Abstract
The mechanisms by which a small percentage of HIV-1 infected individuals known as elite suppressors or controllers are able to control viral replication are not fully understood. Early cases of viremic control were attributed to infection with defective virus, but subsequent work has demonstrated that infection with a defective virus is not the exclusive cause of control. Replication-competent virus has been isolated from patients who control viral replication, and studies have demonstrated that evolution occurs in plasma virus but not in virus isolates from the latent reservoir. Additionally, transmission pair studies have demonstrated that patients infected with similar viruses can have dramatically different outcomes of infection. An increased understanding of the viral factors associated with control is important to understand the interplay between viral replication and host control, and has implications for the design of an effective therapeutic vaccine that can lead to a functional cure of HIV-1 infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Typically, Human Immunodeficiency Virus-1 (HIV-1) infection is characterized by very high viral loads in acute infection. During the chronic phase of infection, HIV-1 replication continues, concurrent with a progressive decline in CD4+ T cells counts. Without treatment, chronic progressors (CP) will typically progress to AIDS within 5–10 years. The patients who are placed on an effective antiretroviral therapy (ART) regimen, however, will experience an increase in CD4+ T cell levels and a reduction in the HIV-1 plasma RNA level, usually to undetectable levels (<50 copies/mL).
Long-term nonprogressors (LTNPs) are a subset of HIV-1 infected patients who maintain stable CD4+ T cells counts greater than 500 cells/μL for longer than 7 years, in the absence of ART. Once ultrasensitive assays to detect the HIV-1 plasma RNA levels were developed, it became clear that LTNPs were a phenotypically diverse population comprised of individuals with varying HIV-1 plasma RNA levels. Elite controllers (EC) or suppressors (ES) are distinct from LTNPs in that they are defined by the level of HIV-1 RNA plasma levels rather than their CD4+ T cell counts. These remarkable individuals maintain HIV-1 plasma RNA levels below the limit of detection of standard commercial assays (<50 copies/mL) in the absence of ART, and represent less than 1 % of the total HIV-1 infected population [1].
It is now generally accepted that host factors play a major role in elite control. The HLA-B*57 and B*27 alleles are over represented in EC [2–8], and these two alleles along with a polymorphism in the promoter of HLA-C have been associated with slow progression in multiple GWAS studies [9–14]. HLA class I proteins are involved in presentation of peptides to CD8+ T cells, and this may explain why HIV-specific CD8+ T cells from EC are more effective at controlling HIV-1 replication in vitro than CD8+ T cells from patients with progressive disease [2, 15–18]. These data complement studies in the SIV model of elite control where depletion of CD8+ T cells in EC monkeys results in virologic breakthrough [19, 20].
The role of other host factors in elite control is more controversial. EC do not have elevated titers of neutralizing antibodies to autologous virus [21], but while one study suggested that these patients may have higher levels of antibody-dependant cell-mediated cytotoxicity (ADCC) than CP [22], a recent study found no differences in ADCC levels between EC and patients with progressive disease [23]. Similarly, while some studies have suggested that CD4+ T cells from EC may be less susceptible to HIV-1 infection [24, 25], other studies have shown that CD4+ T cells from many EC are fully susceptible to infection [26–28].
The viral factors that are associated with elite control are poorly understood. Initial reports suggested that EC were infected with defective virus, including viruses that harbored large deletions or difficult to revert polymorphisms. However, many of these studies looked at sequence analysis of proviral genes alone, and so the effect that these mutations or deletions had on the overall viral fitness was unclear [29–38]. Additionally, it was not clear whether the observed replication defects were due to infection with a defective virus, or whether the reduction in fitness was a result of a highly active immune response in patients that controlled viral replication. Using chimerical virus systems, individual HIV-1 viral genes were cloned from patients and the genes isolated from EC were seen to be less fit compared to those from CP [29, 34, 39]. Subsequently, replication-competent virus was successfully isolated from EC, indicating that some EC were, in fact, infected with virus that replicated effectively in vitro [26, 40, 41]. Full genome sequence analysis also indicated that the replication-competent virus isolated from some patients contained no large-scale deletions or gross mutations [40], and transmission pair studies have demonstrated that infection with genetically similar, replication-competent viruses can result in drastically different clinical outcomes of infection [42, 43]. The data indicate that infection with defective virus is not the exclusive cause of elite control and that some EC are infected with virus that is able to cause pathogenic disease in vivo. This has been shown definitively in the macaque model of elite control [44], where some monkeys control fully pathogenic laboratory SIV isolates through CD8+ T cell responses [19, 20].
Here, we will discuss key viral factors that are associated with the control of HIV-1 viral replication in LTNPs and EC. We will focus on the fitness of the infecting virus (summarized in Table 1), latency, and residual viremia, and the implications these factors have on the host response to infection. Additionally, we will review the understanding of latency and residual viremia in EC. An increased understanding of the viral factors of elite control has important implications about the nature of the host control of replication, the design of a therapeutic vaccine, and the development of effective eradication strategies.
Viral fitness: cause or effect of elite control?
Initially, it was believed that LTNPs were infected with a defective viral strain. The earliest evidence to support this hypothesis was described in the Sydney Blood Bank Cohort. In this cohort, 7 patients were infected via blood transfusion-transmission of HIV-1 from a single infected blood donor. All patients were observed to have no decline in CD4+ T cells counts and none had classical, AIDS-defining symptoms. Full genome sequencing was performed on viruses that were isolated from each patient, and a large deletion in nef and the U3 region of the long terminal repeat was identified in the viruses from all the patients [32]. nef has been shown to be important for HIV-1 replication in vitro and was shown to be required for progression in SIV monkey models of disease [35]. Subsequently, various studies identified defects in nef and other HIV-1 genes from EC and LTNPs [36, 37]. In one study, mutations in nef were identified in the majority of the five LTNPs that were studied [37]. Another case study also documented an LTNP who has been asymptomatic for 20 years and harbored a virus with a large deletion in nef [36]. These studies and others provide evidence for the importance of nef in HIV-1 pathogenesis and clearly indicate that deletions in nef can increase the likelihood for elite control.
Mutations and defects in other genes have also been implicated in control of HIV-1. In one such study, rare, difficult to revert polymorphisms in key genes were hypothesized to result in control of HIV-1 replication [30]. Furthermore, chimeric viruses containing rev, gag-pol, and vif genes from EC viral isolates were observed to have a reduced replication capacity compared to similar viruses with viral genes isolated from CPs [29, 34, 39]. More recently, it was demonstrated that some EC/viremic controllers (VC) were infected with virus containing drug resistance mutations and/or escape mutations implying that patients were more likely to control viral infection if they were infected with an attenuated viral variant [45].
These data suggest that infection with defective virus, due to mutations or deletions in key genes, can increase the likelihood of elite control. However, studies that have sequenced proviral genes from EC have reached different conclusions. In a cohort of 95 EC, plasma and proviral clones were amplified from a majority of the patients, and a high incidence of drug resistance mutations was not reported [46]. In other studies, escape mutations were rarely seen in proviral gag [47] and nef [48] clones. Furthermore, another study demonstrated that the presence of escape mutations could not explain control in patients with low level viremia [49]. Therefore, while, in some cases, infection with attenuated isolates can lead to control of HIV-1 replication, it appears that this is not the cause of elite control in many patients.
More recently, studies have suggested that some EC are not infected with defective virus. Using a highly sensitive, limiting dilution co-culture assay, replication-competent virus was isolated from a cohort of EC. These viral isolates were shown to replicate as well as laboratory X4 and R5 benchmark replication strains [40]. This study demonstrated, for the first time, that some EC were infected with full replication-competent virus. Full genome sequence analysis identified no deletions or mutations that were associated with elite control [40]. In another study, replication-competent virus was isolated from EC CD4+ T cells after mitogen or IL-7 stimulation and subsequent sequence analysis indicated no deletions or insertions in the vpr, vpu, or nef genes [41]. A recent study demonstrated that stimulation of CD4+ T cells from EC rarely resulted in viral outgrowth, but viral outgrowth was robust in many cases were it occurred. Viral outgrowth was more commonly observed in ART-treated patients and CP when compared to EC [26], which is consistent with studies showing that the frequency of infected CD4+ T cells was much lower than the frequency usually seen in CP [40].
Most convincingly, studies that analyzed HIV-1 transmission pairs have supported the hypothesis that, in some cases, host factors predominately dictate control of viral replication [42, 43]. In these transmission pair studies, individuals were observed to transmit highly similar viruses to their partner, yet each patient had strikingly different clinical outcomes of infection. In a transmission pair study that analyzed two HLA-B*57 positive patients, fitness of the virus isolated from the EC was observed to be reduced compared to the isolates from the CP transmission partner. While the reduction in fitness was hypothesized to be a result of escape mutations that had an attenuating effect on viral fitness [42], it was also possible that the virus transmitted to the EC contained the T242N escape mutation that has previously been reported to result in a reduction in viral fitness [50, 51]. Infection with this attenuated isolate may have contributed to elite control. However, in two other cases where virus was transmitted between a patient who controlled viral replication and a CP, viral fitness was observed to be the same between all patients [43]. Thus, deficiencies in viral replication were not the root cause of control of viral replication in these cases. This suggests that fully replication-competent virus can sometimes be controlled by the immune system, and, in some cases, selective pressure from HIV-specific CD8+ T cells forces and maintains attenuating escape mutations that may be playing a significant role in the control of infection.
Latency, residual viremia, and evolution in elite controllers
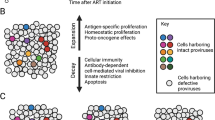
As a consequence of the normal physiology of CD4+ T cells, latency is established early in viral infection. These latently infected cells represent a major barrier to eradication in HIV-1 infected individuals using current strategies for treatment of infection [52]. A limiting dilution, co-culture assay that approximates the number of infectious, resting CD4+ T cells in a patient likely reflects the most accurate measure to quantify latently infected cells [53]. Using this assay, EC were observed to have a one and a half log lower median infectious units per millions cells (IUPM) compared to CP [40]. EC have been shown to have significantly lower levels of integrated proviral DNA [54] compared to CPs, and a recent study that looked at four unique EC with weakly reactive western blots, found that these patients had markedly lower levels of total and integrated proviral DNA compared to conventional EC. These data suggest that the control of HIV-1 replication varies between EC [55]. One study used a transcription-mediated amplification assay to assess cell-associated RNA in PBMCs and found detectable levels of RNA in 25 out 29 EC. This suggests that some level of HIV-1 transcription may be occurring in EC [56]. The reduction in the frequency of resting CD4+ T cells in EC may be a result of lower levels of HIV-1 RNA during the acute phase of infection [57, 58], which could limit the seeding of the latent reservoir (Fig. 1).
Models to explain the seeding of the latent reservoir: comparison between EC and CP. Top panel CP natural history: HIV-1 infection is typical characterized by robust viral replication during acute infection. As HIV-1 plasma RNA levels increase (blue line), there is a parallel increase in the number of latently infected cells (infectious units per million, IUPM; red line). A drop in both the HIV-1 plasma RNA levels and IUPM occurs with the initiation of the acquired immune response, likely due to CTL pressure. Without ART, viral replication continues, and escape mutations occur early (dotted red and blue lines). High levels of replication result in an equilibrium between seeding of the latent reservoir and reactivation of the latent reservoir (red and blue solid arrows), thus resulting in the reseeding of the latent reservoir with mutated virus. Upon the initiation of ART therapy, HIV-1 plasma RNA levels fall to undetectable levels, in concert with a decline in IUPM. ART halts ongoing replication, but reactivation of the latent reservoir results in the release of low levels of virus with escape mutations (red dashed arrow). Bottom panel EC natural history. During acute infection, HIV-1 plasma RNA level increases, but has been documented to be lower in EC compared to CP (blue line). The frequency of latently infected CD4+ T cells increases in parallel, but to levels that are lower compared to CP (red line). CTL pressure reduced viral replication to below the limit of detection, and there is limited seeding of the latent reservoir, resulting in a reduced IUPM in EC compared to CP. Escape occurs early in infection in EC, but there is limited seeding of the latent reservoir due to CTL pressure (blue dashed arrow). The majority of sequences in the latent reservoir do not contain escape mutations, as the seeding of the latent reservoir is limited by CTL pressure
Several studies have used highly sensitive RT-PCR assays to quantify the level of HIV-1 RNA in the plasma of CP and EC. While EC are typically thought to have undetectable levels of HIV-1 plasma RNA, the use of single copy assays to quantify down to 1 copy of HIV-1 RNA per mL of plasma has allowed a better understanding of the level of residual viremia in these patients. Incredibly, in multiple studies, EC were seen to have levels of virus in the plasma that were equal to those seen in ART-treated individuals [2, 56, 59, 60]. Remarkably, a significant number of patients had less than 1 copy of HIV-1 RNA per mL of plasma. An analysis of the residual viremia in EC can provide information about the nature of elite control and the effect that immune pressure has on the virus. In comparison to ART-treated patients, who were not observed have ongoing rounds of replication in multiple phylogenetic studies [61, 62], evidence for ongoing replication has been documented in EC [63–65]. Using a highly sensitive RT-PCR-based assay, O’Connell and colleagues amplified and sequenced gag clones from the proviral and plasma compartments at multiple time points. After phylogenetic analysis, a clear discordance between the plasma and proviral sequences was observed (representative data in Fig. 2) [64]. All the plasma sequences were observed to have mutations in HLA-B*57 restricted epitopes, whereas these escape mutations were rarely observed in the proviral compartment. Additionally, the proviral sequences were ancestral to the plasma sequences. While there was evidence of synonymous evolution of clones that were amplified from the plasma, there was no evidence of evolution in the proviral gag clones that were amplified from resting CD4+ T cells. [64]. This argues that, while ongoing viral replication occurs in the compartment that produces the low levels of virus present in the plasma, the plasma virions are not reseeding the latent reservoir of EC to a significant degree (Fig. 1).
Discordance between plasma and proviral sequences: evidence for ongoing replication in EC. Representative phylogenetic data from an EC demonstrating the discordance between plasma and proviral sequences as identified by limiting dilution PCR to obtain clonal gag sequences. All sequences were estimated using a classical approach using the maximum likelihood analysis. Clonal sequences from resting CD4+ T cells, activated CD4+ T cells, and plasma sequences were amplified [64]. A clear discordance between the proviral compartments in resting CD4+ T cells (circles) and activated CD4+ T cells (squares) can be observed compared to the plasma viral sequences (triangles) over a period of 6 years. The proviral compartment cluster together, with the plasma virions showing evidence of ongoing replication
Similar results were obtained in both a companion study that focused on analyzing the evolution of the nef gene [65] and a study that analyzed the evolution of clonal sequences of RT-pol and env genes from EC [63]. Interestingly, viral evolution was observed in patients with and without previously described protective HLA alleles, and evolution was found to be significantly lower in EC compared to CPs who were not on ART [63]. In the context of low level ongoing viral replication, the fact that synonymous changes were commonly observed over time in the plasma virus suggests that the virus achieved an optimal balance between immune evasion and fitness [63, 64, 66]. The discordance between escape mutations in the plasma virus in comparison to the proviral clones suggests that many EC are infected with virus that contains a wild-type sequence in many epitopes, and this virus seeds the latent reservoir early in infection. Selective pressure from CTL results in the development of escape mutations, but this occurs when the viral load is too low to lead to efficient entry into the latent reservoir. While there is minimal ongoing replication in the latent reservoir, there are probably other compartments where low level viral replication occurs. The source of this ongoing replication is unknown, but a recent study demonstrated that monocytes are not an important reservoir in EC [67].
The level of viral replication in EC is much lower than the levels seen in viremic CPs. Therefore, the virus in EC does not evolve to achieve similar levels of fitness in EC. This is best illustrated by a longitudinal study of viral fitness in HLA-B*57 LTNPs and CPs. Shortly after infection, virus isolated from PBMCs in both groups of patients had attenuating escape mutations that were present in gag and resulted in low viral fitness. While this low fitness virus was maintained during chronic infection in LTNPs, the fitness of the virus increased significantly over time in CPs. These data could explain why plasma gag clones from HLA-B*57 CPs are more likely to contain compensatory, fitness-restoring mutations compared to gag clones amplified from HLA-B*57 EC [68]. Thus, the maintenance of attenuating mutations that have a significant reduction in viral fitness early in infection coupled with the lack of compensatory mutations due to limited viral evolution may partially explain the control of viral replication in EC. However, unlike viremic LTNPs and CPs, HLA-B*57 EC also maintain virus that does not contain attenuating escape mutations in the latent reservoir. Thus, EC maintain control over two distinct types of virus; replication-competent wild-type virus that is archived in a low frequency of latently infected CD4+ T cells, and attenuated escape mutants that can be found at very low levels in plasma.
These attenuated plasma virions in EC have been analyzed in several studies where either the RT-pol [69], gag [38, 45] or env clones [70] from EC were isolated and cloned into an NL4-3 backbone. The relative replicative capacity of these clones was found to significantly lower than chimeric clones from CPs. Attenuating mutations were correlated with protective HLA epitopes [69].
These studies are limited by the fact that they look only at single viral genes in isolation. Thus, compensatory or detrimental mutations elsewhere in the genome are unaccounted for, and complete viral variants are not compared. More importantly, plasma viruses are subject to selective pressure and may not be representative of the original infecting virus. Mutations in plasma viruses in gag [47], nef [48], and env [21] have been identified that are very rare in the CD4+ T cell proviral compartment. For example, the attenuating T242N mutation was found in almost all plasma sequences from EC, but was not equally reflected in the proviral compartment [47]. A similar discordance was observed in nef in another study [48]. Most convincingly, in a recent study, replication-competent virus was isolated from activated and resting CD4+ T cells. The isolate from the activated CD4+ T cells resembled plasma virus and contained multiple escape mutations in gag. In contrast, the virus cultured from resting CD4+ T cells resembled proviral clones and did not contain escape mutations. In a fitness assay, the virus containing the escape mutants was significantly less fit than a reference laboratory strain, whereas the archived virus from resting CD4+ T cells had no evidence of attenuation (Fig. 3) [71]. These data indicate that the virus that this patient was infected with did not possess escape mutations or viral attenuation, instead these mutations were acquired over time as a result of selective pressure exerted by HIV-specific CTL. Therefore, while informative, the analysis of plasma clones alone may not provide conclusive evidence to support or refute the relationship between viral fitness and elite control, and nor do they represent an accurate representation of the original infecting virus. Studies looking at replication-competent virus isolated from resting CD4+ T cells are needed for this purpose.
Understanding the relationship between the latent reservoir and the plasma virus in EC. a The latent reservoir represents a major barrier to eradication, and resting CD4+ T cells remain in a resting quiescent state. Integrated provirus remains silenced by poorly understood mechanisms, but minimal evolution occurs within this compartment. Upon immune activation, virus is released from these latently infected cells, and nonsynonymous mutations likely result in virions that escape immune pressure. Continuous, ongoing replication occurs in EC, the location of the replication is unknown but may be represented by either activated CD4+ T cells, the gut-associated lymphoid tissue or other lymphoid organs, or the central nervous system. Plasma virions with escape mutations are not commonly represented in the latent reservoir, thus the source of these viruses are currently unknown. It has been shown that evolution occurs during low level ongoing replication, but is most commonly characterized by synonymous changes. The production of virus from this unknown compartment results in low level viremia that may re-infect the latent reservoir at very low levels or contribute to ongoing replication. b Representative data showing that there are clear differences in fitness between isolates cultured from resting CD4+ T cells containing wild type sequence and escape mutants cultured from activated CD4+ T cells [71]. Primary CD4+ T cells were infected, and viral production was measured by p24 ELISA. These data indicate that the latent virus (blue) is more fit compared to the escape mutant (red line), likely due to the attenuating effect of the escape mutations. Thus, it is likely that plasma virions that contain similar escape mutations do not accurately reflect the fitness of the infecting virus that is archived in the latent reservoir
Concluding remarks
An increased understanding of the viral factors that influence elite control is still necessary to provide information on the nature and mechanisms of control of HIV-1 replication. While it is clear that, in some cases, EC are infected with defective viral strains, there is abundant evidence to suggest that this is not the sole explanation for elite control. Infection with an attenuated virus can increase the likelihood of control of viral replication, but it is probable that, in some cases, a combination of host and viral factors are required to fully suppress viral replication and, in others, host factors alone lead to the control of fully pathogenic virus. Comparing EC with chronically infected and ART-treated patients is informative, but it is clear that differences in the levels of viral replication early in infection may result in irrevocable alteration in both the host response to viral infection and the replicative capacity of the infecting virus. Thus, comparisons made between these patient populations are imperfect and must reflect these limitations. It is clear that the analysis of plasma virions and relying on sequence analysis alone are not sufficient. The isolation and amplification of replication-competent viruses from EC is paramount in furthering our understanding of viral factors of control.
Ultimately, EC represent a unique model system where the host immune response to HIV-1 infection can be studied. This is not a perfect model for control of HIV-1 replication since, in some cases, immune activation [72, 73], declining CD4+ T cells counts [59, 72–75], and spontaneous virologic breakthrough [76] have been reported; however, it appears that the majority of EC have maintained stable CD4+ T cell counts and control of viral replication for more than 20 years. Because of the inability of the host response to eliminate latently infected HIV-1 cells, EC can and should be used as model system for a functional cure where the latent reservoir is contained rather than eradicated. Additionally, because it has been shown that some, if not a majority, of EC are infected with fully replication-competent virus, the study of host factors and their contributions toward an effective immune response can lead to the development of novel strategies for an effective HIV-1 therapeutic vaccine.
References
Ockulicz J, Lambotte O (2011) Epidemiology and clinical characteristics of elite controllers. Curr Opin HIV AIDS 6(3):163–168
Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, Rood JE, Berkley AM, Sacha JB, Cogliano-Shutta NA, Lloyd M, Roby G, Kwan R, McLaughlin M, Stallings S, Rehm C, O’Shea MA, Mican J, Packard BZ, Komoriya A, Palmer S, Wiegand AP, Maldarelli F, Coffin JM, Mellors JW, Hallahan CW, Follman DA, Connors M (2008) Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009–1021
Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, Hallahan CW, Selig SM, Schwartz D, Sullivan J, Connors M (2000) HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci USA 97:2709–2714
Lambotte O, Boufassa F, Madec Y, Nguyen A, Goujard C, Meyer L, Rouzioux C, Venet A, Delfraissy JF and SEROCO-HEMOCO Study Group (2005) HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis 41:1053–1056
Emu B, Sinclair E, Hatano H, Ferre A, Shacklett B, Martin JN, McCune JM, Deeks SG (2008) HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J Virol 82:5398–5407
Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, Baker B, Trocha A, Rosenberg R, Mackey E, Ueda P, Lu Z, Cohen D, Wrin T, Petropoulos CJ, Rosenberg ES, Walker BD (2008) Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis 197:563–571
Han Y, Lai J, Barditch-Crovo P, Gallant JE, Williams TM, Siliciano RF, Blankson JN (2008) The role of protective HCP5 and HLA-C associated polymorphisms in the control of HIV-1 replication in a subset of elite suppressors. AIDS 22:541–544
Sajadi MM, Constantine NT, Mann DL, Charurat M, Dadzan E, Kadlecik P, Redfield RR (2009) Epidemiologic characteristics and natural history of HIV-1 natural viral suppressors. J Acquir Immune Defic Syndr 50:403–408
Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, Zhang K, Gumbs C, Castagna A, Cossarizza A, Cozzi-Lepri A, De Luca A, Easterbrook P, Francioli P, Mallal S, Martinez-Picado J, Miro JM, Obel N, Smith JP, Wyniger J, Descombes P, Antonarakis SE, Letvin NL, McMichael AJ, Haynes BF, Telenti A, Goldstein DB (2007) A whole-genome association study of major determinants for host control of HIV-1. Science 317:944–947
International HIV Controllers Study, Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, Ripke S, Brumme CJ, Pulit SL, Carrington M, Kadie CM, Carlson JM, Heckerman D, Graham RR, Plenge RM, Deeks SG, Gianniny L, Crawford G, Sullivan J, Gonzalez E, Davies L, Camargo A, Moore JM, Beattie N, Gupta S, Crenshaw A, Burtt NP, Guiducci C, Gupta N, Gao X, Qi Y, Yuki Y, Piechocka-Trocha A, Cutrell E, Rosenberg R, Moss KL, Lemay P, O’Leary J, Schaefer T, Verma P, Toth I, Block B, Baker B, Rothchild A, Lian J, Proudfoot J, Alvino DM, Vine S, Addo MM, Allen TM, Altfeld M, Henn MR, Le Gall S, Streeck H, Haas DW, Kuritzkes DR, Robbins GK, Shafer RW, Gulick RM, Shikuma CM, Haubrich R, Riddler S, Sax PE, Daar ES, Ribaudo HJ, Agan B, Agarwal S, Ahern RL, Allen BL, Altidor S, Altschuler EL, Ambardar S, Anastos K, Anderson B, Anderson V, Andrady U, Antoniskis D, Bangsberg D, Barbaro D, Barrie W, Bartczak J, Barton S, Basden P, Basgoz N, Bazner S, Bellos NC, Benson AM, Berger J, Bernard NF, Bernard AM, Birch C, Bodner SJ, Bolan RK, Boudreaux ET, Bradley M, Braun JF, Brndjar JE, Brown SJ, Brown K, Brown ST, Burack J, Bush LM, Cafaro V, Campbell O, Campbell J, Carlson RH, Carmichael JK, Casey KK, Cavacuiti C, Celestin G, Chambers ST, Chez N, Chirch LM, Cimoch PJ, Cohen D, Cohn LE, Conway B, Cooper DA, Cornelson B, Cox DT, Cristofano MV, Cuchural G, Jr, Czartoski JL, Dahman JM, Daly JS, Davis BT, Davis K, Davod SM, DeJesus E, Dietz CA, Dunham E, Dunn ME, Ellerin TB, Eron JJ, Fangman JJ, Farel CE, Ferlazzo H, Fidler S, Fleenor-Ford A, Frankel R, Freedberg KA, French NK, Fuchs JD, Fuller JD, Gaberman J, Gallant JE, Gandhi RT, Garcia E, Garmon D, Gathe JC, Jr, Gaultier CR, Gebre W, Gilman FD, Gilson I, Goepfert PA, Gottlieb MS, Goulston C, Groger RK, Gurley TD, Haber S, Hardwicke R, Hardy WD, Harrigan PR, Hawkins TN, Heath S, Hecht FM, Henry WK, Hladek M, Hoffman RP, Horton JM, Hsu RK, Huhn GD, Hunt P, Hupert MJ, Illeman ML, Jaeger H, Jellinger RM, John M, Johnson JA, Johnson KL, Johnson H, Johnson K, Joly J, Jordan WC, Kauffman CA, Khanlou H, Killian RK, Kim AY, Kim DD, Kinder CA, Kirchner JT, Kogelman L, Kojic EM, Korthuis PT, Kurisu W, Kwon DS, LaMar M, Lampiris H, Lanzafame M, Lederman MM, Lee DM, Lee JM, Lee MJ, Lee ET, Lemoine J, Levy JA, Llibre JM, Liguori MA, Little SJ, Liu AY, Lopez AJ, Loutfy MR, Loy D, Mohammed DY, Man A, Mansour MK, Marconi VC, Markowitz M, Marques R, Martin JN, Martin HL, Jr, Mayer KH, McElrath MJ, McGhee TA, McGovern BH, McGowan K, McIntyre D, Mcleod GX, Menezes P, Mesa G, Metroka CE, Meyer-Olson D, Miller AO, Montgomery K, Mounzer KC, Nagami EH, Nagin I, Nahass RG, Nelson MO, Nielsen C, Norene DL, O’Connor DH, Ojikutu BO, Okulicz J, Oladehin OO, Oldfield EC, 3rd, Olender SA, Ostrowski M, Owen WF, Jr, Pae E, Parsonnet J, Pavlatos AM, Perlmutter AM, Pierce MN, Pincus JM, Pisani L, Price LJ, Proia L, Prokesch RC, Pujet HC, Ramgopal M, Rathod A, Rausch M, Ravishankar J, Rhame FS, Richards CS, Richman DD, Rodes B, Rodriguez M, Rose RC, 3rd, Rosenberg ES, Rosenthal D, Ross PE, Rubin DS, Rumbaugh E, Saenz L, Salvaggio MR, Sanchez WC, Sanjana VM, Santiago S, Schmidt W, Schuitemaker H, Sestak PM, Shalit P, Shay W, Shirvani VN, Silebi VI, Sizemore JM, Jr, Skolnik PR, Sokol-Anderson M, Sosman JM, Stabile P, Stapleton JT, Starrett S, Stein F, Stellbrink HJ, Sterman FL, Stone VE, Stone DR, Tambussi G, Taplitz RA, Tedaldi EM, Telenti A, Theisen W, Torres R, Tosiello L, Tremblay C, Tribble MA, Trinh PD, Tsao A, Ueda P, Vaccaro A, Valadas E, Vanig TJ, Vecino I, Vega VM, Veikley W, Wade BH, Walworth C, Wanidworanun C, Ward DJ, Warner DA, Weber RD, Webster D, Weis S, Wheeler DA, White DJ, Wilkins E, Winston A, Wlodaver CG, van’t Wout A, Wright DP, Yang OO, Yurdin DL, Zabukovic BW, Zachary KC, Zeeman B and Zhao M (2010) The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330:1551–1557
Catano G, Kulkarni H, He W, Marconi VC, Agan BK, Landrum M, Anderson S, Delmar J, Telles V, Song L, Castiblanco J, Clark RA, Dolan MJ, Ahuja SK (2008) HIV-1 disease-influencing effects associated with ZNRD1, HCP5 and HLA-C alleles are attributable mainly to either HLA-A10 or HLA-B*57 alleles. PLoS One 3:e3636
Dalmasso C, Carpentier W, Meyer L, Rouzioux C, Goujard C, Chaix ML, Lambotte O, Avettand-Fenoel V, Le Clerc S, de Senneville LD, Deveau C, Boufassa F, Debre P, Delfraissy JF, Broet P, Theodorou I, ANRS Genome Wide Association 01 (2008) Distinct genetic loci control plasma HIV-RNA and cellular HIV-DNA levels in HIV-1 infection: the ANRS genome wide association 01 study. PLoS One 3:e3907
Limou S, Le Clerc S, Coulonges C, Carpentier W, Dina C, Delaneau O, Labib T, Taing L, Sladek R, Deveau C, Ratsimandresy R, Montes M, Spadoni JL, Lelievre JD, Levy Y, Therwath A, Schachter F, Matsuda F, Gut I, Froguel P, Delfraissy JF, Hercberg S, Zagury JF and ANRS genomic group (2009) Genomewide association study of an AIDS-nonprogression cohort emphasizes the role played by HLA genes (ANRS Genomewide Association Study 02). J Infect Dis 199:419–426
van Manen D, Kootstra NA, Boeser-Nunnink B, Handulle MA, van’t Wout AB, Schuitemaker H (2009) Association of HLA-C and HCP5 gene regions with the clinical course of HIV-1 infection. AIDS 23:19–28
Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, Van Baarle D, Kostense S, Miedema F, McLaughlin M, Ehler L, Metcalf J, Liu S, Connors M (2002) HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol 3:1061–1068
Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, Kovacs CM, Rodriguez B, Sieg SF, Teixeira-Johnson L, Gudonis D, Goepfert PA, Lederman MM, Frank I, Makedonas G, Kaul R, Walker BD, Betts MR (2010) Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog 6:e1000917
Saez-Cirion A, Sinet M, Shin SY, Urrutia A, Versmisse P, Lacabaratz C, Boufassa F, Avettand-Fenoel V, Rouzioux C, Delfraissy JF, Barre-Sinoussi F, Lambotte O, Venet A, Pancino G and ANRS EP36 HIV Controllers Study Group (2009) Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J Immunol 182:7828–7837
Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, Barre-Sinoussi F, Delfraissy JF, Sinet M, Pancino G, Venet A and Agence Nationale de Recherches sur le Sida EP36 HIV Controllers Study Group (2007) HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci USA 104:6776–6781
Pandrea I, Gaufin T, Gautam R, Kristoff J, Mandell D, Montefiori D, Keele BF, Ribeiro RM, Veazey RS, Apetrei C (2011) Functional cure of SIVagm infection in rhesus macaques results in complete recovery of CD4+ T cells and is reverted by CD8+ cell depletion. PLoS Pathog 7:e1002170
Friedrich TC, Valentine LE, Yant LJ, Rakasz EG, Piaskowski SM, Furlott JR, Weisgrau KL, Burwitz B, May GE, Leon EJ, Soma T, Napoe G, Capuano SV 3rd, Wilson NA, Watkins DI (2007) Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J Virol 81:3465–3476
Bailey JR, Lassen KG, Yang HC, Quinn TC, Ray SC, Blankson JN, Siliciano RF (2006) Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J Virol 80:4758–4770
Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX, Parks RJ, Hicks CB, Owzar K, Tomaras GD, Montefiori DC, Haynes BF, Delfraissy JF (2009) Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 23:897–906
Smalls-Mantey A, Doria-Rose N, Klein R, Patamawenu A, Migueles SA, Ko SY, Hallahan CW, Wong H, Liu B, You L, Scheid J, Kappes JC, Ochsenbauer C, Nabel GJ, Mascola JR and Connors M (2012) Antibody-dependent cellular cytotoxicity against primary HIV-infected CD4+ T cells is directly associated with the magnitude of surface IgG binding. J Virol [Epub ahead of print]
Chen H, Li C, Huang J, Cung T, Seiss K, Beamon J, Carrington MF, Porter LC, Burke PS, Yang Y, Ryan BJ, Liu R, Weiss RH, Pereyra F, Cress WD, Brass AL, Rosenberg ES, Walker BD, Yu XG, Lichterfeld M (2011) CD4+ T cells from elite controllers resist HIV-1 infection by selective upregulation of p21. J Clin Invest 121:1549–1560
Saez-Cirion A, Hamimi C, Bergamaschi A, David A, Versmisse P, Melard A, Boufassa F, Barre-Sinoussi F, Lambotte O, Rouzioux C, Pancino G, ANRS CO18 Cohort (2011) Restriction of HIV-1 replication in macrophages and CD4+ T cells from HIV controllers. Blood 118:955–964
Julg B, Pereyra F, Buzon MJ, Piechocka-Trocha A, Clark MJ, Baker BM, Lian J, Miura T, Martinez-Picado J, Addo MM, Walker BD (2010) Infrequent recovery of HIV from but robust exogenous infection of activated CD4+ T cells in HIV elite controllers. Clin Infect Dis 51:233–238
O’Connell KA, Rabi SA, Siliciano RF, Blankson JN (2011) CD4+ T cells from elite suppressors are more susceptible to HIV-1 but produce fewer virions than cells from chronic progressors. Proc Natl Acad Sci USA 108:E689–E698
Rabi SA, O’Connell KA, Nikolaeva D, Bailey JR, Jilek BL, Shen L, Page KR, Siliciano RF, Blankson JN (2011) Unstimulated primary CD4+ T cells from HIV-1-positive elite suppressors are fully susceptible to HIV-1 entry and productive infection. J Virol 85:979–986
Alexander L, Aquino-DeJesus MJ, Chan M, Andiman WA (2002) Inhibition of human immunodeficiency virus type 1 (HIV-1) replication by a two-amino-acid insertion in HIV-1 Vif from a nonprogressing mother and child. J Virol 76:10533–10539
Alexander L, Weiskopf E, Greenough TC, Gaddis NC, Auerbach MR, Malim MH, O’Brien SJ, Walker BD, Sullivan JL, Desrosiers RC (2000) Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J Virol 74:4361–4376
Calugi G, Montella F, Favalli C, Benedetto A (2006) Entire genome of a strain of human immunodeficiency virus type 1 with a deletion of nef that was recovered 20 years after primary infection: large pool of proviruses with deletions of env. J Virol 80:11892–11896
Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, McPhee DA, Greenway AL, Ellett A, Chatfield C, Lawson VA, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan JS, Cunningham A, Dwyer D, Dowton D, Mills J (1995) Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988–991
Huang Y, Zhang L, Ho DD (1998) Characterization of gag and pol sequences from long-term survivors of human immunodeficiency virus type 1 infection. Virology 240:36–49
Iversen AK, Shpaer EG, Rodrigo AG, Hirsch MS, Walker BD, Sheppard HW, Merigan TC, Mullins JI (1995) Persistence of attenuated rev genes in a human immunodeficiency virus type 1-infected asymptomatic individual. J Virol 69:5743–5753
Kestler HW 3rd, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC (1991) Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651–662
Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC (1995) Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med 332:228–232
Mariani R, Kirchhoff F, Greenough TC, Sullivan JL, Desrosiers RC, Skowronski J (1996) High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J Virol 70:7752–7764
Miura T, Brockman MA, Schneidewind A, Lobritz M, Pereyra F, Rathod A, Block BL, Brumme ZL, Brumme CJ, Baker B, Rothchild AC, Li B, Trocha A, Cutrell E, Frahm N, Brander C, Toth I, Arts EJ, Allen TM, Walker BD (2009) HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte (corrected) recognition. J Virol 83:2743–2755
Huang Y, Paxton WA, Wolinsky SM, Neumann AU, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstman K, Erickson D, Dragon E, Landau NR, Phair J, Ho DD, Koup RA (1996) The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med 2:1240–1243
Blankson JN, Bailey JR, Thayil S, Yang HC, Lassen K, Lai J, Gandhi SK, Siliciano JD, Williams TM, Siliciano RF (2007) Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol 81:2508–2518
Lamine A, Caumont-Sarcos A, Chaix ML, Saez-Cirion A, Rouzioux C, Delfraissy JF, Pancino G, Lambotte O (2007) Replication-competent HIV strains infect HIV controllers despite undetectable viremia (ANRS EP36 study). AIDS 21:1043–1045
Bailey JR, O’Connell K, Yang HC, Han Y, Xu J, Jilek B, Williams TM, Ray SC, Siliciano RF, Blankson JN (2008) Transmission of human immunodeficiency virus type 1 from a patient who developed AIDS to an elite suppressor. J Virol 82:7395–7410
Buckheit RW 3rd, Allen TG, Alme A, Salgado M, O’Connell KA, Huculak S, Falade-Nwulia O, Williams TM, Gallant JE, Siliciano RF, Blankson JN (2012) Host factors dictate control of viral replication in two HIV-1 controller/chronic progressor transmission pairs. Nat Commun 3:716
Mudd PA, Watkins DI (2011) Understanding animal models of elite control: windows on effective immune responses against immunodeficiency viruses. Curr Opin HIV AIDS 6:197–201
Miura T, Brumme ZL, Brockman MA, Rosato P, Sela J, Brumme CJ, Pereyra F, Kaufmann DE, Trocha A, Block BL, Daar ES, Connick E, Jessen H, Kelleher AD, Rosenberg E, Markowitz M, Schafer K, Vaida F, Iwamoto A, Little S, Walker BD (2010) Impaired replication capacity of acute/early viruses in persons who become HIV controllers. J Virol 84:7581–7591
Miura T, Brockman MA, Brumme CJ, Brumme ZL, Carlson JM, Pereyra F, Trocha A, Addo MM, Block BL, Rothchild AC, Baker BM, Flynn T, Schneidewind A, Li B, Wang YE, Heckerman D, Allen TM, Walker BD (2008) Genetic characterization of human immunodeficiency virus type 1 in elite controllers: lack of gross genetic defects or common amino acid changes. J Virol 82:8422–8430
Bailey JR, Williams TM, Siliciano RF, Blankson JN (2006) Maintenance of viral suppression in HIV-1-infected HLA-B*57 + elite suppressors despite CTL escape mutations. J Exp Med 203:1357–1369
Bailey JR, Brennan TP, O’Connell KA, Siliciano RF, Blankson JN (2009) Evidence of CD8+ T-cell-mediated selective pressure on human immunodeficiency virus type 1 nef in HLA-B*57+ elite suppressors. J Virol 83:88–97
Durand CM, O’Connell KA, Apuzzo LG, Langan SJ, Imteyaz H, Ahonkhai AA, Ceccato CM, Williams TM, Margolick JB, Blankson JN (2010) HIV-1 Gag evolution in recently infected human leukocyte antigen-B*57 patients with low-level viremia. AIDS 24:2405–2408
Martinez-Picado J, Prado JG, Fry EE, Pfafferott K, Leslie A, Chetty S, Thobakgale C, Honeyborne I, Crawford H, Matthews P, Pillay T, Rousseau C, Mullins JI, Brander C, Walker BD, Stuart DI, Kiepiela P, Goulder P (2006) Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J Virol 80:3617–3623
Leslie AJ, Pfafferott KJ, Chetty P, Draenert R, Addo MM, Feeney M, Tang Y, Holmes EC, Allen T, Prado JG, Altfeld M, Brander C, Dixon C, Ramduth D, Jeena P, Thomas SA, St. John A, Roach TA, Kupfer B, Luzzi G, Edwards A, Taylor G, Lyall H, Tudor-Williams G, Novelli V, Martinez-Picado J, Kiepiela P, Walker BD, Goulder PJ (2004) HIV evolution: CTL escape mutation and reversion after transmission. Nat Med 10:282–289
Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF (1997) Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300
Siliciano JD, Siliciano RF (2005) Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol 304:3–15
Graf EH, Mexas AM, Yu JJ, Shaheen F, Liszewski MK, Di Mascio M, Migueles SA, Connors M, O’Doherty U (2011) Elite suppressors harbor low levels of integrated HIV DNA and high levels of 2-LTR circular HIV DNA compared to HIV+ patients on and off HAART. PLoS Pathog 7:e1001300
Mendoza D, Johnson SA, Peterson BA, Natarajan V, Salgado M, Dewar RL, Burbelo PD, Doria-Rose NA, Graf EH, Greenwald JH, Hodge JN, Thompson WL, Cogliano NA, Chairez CL, Rehm CA, Jones S, Hallahan CW, Kovacs JA, Sereti I, Sued O, Peel SA, O’Connell RJ, O’Doherty U, Chun T, Connors M, Migueles SA (2012) Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood 119(20):4645–4655
Hatano H, Delwart EL, Norris PJ, Lee TH, Dunn-Williams J, Hunt PW, Hoh R, Stramer SL, Linnen JM, McCune JM, Martin JN, Busch MP, Deeks SG (2009) Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol 83:329–335
Altfeld M, Addo MM, Rosenberg ES, Hecht FM, Lee PK, Vogel M, Yu XG, Draenert R, Johnston MN, Strick D, Allen TM, Feeney ME, Kahn JO, Sekaly RP, Levy JA, Rockstroh JK, Goulder PJ, Walker BD (2003) Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS 17:2581–2591
Goujard C, Chaix ML, Lambotte O, Deveau C, Sinet M, Guergnon J, Courgnaud V, Rouzioux C, Delfraissy JF, Venet A, Meyer L and Agence Nationale de Recherche sur le Sida PRIMO Study Group (2009) Spontaneous control of viral replication during primary HIV infection: when is “HIV controller” status established? Clin Infect Dis 49:982–986
Pereyra F, Palmer S, Miura T, Block BL, Wiegand A, Rothchild AC, Baker B, Rosenberg R, Cutrell E, Seaman MS, Coffin JM, Walker BD (2009) Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis 200:984–990
Dinoso JB, Kim SY, Siliciano RF, Blankson JN (2008) A comparison of viral loads between HIV-1-infected elite suppressors and individuals who receive suppressive highly active antiretroviral therapy. Clin Infect Dis 47:102–104
Bailey JR, Sedaghat AR, Kieffer T, Brennan T, Lee PK, Wind-Rotolo M, Haggerty CM, Kamireddi AR, Liu Y, Lee J, Persaud D, Gallant JE, Cofrancesco J Jr, Quinn TC, Wilke CO, Ray SC, Siliciano JD, Nettles RE, Siliciano RF (2006) Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol 80:6441–6457
Brennan TP, Woods JO, Sedaghat AR, Siliciano JD, Siliciano RF, Wilke CO (2009) Analysis of human immunodeficiency virus type 1 viremia and provirus in resting CD4+ T cells reveals a novel source of residual viremia in patients on antiretroviral therapy. J Virol 83:8470–8481
Mens H, Kearney M, Wiegand A, Shao W, Schonning K, Gerstoft J, Obel N, Maldarelli F, Mellors JW, Benfield T, Coffin JM (2010) HIV-1 continues to replicate and evolve in patients with natural control of HIV infection. J Virol 84:12971–12981
O’Connell KA, Brennan TP, Bailey JR, Ray SC, Siliciano RF, Blankson JN (2010) Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus. J Virol 84:7018–7028
Salgado M, Brennan TP, O’Connell KA, Bailey JR, Ray SC, Siliciano RF, Blankson JN (2010) Evolution of the HIV-1 nef gene in HLA-B*57 positive elite suppressors. Retrovirology 7:94
Salgado M, Rabi SA, O’Connell KA, Buckheit RW 3rd, Bailey JR, Chaudhry AA, Breaud AR, Marzinke MA, Clarke W, Margolick JB, Siliciano RF, Blankson JN (2011) Prolonged control of replication-competent dual- tropic human immunodeficiency virus-1 following cessation of highly active antiretroviral therapy. Retrovirology 8:97
Spivak AM, Salgado M, Rabi SA, O’Connell KA, Blankson JN (2011) Circulating monocytes are not a major reservoir of HIV-1 in elite suppressors. J Virol 85:10399–10403
Schneidewind A, Brockman MA, Yang R, Adam RI, Li B, Le Gall S, Rinaldo CR, Craggs SL, Allgaier RL, Power KA, Kuntzen T, Tung CS, LaBute MX, Mueller SM, Harrer T, McMichael AJ, Goulder PJ, Aiken C, Brander C, Kelleher AD, Allen TM (2007) Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J Virol 81:12382–12393
Brumme ZL, Li C, Miura T, Sela J, Rosato PC, Brumme CJ, Markle TJ, Martin E, Block BL, Trocha A, Kadie CM, Allen TM, Pereyra F, Heckerman D, Walker BD, Brockman MA (2011) Reduced replication capacity of NL4-3 recombinant viruses encoding reverse transcriptase-integrase sequences from HIV-1 elite controllers. J Acquir Immune Defic Syndr 56:100–108
Lassen KG, Lobritz MA, Bailey JR, Johnston S, Nguyen S, Lee B, Chou T, Siliciano RF, Markowitz M, Arts EJ (2009) Elite suppressor-derived HIV-1 envelope glycoproteins exhibit reduced entry efficiency and kinetics. PLoS Pathog 5:e1000377
O’Connell KA, Hegarty RW, Siliciano RF, Blankson JN (2011) Viral suppression of multiple escape mutants by de novo CD8+ T cell responses in a human immunodeficiency virus-1 infected elite suppressor. Retrovirology 8:63
Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, Hsue P, Emu B, Krone M, Lampiris H, Douek D, Martin JN, Deeks SG (2008) Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 197:126–133
Andrade A, Bailey JR, Xu J, Philp FH, Quinn TC, Williams TM, Ray SC, Thomas DL, Blankson JN (2008) CD4+ T cell depletion in an untreated HIV type 1-infected human leukocyte antigen-B*5801-positive patient with an undetectable viral load. Clin Infect Dis 46:e78–e82
Sedaghat AR, Rastegar DA, O’Connell KA, Dinoso JB, Wilke CO, Blankson JN (2009) T cell dynamics and the response to HAART in a cohort of HIV-1-infected elite suppressors. Clin Infect Dis 49:1763–1766
Okulicz JF, Marconi VC, Landrum ML, Wegner S, Weintrob A, Ganesan A, Hale B, Crum-Cianflone N, Delmar J, Barthel V, Quinnan G, Agan BK, Dolan MJ and Infectious Disease Clinical Research Program (IDCRP) HIV Working Group (2009) Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis 200:1714–1723
Bailey JR, Zhang H, Wegweiser BW, Yang HC, Herrera L, Ahonkhai A, Williams TM, Siliciano RF, Blankson JN (2007) Evolution of HIV-1 in an HLA-B*57-positive patient during virologic escape. J Infect Dis 196:50–55
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buckheit III, R.W., Salgado, M., Martins, K.O. et al. The implications of viral reservoirs on the elite control of HIV-1 infection. Cell. Mol. Life Sci. 70, 1009–1019 (2013). https://doi.org/10.1007/s00018-012-1101-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1101-7