Abstract
Intervention strategies for obesity are global issues that require immediate attention. The objective of this study was to assess the possibility that Clostridium butyricum and its potential components could reduce lipogenesis. Co-culture experiments of Caco-2 cells and 1 × 106, 1 × 107, and 1 × 108 CFU/ml of C. butyricum were set up to monitor the cytotoxicity of C. butyricum and the changes of angiopoietin-like protein 4 (ANGPTL4) mRNA expression. It was found that cell viability was not affected by C. butyricum, and ANGPTL4 mRNA expression in Caco-2 cells was highly induced by 1 × 107 CFU/ml of C. butyricum. Co-culture experiment of Caco-2 cells and potential components of C. butyricum were set up to monitor any ensuing alteration in ANGPTL4. It was observed that bacterial wall components and potentially secreted factors from C. butyricum could induce ANGPTL4 mRNA expression and protein secretion. To determine whether butyrate could affect the ANGPTL4 production in Caco-2 cells, the role of monocarboxylate transporter 1 (MCT1) in mediating potentially secreted factors from C. butyricum-induced ANGPTL4 production in Caco-2 cells and the effect of 0.1 mM of butyrate on ANGPTL4 production in Caco-2 cells were investigated. It is confirmed that butyrate was the factor secreted by C. butyricum to stimulate ANGPTL4 production. Besides, the soluble factors secreted by live C. butyricum-Caco-2 cells interaction, bacterial wall components-Caco-2 cells interaction, and the main metabolites butyrate-Caco-2 cells interaction reduced lipogenic gene expression in HepG2 cells. In conclusion, 1 × 107 CFU/ml of C. butyricum could reduce lipogenesis through the bacterial wall components and the metabolites such as butyrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past decade, there is a growing speculation that intestinal microbiota can influence fat storage (Greiner and Bäckhed 2011). This notion has spurred the search for circulating factors that communicate between the intestinal microbiota and other parts of the body. One factor that was found is angiopoietin-like protein 4 (ANGPTL4), which is a circulating lipoprotein lipase (LPL) inhibitor and plays a key role in regulating deposition of triglycerides in adipocytes (Yoshida et al. 2002; Sukonina et al. 2006). During recent years, ANGPTL4 has been widely investigated as a multifunctional signal protein, mainly expressed in the intestine, liver, adipose tissue, skeletal muscle, brain, and thyroid tissue (Kersten et al. 2009). Recent researches have revealed that the intestinal microbiota can directly (by cell contact) or indirectly (by metabolite or secretion factors) modulate ANGPTL4 mRNA expression and protein secretion by intestinal epithelial cells (Grootaert et al. 2011). Therefore, more and more attention was paid to supplementation of probiotics and their metabolites to modulate ANGPTL4 production by intestinal epithelial cells
Clostridium butyricum is a butyric acid-producing, spore-forming, gram-positive anaerobe, which is found in soil and in the intestines of healthy humans and animals (Murayama et al. 1995; Nakanishi and Tanaka 2010). Previous studies demonstrated that C. butyricum could influence fat storage of animals (Yang et al. 2010; Zhang et al. 2011; Zhao et al. 2013). However, information is lacking on the circulating factors that communicate between C. butyricum and animal body tissue. Therefore, the objective of this study was to assess the possibility that C. butyricum and its potential components could target the fat storage regulator ANGPTL4 and, as a consequence, execute its modulatory effects.
Materials and methods
Cell culture
The human colon carcinoma cell line Caco-2 (ATCC HTB-37) and human hepatoma cell line HepG2 (ATCC HB-8065) were obtained from Beijing Jin Zijing Biological Pharmaceutical Technology Co. Ltd., Beijing, China. Cells were routinely grown in minimum essential medium (MEM) (Macgene, Beijing, China) supplemented with 10 % v/v fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA), 100 U/ml penicillin, and 100 ug/ml streptomycin. Cells were maintained in culture at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air. The culture medium was changed every other day.
Bacterial strains and culture conditions
The C. butyricum B1 (CGMCC 4845) used in this study was provided by Beijing Gold-Tide Biotechnology Co. Ltd., Beijing, China. It was cultured in Reinforced Clostridium Medium (RCM) (Beijing Land Bridge Technology Co. Ltd., Beijing, China) at 37 °C in an anaerobic environment. Before stimulation assays, the live C. butyricum were washed twice with PBS (centrifugation at 4,000 × g for 5 min) and resuspended in FBS and antibiotic-free MEM at the density of 1 × 108 CFU/ml.
Cell viability assay
The cell viability was evaluated by cell counting kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan). Caco-2 cells were seeded in 96-well culture plates (6 × 103 cells per well) and were maintained at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air until 70 to 80 % confluence. Twenty-four hours before stimulation, the confluent cells were washed and cultured in fresh medium without FBS and antibiotic. Caco-2 cells were treated with 100 μl of MEM or C. butyricum suspensions at designated concentration (1 × 106, 1 × 107, and 1 × 108 CFU/ml). After 2 h incubation, the cells were washed twice with PBS. Ten microliter CCK-8 and 100 μl MEM were added to each well and incubated for an additional 4 h at 37 °C. The optical density (OD) of each well at 450 nm was recorded on a Microplate Reader (ELx50, BioTek Instruments Inc., Winooski, VT, USA). The cell viability (% of control) is expressed as the percentage of (ODtest − ODblank) / (ODcontrol − ODblank), where ODtest is the optical density of the cells exposed to C. butyricum sample, ODcontrol is the optical density of the control sample, and ODblank is the optical density of the wells without Caco-2 cells. The experiment was performed three times.
Treatment of Caco-2 cells with live C. butyricum
Caco-2 cells were seeded in 6-well culture plates (Costar, Cambridge, MA, USA) and were maintained at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air until 80 to 90 % confluence. Twenty-four hours before stimulation, the confluent cells were washed and cultured in fresh medium without FBS and antibiotic. Caco-2 cells were treated with 2 ml of MEM or C. butyricum suspensions at designated concentration (1 × 106, 1 × 107, and 1 × 108 CFU/ml) and incubated in 5 % CO2 at 37 °C for 2 h. The experiment was terminated by thoroughly washing the plates with ice-cold PBS, and the cells were harvested for real-time PCR analysis. The experiment was performed three times.
Caco-2 cells treated with live and heat-inactivated C. butyricum and prefermented minimum essential medium
To obtain dead bacterial cells, 1 × 107 CFU/ml of C. butyricum was washed twice with PBS (centrifugation at 4,000 × g for 5 min). The pellet was incubated in a hot water bath at 100 °C for 30 min and resuspended in FBS and antibiotic-free MEM. To obtain prefermented MEM, an equal amount of living bacteria was suspended in MEM and incubated at 37 °C for 2 h without being in contact with Caco-2 cells. Thereafter, the suspension was centrifuged (5 min, 4,000 × g), and the supernatant was sterilized with a 0.22-μm filter (Millipore, Billerica, MA, USA) and retained as the prefermented MEM. Fetal bovine serum and antibiotic-free MEM, MEM with live C. butyricum, MEM with heat-inactivated C. butyricum, and cell-free MEM prefermented with C. butyricum were added to the Caco-2 cells and incubated for 2 h at 37 °C and in the presence of 5 % CO2 (denoted as control, active Cb, inactive Cb, and prefermented). The culture medium were collected and analyzed for ANGPTL4 by enzyme-linked immunosorbent assay (ELISA), and the same cells (after washed with ice-cold PBS) from the culture medium were harvested for real-time PCR analysis. The experiment was performed three times.
The role of monocarboxylate transporter 1 in modulating angiopoietin-like protein 4 production in Caco-2 cells exposed to prefermented minimum essential mediumsina transient transfection
One day before transfection, Caco-2 cells (5 × 105 cells per well) were allowed to attach and grow in 6-well culture plates (Costar). When the plate cells in medium without antibiotics were 60–80 % confluent, Silencer® Select Negative Control #1 siRNA (Ambion, Austin, TX, USA) or monocarboxylate transporter 1 (MCT1) Silencer® Select Validated small interfering RNA (siRNA, s579) was transfected into cells with Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) (denoted as siNC and siMCT1, respectively). After 48 h, cells were treated with prefermented MEM and assayed for transfection efficiency by real-time PCR.
Stimulation of cells
Prefermented MEM was obtained using the same procedure as described above. Caco-2 cells were treated with 2 ml of MEM or prefermented MEM and incubated in 5 % CO2 at 37 °C for 2 h. The culture medium were collected and analyzed for ANGPTL4 by ELISA, and the same cells (after washed with ice-cold PBS) from the culture medium were harvested for real-time PCR analysis. The experiment was performed three times.
Treatment of Caco-2 cells with butyrate
Caco-2 cells were treated with 2 ml of FBS and antibiotic-free MEM, MEM with 0.1 mM of sodium butyrate (Sigma, St. Louis, MO, USA) (equal to the amount of butyrate in cell-free MEM prefermented with 1 × 107 CFU/ml of C. butyricum) and cell-free MEM prefermented with 1 × 107 CFU/ml of C. butyricum, and incubated in 5 % CO2 at 37 °C for 2 h. The culture medium were collected and analyzed for ANGPTL4 by ELISA, and the same cells (after washed with ice-cold PBS) from the culture medium were harvested for real-time PCR analysis. The experiment was performed three times.
HepG2 cells treated with conditioned media from C. butyricum interacting with Caco-2 cells
Preparation of conditioned media
Caco-2 cells were treated with 2 ml of FBS and antibiotic-free MEM, MEM with live C. butyricum, MEM with heat-inactivated C. butyricum, and MEM with 0.1 mM of sodium butyrate respectively and incubated in 5 % CO2 at 37 °C for 2 h. Thereafter, the culture medium was filtered (pore size, 0.22 μm) and retained as the conditioned media from Caco-2 cells, conditioned media from live C. butyricum interacting with Caco-2 cells, conditioned media from heat-inactivated C. butyricum interacting with Caco-2 cells, and conditioned media from butyrate interacting with Caco-2 cells respectively.
Stimulation of HepG2 cells
The conditioned media from Caco-2 cells, conditioned media from live C. butyricum interacting with Caco-2 cells, conditioned media from heat-inactivated C. butyricum interacting with Caco-2 cells, and conditioned media from butyrate interacting with Caco-2 cells obtained above were added to the HepG2 cells respectively and incubated for 2 h at 37 °C and in the presence of 5 % CO2 (denoted as Control, Active Cb, Inactive Cb, and Butyrate, respectively). The HepG2 cells (after washed with ice-cold PBS) from the culture medium were harvested for real-time PCR analysis. The experiment was performed three times.
Real-time quantitative PCR
Total RNA of the cells was extracted using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The RNA concentration and purity were determined by measuring the absorbance at 260 and 280 nm, and the RNA integrity was assessed via agarose gel electrophoresis. For the production of cDNA, 400 ng of total RNA was reverse transcribed with PrimeScriptTM RT Master Mix (Perfect Real Time) (TaKaRa, Dalian, China) according to the manufacturer’s instructions. All of the cDNA preparations were stored frozen at −30 °C until further use. A real-time quantitative PCR assay was performed with the 7500 Real Time PCR Systems (Applied Biosystems, Foster City, CA, USA) according to the optimized PCR protocols using SYBR® Premix Ex Taq TM (TaKaRa). The thermocycle protocol consisted of 30 s initial denaturation at 95 °C, followed by 40 cycles of 5 s denaturation at 95 °C, and 34 s annealing/extension at 60 °C. The gene-specific primers for ANGPTL4, MCT1, fatty acid synthase (FASN), malic enzyme 1 (ME1), acetyl-CoA carboxylase α (ACACA), sterol regulatory element binding protein 1 (SREBP-1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are listed in Table 1. Standard curves were derived from serial dilutions of samples. To confirm amplification specificity, the PCR products from each primer pair were subjected to a melting curve analysis and subsequent agarose gel electrophoresis. The ΔΔCt method was used to estimate mRNA abundance. Glyceraldehyde-3-phosphate dehydrogenase was used as the internal reference gene, and the mRNA expression of target genes was normalized to GAPDH mRNA expression. All the samples were analyzed in triplicates, and the mean values of these measurements were used for calculations of mRNA expression.
ELISA
Human ANGPTL4 level in the culture medium was measured by a specific ELISA kit (Abcam, Cambridge, UK) according to the manufacturer’s instructions.
SCFA analysis
Short-chain fatty acid (acetate, propionate, and butyrate) concentrations in cell-free MEM prefermented with 1 × 107 CFU/ml of C. butyricum were measured using gas chromatography as described by Li and Meng (2006).
Data calculations and statistical analyses
All data were subjected to analysis of variance (ANOVA) using the general liner model (GLM) procedure of Statistical Analysis System (SAS) 8.1 software (SAS Institute, Inc., Cary, NC, USA). Data are expressed as means ± standard error (SE). The significance of differences among treatments was tested by Duncan’s multiple range test. A level of P < 0.05 was used as the criterion for statistical significance.
Results
The cytotoxicity of C. butyricum in Caco-2 cells
The cell viability was assayed to estimate the cytotoxicity of C. butyricum quantitatively by CCK-8 assay in which the formation of formazan dye depends on the mitochondria activity. As shown in Fig. 1, no difference was observed in the viability of Caco-2 cells after exposure to C. butyricum at the concentration of 0, 1 × 106, 1 × 107, and 1 × 108 CFU/ml for 2 h. This result demonstrated that C. butyricum supplemented at the concentration of 1 × 106, 1 × 107, and 1 × 108 CFU/ml to Caco-2 cells for 2 h did not negatively affect the cell viability. Therefore, the above concentrations of C. butyricum were applied in the following experiment.
Angiopoietin-like protein 4 mRNA expression in Caco-2 cells after exposed to different concentrations of C. butyricum
To investigate whether C. butyricum could regulate ANGPTL4 mRNA expression in Caco-2 cells, a stimulation assay was performed, as described in the “Materials and methods.” Figure 2 showed that Caco-2 cells after exposed to 1 × 107 CFU/ml of C. butyricum for 2 h had higher (P < 0.05) ANGPTL4 mRNA level than control cells. However, no difference in ANGPTL4 mRNA level was observed among 1 × 106, 1 × 107, and 1 × 108 CFU/ml of C. butyricum-supplemented cells.
Angiopoietin-like protein 4 (ANGPTL4) mRNA expression in Caco-2 cells after exposure to 0, 1 × 106, 1 × 107, or 1 × 108 CFU/ml of C. butyricum for 2 h. Value of each treatment is the mean of three independent experiments, and the vertical bar represents standard error. Means with different letters differ significantly (P < 0.05)
Angiopoietin-like protein 4 in Caco-2 cells after exposed to live and heat-inactivated C. butyricum and prefermented minimum essential medium
To disclose the mechanism of ANGPTL4 induction, potential components of C. butyricum were separated and investigated individually. Caco-2 cells of active Cb, inactive Cb, and prefermented had higher (P < 0.05) ANGPTL4 mRNA expression than control cells (Fig. 3a). Besides, among the groups supplemented with potential components of C. butyricum, cells of inactive Cb appeared to contain the highest (P < 0.05) ANGPTL4 mRNA level. The ANGPTL4 protein secretion in the culture medium of active Cb, inactive Cb, and prefermented cells were higher (P < 0.05) than those of cells in control, whereas this protein secretion was similar among active Cb, inactive Cb, and prefermented (Fig. 3b).
Angiopoietin-like protein 4 (ANGPTL4) in Caco-2 cells after exposed to minimum essential medium (MEM) (Control), MEM with 1 × 107 CFU/ml of live C. butyricum (Active Cb), MEM with 1 × 107 CFU/ml of heat-inactivated C. butyricum (Inactive Cb), or cell-free MEM prefermented with 1 × 107 CFU/ml of C. butyricum (Prefermented) for 2 h. a ANGPTL4 mRNA expression in Caco-2 cells. b ANGPTL4 concentration in medium of Caco-2 cells. Value of each treatment is the mean of three independent experiments, and the vertical bar represents standard error. Means with different letters differ significantly (P < 0.05)
Silence of monocarboxylate transporter 1 interrupted prefermented minimum essential medium-induced angiopoietin-like protein 4 production in Caco-2 cells
Monocarboxylate transporter 1 (MCT1) has been demonstrated to mediate the entry of butyrate in Caco-2 cells (Hadjiagapiou et al. 2000). In the current study, ANGPTL4 production in Caco-2 cells was found to be stimulated by factors secreted by C. butyricum. To determine whether butyrate, which is one of the main metabolic products of C. butyricum, could affect the ANGPTL4 production in Caco-2 cells, the role of MCT1 in mediating prefermented MEM-induced ANGPTL4 production in Caco-2 cells was investigated. Transfection of Caco-2 cells with MCT1 siRNA effectively silenced (P < 0.05) MCT1 mRNA expression (Fig. 4a). ANGPTL4 mRNA expression and protein secretion in siNC-transfected Caco-2 cells were found to be stimulated (P < 0.05) by cell-free MEM prefermented with C. butyricum (Fig. 4b, c). However, prefermented MEM-induced ANGPTL4 mRNA expression and protein secretion in siMCT1-transfected Caco-2 cells was close to MEM-induced ANGPTL4 production in siNC- and siMCT1-transfected Caco-2 cells (Fig. 4b, c). The results of this study demonstrated the necessity of MCT1 in cell-free MEM prefermented with C. butyricum-induced ANGPTL4 mRNA expression and protein secretion, indicating that butyrate could be the main factor secreted by C. butyricum to stimulate ANGPTL4 production.
Induction of angiopoietin-like protein 4 (ANGPTL4) in Caco-2 cells by cell-free minimum essential medium (MEM) prefermented with 1 × 107 CFU/ml of C. butyricum is mediated by monocarboxylate transporter 1 (MCT1). Caco-2 cells were transfected with negative control siRNA (siNC) or MCT1 siRNA (siMCT1) for 48 h prior to stimulation. ANGPTL4 was assayed after a 2-h MEM (C) or prefermented MEM (T) treatment. a MCT1 mRNA expression in Caco-2 cells. b ANGPTL4 mRNA expression in Caco-2 cells. c ANGPTL4 concentration in medium of Caco-2 cells. Value of each treatment is the mean of three independent experiments, and the vertical bar represents standard error. Means with different letters differ significantly (P < 0.05)
Butyrate stimulate angiopoietin-like protein 4 synthesis in Caco-2 cells
Previously, ANGPTL4 production in Caco-2 cells was found to be stimulated by cell-free MEM prefermented with C. butyricum which was mediated by MCT1. We hypothesized that one of the major factors corresponded to butyrate, which is the main substance produced by C. butyricum. Accordingly, we assayed the ANGPTL4 mRNA expression and protein secretion in Caco-2 cells after exposed to FBS and antibiotic-free MEM, MEM with 0.1 mM of butyrate (equal to the amount of butyrate in cell-free MEM prefermented with 1 × 107 CFU/ml of C. butyricum), and cell-free MEM prefermented with 1 × 107 CFU/ml of C. butyricum for 2 h. Caco-2 cells with sodium butyrate and prefermented MEM for 2 h resulted in increased (P < 0.05) ANGPTL4 mRNA expression and protein secretion compared to control cells (Fig. 5a, b). However, no difference in ANGPTL4 production was observed between sodium butyrate and prefermented MEM stimulated cells. All of the above results provided further evidence that butyrate was the factor secreted by C. butyricum that stimulate ANGPTL4 production.
Angiopoietin-like protein 4 (ANGPTL4) in Caco-2 cells after exposed to minimum essential medium (MEM) (Control), MEM with butyrate which is equal to the amount of butyrate in cell-free MEM prefermented with 1 × 107 CFU/ml of C. butyricum (Butyrate), or cell-free MEM prefermented with 1 × 107 CFU/ml of C. butyricum (Prefermented) for 2 h. a ANGPTL4 mRNA expression in Caco-2 cells. b ANGPTL4 concentration in medium of Caco-2 cells. Value of each treatment is the mean of three independent experiments, and the vertical bar represents standard error. Means with different letters differ significantly (P < 0.05)
Conditioned media from C. butyricum interacting with Caco-2 cells stimulate lipid metabolism gene expression in HepG2 cells
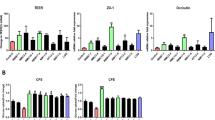
HepG2 cells after exposed to conditioned media from live C. butyricum interacting with Caco-2 cells for 2 h appeared to contain the lowest (P < 0.05) FASN, ME1, ACACA, and SREBP-1 mRNA level (Fig. 6a–d). The FASN, ME1, ACACA, and SREBP-1 mRNA expression of inactive Cb HepG2 cells and FASN, ACACA, and SREBP-1 mRNA expression of butyrate HepG2 cells were lower (P < 0.05) than those of HepG2 cells in control. However, no difference was observed in ME1, ACACA, and SREBP-1 mRNA expression between active Cb and inactive Cb HepG2 cells and FASN, ACACA, and SREBP-1 mRNA expression between active Cb and butyrate HepG2 cells.
Lipid metabolism gene expression in HepG2 cells after exposed to conditioned media from Caco-2 cells (Control), conditioned media from live C. butyricum interacting with Caco-2 cells (Active Cb), conditioned media from heat-inactivated C. butyricum interacting with Caco-2 cells (Inactive Cb), or conditioned media from butyrate interacting with Caco-2 cells (Butyrate) for 2 h. a FASN mRNA expression in HepG2 cells. b ME1 mRNA expression in HepG2 cells. c ACACA mRNA expression in HepG2 cells. d SREBP-1 mRNA expression in HepG2 cells. Value of each treatment is the mean of three independent experiments, and the vertical bar represents standard error. Means with different letters differ significantly (P < 0.05)
Discussion
Obesity has increased dramatically during the past decades and has now reached epidemic proportions in both developed and developing countries. The increase in obesity is associated with corresponding increases in type 2 diabetes, hypertension, cardiovascular disease, and cancer (Allison et al. 1999; Greiner and Bäckhed 2011). Recently, the use of probiotics as lipid metabolism regulators has gained increasing interest because of the global trend of human obesity. In vitro and in vivo studies have reported that Lactobacillus could reduce the fat storage (Aronsson et al. 2010; Huang and Zheng 2010). Likewise, in vitro study has shown that Enterococcus faecalis and Bacteroides thetaiotaomicron could increase the ANGPTL4 production in gut epithelial cell lines, thereby decreasing fat storage in the host (Grootaert et al. 2011). However, information about the effects of C. butyricum on human colon carcinoma cell line is lacking. Angiopoietin-like protein 4 has been proposed as a circulating mediator between the gut microbiota and fat storage. It is released as a 50-kDa prohormone that is subsequently cleaved into N- and C-terminal fragments. The N-terminal fragment of ANGPTL4 blocks activity of LPL, which catalyzes uptake of circulating lipids into tissues (Yoshida et al. 2002). Hence, it is well-recognized that ANGPTL4 is an indicator that could reflect the effects of C. butyricum on lipid metabolism.
In the stimulation assay, the transcription of ANGPTL4 in Caco-2 cells was highly induced by 1 × 107 CFU/ml of C. butyricum. The finding was consistent with that reported by Aronsson et al. (2010), who also observed increased ANGPTL4 mRNA expression in several colonic cell lines including HCT116, HT-29, LoVo, and SW480 cells as a result of supplementation of Lactobacillus F19, Lactobacillus rhamnosus GG, and Bifidobacterium lactis 12 at the rate of 107/ml. Incubation of colonic cell lines with E. faecalis, isolated from newborn babies, also reported to result in increased transcription of ANGPTL4 (Are et al. 2008). However, the mechanism by which the C. butyricum increased the ANGPTL4 mRNA expression in Caco-2 cells had not been disclosed. The ability of heat-inactivated C. butyricum and cell-free MEM prefermented with 1 × 107 CFU/ml of C. butyricum to generate a response that showed the bacterial wall components and the factors secreted by C. butyricum were the modulators for ANGPTL4 production, but little information is on the effects of different components of C. butyricum on ANGPTL4 mRNA expression and protein secretion. Aronsson et al. (2010) reported that bacterial wall components of Lactobacillus F19 were not the stimuli of ANGPTL4 production in colon cancer cells. The different bacterial wall components of the different types of bacteria could contribute to the different consequence. Aronsson et al. (2010) also observed that ANGPTL4 was upregulated in colon cancer cells by a secretion product of Lactobacillus F19. Butyrate is one of the major metabolites of normal intestinal microbiota. When measuring the SCFA content in cell-free MEM prefermented with 1 × 107 CFU/ml of C. butyricum, none of the tested SCFAs was detected, except for butyrate. Previous studies have suggested that MCT1 represents one of the major routes for butyrate transport in Caco-2 cells (Hadjiagapiou et al. 2000). In this study, when MCT1 was abrogated by siRNA, induction of ANGPTL4 by potentially secreted factors from C. butyricum was considerably compromised. This implies that the ANGPTL4 production in Caco-2 cells stimulated by factors secreted by C. butyricum is in a MCT1-dependent manner. It indicated that butyrate was one of the major effective factors modulating ANGPTL4 production. To further confirm that butyrate is the factor secreted by C. butyricum that stimulate ANGPTL4 production in Caco-2 cells, ANGPTL4 mRNA expression and protein secretion in Caco-2 cells were measured after exposed to FBS and antibiotic-free MEM, MEM with 0.1 mM of butyrate which is equal to the amount of butyrate in cell-free MEM prefermented with 1 × 107 CFU/ml of C. butyricum, and cell-free MEM prefermented with 1 × 107 CFU/ml of C. butyricum for 2 h. The similar production of ANGPTL4 in Caco-2 cells between butyrate and metabolites of C. butyricum supplementation groups finally confirmed that butyrate is one main factor secreted by C. butyricum that stimulate ANGPTL4 production.
In human, de novo lipogenesis occurs primarily in the liver, and soluble factors in the intestine can in part modulate hepatic lipogenesis (Greiner and Bäckhed 2011). There is considerable evidence that ACACA plays a role in the regulation of fatty acid biosynthesis in animal tissues and is generally considered as a rate-limiting enzyme of lipogenesis in animals (Numa et al. 1970). Fatty acid synthase, which catalyzes the last step in the lipogenic pathway, is also a key determinant for the maximal capacity of a tissue to synthesize fatty acid. Furthermore, FASN and ACACA seem to be coordinately regulated (Toussant et al. 1981). Nicotinamide adenine dinucleotide phosphate (NADPH) is a coenzyme required for the reductive biosynthesis of fatty acid, and ME1 is recognized to be the main enzyme involved in supplying NADPH (Wise and Ball 1964; Young et al. 1964). In addition, SREBP-1 is an accessory transcription factor that plays an active and central role in the lipid metabolism genes such as ACACA and FASN regulation (Ryan et al. 2011). Therefore, it is well-recognized that FASN, ME1, ACACA, and SREBP-1 are four main indicators that reflect the de novo lipogenesis. The lower FASN, ME1, ACACA, and SREBP-1 mRNA levels in HepG2 cells exposed to conditioned media from live C. butyricum interacting with Caco-2 cells in this study suggesting that soluble factors secreted by the interaction between C. butyricum and Caco-2 cells can reduce lipogenesis in the liver. In order to address the mechanism of action of C. butyricum, lipid metabolism gene expression in HepG2 cells in response to the soluble factors secreted by different components of C. butyricum-Caco-2 cells interaction were monitored. Both conditioned media from heat-inactivated C. butyricum interacting with Caco-2 cells and conditioned media from butyrate interacting with Caco-2 cells reduced lipid metabolism gene expression in HepG2 cells, as did conditioned media from live C. butyricum interacting with Caco-2 cells. These results indicated that the reduced lipogenesis in the HepG2 cells by conditioned media from live C. butyricum interacting with Caco-2 cells is attributed to the soluble factors secreted by both the bacterial wall components-Caco-2 cells interaction and the main metabolites butyrate-Caco-2 cells interaction. However, the mechanisms by which the soluble factors decreased the lipid metabolism gene expression in the liver are not clear.
In conclusion, 1 × 107 CFU/ml of C. butyricum decreased lipogenesis by increasing ANGPTL4 production in human colon carcinoma cell line Caco-2 and decreasing lipogenic genes expression in human hepatoma cell line HepG2. The reduced lipogenesis by C. butyricum supplementation is attributed to both the bacterial wall components and the metabolites such as butyrate.
References
Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB (1999) Annual deaths attributable to obesity in the United States. JAMA 282:1530–1538. doi:10.1001/jama.282.16.1530
Are A, Aronsson L, Wang S, Greicius G, Lee YK, Gustafsson JA, Pettersson S, Arulampalam V (2008) Enterococcus faecalis from newborn babies regulate endogenous PPARγ activity and IL-10 levels in colonic epithelial cells. Proc Natl Acad Sci U S A 105:1943–1948. doi:10.1073/pnas.0711734105
Aronsson L, Huang Y, Parini P, Korach-André M, Håkansson J, Gustafsson JÅ, Pettersson S, Arulampalam V, Rafter J (2010) Decreased fat storage by Lactobacillus paracasei is associated with increased levels of angiopoietin-like 4 protein (ANGPTL4). PLoS ONE 5:e13087. doi:10.1371/journal.pone.0013087
Crane JD, Devries MC, Safdar A, Hamadeh MJ, Tarnopolsky MA (2010) The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J Gerontol A Biol Sci Med Sci 65:119–128. doi:10.1093/gerona/glp179
Gonçalves P, Araújo JR, Pinho MJ, Martel F (2009) Modulation of butyrate transport in Caco-2 cells. Naunyn Schmiedebergs Arch Pharmacol 379:325–336. doi:10.1007/s00210-008-0372-x
Greiner T, Bäckhed F (2011) Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol Metab 22:117–123. doi:10.1016/j.tem.2011.01.002
Grootaert C, Van de Wiele T, Van Roosbroeck I, Possemiers S, Vercoutter-Edouart AS, Verstraete W, Bracke M, Vanhoecke B (2011) Bacterial monocultures, propionate, butyrate and H2O2 modulate the expression, secretion and structure of the fasting-induced adipose factor in gut epithelial cell lines. Environ Microbiol 13:1778–1789. doi:10.1111/j.1462-2920.2011.02482.x
Hadjiagapiou C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K (2000) Mechanism(s) of butyrate transport in Caco-2 cells: role of monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol 279:G775–G780
Huang Y, Zheng Y (2010) The probiotic Lactobacillus acidophilus reduces cholesterol absorption through the down-regulation of Niemann-Pick C1-like 1 in Caco-2 cells. Br J Nutr 103:473–478. doi:10.1017/S0007114509991991
Kersten S, Lichtenstein L, Steenbergen E, Mudde K, Hendriks HF, Hesselink MK, Schrauwen P, Müller M (2009) Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler Thromb Vasc Biol 29:969–974. doi:10.1161/ATVBAHA.108.182147
Li Y, Meng Q (2006) Effect of different types of fibre supplemented with sunflower oil on ruminal fermentation and production of conjugated linoleic acids in vitro. Arch Anim Nutr 60:402–411. doi:10.1080/17450390600884401
Murayama T, Mita N, Tanaka M, Kitajo T, Asano T, Mizuochi K, Kaneko K (1995) Effects of orally administered Clostridium butyricum MIYAIRI 588 on mucosal immunity in mice. Vet Immunol Immunopathol 48:333–342. doi:10.1016/0165-2427(95)05437-B
Nakanishi S, Tanaka M (2010) Sequence analysis of a bacteriocinogenic plasmid of Clostridium butyricum and expression of the bacteriocin gene in Escherichia coli. Anaerobe 16:253–257. doi:10.1016/j.anaerobe.2009.10.002
Numa S, Nakanishi S, Hashimoto T, Iritani N, Okazaki T (1970) Role of acetyl coenzyme A carboxylase in the control of fatty acid synthesis. Vitam Horm 28:213–243. doi:10.1016/S0083-6729(08)60895-X
Ryan MC, Desmond PV, Slavin JL, Congiu M (2011) Expression of genes involved in lipogenesis is not increased in patients with HCV genotype 3 in human liver. J Viral Hepat 18:53–60. doi:10.1111/j.1365-2893.2010.01283.x
Sukonina V, Lookene A, Olivecrona T, Olivecrona G (2006) Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci U S A 103:17450–17455. doi:10.1073/pnas.0604026103
Toussant MJ, Wilson MD, Clarke SD (1981) Coordinate suppression of liver acetyl-CoA carboxylase and fatty acid synthetase by polyunsaturated fat. J Nutr 111:146–153
Wise EM, Ball EG (1964) Malic enzyme and lipogenesis. Proc Natl Acad Sci U S A 52:1255–1263. doi:10.1073/pnas.52.5.1255
Yang X, Zhang B, Guo Y, Jiao P, Long F (2010) Effects of dietary lipids and Clostridium butyricum on fat deposition and meat quality of broiler chickens. Poult Sci 89:254–260. doi:10.3382/ps.2009-00234
Yoshida K, Shimizugawa T, Ono M, Furukawa H (2002) Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J Lipid Res 43:1770–1772. doi:10.1194/jlr.C200010-JLR200
Young JW, Shrago E, Lardy HA (1964) Metabolic control of enzymes involved in lipogenesis and gluconeogenesis. Biochemistry 3:1687–1692. doi:10.1021/bi00899a015
Zhang B, Yang X, Guo Y, Long F (2011) Effects of dietary lipids and Clostridium butyricum on serum lipids and lipid-related gene expression in broiler chickens. Animal 5:1909–1915. doi:10.1017/S1751731111001066
Zhao X, Guo Y, Guo S, Tan J (2013) Effects of Clostridium butyricum and Enterococcus faecium on growth performance, lipid metabolism and cecal microbiota of broiler chickens. Appl Microbiol Biotechnol 97:6477–6488. doi:10.1007/s00253-013-4970-2
Zhu J, Cui G, Chen M, Xu Q, Wang X, Zhou D, Lv S, Fu L, Wang Z, Zuo J (2013) Expression of R132H mutational IDH1 in human U87 glioblastoma cells affects the SREBP1a pathway and induces cellular proliferation. J Mol Neurosci 50:165–171. doi:10.1007/s12031-012-9890-6
Acknowledgments
This work was supported by Chinese Universities Scientific Fund and the Ministry of Education of the People’s Republic of China (grant number 2013RC004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, X., Guo, Y., Liu, H. et al. Clostridium butyricum reduce lipogenesis through bacterial wall components and butyrate. Appl Microbiol Biotechnol 98, 7549–7557 (2014). https://doi.org/10.1007/s00253-014-5829-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5829-x