1007/s12602-024-10227-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-024-10227-1