Abstract
Inflammatory bowel diseases (IBD), including Crohn’s disease, ulcerative colitis, and pouchitis, are chronic, relapsing intestinal inflammatory disorders mediated by dysregulated immune responses to resident microbiota. Current standard therapies that block immune activation with oral immunosuppressives or biologic agents are generally effective, but each therapy induces a sustained remission in only a minority of patients. Furthermore, these approaches can have severe adverse events. Recent compelling evidence of a role of unbalanced microbiota (dysbiosis) driving immune dysfunction and inflammation in IBD supports the therapeutic rationale for manipulating the dysbiotic microbiota. Traditional approaches using currently available antibiotics, probiotics, prebiotics, and synbiotics have not produced optimal results, but promising outcomes with fecal microbiota transplant provide a proof of principle for targeting the resident microbiota. Rationally designed oral biotherapeutic products (LBPs) composed of mixtures of protective commensal bacterial strains demonstrate impressive preclinical results. Resident microbial-based and microbial-targeted therapies are currently being studied with increasing intensity for IBD primary therapy with favorable early results. This review presents current evidence and therapeutic mechanisms of microbiota modulation, emphasizing clinical studies, and outlines prospects for future IBD treatment using new approaches, such as LBPs, bacteriophages, bacterial function-editing substrates, and engineered bacteria. We believe that the optimal clinical use of microbial manipulation may be as adjuvants to immunosuppressive for accelerated and improved induction of deep remission and as potential safer solo approaches to sustained remission using personalized regimens based on an individual patient’s microbial profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Akihiko Oka

R. Balfour Sartor
Introduction
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD), ulcerative colitis (UC), and pouchitis, are chronic intestinal inflammatory disorders characterized by dysregulated immune responses to enteric resident microbiota in genetically susceptible hosts [1,2,3]. Based on the requirement of microbiota colonization to develop colitis in germ-free (GF) susceptible rodents [4,5,6,7], gut microbiota play a crucial role in the pathogenesis of IBD [1, 3, 8]. Microbiota include potentially pathogenic microbes driving inflammation (pathobionts), as well as potentially beneficial microbes inducing protective immune responses (commensals) [1, 9, 10]. However, most IBD patients exhibit unbalanced gut microbiota profiles (dysbiosis), with expanded potentially pathogenic Proteobacteria (especially Enterobacteriaceae that include E. coli and Klebsiella), Fusobacteria, Ruminococcus gnavus, and Candida tropicalis [11] and reduced potentially protective Firmicutes (especially Faecalibacterium prausnitzii, Ruminococci, and Clostridium clusters IV and XIVa) [12, 13] (Table 1). The immunologic consequences of dysbiosis and its causal role in experimental colitis provide a strong rationale for therapeutically modifying the enteric microbiota in patients with IBD [1, 3, 8, 14]. Current primary therapies in IBD, such as corticosteroids, methotrexate, 5-aminosalicylic acid (5-ASA), JAK inhibitors, anti-tumor necrosis factor (TNF)-α, anti-interleukin (IL)-12p40 antibody, and anti-integrin antibodies and surgical resection, mostly target effector immune responses [15,16,17]. These therapies can induce remission in many IBD patients, but can have severe adverse events with impaired quality of life (QOL). Microbiota-based therapies, including fecal microbiota transplant (FMT), probiotics, and prebiotics, are suggested to be safe and can potentially correct the dysbiosis driving the dysregulated immune response [1, 3, 18]. Recent success of FMT in recurrent or refractory Clostridium difficile infection (rCDI) [19] achieved a major breakthrough in microbial-based therapy, which is being studied with increasing intensity as IBD primary therapy with favorable reported results [20]. In response to this trend, the United States Food and Drug Administration (FDA) created a new category, live biotherapeutic products (LBPs), for ‘live organisms, such as bacteria, which are applicable to the prevention, treatment, or cure of a disease or condition of human beings’ and issued a guidance for clinical trials [21]. This review provides an overview of current microbial-based and microbiota-targeted therapies (Tables 2, 3, 4, 5) and prospects for future treatments in IBD (Table 6) (Fig. 1).
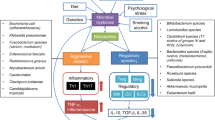
Graphic overview. The concept of manipulating microbiota to correct dysbiosis is a relatively new approach to treating inflammatory bowel disease (IBD). This review updates the status of current microbial-based and microbial-targeted therapies and prospects for future treatments in IBD. Tx, therapy; 5-ASA, 5-aminosalicylic acid; MTX, methotrexate; JAK, Janus kinase; IL, interleukin; TNF, tumor necrosis factor; SCFA, short-chain fatty acid; PXR, pregnane X receptor; PPAR, peroxisome proliferator-activated receptor; Treg, regulatory T cell; GOS, galacto-oligosaccharide; FOS, fructo-oligosaccharide; GBF, germinated barley foodstuff; OI, oligofructose-enriched inulin; BGS, bifidogenic growth stimulator; FODMAP, fermentable oligosaccharide disaccharide, monosaccharide, and polyol; FMT, fecal microbiota transplant; LBP, live biotherapeutic product; PolyP, polyphosphate; KFXL, Kangfuxin liquid; path, pathogenic; AIEC, adherent-invasive Escherichia coli, Images of antibiotics and prebiotics are adopted from KEGG. Image of prebiotic diet is adopted from Monash University (https://www.monashfodmap.com/blog/a-low-fodmap-mediterranean-style-diet/). Red: aggressive microbial species and cells, blue: protective microbial species and cells
Microbiota in IBD: The Rationale for Therapeutic Microbial Manipulation
In general, ‘microbiota (or microbes)’ includes bacteria, fungi, and viruses (mostly bacteriophages) while ‘microbiome’ refers to microbiota and their genes and metabolites [1, 22, 23]. A huge number of microbial cells in the distal intestine (1014 bacteria/g), species (approximately equal to human cells), genes (outnumber human genes by 100-fold), bacteriophages (outnumber bacteria by tenfold), and their weight (1–2 kg) [13, 22, 24, 25] are considered a ‘superorganism’ and ‘forgotten organ’ [26, 27]. The colonic lumen contains the densest bacteria concentration in the human body (1011–1014 bacteria/g), followed by oral (108/g), ileum (107–108/g), jejunum (104/g), duodenum (103/g), and stomach (101/g) [22, 23, 28]. An individual’s enteric bacterial composition varies greatly, and each individual harbors 100–150 diverse intestinal species [22, 29]. This diversity allows humans to obtain a variety of benefits, such as digesting various foods (especially fiber), producing vitamins and other protective metabolites, activating homeostatic gut and systemic immune responses, and preventing colonization by exogenous pathogens [1]. However, the diversity of bacteria in IBD patients is significantly decreased [12, 13, 30, 31], whereas fungi and bacteriophages are expanded [11, 31,32,33]. Furthermore, the composition and function of enteric microbiota in IBD patients are frequently disrupted, characterized by expanded potentially pathogenic microbes and reduced protective microbes producing short-chain fatty acids (SCFAs) [12, 13, 34,35,36,37,38,39]. This microbial imbalance, termed as ‘dysbiosis,’ was first noted in the intestine of IBD patients [12, 13, 30, 34,35,36,37,38], but recently oral dysbiosis is also reported [40,41,42], the latter indicating that dysbiosis can be independent of local inflammatory processes. Although more careful assessment is needed in various patient subsets using modern detection techniques, consistent changes occur in CD and UC (Table 1). The link between this dysbiosis and gut inflammation is supported by many experimental studies. CD-associated adherent-invasive Escherichia coli (AIEC) invades epithelial cells and replicates within macrophages and can cause chronic experimental colitis [43, 44]. Another Enterobacteriaceae, Klebsiella pneumoniae isolated from a CD patient, induces experimental colitis with high Th1 response compared to other control strains and species [42]. Fusobacterium varium strains from UC patients invade epithelial cells compared to strains from healthy controls and induce experimental colitis [45]. Alternatively, certain Clostridium species and F. prausnitzii are putative anti-inflammatory microbes. Clostridia are dominant intestinal microbes, accounting for over 60% of mucosa-associated bacteria [46]. A subset of resident Clostridium species produce SCFAs and can induce colonic regulatory T cells (Tregs) or IL-10-producing B cells and macrophages to protect against experimental colitis [47,48,49,50] with reduction in the abundance of Enterobacteriaceae [50]. F. prausnitzii another major SCFA producer induces IL-10 production by human and murine dendritic cells [51]. Indeed, IBD-derived fecal bacteria stool did not induce colonic Treg in GF mice [9]. Interestingly, most expanded bacteria in IBD are aerotolerant species (aerobes or facultative anaerobes), such as E. coli, F. varium, Haemophilus, Enterococcus faecalis, and Neisseriaceae. In contrast, the majority of reduced bacteria are obligate anaerobes, such as Clostridium clusters IV, XIVa, XVIII, and F. prausnitzii. This trend gives rise to the ‘oxygen hypothesis’ wherein disruption in anaerobiosis indicates to a role for oxygen in intestinal dysbiosis [52]. Recent studies support this hypothesis by showing that Clostridium strains inhibit dysbiotic Enterobacteriaceae expansion by reducing luminal oxygen via activation of epithelial PPAR-γ [53, 54]. Notably, decreased PPAR-γ gene expression is associated with IBD pathogenesis [55]. Dysbiosis of fungi and bacteriophages in IBD were also noted recently [31,32,33] with interactions between C. tropicalis, E. coli, and Serratia marcescens [11]. Further investigations may determine the significance of elevated anti-fungus antibody in many CD patients [56]. A causal association between dysbiosis and IBD is further supported by results from recent FMT trials, as a shift of the recipient’s dysbiotic microbiota toward the donor’s non-dysbiotic microbiota is associated with clinical response [57,58,59,60]. In addition to its causal role in driving inflammation, microbiota influence efficacy of certain immunomodulatory therapies, including anti-TNF-α [61, 62], steroids [63], and PD-1-based treatments [64]. Understanding microbial dynamics is necessary for optimal current and future IBD therapies, particularly personalized management. Technologic developments and ongoing human microbiome projects have improved the culture of previously ‘unculturable’ human microbiota [65], access to more extensive multi-omics databases [66], and gene catalogues established by metagenomic sequencing [12, 24].
Antibiotics
Antibiotics, antimicrobial substances active against bacteria, are widely used treat complications of IBD (bacteremia, abscess, opportunistic, and surgical site infections) [1]. Antibiotics are also used as primary therapy for inducing or maintaining remission based on the hypothesis that certain bacteria cause IBD, the pathologic similarities between CD and Mycobacterium avium subspecies paratuberculosis infection and isolation of this organism in some CD patients [67]. IBD is considered to be caused by intricately intertwined gut microbiota, host genes, immune system, and environmental factors rather than a specific infectious colitis [1, 3]. However, as potential pathobionts are expanded in dysbiotic IBD intestines, targeted antibiotic therapy is a rational strategy. Unfortunately, most antibiotics decrease overall bacterial diversity and inhibit not only pathobionts but also beneficial bacteria, which can lead to overgrowth of pathogenic bacteria (C. difficile), fungi (candida), and bacteriophages [32]. Despite their inhibitory effects, some antibiotics increase protective bacteria [68,69,70] and modulate host immune functions [71]. This section updates clinical efficacy of antibiotics in IBD (Table 2) and their therapeutic mechanisms.
Ulcerative Colitis
Two meta-analyses of antibiotic therapy for active UC demonstrated improved remission rates overall (64% vs 48% placebo) [72, 73]. With a broad variety of different agents and protocols (vancomycin, metronidazole, tobramycin, ciprofloxacin, amoxicillin, ethambutol, tetracycline, and rifamycin), it is difficult to choose optimal antibiotic agents. Of note, all randomized controlled trials (RCTs) using intravenous antibiotics failed to achieve therapeutic benefit over control treatment. In contrast, most oral antibiotics achieved clinical response except for 2 RCTs of ciprofloxacin. Two RCTs of a promising 2-week triple antibiotic primary therapy cocktail including oral amoxicillin, tetracycline, and metronidazole (ATM) showed significantly improved remission rates, clinical, and endoscopic scores [74, 75]. This regimen, designed based on susceptibility testing of F. varium [75], significantly reduced mucosal F. varium abundance in Japanese UC patients [76]. Further, a new RCT of the ATM triple cocktail versus AT cocktail, excluding metronidazole, has been initiated (ClinicalTrials.gov identifier: NCT03986996), given metronidazole’s potential negative effect on gut barrier function and poor patient acceptance. Only a few reports address the long-term outcomes of antibiotics: One trial reported that 7 days of oral tobramycin significantly improved remission rates at 1 week (74% vs 43% placebo) [77], but no statistical difference in relapse rates at 2-year follow-up (24% vs 12%) [78]. Another trial showed 6-month oral ciprofloxacin improved endoscopic and histological appearances at early 3 month, but the benefit disappeared by 6 and 12 months [79]. In contrast, the ATM cocktail therapy demonstrated significantly higher remission rates and lower clinical and endoscopic scores at both intermediate (3–5 months) and long-term (12–14 months) follow-ups [74, 75].
Pouchitis
All three RCTs (including metronidazole, ciprofloxacin, and rifaximin) showed therapeutic benefit, matching widespread clinical use. Three meta-analyses including RCTs and cohort studies support the favorable results [80,81,82]. A meta-analysis concludes that antibiotics and biologics (anti-TNF-α) are more beneficial for chronic refractory pouchitis than are corticosteroids, bismuth, elemental diet, and tacrolimus [81]. Furthermore, ciprofloxacin is suggested to be more effective than metronidazole [82]. Ciprofloxacin reduced Clostridium perfringens and E. coli and did not affect abundance of anaerobic bacteria, while metronidazole reduced C. perfringens, but not E. coli, and reduced anaerobic bacteria in a cohort study [83], indicating that ciprofloxacin is more active against pathogenic species and less harmful to beneficial species. Major concerns with sustained or intermittent use of antibiotics include antibiotic resistance and side effects, such as tendon rupture with ciprofloxacin and peripheral neuropathy with metronidazole. Regarding antibiotic dependency observed in many pouchitis cases, a recent clinical trial proposed a unique hypothesis that antibiotics enrich antibiotic resistance in nonpathogenic species, which might prevent colonization with pathogenic species as long as antibiotics are used [84]. Based on this, a new RCT is currently underway that alternates antibiotics short term with dietary interventions to support growth of beneficial species to avoid progression to antibiotic-dependent disease (NCT04082559).
Crohn’s Disease
A meta-analysis of studies designed to maintain remission after surgical resections demonstrates significant benefit of antibiotics alone and as adjuvants to immunomodulators (azathioprine or 6-mercaptopurine) or anti-TNF-α therapies [85]. However, three meta-analyses suggest that the benefit of antibiotics is weak for overall treatment of CD [80, 86, 87]. However, anti-Mycobacterium agents (especially rifamycin-containing regimens) demonstrate some benefit for inducing remission [73, 88] but do not induce a sustained remission to support clearance of a pathogen [89, 90]. Long-term responses have been studied in a few reports, indicating that the protective effects of antibiotics wane over time; Aberra et al. suggested efficacy over 60 days [90, 91]. Multiple additional RCTs of rifamycin for active CD (NCT02240108, NCT00603616, NCT02240121, NCT02620007) and prevention of postoperative CD recurrence (NCT03185624, NCT03185611) are ongoing (Table 6) or recently completed (NCT01951326). One of these trials specifically targets CD-associated E. coli-colonized patients (NCT02620007). Other agents, including ciprofloxacin and metronidazole alone or in combination, also show remission induction [72, 73]. Given the fungal dysbiosis in CD, an ongoing RCT investigates whether addition antifungal fluconazole to an antibiotic cocktail can improve remission rates (NCT02765256). For specific conditions such as anal or internal fistula, ciprofloxacin and metronidazole reduce drainage [73], improve symptoms, and improve fistula closure rates [92,93,94]. For abscesses, the first choice is surgical treatment, but frequently emergency surgery can be avoided by antibiotics and percutaneous drainage [95]. Although meta-analyses of antibiotics in IBD are quite positive, clinical use of these agents is largely restricted to patients with active pouchitis and septic complications of CD. This disparity is in part due to publication bias that favors publication of positive results.
Therapeutic Mechanisms
Several mechanisms mediate therapeutic actions of antibiotics. (1) Inhibiting pathobionts Each antibiotic has a unique spectrum against bacteria, and most antibiotics inhibit pathogenic species and decrease overall bacterial diversity. Long-term metronidazole eliminates Bacteroides, with bacterial concentrations correlated with disease activity [96]. Ciprofloxacin is effective against enteric pathogens and most Gram-negative Enterobacteriaceae. Although rifamycin does to alter the overall microbiota composition in IBD patients [71], it reduces bacterial attachment [71]. (2) Increasing beneficial bacteria Despite many antibiotics reducing beneficial species, such as F. prausnitzii [84], some antibiotics can increase protective species. For example, rifamycin increases Lactobacillus [70], Bifidobacterium, and F. prausnitzii [69]. (3) Modifying bacterial metabolites Shifts in microbiota composition alter microbial metabolites, with increased SCFAs and other beneficial products [12, 69] that correlate with clinical response in IBD patients [69, 97]. (4) Immunomodulatory effect Rifamycins, ciprofloxacin, metronidazole, and macrolides have mucosal immunomodulatory effects [71, 98,99,100]. Specifically, rifaximin is a gut-specific agonist of the human pregnane X receptor (PXR) that helps maintain mucosal homeostasis [71, 99].
Clinical Concerns
Safety
In clinical trials, IBD patients exhibited no increased risk of severe adverse events with antibiotics compared to placebo [86], but safety issues must be considered. Anti-Mycobacterium therapy has more frequent adverse events, such as rashes and skin pigmentation, but not increased withdrawal rate [88]. Long-term use of metronidazole can cause peripheral neuropathy [80, 101].
Risk of Resistance
Probably due to higher antibiotic exposure, the prevalence rates of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and extended-spectrum beta-lactamases (ESBL)-producing E. coli are significantly higher among IBD patients [102].
Risk of CDI
Antibiotics increase CDI in IBD patients [103] through decreased lactate-producing bacteria numbers and increased succinic acid [104] although others reported rare CDI in CD patients [105]. Antibiotic-resistant probiotics may prevent CDI after antibiotic therapy [106].
Effective Protocols
While oral antibiotics appear to be effective as adjunctive therapy for IBD flares based on their direct effects on luminal bacteria and mucosal immune function, the benefits of intravenous antibiotics are not proven [107]. For fulminant colitis, such as toxic megacolon at risk of severe bacteremia, especially when receiving corticosteroids, intravenous broad-spectrum antibiotics appear to be reasonable [108]. The most effective therapy duration remains unclear, but Ledder provides recommendations [109]. The short-term benefit (induction of remission) of antibiotics is promising, while the long-term benefits (maintenance) appear low with increased toxicity or antibiotic-resistant bacteria [78, 90, 91]. Sequential maintenance approaches, such as protective nutrients, probiotics, or FMT, need to be considered after induction of remission with antibiotics.
Risk of Dysbiosis
Compelling epidemiologic evidence indicates that multiple early childhood exposures to antibiotics carry higher risk of developing CD [1]. It is unclear whether this risk is due to antibiotics themselves, an infection that required antibiotic use, or early IBD symptoms.
Conclusions
Despite the many different antibiotics, protocols, and endpoint assessments in clinical trials with publication bias, oral antibiotics provide a promising primary or adjuvant therapy for inducing remission of IBD. Specific pathobiont-targeted strategy has recently emerged as an area of interest (F. varium, AIEC, C. perfringens), supporting future personalized antibiotic use. For active UC, the oral ATM cocktail is promising. For active pouchitis, ciprofloxacin > metronidazole is effective. For active CD, rifamycins are promising. Given the negative potential effects of long-term use such as host toxicity and antibiotic resistance, short-term use followed by alternative maintenance therapies, such as probiotics, prebiotics, diet, and standard immunotherapies, should be considered.
Standard Probiotics and LBPs Using Resident Protective Microbiota
Probiotics are living microorganisms such as bacteria or yeasts with beneficial health effects [110], which included LBP [21]. Since Metchnikoff first published the concept of probiotics (yogurt containing Lactobacillus bulgaricus) in 1907, many probiotic strains have been studied in clinical trials including IBD [108, 110] (Table 3). Probiotic strains used in IBD trials have mostly belonged to two genera, Bifidobacterium and Lactobacillus, and isolated from limited sources (yogurt, milk, etc.) [108, 110]. Recently, a variety of LBP candidates (Clostridium, Firmicutes spores, Bacteroides, Roseburia) isolated from healthy human microbiota have been investigated [1, 3].
Ulcerative Colitis
A meta-analysis of 18 RCTs in UC patients, including pediatric, demonstrates therapeutic benefit over placebo [111]. Also another meta-analysis including Chinese-based RCTs supports the use of adjuvant probiotics with 5-ASA in active UC [112]. Multiple strains have been investigated with favorable results (Table 3). A systematic sub-analysis suggests Bifidobacterium-containing probiotics significantly benefit active UC [113]. Because different strains have different metabolomic and immunomodulatory activities and provide complementary help, a cocktail of different strains may be more efficient than a single strain. Indeed, VSL#3, a cocktail of 8 strains, Lactobacillus casei, L. plantarum, L. acidophilus, L. delbrueckii subspecies bulgaricus, Bifidobacterium longum, B. breve, B. infantis, and Streptococcus salivarius subspecies thermophilus, improved remission and relapse rates [111, 112]. Additional RCTs of VSL#3 (NCT03415711) and L. rhamnosus (NCT04102852) in active UC are underway. More recently, a SERES Therapeutics (Boston, MA) cocktail of purified Firmicutes spore (SER-287) from feces of healthy screened donors was tested in a Phase 1B RCT in active UC [114,115,116]. Treatment arms included 6 days of vancomycin pre-treatment followed by 8 weeks of SER-287 either daily or weekly or placebo pre-treatment followed by weekly SER-287 versus placebo/placebo. Vancomycin improves engraftment of microbes from SER-287 [114] and improved remission rates (placebo/placebo daily: 0%, vanco/SER-287 daily: 40%, placebo/SER-287 weekly: 13.3%, vanco/SER-287 weekly: 17.7%) and endoscopic scores [115]. SER-287-treated remitters exhibited widespread transcriptional shifts from baseline, with by decreased expression of inflammatory genes and increased expression of homeostatic mediators [116]. These promising results led to a Phase 2B, 3-arm RCT in active UC (NCT03759041). Based on improved engraftment of SER-287 by vancomycin pre-treatment, two patient groups receive different doses of SER-287, both following short courses of oral vancomycin. In pediatric UC, 2 RCTs demonstrated that oral VSL#3 [117] or rectal L. reuteri ATCC55730 [118] significantly improved clinical and endoscopic scores. Long-term follow-up data are limited, but a 2-year follow-up showed promising results [119].
Pouchitis
Meta-analyses indicate that probiotics significantly induce remission and prevent relapse in pouchitis [80, 120, 121]. A RCT of oral C. butyricum MIYAIRI showed improved relapse rates (11% vs 50%) [122]. Gionchetti and colleagues reported strikingly decreased relapses (15% vs 100% placebo) in recurrent pouchitis after 9 months of oral VSL#3 therapy [123], but these positive results were not replicated in the USA [124]. In naïve ileal pouches within a year after surgery, VSL#3 prevented onset of pouchitis over placebo (10% vs 40%, P < 0.05) [125]. Microbial analysis revealed that the probiotic enriched lactobacilli and bifidobacteria and increased bacteria diversity while reducing fungal diversity [126]. In contrast, probiotics containing Lactobacillus and Bifidobacterium did not improve pouch dysfunction nor pouchitis activity [127].
Crohn’s Disease
In contrast to UC and pouchitis, several meta-analyses in CD suggest very weak or no benefit of standard probiotics [111, 128] with benefits limited to maintaining remission after surgery [111, 129, 130]. However, there is a strong strain-specific effect [131]; VSL#3 improved endoscopic features [129, 130] and decreased mucosal inflammatory cytokine levels [129]. E. coli Nissle 1917 and other Lactobacillus strains tested in RCTs lacked benefit [111, 128,129,130, 132], but significantly induced Treg numbers in peripheral blood [132]. Bifidobacterium strains, some of which are included in VSL#3, have not been tested alone, but the combination with prebiotics (synbiotics) significantly improved remission rates, clinical activity, and histological scores in CD patients with active disease compared to placebo [133].
Therapeutic Mechanisms
Most orally administered current probiotics pass through, although E. coli Nissle 1917 [134] can colonize the intestine and perform several documented protective functions [135, 136]. Subsets of resident microbiota have extensive evidence of preventing and treating experimental colitis mediated by well-documented mechanisms [1, 48, 50, 137]. Several comprehensive reviews more extensively document protective effects of probiotics and commensal bacteria [135, 136]. The protective mechanisms of traditional probiotics and LBPs include the bacteria themselves (DNA, cytoplasmic, and cell wall contents) and their metabolites, such as organic acids, SCFAs, and lactic acid that stimulate homeostatic immune and mucosal protection [1, 138]. (1) Inhibiting pathobionts Certain protective bacteria inhibit resident potentially pathogenic microbiota, such as Enterobacteriaceae [50, 139], Fusobacterium [50], and Bacteroideceae [140]. Many pathobionts adhere intestinal epithelial cells to induce inflammation, i.e., AIEC and F. varium [45, 141, 142]. Probiotic E. coli Nissle 1917 and L. johnsonii La1 can compete for ecologic niches, epithelial binding, and nutrients with pathobionts and inhibit their adhesion and proliferation [143]. Furthermore, decreased luminal pH by organic acids (SCFAs, etc.) produced by probiotics and protective resident bacteria [144], anti-bacterial peptides (bacteriocins) [145], and bile acids modulated by probiotics [146] can inhibit pathobionts. In addition to these bacterial cross talks, probiotics and resident bacterial species can indirectly (via host cells) affect pathobionts: mucosal PPAR-γ signaling activated by probiotic Clostridium and VSL#3 reduce luminal oxygen and inhibit aerobic Enterobacteriaceae [53, 147]; E. coli Nissle 1917 induces defensin production by epithelial cells through flagellin–Toll-like receptor binding (TLR) [148]. (2) Increasing beneficial resident bacteria Probiotics and LBPs can increase growth of other resident beneficial bacterial species and improve the intestinal ecosystem [50, 123, 149]; increase lactobacilli [126, 139], bifidobacteria [123, 126, 149], S. thermophilus [123], and bacterial diversity [126], while reducing fungal diversity [126]. (3) Improving mucosal barrier function Bifidobacterium strains strengthen epithelial barrier function in UC [150]. SCFAs provide the main energy source of colonic epithelial cells, improve mucosal barrier function, and activate colonic Tregs [151, 152]. However, in clinical studies, some probiotics work without elevating SCFA [153]. Other protective bacterial metabolites include indoles that bind aryl hydrolase receptors, PXR, and sphingolipids [135]. TLR and NOD2-recognition pathways mediate some bacterial protective functions [154]. (4) Mucosal and systemic immunomodulation Probiotics and resident bacteria can induce anti-inflammatory cytokines (IL-10, TGF-β, etc.) and mucosal and systemic regulatory cells (Treg, IgA+, and regulatory B cells) and attenuate inflammatory cytokines (IFN-γ, IL-12p40, TNF-α, etc.) [47, 48, 118, 132, 154,155,156].
Clinical Concerns
Safety
Probiotics are well tolerated with low rates of adverse effects [157, 158] although rare cases of sepsis, endocarditis, and liver abscess with use of Lactobacillus and fungemia by S. boulardii have occurred, primarily in hospitalized and severely ill or immunocompromised patients with intravenous catheters [158].
Optimal Protocols
Resident microbiota compete with exogenous microbes. Therefore, antibiotic pre-treatment seems reasonable to improve engraftment of exogenous probiotics and LBPs. SERES’s RCT demonstrates that pre-treatment with vancomycin enhances engraftment of Firmicutes spores [114, 115]. Some papers suggest that beneficial effects require 106–108 probiotics/g stool [159]. Given that only 20% of probiotic cells survive [160, 161] and stool weight is 1 kg, 5 × 108–1011 probiotic cells administration may be required. However, lower doses may be sufficient for resident LBP strains that proliferate in the intestine. The rectal route (enema) can be considered for pouchitis and pouchitis. D’Inca et al. compared oral versus enema with L. casei GG in active UC and showed a significant advantage of the rectal route for reducing mucosal inflammatory cytokines and modifying the microbiota (Lactobacillus increased, Enterobacteriaceae decreased) [139]. Matthes et al. showed that E. coli Nissle 1917 enemas are effective in a dose-dependent manner in active UC patients [162].
Conclusions
Some standard probiotics benefit UC and pouchitis activities. In contrast, the benefits of probiotics for CD seem to be strain specific and limited in maintaining remission after surgery for CD patients. However, VSL#3 containing Bifidobacterium, Lactobacillus, and S. salivarius has more beneficial effects. These positive reported results are subject to publication bias. Although clinical trials of LBPs are just beginning, providing protective resident microbiota to reverse dysbiosis and restore homeostatic microbial community structure and function is an attractive approach that will be actively investigated in the near future.
Prebiotics
Prebiotics are food ingredients that are selectively fermented by host microbes to confer a health benefit. Examples include dietary fiber and oligosaccharides naturally contained in fruits, vegetables, and grains [163]. IBD patients are traditionally advised to lower their fiber intake and sometimes fast during flares to reduce mechanical stimulation of the damaged mucosa [164]. However, many studies of various dietary fiber and oligosaccharides suggest favorable results as an emerging treatment approaches (Table 4). Their ability to increase potentially beneficial bacteria and beneficial metabolic effects (SCFAs, etc.) has been verified in humans and murine models [163, 165].
Ulcerative Colitis
Many studies focus on QOL, symptoms, and bacterial metabolites in UC treated with various prebiotics. Psyllium, germinated barley foodstuff (GBF), lactulose, and oligofructose-enriched inulin significantly improve QOL and symptoms in UC patients [166,167,168,169]. Intake of psyllium and wheat bran significantly increased fecal butyrate [170, 171]. A large RCT with psyllium demonstrated equivalent effectiveness to 5-ASA to maintain remission in UC [171] and a crossover trial in active UC is underway (NCT03998488). GBF contains low-lignified hemicellulose that is efficiently fermented by colonic microbiota [161, 165]. GBF reduced CRP [172] and improved clinical and endoscopic scores in active UC in an uncontrolled long-term study [173]. Supplemental oligofructose-enriched inulin with 5-ASA significantly reduced fecal calprotectin (fourfold change) at day 7 compared with 5-ASA alone [168]; a RCT is underway (NCT03653481). A RCT of Synergy1, composed of equal proportions of fructo-oligosaccharide and inulin in active UC, has been completed without published results (NCT02093767), and an additional RCT in inactive UC is currently recruiting (NCT02865707). Curcumin, the biologically active component of turmeric with anti-inflammatory and antioxidant effects, can support the growth of protective bacteria [174], so it is a promising prebiotic. A large RCT in UC improved remission rates with clinical and endoscopic scores compared to controls [175]. Additional RCTs of curcumin have recently been registered in pediatric (NCT02277223) and adult (NCT02683759) UC patients. Fucosyllactose modifies microbiota [176]; 2 RCTs are underway (NCT03847467, NCT03847467). Glycomacropeptide can modify microbiota and metabolites, reducing Proteobacteria and increasing SCFAs [177]; the first RCT is recruiting UC patients (NCT02825914).
Pouchitis
Two crossover studies focused on disease activity. Three weeks of inulin supplementation significantly improved clinical and histological scores from baselines associated with increased butyrate levels and reduced pH, B. fragilis and increased secondary bile acids levels [178]. However, the same treatment protocol failed to show significant benefit although a slightly increased butyrate level correlated with reduced disease activity [179]. One possible explanation is variable microbiota in individuals, since efficacy of prebiotics can depend on abundance of resident Bifidobacteria [180].
Crohn’s Disease
Restricted dietary fiber did not improve symptoms need for surgery or hospitalization in CD patients [181]. On the contrary, fiber-rich diets significantly reduced surgery in active CD [182] and prevented relapse during remission [183]. Two recent RCTs using oligofructose-enriched inulin inhibited disease activity of active CD associated with increased SCFAs [184] and B. longum and reduced R. gnavus, a potential pathogen in CD [185]. An additional RCT is underway (NCT03653481). 2 RCTs are underway (NCT03847467, NCT03847467) testing fucosyllactose a prebiotic that modifies microbiota [176].
Therapeutic Mechanisms
Prebiotics are substrates fermented by resident microbiota to organic acids (SCFAs), CO2, H2, and methane gas [163, 165]. (1) Increasing beneficial bacteria Prebiotics enriched Bifidobacteria [185, 186], lactobacilli [186], F. prausnitzii [187], and Clostridium clusters IV and XIVa [188]. (2) Inhibiting pathobionts Prebiotics can decrease Proteobacteria [177], Bacteroides [178, 186], R. gnavus [185], and Candida [186]. (3) Improving mucosal barrier SCFAs improve mucosal barrier function by providing a key nutrient for colonic epithelial cells [47, 48, 135], while inulin prevents mucus defects [189]. (4) Mucosal and systemic immunomodulation Some prebiotics induce Tregs [47, 48, 135], likely through SCFA production, and intestinal IgA [190]. (5) Absorption of toxic substances Dietary fiber can adsorb toxic substances, cholesterol, bile acids and provide bacterial scaffolds to benefit inflammation [191].
Clinical Concerns
Safety
Because prebiotics derive from natural foods, prebiotics are considered to be safe [166, 167, 169, 192]. There were no severe adverse events reported in RCT, although a few food-allergy events occurred [192, 193]. Of note, psyllium may cause gastrointestinal obstruction, especially at stenotic sites [192, 193], and has not used in CD trials.
Tolerability
Clinical use is limited by high participant dropout due to bloating and discomfort among IBD patients [184].
Conclusions
Improved beneficial bacteria community structure and metabolism by prebiotics are documented in human volunteers and IBD patients, but relatively few clinical RCTs have been conducted. Although more high-quality and disease activity-focused clinical studies are needed, prebiotic therapy is a promising safe and physiologic treatment and maintenance approach to IBD, perhaps in combination with LBPs.
Prebiotic Diets
Diet greatly affects microbiota composition and metabolism, and IBD dysbiosis is associated with diet [194,195,196]. Many rigorously designed RCTs have been newly registered. Exclusive enteral nutrition (EEN) is used as first-line therapy for inducing remission in CD with mucosal healing and histological improvement [194, 197]. This approach is most widely used in pediatric patients. Responses may be partially attributed to EEN-mediated microbial changes, despite decreased diversity [32]. The Mediterranean-style diet (MSD), Asian diet, and semi-vegetarian diet increase beneficial bacteria [198], potentially reduce pathobionts [199], and may benefit IBD patients [200], leading to a RCT investigating the effectiveness of MSD in UC (NCT03053713). The specific carbohydrate diet (SCD), consisting of mostly meat, fruits, vegetables, nuts, oils, and honey with the elimination of grains, has shown efficacy in a retrospective IBD study [201] leading to multiple RCTs, investigating its effects microbial profile and clinical outcome (NCT02858557) (NCT02412553) and efficacy in pediatric (NCT02610101, NCT03301311) and adult (NCT03058679, NCT02412553, NCT02858557) IBD and comparison versus MSD (NCT04082559, NCT03058679). Other promising diets are under investigation, such as the fasting-mimicking diet in UC (NCT03165690), Mashiha in IBD (NCT02796339), and the low fermentable oligosaccharide, disaccharide, monosaccharide, and polyol (FODMAP) diet in UC (NCT02469220). Several of these diets, including SCD and low FODMAP, are low in fiber and prebiotics, so they may affect symptoms more than disease efficacy. A better understanding of prebiotics may provide improved advice for patients’ food choices.
Synbiotics
Synbiotics are mixtures of probiotics and prebiotics that beneficially affect the host by improving the survival and implantation of live microbial dietary supplements in the gastrointestinal tract by selectively stimulating the growth and/or by activating the metabolism of one or a limited number of health-promoting bacteria, thus improving host welfare [163]. Given that IBD patients harbor less beneficial intestinal bacteria (Table 1), administration of synbiotics may improve treatment with probiotics or LBPs. Indeed, some papers demonstrated that the benefit of prebiotics depends on baseline abundance of resident protective species [180]. However, clinical studies of synbiotics are limited (Table 4). Ishikawa et al. demonstrated that bifidobacterial strains plus galacto-oligosaccharide synbiotics improved endoscopic scores and decreased inflammatory markers in treated UC patients [202]. Furrie et al. detected higher numbers of total bifidobacteria on the mucosal surface in active UC patients fed a synbiotic containing B. longum and Synergy1 than in those taking placebo [203]. In CD, B. longum plus Synergy1 was effective [133]. Overall, prebiotic therapy appears safe and promising, but RCTs are needed to assess the efficacy of dietary/prebiotic interventions. The concept of combined therapies is supported by observations that partial EN plus an exclusion diet high in fiber and fresh fruits and vegetables was better tolerated and induced a more sustained remission in pediatric CD patients compared with standard EEN therapy [197].
FMT
After the breakthrough success of FMT therapy in rCDI in 2013 [19], several accessible fecal banks have been established (OpenBiome, etc.) and multiple clinical studies have been performed in IBD patients. This section updates FMT clinical trials in IBD (Table 5), which have been extensively reviewed [57, 204, 205].
Ulcerative Colitis
After initial success of FMT for induction of remission in UC in 1989 [206], four RCTs and several case series have provided promising results. Three out of four RCTs demonstrated a significantly improved clinical, endoscopic, and histological scores [58, 59, 207] although clinical response rates (24–32%) are not as dramatic as in FMT for rCDI (93%). In the unsuccessful RCT [60], the FMT group showed a higher clinical remission rate over controls (41% vs 25%) without statistical significance, likely due to limited subject numbers. However, clinical efficacy was strikingly different with different donors [58]. Indeed, pooled analyses show effectiveness of FMT for active UC [57, 204, 205]. Bacterial taxa analyses revealed that FMT significantly improved bacterial diversity, which correlates with clinical responses [59, 204]. Interestingly, several bacteria taxa were associated with remission after FMT, such as Clostridium clusters IV and XVIII, while the presence of Proteobacteria (Sutterella species) and Fusobacterium species was associated with lack of remission [59]. Ishikawa et al. modified the Fusobacterium-targeted antibiotic ATM cocktail (tetracycline replaced by fosfomycin, AFM cocktail) and showed that the pre-AFM + FMT combination improved outcomes [208]. Moreover, they showed that the reduced abundance of Bacteroidetes by AFM antibiotics pre-treatment was clearly restored in FMT responders, but not non-responders. Bacteroidetes is one of the symbiotic taxa [209], which can inhibit C. perfringens [145] and induce Treg [47, 48, 137]. Two RCTs investigating antibiotics prior to FMT are currently underway (NCT02606032, NCT02033408). Based on limited long-term follow-up reports, the effects of FMT seem to gradually decrease over 3 months [210, 211]. However, some responders exhibit long-term remission (> 1–2 years) [207, 212]. Multiple RCTs are underway in several countries.
Pouchitis
Herfarth et al. [213] demonstrated the difficulty of engraftment of FMT: One out of six patients showed successful engraftment and remission. This could be due to several factors, including donor selection, the dose, frequency and route of administration of FMT, and the pouch microenvironment. Pouches are constructed from the small intestine where potentially beneficial Firmicutes bacteria such as Clostridia are rarely detectable in normal conditions. Of note, Stallmach et al. [214] showed impressive clinical benefits and engraftment by multiple FMT in antibiotic-refractory pouchitis patients: All 5 patients who received FMT achieved clinical response (4/5 remission) and 3/5 patients maintained remission with sequential FMTs. A RCT is currently recruiting (NCT02049502).
Crohn’s Disease
Although some case series showed less benefit of FMT in CD patients compared with UC [212, 215], many promising case reports and series describe induction of CD remission [204]. Meta-analysis of 6 prospective and uncontrolled trials [204] shows 52% clinical remission rate with publication bias. For adult CD, 58–87% clinical responses were reported [216, 217]. Responders showed greater improvement in microbial diversity with a significant shift in fecal microbial composition toward their donor’s profile than non-responders and increased lamina propria Tregs following FMT [216]. FMT via nasogastric tube induced remission in 77.8% of pediatric CD patients 2 weeks after FMT with evidence of engraftment [218]. As seen in UC, responders of FMT in CD showed rapidly improved symptoms and clinical activity several weeks after FMT, but this effect diminished over several months after FMT [216,217,218,219] with return to bacterial composition patterns close to pre-FMT levels [219]. To maintain the clinical benefits from FMT, Li et al. [220] suggested performing the next course of FMT less than 4 months after the previous FMT, based on the large-scale clinical trial. Multiple RCTs are currently underway.
Therapeutic Mechanisms
The therapeutically relevant components of FMT remain elusive [221]. Increased bacterial diversity is clearly associated with successful response of FMT in IBD [59, 204, 216]. Further, the recipient microbiota after successful FMT resemble donor microbiota, likely due to implantation of donor bacteria and/or donor feces promoting growth of resident bacteria that resemble the donor’s species [221]. Although several potentially relevant species are reported [59], more data are required to support the protective species. FMT is a complex material containing bacteriophages, fungi, and metabolites as well as bacteria [59]. Given the therapeutic benefits of filtrated FMT in rCDI studies [222], cell-free components (bacteriophages and metabolites) in FMT need to be included as research targets. A RCT of filtrated FMT in UC has been registered (NCT03843385).
Clinical Concerns
Safety
FMT is safe and well tolerated in IBD clinical trials [57, 204, 223,224,225]. Importantly, two bacteremias (one death) by ESBL E. coli were reported in immunocompromised patients who received donor stool harboring these strains [226]. Exclusion criteria now include ESBL-producing species. Fecal banks are one source for donor stool.
Effective Donor
FMT success depends on microbial diversity and composition of the donor’s stool, leading to the proposed existence of FMT ‘super donors’ [58, 212, 227]. The optimal microbial characteristics of donor feces have not defined in IBD. A family member is often chosen as a donor. Because siblings and relatives share similar gut microbiota because of similar lifestyles, diets, and genetics [228, 229], they may not be optimal donors if the goal is to modulate the recipient’s microbial composition. Switching donors rescued non-responders in a rCDI trial [19]. Whether donors can be optimized by various methods is under investigation.
Optimal Protocol
Engraftment is important factor of efficacy in FMT [230]. A 2018 meta-analysis of FMT protocols indicated that fresh or frozen donor stools, delivery route, and antibiotic pre-treatment have no impact on FMT efficacy in IBD [205]. Multiple administrations appear more effective rather than single FMT [205]. Costello et al. [207] demonstrated marked benefit of anaerobically prepared FMT, while Cui et al. [217] established a laboratory preparation of fecal materials. Vermeire et al. [212] demonstrated that increased CRP levels at week 2 were an early marker of failure, which could allow early rescue therapy in those IBD patients that will not benefit from FMT or guide repeat FMT with a different donor. Because mucosal inflammation reduces microbial diversity and increases pathobionts [231], pre-treatment with immunosuppression to reduce local inflammation and antibiotics to eliminate competing native microbiota may improve engraftment of beneficial species.
Conclusions
Successful FMT has been reported primarily in UC patients. A few positive results exist for CD and pouchitis from case reports and open-label studies. Ongoing multiple RCTs and efforts to optimize protocols, engraftment, donor and recipient selection, and matching the optimal donor with individual recipients based on microbial sequencing could improve FMT as a primary therapy. However, we continuously need to consider possible transmission of ‘undefined’ infectious agents in human stools, in contrast to the safety of defined therapeutic LBP cocktails.
Emerging Options (Bugs as Drugs)
This section discusses recent and ongoing preclinical studies, technologies, and emerging therapeutic concepts [1,2,3].
Rationally Defined Human-Derived Bacterial Consortia—LBPs
A potentially better, more consistent therapeutic approach uses well-characterized, rationally defined and orally delivered LBPs from resident bacterial species from the intestine of healthy subjects. The most advanced LBP for IBD investigation is a Clostridium cocktail. Atarashi et al. [48] isolated 17 Clostridium strains from healthy human stool screened for induction of FOXP3+ murine CD4+ Tregs. These strains protected several experimental colitis models with high production of SCFAs and induction of colonic IL-10-producing Tregs [48]. All 17 strains belong to Clostridium clusters IV, XIVa, or XVIII, which are reduced in IBD patients [13, 30]. Administering these strains is designed to restore a normal ecology in IBD patients [232]. Based on these results [40] and mechanistic preclinical studies that identified additional mechanisms beyond the Treg and SCFA pathways, such as correcting dysbiosis and altering non-SCFAs metabolites [50], Janssen Research & Development and Vedanta initiated a Phase 1 clinical study in healthy volunteers (NCT03723746). Many other LBPs based on different in vitro and in vivo screening methods are in development and should reach clinical trials soon.
Screening LBPs
Choosing protective resident bacterial strains has been performed in vivo by reductionist [48] and combinatorial [233] approaches in gnotobiotic mice by screening for Treg activation and in vitro with cell lines and human blood lymphocytes [234]. However, results of in vitro studies do not always predict in vivo effects [235]. Peran et al. [236] showed that a specific strain of Lactobacillus salivarius prevented colitis in a TNBS rat model. This strain was selected from 30 laboratory strains for eliciting the highest IL-10/IL-12 and IL-10/TNFα ratios in macrophages. Unfortunately, no strains exhibiting a moderate or low IL-10/IL-12 profile were included in the in vivo study. Similarly strains ranked on their induction of in vitro IL-10/IL-12 cytokine induction closely match their in vivo attenuation of experimental colitis [234, 237]. Our group established a novel in vivo/in vitro combined method using gnotobiotic IL-10-reporter mice to measure individual resident bacterial strain induction of IL-10/IFNγ ratios, with high ratio strains preventing and reversing experimental colitis induced by low IL-10/IFNγ-inducing strains [10].
Substrates from Microbiota
Although SCFAs are an important anti-inflammatory substrate in microbe–microbe and microbe–host interactions [144], many other candidate bacterial metabolites affect microbiota and host responses to attenuate mucosal inflammation. Because microbial-based therapies have strong strain- and donor-specific effects [131], purified substrates from defined microbes may provide more consistent results. Examples of established protective bioactive substances produced by probiotic and resident bacteria include p75/p40 from L. rhamnosus that acts through an EGFR-dependent mechanism [238], polyphosphate from lactobacilli [239], lactocepin from VSL#3 [240], polysaccharide-A from B. fragilis [137], an anti-inflammatory protein from F. prausnitzii [241], and kangfuxin liquid extracted from Periplaneta americana dried worms [242]. Most investigators focus on ‘beneficial’ strains to discover a new therapeutic microbial-based tool. In contrast, a unique product from a ‘pathogenic’ E. coli strain, QBECO, is used to immunize hosts (Qu Biologics). Interestingly, purified major macromolecules of an inactivated pathogenic strain of E. coli isolated from a patient with an E. coli infection restored the immune system’s ability to respond productively to invading bacteria in the gastrointestinal tract and rebuild normal barrier function. QBECO treatment improved endoscopic and histological scores in active UC [243] and CD patients cohort [244]. A RCT in CD patients is ongoing [245].
Editing Microbiota
Improved understanding of microbiology and metabolic functions suggests ways to modify or block bacterial functions (enzymes and surface molecules) that provide virulence traits. In mice, tungstate treatment, which inhibits molybdenum-cofactor-dependent microbial respiratory pathways, inhibits Enterobacteriaceae expansion and experimental inflammation [246]. Of note, this effect on microbiota was observed only during inflammation. Additional approaches to selectively inhibit pathobiont numbers and functions in IBD include blocking AIEC epithelial attachment through FimH (NCT03709628) and pathobiont-specific bacteriophages (NCT03808103).
Bacteriophages, Yeasts, and Engineered Bacteria
Virus (mostly bacteriophages targeting specific bacteria) improves intestinal homeostasis and protects against intestinal injury and pathogen infection [247]. This is potentially clinically relevant, since specific bacteria-targeted bacteriophages may act without affecting beneficial resident bacteria. A RCT examining therapeutic effects of a bacteriophage against AIEC is recruiting CD patients (NCT03808103). Interestingly, bacteriophage DNA can induce colitis and activate IFN-γ responses [248], so clinical toxicity must be examined. Probiotic yeast, including Candida glabrata, produces chitin that reduces bacteria/fungus overgrowth and attenuates DSS colitis with activation of PPAR-γ and induction of IL-10 [249, 250]. Although genetically engineered organisms must be carefully handled, several unique bacterial strain expresses anti-inflammatory substrates such as IL-10, IL-35, trefoil factors, and elafin [251,252,253,254].
Screening Patients: Personalized Treatment
Based on the heterogeneity of individual IBD patient’s microbiota and patient therapeutic responses, a pilot RCT investigating effects of personalized microbiota-based therapy (antibiotics and prebiotics) is underway in pouchitis (NCT04082559). As described above, efficacy of probiotics, prebiotics, and FMT (and anti-TNF-α therapy) depends on a patient’s microbiota [255]. Therefore, the best strategy for personalized management of IBD is to identify intestinal microbial profiles prior to beginning therapy or in non-responders [2] to guide optimal microbiome-based therapies.
Conclusions
Microbial-based and microbial-targeted therapies for IBD are emerging with favorable results. The rational for correcting the established dysbiosis in CD, UC, and pouchitis patients is well established. Certain antibiotics are promising short-term primary therapies with relatively safety. However, the risk of resistant bacteria and CDI and uncertain long-term benefit/toxicity profiles limit maintenance use of antibiotics. FMT is also a promising primary therapy with well-designed RCTs underway. However, the risk of transmission of ‘unknown’ pathogens and long-term benefits remain unclear. A major limitation is variable efficacy of different donors. In contrast, LBPs, prebiotics, and diet are well defined, safe for long-term use and could be designed for personalized use based on the microbial community structure of individual recipients. Hopefully, these new generation microbial-related therapies will be validated by high-quality preclinical and clinical trials. The best clinical applications for microbial therapy in IBD remain uncertain. Current studies concentrate on single agents inducing remission of active UC. However, we believe that preventing relapse after achieving clinical remission with corticosteroids or biologic therapies in UC or CD patients or with antibiotics in chronic relapsing or antibiotic-dependent pouchitis might be more important areas to investigate. Other clinical needs possibly fulfilled by microbial-based therapies are use of these agents as adjuncts to standard biologic or immunologic therapies to hasten or increase the frequency of deep remission or to maintain quiescent disease after removing the more toxic immune-suppressing agent. Long-term use of physiologic approaches to restore microbial homeostatic function should be less toxic and more acceptable to patients (and physicians) who are concerned about the risk of infection and neoplasia with sustained immunosuppression. We advocate use of concomitant companion diagnostic tests that profile an individual’s microbiota to guide optimal personalized microbial therapies, determine best timing of intervention, and ultimately prevent disease onset in high-risk individuals.
References
Sartor RB, Wu GD. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology. 2017;152:e4.
Mishima Y, Sartor RB. Manipulating resident microbiota to enhance regulatory immune function to treat inflammatory bowel diseases. J Gastroenterol. 2019;2019:1–11.
Cohen LJ, Cho JH, Gevers D, Chu H. Genetic factors and the intestinal microbiome guide development of microbe-based therapies for inflammatory bowel diseases. Gastroenterology. 2019;156:2174–2189.
Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231.
Stepankova R, Powrie F, Kofronova O, et al. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in SCID mice reconstituted with CD45RBhigh CD4+ T cells. Inflamm Bowel Dis. 2007;13:1202–1211.
Matsumoto S, Okabe Y, Setoyama H, et al. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–78.
Rath HC, Herfarth HH, Ikeda JS, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945–953.
Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633.
Britton GJ, Contijoch EJ, Mogno I, et al. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity. 2019;50:e4.
Liu B, Oka A, Jang J, et al. Protective and potentially aggressive bacterial species occur simultaneously in healthy hosts (abstract). Gastroenterology. 2019;156:S-78.
Hoarau G, Mukherjee PK, Gower-Rousseau C, et al. Bacteriome and mycobiome interactions underscore microbial dysbiosis in familial Crohn’s disease. MBio. 2016;7:1–11.
Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662.
Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785.
Kim SC, Tonkonogy SL, Albright CA, et al. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005;128:891–906.
Ko CW, Singh S, Feuerstein JD, et al. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology. 2019;156:748–764.
Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25.
Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53:305–353.
Laurell A, Sjöberg K. Prebiotics and synbiotics in ulcerative colitis. Scand J Gastroenterol. 2017;52:477–485.
van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415.
Garber K. Drugging the gut microbiome. Nat Biotechnol. 2015;33:228–231.
U. S. Food and Drug Administration. Early clinical trials with live biotherapeutic products: chemistry, manufacturing, and control information; 2016.
Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359.
Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904.
Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65.
Tap J, Mondot S, Levenez F, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009;11:2574–2584.
O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693.
Lederberg J. Infectious history. Science. 2000;288:287–293.
Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340.
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230.
Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392.
Norman JM, Handley SA, Baldridge MT, et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160:447–460.
Lewis JD, Chen EZ, Baldassano RN, et al. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn’s disease. Cell Host Microbe. 2015;18:489–500.
Zwolinska-Wcislo M, Brzozowski T, Budak A, et al. Effect of Candida colonization on human ulcerative colitis and the healing of inflammatory changes of the colon in the experimental model of Colitis ulcerosa. J Physiol Pharmacol. 2009;60:107–118.
Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–211.
Baumgart M, Dogan B, Rishniw M, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. ISME J. 2007;1:403–418.
Giaffer MH, Holdsworth CD, Duerden BI. The assessment of faecal flora in patients with inflammatory bowel disease by a simplified bacteriological technique. J Med Microbiol. 1991;35:238–243.
Mosca A, Leclerc M, Hugot JP. Gut microbiota diversity and human diseases: Should we reintroduce key predators in our ecosystem? Front. Microbiol. 2016;7:1–12.
Ott SJ, Musfeldt M, Wenderoth DF, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693.
Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen Van Zanten SJO. Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J Clin Microbiol. 2006;44:4136–4141.
Xun Z, Zhang Q, Xu T, Chen N, Chen F. Dysbiosis and ecotypes of the salivary microbiome associated with inflammatory bowel diseases and the assistance in diagnosis of diseases using oral bacterial profiles. Front Microbiol. 2018;9:1–17.
Kelsen J, Bittinger K, Pauly-Hubbard H, et al. Alterations of the subgingival microbiota in pediatric Crohn’s disease studied longitudinally in discovery and validation cohorts. Inflamm Bowel Dis. 2015;21:2797–2805.
Atarashi K, Suda W, Luo C, et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017;358:359–365.
Schmitz JM, Tonkonogy SL, Dogan B, et al. Murine adherent and invasive E. coli induces chronic inflammation and immune responses in the small and large intestines of monoassociated IL-10−/− mice independent of long polar fimbriae adhesin A. Inflamm Bowel Dis. 2019;25:875–885.
Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127:412–421.
Ohkusa T, Okayasu I, Ogihara T, Morita K, Ogawa M, Sato N. Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut. 2003;52:79–83.
Van den Abbeele P, Belzer C, Goossens M, et al. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013;7:949–961.
Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341.
Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236.
Hayashi A, Sato T, Kamada N, et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13:711–722.
Oka A, Mishima Y, Bongers G, et al. Tu1844-IL-10-independent protective activities of human-derived clostridium strains in experimental colitis. Gastroenterology. 2018;154:S-1036.
Rossi O, vanBerkel LA, Chain F, et al. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci Rep. 2016;6:18507.
Rigottier-Gois L. Dysbiosis in inflammatory bowel diseases: the oxygen hypothesis. ISME J. 2013;7:1256–1261.
Byndloss MX, Olsan EE, Rivera-Chávez F, et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357:570–575.
Rivera-Chávez F, Zhang LF, Faber F, et al. Depletion of butyrate-producing clostridia from the gut microbiota drives an aerobic luminal expansion of salmonella. Cell Host Microbe. 2016;19:443–454.
Dubuquoy L, Jansson EA, Deeb S, et al. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology. 2003;124:1265–1276.
Quinton JF, Sendid B, Reumaux D, et al. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–791.
Imdad A, Nicholson MR, Tanner-Smith EE, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2018;. https://doi.org/10.1002/14651858.
Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:e6.
Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;6736:1–11.
Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015;149:e4.
Bazin T, Hooks KB, Barnetche T, et al. Microbiota composition may predict anti-Tnf alpha response in spondyloarthritis patients: an exploratory study. Sci Rep. 2018;8:5446.
Aden K, Rehman A, Waschina S, et al. Metabolic functions of gut microbes associate with efficacy of tumor necrosis factor antagonists in patients with inflammatory Bowel diseases. Gastroenterology. 2019;157:e11.
Michail S, Durbin M, Turner D, et al. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm Bowel Dis. 2012;18:1799–1808.
Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97.
Browne HP, Forster SC, Anonye BO, et al. Culturing of “unculturable” human microbiota reveals novel taxa and extensive sporulation. Nature. 2016;533:543–546.
NIH Human Microbiome Project The Inflammatory Bowel Disease Multi’omics Database. https://ibdmdb.org/.
Kirsner JB. Historical aspects of inflammatory bowel disease. J Clin Gastroenterol. 1988;10:286–297.
Perencevich M, Burakoff R. Use of antibiotics in the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:651–664.
Maccaferri S, Vitali B, Klinder A, et al. Rifaximin modulates the colonic microbiota of patients with Crohn’s disease: an in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother. 2010;65:2556–2565.
Gao J, Gillilland MG, Owyang C. Rifaximin, gut microbes and mucosal inflammation: unraveling a complex relationship. Gut Microbes. 2014;5:571–575.
Sartor RB. Review article: the potential mechanisms of action of rifaximin in the management of inflammatory bowel diseases. Aliment Pharmacol Ther. 2016;43:27–36.
Wang S-L, Wang Z-R, Yang C-Q. Meta-analysis of broad-spectrum antibiotic therapy in patients with active inflammatory bowel disease. Exp Ther Med. 2012;4:1051–1056.
Khan KJ, Ullman TA, Ford AC, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:661–673.
Ohkusa T, Kato K, Terao S, et al. Newly developed antibiotic combination therapy for ulcerative colitis: a double-blind placebo-controlled multicenter trial. Am J Gastroenterol. 2010;105:1820–1829.
Ohkusa T, Nomura T, Terai T, et al. Effectiveness of antibiotic combination therapy in patients with active ulcerative colitis: a randomized, controlled pilot trial with long-term follow-up. Scand J Gastroenterol. 2005;40:1334–1342.
Nomura T, Ohkusa T, Okayasu I, et al. Mucosa-associated bacteria in ulcerative colitis before and after antibiotic combination therapy. Aliment Pharmacol Ther. 2005;21:1017–1027.
Burke DA, Axon AT, Clayden SA, Dixon MF, Johnston D, Lacey RW. The efficacy of tobramycin in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 1990;4:123–129.
Lobo AJ, Burke DA, Sobala GM, Axon AT. Oral tobramycin in ulcerative colitis: effect on maintenance of remission. Aliment Pharmacol Ther. 1993;7:155–158.
Turunen UM, Färkkilä MA, Hakala K, et al. Long-term treatment of ulcerative colitis with ciprofloxacin: a prospective, double-blind, placebo-controlled study. Gastroenterology. 1998;115:1072–1078.
Singh S, Stroud AM, Holubar SD, Sandborn WJ, Pardi DS. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. 2015; https://doi.org/10.1002/14651858.
Segal JP, Ding NS, Worley G, et al. Systematic review with meta-analysis: the management of chronic refractory pouchitis with an evidence-based treatment algorithm. Aliment Pharmacol Ther. 2017;45:581–592.
Holubar SD, Cima RR, Sandborn WJ, Pardi DS. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. 2010;2019:CD001176.
Gosselink MP, Schouten WR, Van Lieshout LMC, Hop WCJ, Laman JD, Ruseler-Van Embden JGH. Eradication of pathogenic bacteria and restoration of normal pouch flora: comparison of metronidazole and ciprofloxacin in the treatment of pouchitis. Dis Colon Rectum. 2004;47:1519–1525.
Dubinsky V, Reshef L, Bar N, et al. Predominantly antibiotic-resistant intestinal microbiome persists in patients with pouchitis who respond to antibiotic therapy. Gastroenterology. 2019. https://doi.org/10.1053/j.gastro.2019.10.001.
Singh S, Garg SK, Pardi DS, Wang Z, Murad MH, Loftus EV. Comparative efficacy of pharmacologic interventions in preventing relapse of Crohn’s disease after surgery: a systematic review and network meta-analysis. Gastroenterology. 2015;148:64–76e2.
Townsend CM, Parker CE, MacDonald JK, et al. Antibiotics for induction and maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2019;2:CD012730.
Su JW, Ma JJ, Zhang HJ. Use of antibiotics in patients with Crohn’s disease: a systematic review and meta-analysis. J Dig Dis. 2015;16:58–66.
Patton PH, Parker CE, MacDonald JK, Chande N. Anti-tuberculous therapy for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2016;7:CD000299.
Prantera C, Lochs H, Campieri M, et al. Antibiotic treatment of Crohn’s disease: results of a multicentre, double blind, randomized, placebo-controlled trial with rifaximin. Aliment Pharmacol Ther. 2006;23:1117–1125.
Selby W, Pavli P, Crotty B, et al. Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn’s disease. Gastroenterology. 2007;132:2313–2319.
Aberra FN, Brensinger CM, Bilker WB, Lichtenstein GR, Lewis JD. Antibiotic use and the risk of flare of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2005;3:459–465.
Keshaw H, Foong KS, Forbes A, Day RM. Perianal fistulae in Crohn’s disease: current and future approaches to treatment. Inflamm Bowel Dis. 2010;16:870–880.
Tozer PJ, Burling D, Gupta A, Phillips RKS, Hart AL. Review article: medical, surgical and radiological management of perianal Crohn’s fistulas. Aliment Pharmacol Ther. 2011;33:5–22.
Bernstein LH, Frank MS, Brandt LJ, Boley SJ. Healing of perineal Crohn’s disease with metronidazole. Gastroenterology. 1980;79:599.
Feagins LA, Holubar SD, Kane SV, Spechler SJ. Current strategies in the management of intra-abdominal abscesses in Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9:842–850.
Krook A, Lindström B, Kjellander J, Järnerot G, Bodin L. Relation between concentrations of metronidazole and Bacteroides spp in faeces of patients with Crohn’s disease and healthy individuals. J Clin Pathol. 1981;34:645–650.
Rafii F, Ruseler-Van Embden JG, van Lieshout LM. Changes in bacterial enzymes and PCR profiles of fecal bacteria from a patient with ulcerative colitis before and after antimicrobial treatments. Dig Dis Sci. 1999;44:637–642.
Morikawa K, Watabe H, Araake M, Morikawa S. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother. 1996;40:1366–1370.
Wan YC, Li T, Han Y-D, Zhang H-Y, Lin H, Zhang B. Effect of pregnane xenobiotic receptor activation on inflammatory bowel disease treated with rifaximin. J Biol Regul Homeost Agents. 2015;29:401–410.
Garrido-Mesa N, Camuesco D, Arribas B, et al. The intestinal anti-inflammatory effect of minocycline in experimental colitis involves both its immunomodulatory and antimicrobial properties. Pharmacol Res. 2011;63:308–319.
Shen B, Achkar JP, Lashner BA, et al. A randomized clinical trial of ciprofloxacin and metronidazole to treat acute pouchitis. Inflamm Bowel Dis. 2001;7:301–305.
Leung W, Malhi G, Willey BM, et al. Prevalence and predictors of MRSA, ESBL, and VRE colonization in the ambulatory IBD population. J Crohn’s Colitis. 2012;6:743–749.
Balram B, Battat R, Al-Khoury A, et al. Risk factors associated with clostridium difficile infection in inflammatory bowel disease: a systematic review and meta-analysis. J Crohn’s Colitis. 2019;13:27–38.
Ferreyra JA, Wu KJ, Hryckowian AJ, Bouley DM, Weimer BC, Sonnenburg JL. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe. 2014;16:770–777.
Roy A, Lichtiger S. Clostridium difficile infection: a rarity in patients receiving chronic antibiotic treatment for Crohn’s disease. Inflamm Bowel Dis. 2016;22:648–653.
Goldstein EJC, Johnson SJ, Maziade P, et al. Probiotics and prevention of Clostridium difficile infection. Anaerobe. 2017;45:114–119.
Gupta V, Rodrigues R, Nguyen D, et al. Adjuvant use of antibiotics with corticosteroids in inflammatory bowel disease exacerbations requiring hospitalisation: a retrospective cohort study and meta-analysis. Aliment Pharmacol Ther. 2016;43:52–60.
Chang JC, Cohen RD. Medical management of severe ulcerative colitis. Gastroenterol Clin N Am. 2004;33:235–250.
Ledder O. Antibiotics in inflammatory bowel diseases: Do we know what we’re doing? Transl Pediatr. 2019;8:42–55.
Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62:787–796.
Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol. 2018;233:2091–2103.
Peng L, Zhong Y, Wang A, Jiang Z. Probiotics combined with aminosalicylic acid affiliates remission of ulcerative colitis: a meta-analysis of randomized controlled trial. Biosci Rep. 2019;39:1–12.
Astó E, Méndez I, Audivert S, Farran-Codina A, Espadaler J. The efficacy of probiotics, prebiotic inulin-type fructans, and synbiotics in human ulcerative colitis: a systematic review and meta-analysis. Nutrients. 2019;11:293.
Simmons S, Diao L, O’Brien E, et al. Tu2019—engraftment of ser-287, an investigational microbiome therapeutic, is related to clinical remission in a placebo-controlled, double-blind randomized trial (seres-101) in patients with active mild to moderate ulcerative colitis (UC). Gastroenterology. 2018;154:S1371–S1372.
Bharat M, Curran J, Herfarth HH, et al. 85-SER-287: an investigational microbiome therapeutic, induces remission and endoscopic improvement in a placebo-controlled, double-blind randomized trial in patients with active mild-to-moderate ulcerative colitis. Gastroenterology. 2018;154:S-25.
Diao L, Nnamani MC, OBrien E, et al. 623—Ser-287: an investigational microbiome therapeutic, induces widespread transcriptional changes related to clinical remission in a placebo-controlled, double-blind randomized trial (seres-101) in patients with active mild-to-moderate ulcerative colit. Gastroenterology. 2019;156:130.
Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104:437–443.
Oliva S, Di Nardo G, Ferrari F, et al. Randomised clinical trial: the effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment Pharmacol Ther. 2012;35:327–334.
Palumbo VD, Romeo M, Marino Gammazza A, et al. The long-term effects of probiotics in the therapy of ulcerative colitis: a clinical study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160:372–377.
Ritchie ML, Romanuk TN. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS ONE. 2012;7:e34938.
Dong J, Teng G, Wei T, Gao W, Wang H. Methodological Quality Assessment of meta-analyses and systematic reviews of probiotics in inflammatory bowel disease and pouchitis. PLoS ONE. 2016;11:e0168785.
Yasueda A, Mizushima T, Nezu R, et al. The effect of Clostridium butyricum MIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis. Surg Today. 2016;46:939–949.
Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309.
Shen B, Brzezinski A, Fazio VW, et al. Maintenance therapy with a probiotic in antibiotic-dependent pouchitis: experience in clinical practice. Aliment Pharmacol Ther. 2005;22:721–728.
Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209.
Kühbacher T, Ott SJ, Helwig U, et al. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. 2006;55:833–841.
Bengtsson J, Adlerberth I, Östblom A, Saksena P, Öresland T, Börjesson L. Effect of probiotics (Lactobacillus plantarum 299 plus Bifidobacterium Cure21) in patients with poor ileal pouch function: a randomised controlled trial. Scand J Gastroenterol. 2016;51:1087–1092.
Derwa Y, Gracie DJ, Hamlin PJ, Ford AC. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:389–400.
Fedorak RN, Feagan BG, Hotte N, et al. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13:e2.
Campieri M, Rizzello F, Venturi A, Poggioli G, Ugolini F. Combination of antibiotic and probiotic treatment is efficacious in prophylaxis of post-operative recurrence of Crohn’s disease: a randomized controlled study vs mesalamine. Gastroenterology. 2000;118:A781.
Whelan K, Quigley EMM. Probiotics in the management of irritable bowel syndrome and inflammatory bowel disease. Curr Opin Gastroenterol. 2013;29:184–189.
Lorea Baroja M, Kirjavainen PV, Hekmat S, Reid G. Anti-inflammatory effects of probiotic yogurt in inflammatory bowel disease patients. Clin Exp Immunol. 2007;149:470–479.
Steed H, Macfarlane GT, Blackett KL, et al. Clinical trial: the microbiological and immunological effects of synbiotic consumption—a randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment Pharmacol Ther. 2010;32:872–883.
Joeres-Nguyen-Xuan TH, Boehm SK, Joeres L, Schulze J, Kruis W. Survival of the probiotic Escherichia coli Nissle 1917 (EcN) in the gastrointestinal tract given in combination with oral mesalamine to healthy volunteers. Inflamm Bowel Dis. 2010;16:256–262.
Skelly AN, Sato Y, Kearney S, Honda K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat Rev Immunol. 2019;19:305–323.
Sassone-Corsi M, Raffatellu M. No vacancy: how beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J Immunol. 2015;194:4081–4087.
Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204–12209.
Böhmig GA, Krieger PM, Säemann MD, Wenhardt C, Pohanka E, Zlabinger GJ. n-butyrate downregulates the stimulatory function of peripheral blood-derived antigen-presenting cells: a potential mechanism for modulating T-cell responses by short-chain fatty acids. Immunology. 1997;92:234–243.
D’Incà R, Barollo M, Scarpa M, et al. Rectal administration of Lactobacillus casei DG modifies flora composition and Toll-like receptor expression in colonic mucosa of patients with mild ulcerative colitis. Dig Dis Sci. 2011;56:1178–1187.
Kruis W, Frič P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623.
Neutra MR, Frey A, Kraehenbuhl JP. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–348.
Barnich N, Carvalho FA, Glasser A, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007;117:1566–1574.
Bernet-Camard MF, Liévin V, Brassart D, Neeser JR, Servin AL, Hudault S. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl Environ Microbiol. 1997;63:2747–2753.
Fukuda S, Toh H, Hase K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547.
Crost EH, Pujol A, Ladiré M, et al. Production of an antibacterial substance in the digestive tract involved in colonization-resistance against Clostridium perfringens. Anaerobe. 2010;16:597–603.
Buffie CG, Bucci V, Stein RR, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517:205–208.
Marion-Letellier R, Déchelotte P, Iacucci M, Ghosh S. Dietary modulation of peroxisome proliferator-activated receptor gamma. Gut. 2009;58:586–593.
Petrof EO. Probiotics and gastrointestinal disease: clinical evidence and basic science. Antiinflamm Antiallergy Agents Med Chem. 2009;8:260–269.
Cui HH, Chen CL, De Wang J, et al. Effects of probiotic on intestinal mucosa of patients with ulcerative colitis. World J Gastroenterol. 2004;10:1521–1525.
Duranti S, Gaiani F, Mancabelli L, et al. Elucidating the gut microbiome of ulcerative colitis: bifidobacteria as novel microbial biomarkers. FEMS Microbiol Ecol. 2016;92:1–30.
Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450.
Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573.
Yoshimatsu Y, Yamada A, Furukawa R, et al. Effectiveness of probiotic therapy for the prevention of relapse in patients with inactive ulcerative colitis. World J Gastroenterol. 2015;21:5985–5994.
Mishima Y, Oka A, Liu B, et al. Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells. J Clin Invest. 2019;130:3702–3716.
Pronio A, Montesani C, Butteroni C, et al. Probiotic administration in patients with ileal pouch-anal anastomosis for ulcerative colitis is associated with expansion of mucosal regulatory cells. Inflamm Bowel Dis. 2008;14:662–668.
Hiramatsu Y, Hosono A, Takahashi K, Kaminogawa S. Bifidobacterium components have immunomodulatory characteristics dependent on the method of preparation. Cytotechnology. 2007;55:79–87.
Jia K, Tong X, Wang R, Song X. The clinical effects of probiotics for inflammatory bowel disease: a meta-analysis. Medicine (Baltimore). 2018;97:e13792.
Snydman. The safety of probiotics. Clin Infect Dis. 2008;46:S104–S111.
Charteris WP, Kelly PM, Morelli L, Collins JK. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol. 1998;84:759–768.
Kuisma J, Mentula S, Jarvinen H, Kahri A, Saxelin M, Farkkila M. Effect of Lactobacillus rhamnosus GG on ileal pouch inflammation and microbial flora. Aliment Pharmacol Ther. 2003;17:509–515.
Bezkorovainy A. Probiotics: determinants of survival and growth in the gut. Am J Clin Nutr. 2001;73:399S–405S.
Matthes H, Krummenerl T, Giensch M, Wolff C, Schulze J. Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN). BMC Complement Altern Med. 2010;10:13.
Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412.
Hallert C, Kaldma M, Petersson BG. Ispaghula husk may relieve gastrointestinal symptoms in ulcerative colitis in remission. Scand J Gastroenterol. 1991;26:747–750.
Kanauchi O, Fujiyama Y, Mitsuyama K, et al. Increased growth of Bifidobacterium and Eubacterium by germinated barley foodstuff, accompanied by enhanced butyrate production in healthy volunteers. Int J Mol Med. 1999;3:175–179.
Fujimori S, Gudis K, Mitsui K, et al. A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition. 2009;25:520–525.
Hafer A, Krämer S, Duncker S, Krüger M, Manns MP, Bischoff SC. Effect of oral lactulose on clinical and immunohistochemical parameters in patients with inflammatory bowel disease: a pilot study. BMC Gastroenterol. 2007;7:36.
Casellas F, Borruel N, Torrejón A, et al. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment Pharmacol Ther. 2007;25:1061–1067.
Hanai H, Kanauchi O, Mitsuyama K, et al. Germinated barley foodstuff prolongs remission in patients with ulcerative colitis. Int J Mol Med. 2004;13:643–647.
Hallert C, Björck I, Nyman M, Pousette A, Grännö C, Svensson H. Increasing fecal butyrate in ulcerative colitis patients by diet: controlled pilot study. Inflamm Bowel Dis. 2003;9:116–121.
Fernández-Bañares F, Hinojosa J, Sánchez-Lombraña JL, et al. Randomized clinical trial of Plantago ovata seeds (dietary fiber) as compared with mesalamine in maintaining remission in ulcerative colitis. Spanish Group for the Study of Crohn’s Disease and Ulcerative Colitis (GETECCU). Am J Gastroenterol. 1999;94:427–433.
Faghfoori Z, Shakerhosseini R, Navai L, Somi MH, Nikniaz Z, Abadi A. Effects of an oral supplementation of germinated barley foodstuff on serum CRP level and clinical signs in patients with ulcerative colitis. Heal Promot Perspect. 2014;4:116–121.
Kanauchi O, Mitsuyama K, Homma T, et al. Treatment of ulcerative colitis patients by long-term administration of germinated barley foodstuff: multi-center open trial. Int J Mol Med. 2003;12:701–704.
Ghiamati Yazdi F, Soleimanian-Zad S, van den Worm E, Folkerts G. Turmeric extract: potential use as a prebiotic and anti-inflammatory compound? Plant Foods Hum Nutr. 2019;74:293–299.
Hanai H, Iida T, Takeuchi K, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502–1506.
Salli K, Anglenius H, Hirvonen J, et al. The effect of 2′-fucosyllactose on simulated infant gut microbiome and metabolites; a pilot study in comparison to GOS and lactose. Sci Rep. 2019;9:13232.
Sawin EA, De Wolfe TJ, Aktas B, et al. Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G590–G601.
Welters CFM, Heineman E, Thunnissen FBJM, van den Bogaard AEJM, Soeters PB, Baeten CGMI. Effect of dietary insulin supplementation on inflammation of pouch mucosa in patients with an ileal pouch-anal anastomosis. Dis Colon Rectum. 2002;45:621–627.
Meijer HP, Welters CF, Heineman E, et al. Enteral inulin does not affect epithelial gene expression and cell turnover within the ileoanal pouch. Dis Colon Rectum. 2000;43:1427–1434.
Roberfroid MB, Van Loo JAE, Gibson GR. The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr. 1998;128:11–19.
Levenstein S, Prantera C, Luzi C, D’Ubaldi A. Low residue or normal diet in Crohn’s disease: a prospective controlled study in Italian patients. Gut. 1985;26:989–993.
Heaton KW, Thornton JR, Emmett PM. Treatment of Crohn’s disease with an unrefined-carbohydrate, fibre-rich diet. Br Med J. 1979;2:764–766.
Jones VA, Dickinson RJ, Workman E, Wilson AJ, Freeman AH, Hunter JO. Crohn’s disease: maintenance of remission by diet. Lancet (London, England). 1985;2:177–180.
De Preter V, Joossens M, Ballet V, et al. Metabolic profiling of the impact of oligofructose-enriched inulin in Crohn’s disease patients: a double-blinded randomized controlled trial. Clin Transl Gastroenterol. 2013;4:e30.
Joossens M, De Preter V, Ballet V, Verbeke K, Rutgeerts P, Vermeire S. Effect of oligofructose-enriched inulin (OF-IN) on bacterial composition and disease activity of patients with Crohn’s disease: results from a double-blinded randomised controlled trial. Gut. 2012;61:958.
Ito M, Kimura M, Deguchi Y, Miyamori-Watabe A, Yajima T, Kan T. Effects of transgalactosylated disaccharides on the human intestinal microflora and their metabolism. J Nutr Sci Vitaminol (Tokyo). 1993;39:279–288.
Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2009;101:541–550.
Moens F, De Vuyst L. Inulin-type fructan degradation capacity of Clostridium cluster IV and XIVa butyrate-producing colon bacteria and their associated metabolic outcomes. Benef Microbes. 2017;8:473–490.
Schroeder BO, Birchenough GMH, Ståhlman M, et al. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe. 2018;23:27-40.e7.
Hosono A, Ozawa A, Kato R, et al. Dietary fructooligosaccharides induce immunoregulation of intestinal IgA secretion by murine Peyer’s patch cells. Biosci Biotechnol Biochem. 2003;67:758–764.
Gerber M. Fiber and breast cancer: another piece of the puzzle—but still an incomplete picture. J Natl Cancer Inst. 1996;88:857–858.
Psyllium P. Plantago ovata (psyllium). Altern Med Rev. 2002;7:155–159.
Pittler MH, Schmidt K, Ernst E. Adverse events of herbal food supplements for body weight reduction: systematic review. Obes Rev. 2005;6:93–111.
Lee D, Albenberg L, Compher C, et al. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology. 2015;148:1087–1106.
Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108.
David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563.
Levine A, Wine E, Assa A, et al. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology. 2019;157:e8.
Yu J, Guo H, Xie J, et al. The alternate consumption of quercetin and alliin in the traditional Asian diet reshaped microbiota and altered gene expression of colonic epithelial cells in rats. J Food Sci. 2019;84:678–686.
Benno Y, Endo K, Miyoshi H, Okuda T, Koishi H, Mitsuoka T. Effect of rice fiber on human fecal microflora. Microbiol Immunol. 1989;33:435–440.
Chiba M, Abe T, Tsuda H, et al. Lifestyle-related disease in Crohn’s disease: relapse prevention by a semi-vegetarian diet. World J Gastroenterol. 2010;16:2484–2495.
Obih C, Wahbeh G, Lee D, et al. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition. 2016;32:418–425.
Ishikawa H, Matsumoto S, Ohashi Y, et al. Beneficial effects of probiotic bifidobacterium and galacto-oligosaccharide in patients with ulcerative colitis: a randomized controlled study. Digestion. 2011;84:128–133.
Furrie E, Macfarlane S, Kennedy A, et al. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242–249.
Paramsothy S, Paramsothy R, Rubin DT, et al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2017;11:1180–1199.
Fang H, Fu L, Wang J. Protocol for fecal microbiota transplantation in inflammatory bowel disease: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:8941340.
Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet (London, England). 1989;1:164.
Costello SP, Hughes PA, Waters O, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321:156–164.
Ishikawa D, Sasaki T, Osada T, et al. Changes in intestinal microbiota following combination therapy with fecal microbial transplantation and antibiotics for ulcerative colitis. Inflamm Bowel Dis. 2017;23:116–125.
Lee SM, Donaldson GP, Mikulski Z, Boyajian S, Ley K, Mazmanian SK. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature. 2013;501:426–429.
Wei Y, Gong J, Zhu W, et al. Pectin enhances the effect of fecal microbiota transplantation in ulcerative colitis by delaying the loss of diversity of gut flora. BMC Microbiol. 2016;16:255.
Damman CJ, Brittnacher MJ, Westerhoff M, et al. Low level engraftment and improvement following a single colonoscopic administration of fecal microbiota to patients with ulcerative colitis. PLoS ONE. 2015;10:e0133925.
Vermeire S, Joossens M, Verbeke K, et al. Donor species richness determines Faecal microbiota transplantation success in inflammatory bowel disease. J Crohns Colitis. 2016;10:387–394.
Herfarth H, Barnes EL, Long MD, et al. Combined endoscopic and oral fecal microbiota transplantation in patients with antibiotic-dependent pouchitis: low clinical efficacy due to low donor microbial engraftment. Inflamm Intest Dis. 2019;4:1–6.
Stallmach A, Lange K, Buening J, Sina C, Vital M, Pieper DH. Fecal microbiota transfer in patients with chronic antibiotic-refractory pouchitis. Am J Gastroenterol. 2016;111:441–443.
Wei Y, Zhu W, Gong J, et al. Fecal microbiota transplantation improves the quality of life in patients with inflammatory bowel disease. Gastroenterol Res Pract. 2015;2015:517597.
Vaughn BP, Vatanen T, Allegretti JR, et al. Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn’s disease. Inflamm Bowel Dis. 2016;22:2182–2190.
Cui B, Feng Q, Wang H, et al. Fecal microbiota transplantation through mid-gut for refractory Crohn’s disease: safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol. 2015;30:51–58.
Suskind DL, Brittnacher MJ, Wahbeh G, et al. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflamm Bowel Dis. 2015;21:556–563.
Goyal A, Yeh A, Bush BR, et al. Safety, clinical response, and microbiome findings following fecal microbiota transplant in children with inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:410–421.
Li P, Zhang T, Xiao Y, et al. Timing for the second fecal microbiota transplantation to maintain the long-term benefit from the first treatment for Crohn’s disease. Appl Microbiol Biotechnol. 2019;103:349–360.
Li SS, Zhu A, Benes V, et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science. 2016;352:586–589.
Ott SJ, Waetzig GH, Rehman A, et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology. 2017;152:e7.
Ding X, Li Q, Li P, et al. Long-term safety and efficacy of fecal microbiota transplant in active ulcerative colitis. Drug Saf. 2019;42:869–880.
Goyal A, Yeh A, Bush BR, et al. Safety and efficacy of fecal microbiota transplant in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2016;63:S212.
Uygun A, Ozturk K, Demirci H, et al. Fecal microbiota transplantation is a rescue treatment modality for refractory ulcerative colitis. Medicine (Baltimore). 2017;96:e6479.
DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med.. 2019;21:2043–2050.
Jacob V, Crawford C, Cohen-Mekelburg S, et al. Single delivery of high-diversity fecal microbiota preparation by colonoscopy is safe and effective in increasing microbial diversity in active ulcerative colitis. Inflamm Bowel Dis. 2017;23:903–911.
Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome between lean and obesity twins. Nature. 2009;457:222–227.
Hedin C, van der Gast CJ, Rogers GB, et al. Siblings of patients with Crohn’s disease exhibit a biologically relevant dysbiosis in mucosal microbial metacommunities. Gut. 2016;65:944–953.
Siegmund B. Is intensity the solution for FMT in ulcerative colitis? Lancet (London, England). 2017;389:1170–1172.
Lupp C, Robertson ML, Wickham ME, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129.
Ratner M. Microbial cocktails join fecal transplants in IBD treatment trials. Nat Biotechnol. 2015;33:787–788.
Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med. 2014;6:220ra11.
Foligne B, Nutten S, Grangette C, et al. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol. 2007;13:236–243.
Peña JA, Rogers AB, Ge Z, et al. Probiotic Lactobacillus spp. diminish Helicobacter hepaticus-induced inflammatory bowel disease in interleukin-10-deficient mice. Infect Immun. 2005;73:912–920.
Peran L, Camuesco D, Comalada M, et al. Preventative effects of a probiotic, Lactobacillus salivarius ssp. salivarius, in the TNBS model of rat colitis. World J Gastroenterol. 2005;11:5185–5192.
Alard J, Peucelle V, Boutillier D, et al. New probiotic strains for inflammatory bowel disease management identified by combining in vitro and in vivo approaches. Benef Microbes. 2018;9:317–331.
Yan F, Cao H, Cover TL, et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest. 2011;121:2242–2253.
Segawa S, Fujiya M, Konishi H, et al. Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway. PLoS ONE. 2011;6:e23278.
von Schillde M-A, Hörmannsperger G, Weiher M, et al. Lactocepin secreted by lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe. 2012;11:387–396.
Quévrain E, Maubert MA, Michon C, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii: a commensal bacterium deficient in Crohn’s disease. Gut. 2016;65:415–425.
Li HB, Chen MY, Qiu ZW, et al. Efficacy and safety of Kangfuxin liquid combined with aminosalicylic acid for the treatment of ulcerative colitis: a systematic review and meta-analysis. Med (United States). 2018;97:e10807.
Sham HP, Bazett M, Bosiljcic M, et al. Immune stimulation using a gut microbe-based immunotherapy reduces disease pathology and improves barrier function in ulcerative colitis. Front Immunol. 2018;9:2211.
Bressler B, Bethel KP, Kleef R, et al. Site-specific immunomodulator: a novel treatment for Crohn’s disease. Gastroenterol Res Pract. 2015;2015:231243.
Sutcliffe S, Kalyan S, Pankovich J, et al. Novel microbial-based immunotherapy approach for Crohn’s disease. Front Med. 2019;6:170.
Zhu W, Winter MG, Byndloss MX, et al. Precision editing of the gut microbiota ameliorates colitis. Nature. 2018;553:208–211.
Kernbauer E, Ding Y, Cadwell K. An enteric virus can replace the beneficial function of commensal bacteria. Nature. 2014;12:6.
Gogokhia L, Buhrke K, Bell R, et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe. 2019;25:e8.
Charlet R, Pruvost Y, Tumba G, et al. Remodeling of the Candida glabrata cell wall in the gastrointestinal tract affects the gut microbiota and the immune response. Sci Rep. 2018;8:3316.
Kunyeit L, Kurrey NK, Anu-Appaiah KA, Rao RP. Probiotic yeasts inhibit virulence of non-albicans Candida species. MBio. 2019;10:1–13.
Mohamadzadeh M, Pfeiler EA, Brown JB, et al. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci USA. 2011;108:4623–4630.
Zhang B, Liu Y, Lan X, et al. Oral Escherichia coli expressing IL-35 meliorates experimental colitis in mice. J Transl Med. 2018;16:71.
Riglar DT, Giessen TW, Baym M, et al. Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation. Nat Biotechnol. 2017;35:653–658.
Wells JM, Mercenier A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol. 2008;6:349–362.
Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045S.
Acknowledgments
This research supported by National Institute of Health Grants, P01DK094779, P30DK34987, P40OD010995 to RBS and by the Crohn’s and Colitis Foundation, 407007 to AO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Oka declares no competing financial interests. Dr. Sartor has grant support from Janssen, Gusto Global, SERES, BiomX, and Vedanta and serves on advisory boards for BiomX, Second Genome, Qu Biologics, and Biomica.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oka, A., Sartor, R.B. Microbial-Based and Microbial-Targeted Therapies for Inflammatory Bowel Diseases. Dig Dis Sci 65, 757–788 (2020). https://doi.org/10.1007/s10620-020-06090-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06090-z