Abstract
Endophytic actinomycetes are a rich source of novel antimicrobial compounds. The aim of this study was to evaluate the production of antimicrobial compound by endophytic Streptomyces sp. Av-R5 associated with root of Aloe vera against multidrug-resistant human pathogens. The 16S rRNA sequence of the isolate Av-R5 has been identified as Streptomyces parvulus NBRC 13193T (AB184326) and the sequence was submitted to the National Center for Biotechnology Information (NCBI) GenBank database (accession number KY771080). Streptomyces parvulus Av-R5 grown under submerged fermentation condition optimized by central composite design (glucose 11.16 g/L, soybean meal 10.25 g/L, sodium chloride 11.18 g/L, calcium carbonate 1.32 g/L at pH 7.19 at 31.42 °C with 6.04% seed inoculum for 10 days of incubation) exhibited the highest activity against multidrug-resistant Staphylococcus aureus JNMC-3, Staphylococcus epidermidis JNMC-4, Klebsiella pneumoniae MTCC-3384, Klebsiella pneumoniae JNMC-6, Pseudomonas aeruginosa MTCC-741, Proteus vulgaris JNMC-7, Candida albicans MTCC-183, and Aspergillus niger MTCC-872. The structures of the active compounds were elucidated by UV-Vis spectroscopy, 1H and 13C NMR, FT-IR, and ESIMS. Actinomycin D and actinomycin X0β were detected in crude extracts and major components were eluted by HPLC at 10.96 and 6.81 min, respectively. In this case, a high yield of actinomycin D and actinomycin X0β (400 mg/L) was achieved with Streptomyces parvulus Av-R5, fermented in glucose soybean meal broth media, which can be used in industrial fermentation process to obtain high yields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antibiotic resistance among the microorganisms has become a major challenge to the treatment of infectious diseases. Severe infections caused by bacteria that are resistant to commonly used antibiotics have become a major global healthcare problem in the twenty-first century [1]. It has been reported that endophytic actinomycetes are potential sources of novel natural products like antibiotics, antimycotics, antivirals, immunosuppressant, antioxidants, anticancer compounds, and plant growth hormones which have useful application in medicine, agriculture, and industry [2,3,4]. Endophytic Streptomyces spp. are known to produce different novel antibiotics namely cedarmycin A and B from Streptomyces sp. TP-A0456 associated with a twig of Cryptomeria japonica. Cedarmycin A exhibited antifungal activity against Candida glabrata [5]. Two novel broad-spectrum antibiotics munumbicins E-4 and E-5 have been reported from endophytic Streptomyces NRRL 3052 associated with Kennedia nigriscans. Both compounds exhibited activity against gram-positive and gram-negative bacteria and malarial parasite, Plasmodium falciparum [6]. The intimate association of endophytic actinomycetes with their host plants benefitted the host plant by the production of antimicrobial compounds. These compounds act against pathogenic microorganisms by antibiosis or competition and provide protection to the plants [7]. Endophytic actinomycetes have been identified to protect plants against various soil-borne plant pathogens, including Rhizoctonia solani, Verticillium dahliae, Plectosporium tabacinum, Gaeumannomyces graminis tritici, Fusarium oxysporum, Pythium aphanidermatum, and Colletotrichum orbiculare [8,9,10,11]. Therefore, screening of ethnopharmaceutically important plants for endophytic actinomycetes may yield potential organisms with antimicrobial compounds that can be employed for curing acute and chronic human diseases and may also be used against multidrug-resistant pathogenic strains.
Optimization of culture conditions is essential to achieve high yields of the antimicrobial compounds [4]. Different fermentation conditions for the production of antibiotics by Streptomyces parvulus have been studied from soil sources [12,13,14]. However, optimum fermentation condition for the production of antibiotics by endophytic Streptomyces parvulus has not been studied. The carbon sources like fructose, glucose, glycerol, starch, and sucrose are known to have a profound effect on antibiotic production by different Streptomyces spp. [14, 15], whereas the biosynthesis of many antibiotics by Streptomyces sp. has been reported to inhibit or repress in the presence of glucose, ammonia, and phosphate [12, 15]. Hence, the study has been planned to optimize the fermentation conditions for antibiotic production by endophytic Streptomyces parvulus associated with root of Aloe vera.
In the present study, Streptomyces sp. Av-R5 has been isolated from root of Aloe vera, which exhibits broad-spectrum antibacterial and antifungal activity against multidrug-resistant human pathogens and is a stable high-yield producer of actinomycin D and actinomycin X0β. The polypeptide nature of the antimicrobial compound was ascertained by the treatment with proteolytic enzymes, viz., lysozyme, proteinase K, and trypsin. Fermentation was conducted using a one factor at a time method. However, these methods frequently fail to locate the combined effect of factors. Hence, response surface methodology has been employed to optimize the optimum concentration of nutrients and the fermentation conditions for enhanced production of antimicrobial compound in submerged fermentation.
Materials and Methods
Isolation of Endophytic Actinomycetes
The endophytic actinomycete Av-R5 was isolated from medicinal herb Aloe vera (L.) Burm. F. growing in the garden of School of Studies in Life Science, Pt. Ravishankar Shukla University, Raipur, Chhattisgarh, India (latitude 21.1797° N, longitude 81.7787° E). Endophytic actinomycetes were isolated by the five-step surface sterilization process on starch casein agar medium (w/v) (g/L) (soluble starch 10 g, K2HPO4 2 g, KNO3 2 g, NaCl 2 g, casein 0.3 g, MgSO4 0.05 g, CaCO3 0.02 g, FeSO4 0.01 g; pH 7.2) supplemented with nalidixic acid (50 mg/L) and nystatin (50 mg/L) to suppress the growth of bacteria and fungi, respectively [16]. The plates were incubated at 28 ± 2 °C for 21 days. After attaining visible powdery growth, colonies were transferred on starch casein agar slants for storage and preservation.
Characterization and Identification of Endophytic Actinomycete Av-R5
Cultural, biochemical, and physiological characteristics of isolate Av-R5 were examined as per international guidelines [17]. Morphological identification of the isolate was done by cover slip culture method [18]. The ability of isolate for utilization of different carbon and nitrogen sources was carried out by growing the isolate in basal mineral salt agar medium (g/L) [(NH4)2SO4 2.64 g, KH2PO4 2.38 g, K2HPO4.2H2O 5.65 g, MgSO4.7H2O 1 g; agar 15 g; trace salt solution 1 mL; pH 6.8–7.0] at 1.0% (w/v) concentration, and results were recorded after 7, 14, and 21 days using glucose and proline as positive control and carbon source and nitrogen source-free medium as negative control [19].
For molecular identification of the potent antibiotic-producing endophytic actinobacterial isolate Av-R5 from root of Aloe vera, 16S rRNA gene was amplified using the universal primers of 27f (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492r (5′-GGTTACCTTGTTACGACTT-3′). The sequenced 16S rRNA gene of the strain Av-R5 was aligned with the nucleotide sequences of Streptomyces genera in EzTaxon database (http://www.eztaxon.org/). Sequences with more than 98% homology were considered for molecular taxonomy analysis. Multiple alignments with sequences of the most closely related Streptomyces and calculations of level of sequence similarity were carried out using EzEditor software [20] (http://www.ezbiocloud.net/sw/ezeditor). The phylogenetic tree was constructed using the neighbor-joining method with bootstrap testing of 1000 replicates in MEGA 5.0 software [21]. The 16S rRNA sequence of the isolate Av-R5 has been submitted in the GenBank database of National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov).
Pathogenic Microorganisms and Their Susceptibility to Antibiotics
Bacterial and fungal pathogens were procured from the Institute of Microbial Technology (IMTECH), Chandigarh, India. They included Bacillus cereus MTCC-430 and Staphylococcus aureus MTCC-96 (resistant to amoxicillin); Bacillus subtilis MTCC-441 and Staphylococcus epidermidis MTCC-435; Escherichia coli MTCC-1687 (resistant to penicillin); Klebsiella pneumoniae MTCC-3384 (resistant to amoxicillin, chloramphenicol and penicillin); Proteus vulgaris MTCC-744 (resistant to amoxicillin and penicillin); Pseudomonas aeruginosa MTCC-741 (resistant to amoxicillin, chloramphenicol, penicillin, and tetracycline); Candida albicans MTCC-183 and Aspergillus niger MTCC-872 (resistant to fuconazole and ketoconazole). Clinical human pathogenic bacteria were procured from Pandit Jawaharlal Nehru Medical College (JNMC), Raipur, Chhattisgarh, India. They included Bacillus cereus JNMC-1 and Bacillus subtilis JNMC-2 (resistant to gentamycin and tetracycline); Staphylococcus aureus JNMC-3 (resistant to amoxicillin, chloramphenicol, and penicillin); Staphylococcus epidermidis JNMC-4 and Escherichia coli JNMC-5 (resistant to amoxicillin, chloramphenicol, and tetracycline); Klebsiella pneumoniae JNMC-6 (resistant to chloramphenicol, gentamycin, penicillin, streptomycin, and tetracycline); Proteus vulgaris JNMC-7 (resistant to amoxicillin, gentamycin, penicillin, and tetracycline). Antibiotic susceptibility of pathogenic organisms was tested by disc diffusion method. Antibiotic disc (Hi-Media, Mumbai, India) was used in the study at 10 mcg concentration. The antibiotic discs were placed on Muller-Hinton agar plates previously seeded with 16–18-h-old bacterial cultures (108 CFU/mL) grown in Muller-Hinton broth. Plates were incubated at 37 °C for 24 h. The zones of inhibition were measured to the nearest millimeter.
Optimization of Culture Condition for Antimicrobial Compound Production
The selection of suitable medium for antimicrobial compound production by endophytic Streptomyces sp. Av-R5 was carried out by growing Streptomyces sp. Av-R5 in ten different culture media (w/v) (Hi-Media, Mumbai, India), viz., Czapek-Dox broth (CZB), glycerol asparagine broth (ISP-5), glucose soybean meal broth (GSB), inorganic salt starch broth (ISP-4), nutrient broth (NB), potato dextrose broth (PDB), sabouraud broth (SB), soybean meal broth (X-media), starch casein nitrate broth (SCNB), and yeast extract malt extract broth (ISP-2). Fifty milliliters of the above broth culture medium in a 150-mL conical flask was inoculated with 1 mL of Streptomyces sp. Av-R5 seed culture and incubated at 28 ± 2 °C for 14 days. After incubation, the filtrate was separated from the culture broth by Whatman no. 1 filter paper and assessed against Bacillus cereus MTCC-430 for antimicrobial activity. Endophytic Streptomyces sp. Av-R5 exhibited the highest antibacterial activity against Bacillus cereus MTCC-430; hence, this organism was selected as an indicator organism to study the antimicrobial compound production. The medium in which the Streptomyces sp. Av-R5 exhibited maximum zone of inhibition against Bacillus cereus MTCC-430 was used for further study.
The effect of different inoculum volumes (v/v) (1–10%), incubation time (1 to 14 days), pH (4–10), and temperature (28–40 °C) on production of antimicrobial compound was determined by adding Streptomyces sp. Av-R5 seed inoculum to another flask containing 50 mL of growth medium. The flasks were incubated at 28 ± 2 °C for 14 days. Culture filtrate was then assessed for antibacterial activity. In order to determine the effect of different nutrient supplements on antimicrobial compound production, the broth was amended with various sugars (1% w/v: glucose, sorbitol, lactose, maltose, sucrose, fructose, glycerol, starch, xylose, and mannitol), and organic and inorganic nitrogen sources (1% w/v: soybean meal, urea, peptone, yeast extract, malt extract, beef extract, sodium nitrate, potassium nitrate, ammonium sulfate, and ammonium nitrate). All the experiments were conducted in duplicate plates, having 100 μL of culture filtrate of isolate Av-R5 in four wells. In order to measure the dry cell weight (g/L), mycelia were harvested and dried on a pre-weighed Whatman no. 1 filter paper at 80 °C for 24 h. The difference between the final and initial weight provided the dry weight of mycelia.
Central composite design (CCD) was employed in order to optimize the optimum environmental conditions, viz., temperature, pH, incubation time, inoculum volume, and nutritional conditions, viz., glucose, soybean meal, calcium carbonate, and sodium chloride for enhanced production of antimicrobial compound by endophytic Streptomyces sp. Av-R5. The model was studied within five coded levels designated as (−α), (−1), (0), (+1), and (+α) with 30 experimental runs (Online Resource 1–2). The model was validated by growing the endophytic Streptomyces sp. Av-R5 under CCD-optimized media (glucose soybean meal broth) and un-optimized media (starch casein nitrate broth). The broths were centrifuged at 4000g for 10 min, and the supernatant was tested against Bacillus cereus MTCC-430 by agar well diffusion method. Antimicrobial activity was expressed in terms of inhibition zone diameter (mm).
Submerged Fermentation and Production of Antimicrobial Compound
A stock culture of endophytic Streptomyces sp. Av-R5 was grown and maintained on glucose soybean meal agar (GSB) slant. The stock culture was prepared by transferring 5 mL of sterile water into 14-day-old well-sporulated slant of isolate Av-R5; the surface of the medium was scrapped with a sterile inoculating needle and transferred into 45 mL of seed medium with the same components as the agar slant. The seed culture medium was incubated at 28 °C for 48 h [22]. Six milliliters (6%) of the inoculum from seed culture medium in each flask after incubation was aseptically transferred into a lot of 1000-mL Erlenmeyer flasks (15 nos.) containing 300 mL of production medium optimized by central composite design: glucose 11.16 g/L, soybean meal 10.25 g/L, calcium carbonate 1.32 g/L, and sodium chloride 11.18 g/L in each flask. The flasks were incubated at 31.42 °C for 10 days in static condition. Aliquots (2 mL) of cultures were collected after every 4 days of incubation and checked for antibiotic production by evaporation in a rotary evaporator at 40 °C. After fermentation, the medium was centrifuged at 4000g for 10 min and the supernatant was extracted with ethyl acetate in the ratio of 1:1 (v/v). Gentle mixing was done for 15 min using separating funnel and allowed to stand for 30 min to separate the organic phase from the aqueous phase. Both the phases were checked for antimicrobial activity against Bacillus cereus MTCC-430 by agar well diffusion method and concentrated in a rotary vacuum evaporator at 40 °C. A total of 2.5 g dark reddish orange-colored active crude was collected from 5 L of fermentation broth.
Purification and Bioactivity of Antimicrobial Compound
To check the antimicrobial compound clearly by bioautography, the crude antibiotic was partially purified by thin layer chromatography (TLC) by using three different solvent systems, viz., n-butanol:ethyl acetate:water (v/v 9:9:1), n-hexane:ethyl acetate (v/v 9:1), and chloroform:methanol (v/v 6:4) as a mobile phase on a pre-coated silica gel aluminum plate (Merck 60F-254; 0.5 mm thick, Germany). The sample was loaded using a capillary tube 1.5 cm above from the bottom of TLC plate in a row along a line and the spot was left to dry. The TLC plate was placed vertically in a glass jar containing the solvents and incubated at room temperature for 30 min. When the solvent reached near the top, the plate was taken out and dried. Separated component was visualized under visible and ultraviolet light (254 and 366 nm), and their Rf value was calculated. For bioautography analysis, the developed TLC plate was dried overnight and the bands were scraped out separately and dissolved in ethyl acetate and centrifuged at 4000g for 10 min. Supernatant was subjected to antimicrobial activity assay against multidrug-resistant pathogens by agar well diffusion method. Further, purity of the TLC-purified antimicrobial compound was analyzed by preparative reverse phase HPLC (Shimadzu). Antimicrobial compound was dissolved in methanol (1.5 mg/mL) and was subjected to chromatography on a silica gel column Hypersil BDS C18 (150 × 4.6 mm, 5 μ pore size); injection volume was 20 μL. The mobile phase was solvent A, acetonitrile, and solvent B, 5 mM ammonium acetate, in water with the gradient of 95–10% for 0.01–10.0 min and 10–10% for 10–30 min, with a flow rate of 1 mL/min and total run time of 30 min. Purified compound was evaluated for its minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) against multidrug-resistant human pathogens using Mueller-Hinton broth (Standard NCCL method 2000). Dilution range of the antimicrobial compound taken was from 1 to 0.0315 mg/mL.
Structure Elucidation and Identification of the Active Compound
The structure of the active compounds isolated from HPLC was elucidated by UV-Vis spectroscopy, 1H and 13C NMR, FT-IR, and ESI-MS. The ultraviolet and visible spectra of the active compounds were recorded using ethyl acetate (0.01 mg/mL) in UV-visible spectrophotometer (JASCO, UV-630). Different bioactive compounds have different absorption spectra in UV and visible light that enable the characterization of antimicrobial compound. The active compound was scanned over a range of wavelength (200–800 nm). FT-IR spectrum of the antimicrobial compound was recorded using JASCO FT-IR model no. 4100 at 400–4000 cm−1 using KBr pellet technique. The 1H and 13C NMR spectrum of the antimicrobial compound was determined in JEOL 400-MHz using CDCl3 as a solvent. Mass spectrum of the antimicrobial compound was recorded using Shimadzu LCMS with APCI and ESI probes (model LC-2010EV). Electrospray ionization was operated in the positive and negative ion modes and mass spectra were recorded over a range of 100–1300 m/z.
Stability of Antimicrobial Compound in the Presence of Proteolytic Enzymes
The sensitivity of the purified antibiotic to denaturation by the enzymes (w/v) was tested with trypsin, proteinase K, and lysozyme (Hi-Media, Mumbai, India) [23, 24]. All the enzymes were dissolved in distilled water at concentration 1 mg/mL. One hundred microliters of the antimicrobial solution (1 mg/mL) was mixed with 100 μL enzyme and incubated at 37 °C for 1 h. The antibiotic solution without any enzymes was used as control, and antibacterial activity of the mixture was tested against Bacillus cereus MTCC-430 by agar well diffusion method.
Residual activity of antimicrobial compound was calculated using the equation:
Where,
- RA:
-
Residual activity
- H T :
-
Mean inhibition halo of extract after treatment (mm)
- H C :
-
Mean inhibition halo of the control (mm)
- 6:
-
Diameter of the well (mm)
Statistical Analysis
Optimization for the production of antimicrobial compound by endophytic Streptomyces sp. Av-R5 was analyzed by SPSS software package (version 16.0). Means of the eight measurements of inhibition zone diameter for each experiment were compared using Duncan’s Multiple Range Test (DMRT) at (p ≤ 0.05). Central composite design (CCD) and statistical analysis of multiple factors were carried out with the Design Expert software package (version 9.0.1, State-Ease Inc., USA). Model was analyzed by ANOVA, and the quality of polynomial model equation was assessed by the coefficient of determination (R2). The significance of regression coefficient was assessed based on p value.
Results
Characterization and Identification of Endophytic Actinobacterial Isolate Av-R5
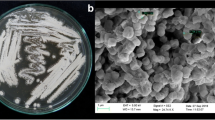
The isolate Av-R5 exhibited 5.4 g/L biomass on starch casein nitrate broth (SCA), 7.4 g/L on oatmeal broth (ISP-3), 8.4 g/L on inorganic salt starch broth (ISP-4), 2.4 g/L on glycerol asparagine broth (ISP-5), 2.5 g/L on tyrosine broth (ISP-7), and 2.2 g/L biomass on yeast extract malt extract broth (ISP-2) medium. It showed gray-colored aerial mycelium and orange yellow-colored substrate mycelium in all types of medium. In addition, the isolate Av-R5 produced yellow-colored pigment on all types of medium. Aerial filaments showed Retinaculum Apertum type of spore chain, open loops with extended spirals of wide diameter structure. The isolate Av-R5 was able to degrade starch, lecithin, lipid, and pectin. It showed positive reaction for catalase, nitrate reduction, hippurate hydrolysis, and xanthine degradation. The isolate was able to grow at 1–10% NaCl and in the presence of 0.01% sodium azide, 0.1% phenol, and 0.01% potassium tellurite. It was able to grow at temperatures between 28 and 37 °C and utilize l-valine and l-histidine as a nitrogen source. Similarly, the isolate was able to utilize sucrose, meso-inositol, mannitol, l-rhamnose, raffinose, d-melezitose, adonitol, d-melibiose, dextran, and xylitol as a carbon source. The nucleotide sequence of 16S rRNA and the phylogenetic tree generated from representative strains of the related genera showed that endophytic Streptomyces sp. Av-R5 had high levels of sequence similarity to species of Streptomyces parvulus NBRC 13193T (AB184326). The 16S rRNA analysis revealed that endophytic Streptomyces sp. Av-R5 is phylogenetically closely related to Streptomyces parvulus (the sequence similarity levels were 100%). Nearly complete (1476 base pair) 16S rRNA sequence of endophytic Streptomyces sp. Av-R5 has been submitted to the GenBank database of National Center for Biotechnology Information (NCBI) (accession number KY771080).
Optimization of Culture Condition for Antimicrobial Compound Production
Optimization of culture conditions for the production of antimicrobial compound by endophytic Streptomyces parvulus Av-R5 is given in Fig. 1. Antimicrobial compound production by Streptomyces parvulus Av-R5 was carried out on ten different types of media in a submerged culture for 14 days. Among them, glucose soybean meal broth media composed of (w/v) (g/L) glucose 10 g, soybean meal 10 g, CaCO3 1 g, NaCl 10 g, pH 7.5, and X-media composed of (w/v) (g/L) soybean meal 10 g, glucose 10 g, CaCO3 3 g, MgSO4.7H2O 0.5 g, (NH4)2HPO4 0.5 g, NaCl 3 g, and K2HPO4 1 g, pH 7.5, were found to be the best media for biomass as well as antimicrobial compound production (p < 0.001). Minimum production of biomass and antimicrobial compound was achieved using Czapek-Dox broth (CZB) medium. However, antimicrobial compound production was totally inhibited in the medium potato dextrose broth (PDB), sabouraud broth (SB), and yeast extract malt extract broth (ISP-2). These results suggested that the variation in antimicrobial compound production and biomass production among different media might be the difference in pH and the composition of media.
a Selection of media: 1 Czapek-Dox broth (CZB), 2 glycerol asparagine broth (ISP-5), 3 glucose soybean meal broth (GSB), 4 inorganic salt starch broth (ISP-4), 5 nutrient broth (NB), 6 potato dextrose broth (PDB), 7 sabouraud broth (SB), 8 soybean meal broth (X-media), 9 starch casein nitrate broth (SCNB), 10 yeast extract malt extract broth (ISP-2). b Effect of carbon sources: 1 fructose, 2 glucose, 3 glycerol, 4 lactose, 5 maltose, 6 mannitol, 7 sorbitol, 8 starch, 9 sucrose, 10 xylose. c Effect of nitrogen sources: 1 ammonium nitrate, 2 ammonium sulfate, 3 beef extract, 4 malt extract, 5 peptone, 6 potassium nitrate, 7 sodium nitrate, 8 soybean meal, 9 urea, 10 yeast extract. d Effect of different percent of glucose (w/v) (0.5–8%). e Effect of different percents of soybean meal (w/v) (0.5–8%). f Effect of inoculum volume (v/v) (1–10%). g Effect of pH. h Effect of temperature. i Growth curve and incubation time for optimum production of antimicrobial compound by endophytic Streptomyces parvulus Av-R5. DCW, dry cell weight (values with different alphabet labels are statistically significant at p < 0.001, based on DMRT)
Among the different carbon sources tested, maximum production of biomass and antimicrobial compound by Streptomyces parvulus Av-R5 was found when the isolate was cultivated in the media having glucose as the carbon source. In contrast, low quantity was observed with glycerol, maltose, and mannitol. Although addition of other carbon sources did not inhibit the antibiotic production, the activity was higher with glucose. The nature and amount of nitrogen sources are considered as direct precursors for antibiotic biosynthesis. It was observed that organic nitrogen sources are easily assimilated by Streptomyces parvulus Av-R5 than inorganic nitrogen sources. Soybean meal was found to be the best for biomass and antimicrobial compound production. Antibiotic production was completely inhibited in the presence of peptone. In order to study the optimum concentration of glucose and soybean meal for antimicrobial compound production, Streptomyces parvulus Av-R5 was inoculated in the medium supplemented with different concentrations of glucose and soybean meal. Antibiotic concentration significantly (p < 0.001) increased with increasing the concentration of glucose and soybean meal up to 2%, above which the antibiotic production gradually decreased (Fig. 1d and e). However, in the presence of higher concentrations of glucose and soybean meal (0.5 to 8%), the yield of biomass continuously increased from 5.3 to 9.2 g/L and from 2.8 to 8.2 g/L, respectively. The study of environmental conditions on antimicrobial compound production by Streptomyces parvulus Av-R5 revealed maximum biological activity using 6% seed inoculum at pH 7 on day 8 of incubation at 28 °C. Further increases in the incubation time resulted in gradual decrease in antimicrobial activity (p < 0.001). The reduction in antimicrobial activity after an optimum incubation time in static cultures could be a natural phenomenon related to depletion of nutrients and accumulation of harmful by-products.
The results of response surface methodology (RSM) experiment for studying the effect of independent physical variables on antibiotic synthesis by endophytic Streptomyces parvulus Av-R5 grown on glucose soybean meal broth media showed that the maximum production of antibiotics was achieved at run nos. 7, 10, 19, 20, and 33 when temperature 32.5 °C, pH 7.25, incubation time 9 days, and inoculum size 5.5%, while the minimum production of antibiotic was observed in run no. 21 at temperature 37.0 °C, pH 7.25, incubation time 9 days, and inoculum size 5.5%. The goodness of the model was checked by the coefficient of determination (R2). In the present study, R2 value of 0.98 indicated that the response model can explain 98% of the total variation. The response surface contour plots were employed in order to determine the degree of interaction between environmental factors that have the most significant effect on antibiotic production by Streptomyces parvulus Av-R5 (Fig. 2a). A significant interaction was found between the temperature and inoculum size (p < 0.0018). Maximum production of antibiotic was observed at lower temperatures (between 32.5 and 30.5 °C) and higher inoculums (between 5.5 and 6.0%). Similarly, the decrease in pH (between 7.25 and 7.17) and increase in inoculum size (between 5.50 and 6.08%) gave higher antibiotic production (p < 0.0005). The final optimized environmental conditions obtained after CCD for maximum antibiotic production by Streptomyces parvulus Av-R5 were temperature 31.42 °C, pH 7.19, incubation time 9.44 days, and inoculum size 6.04% (Online Resource 1).
a Contour plot showing significant interaction of physical variables between the following: (1) temperature and inoculum size (p < 0.0018) and (2) pH and inoculum size (p < 0.0005) on actinomycin production by endophytic Streptomyces parvulus Av-R5. b Contour plot showing significant interaction of nutritional variables between the following: (1) glucose and soybean meal (p < 0.0002), (2) glucose and calcium carbonate (p < 0.0451), (3) soybean meal and sodium chloride (p < 0.0048), and (4) calcium carbonate and sodium chloride (p < 0.0348) on actinomycin production by endophytic Streptomyces parvulus Av-R5
The optimized environmental conditions were further maintained at a constant level for the optimization of nutritional conditions for maximum production of antibiotics by Streptomyces parvulus Av-R5. The maximum production of antibiotics was obtained (run no. 22) in medium containing glucose 10.25 g/L, soybean meal 10.25 g/L, calcium carbonate 1.32 g/L, and sodium chloride 10.25 g/L (Online Resource 2), while the minimum production of antibiotics was obtained (run no. 13) in medium containing glucose 11.50 g/L, soybean meal 11.50 g/L, calcium carbonate 1.15 g/L, and sodium chloride 14.00 g/L. In the present study, the coefficient of determination (R2) was found to be 0.93, which indicated that the response model can explain 93% of the total variation. Figure 2b shows the significant interaction between glucose and soybean meal (p < 0.0002). It is clear from the figure that a higher concentration of glucose (between 10.25 and 11.25 g/L) and a lower concentration of soybean meal (between 11.25 and 10.25 g/L) increase the production of antibiotics. Similarly, higher production of antibiotics was observed when the concentration of glucose was found to be 11.25 g/L and calcium carbonate 1.14 g/L (p < 0.0451). Further, increase or decrease in their concentrations resulted in concentration-dependent reduction in the production of antibiotics. The interaction between sodium chloride and soybean meal shows that the lower concentration of soybean meal (between 11.25 and 10.25 g/L) and higher concentration of sodium chloride (between: 10.25 and 11.25 g/L) significantly (p < 0.0048) enhance the production of antibiotics. In addition, higher synthesis of antibiotics was observed when the concentration of calcium carbonate was 1.14 g/L and that of sodium chloride was 11.25 g/L (p < 0.0348). Further, increase or decrease in their concentrations was gradually found to decrease the production. The final optimized medium by CCD for maximum production of antibiotics by Streptomyces parvulus Av-R5 was composed of glucose 11.16 g/L, soybean meal 10.25 g/L, calcium carbonate 1.32 g/L, and sodium chloride 11.18 g/L.
Fermentation and Production of Antimicrobial Compound
The Streptomyces parvulus Av-R5 showed the highest biomass production and antimicrobial activity in glucose soybean meal broth having medium composed of glucose 11.16 g/L, soybean meal 10.25 g/L, sodium chloride 11.18 g/L, and calcium carbonate 1.32 g/L at pH 7.19 at 31.42 °C with 6.04% seed inoculum for 10 days of incubation. Antimicrobial compound and biomass production by the isolate was observed up to 14 days of incubation. The antibiotic production by Streptomyces parvulus Av-R5 in culture broth was begun to start after 2 days and reached maximum on the 10th day of incubation. Further increase in the incubation time was gradually found to decrease the production. However, mycelium growth of isolate gradually increased up to 14 days of incubation. These results suggested that the antibiotic production by Streptomyces parvulus Av-R5 was started at the early logarithmic phase of growth and reached the maximum at the late exponential or stationary phase of growth. From 5 L fermentation broth, red orange-colored active compound (400 mg/L) was obtained after concentration in rotary evaporator at 40 °C.
Purification and Antimicrobial Activity of the Compound Against Multidrug-Resistant Pathogens
Purification and isolation of antibiotics from endophytic Streptomyces parvulus Av-R5 associated with root of Aloe vera are shown in Online Resource 3. Separation of active compound from the crude extract was carried out by thin-layer chromatography. The crude antibiotic was dissolved in ethyl acetate and spotted on TLC plate. Among the different solvent systems used to separate the antibiotic, n-butanol:ethyl acetate:water (v/v 9:9:1) was found to be the best. A single separated band was observed with the Rf value 0.64 (UV active). The active spot is confirmed by testing the compound (1 mg/mL) against multidrug-resistant pathogens. The TLC-purified antimicrobial compound has been applied in the reverse phase HPLC (Shimadzu) to further check the purity of antimicrobial compound. Two compounds with antimicrobial activity were separated by the preparative reverse phase HPLC with the retention times of 10.96 and 6.81 min, respectively (Online Resource 4). The percentages of the first and second compounds were 64.128 and 6.489, respectively. The two major peaks eluted separately, and on further analysis were found to be active against multidrug-resistant gram-positive, gram-negative, and fungal pathogens (Table 1). The purified antibiotic exhibited activity against multidrug-resistant pathogens (resistant to at least three different classes of antibiotics), viz., Staphylococcus aureus JNMC-3, Staphylococcus epidermidis JNMC-4, Klebsiella pneumoniae MTCC-3384, Klebsiella pneumoniae JNMC-6, Pseudomonas aeruginosa MTCC-741, and Proteus vulgaris JNMC-7. In addition, the compound showed antifungal activity against fuconazole- and ketoconazole-resistant Candida albicans MTCC-183 and Aspergillus niger MTCC-872. The minimum bactericidal concentration (MBC) was also determined by subculturing the contents of the tube of MIC showing no growth onto antibiotic-free liquid medium and examining for bacterial growth (Table 1). The MBC values were found to be higher than the MIC against Bacillus cereus MTCC-430, Bacillus subtilis MTCC-441, Staphylococcus aureus MTCC-96, Pseudomonas aeruginosa MTCC-741, Bacillus subtilis JNMC-2, and Staphylococcus epidermidis JNMC-4. As the MBC was higher than the MIC against particular organisms, it is inferred that the compound has bacteriostatic nature against these organisms not bactericidal. The MBC value of the compound against all the tested multidrug-resistant human pathogens was 1 mg/mL. However, the compound showed a higher MBC value (> 1 mg/mL) against Aspergillus niger MTCC-872.
Structure Elucidation and Identification of Antibiotic from Streptomyces sp. Av-R5
Active compounds were isolated from glucose soybean meal broth medium and two bioactive compounds were obtained. Their structures were elucidated by UV-Vis, 1H NMR, 13C NMR, FT-IR, and ESI-MS analyses (Online Resource 5–10). The compounds showed typical UV-Vis spectra with maximum absorbance at 433.5, 263.2, and 201.3 nm, similar to those of known actinomycins. ESI-MS of isolated compounds revealed molecular ion peaks at 1255.60 m/z [M+H] + for compound 1 and 1272.6 m/z [M+H] + for compound 2. The molecular weights of the compounds were identical to those of actinomycin D and actinomycin X0β, respectively. From 1H NMR and 13C NMR and by comparison with the previously reported data of actinomycins [25, 26], the structures of compounds 1 and 2 were confirmed to be actinomycin D and actinomycin X0β (Table 2).
Production of Actinomycin D and Actinomycin X0β by Streptomyces sp. Av-R5
Actinomycin D and actinomycin X0β were detected in crude extracts, and these major components were eluted by HPLC at 10.96 and 6.81 min, respectively. The antibiotics produced were quantified in GSB broth cultures at an interval of 4 days (Fig. 3). The yields of actinomycin D and actinomycin X0β from glucose soybean meal broth medium were 360 mg/L after 4 days of incubation and 400 mg/L after 8 and 12 days of incubation, respectively.
Enzymatic Stability of Antimicrobial Compounds
The effect of enzyme on activity of antimicrobial compound was checked by mixing the antimicrobial compound and enzymes (w/v) (Fig. 4). The inhibition zone diameters were found to be 27.25, 27.00, 26.50, and 25.50 mm for control, lysozyme, proteinase K, and trypsin, respectively. Metabolite treated with enzymes retained 98.82, 96.47, and 91.76% of the antibacterial activity and non-treated (control) retained 100% antibacterial activity when incubated at 37 °C for 1 h. The statistical analysis revealed that treatment with enzymes significantly reduced the antibacterial activity at p < 0.043. Hence, the active metabolite has peptide bonds, that when hydrolyzed lead to the reduction in the residual activity.
Discussion
Endophytic Streptomyces spp. have been studied significantly due to their very high capability of producing novel antibiotics [27, 28]. The metabolic and physiological differences between endophytic Streptomyces spp. and free-living Streptomyces species arising from different conditions in soil to host plants would result in that these endophytic Streptomyces species produce a higher amount of natural products applicable in medicine, agriculture, and industry [6, 29]. In the present study, Streptomyces parvulus NBRC 13193T (AB184326) has been isolated from root of Aloe vera by surface sterilization method on starch casein nitrate agar medium. Streptomyces parvulus as an endophyte has also been reported from stem of the medicinal plant, Dracaena cochinchinensis [30]. Recently, two strains of Streptomyces parvulus NBRC 13193T (99.73%) and Streptomyces parvulus 12811T (100%) have been reported from stem and root parts of the medicinal plant Dracaena cochinchinensis [29]. In this study, Streptomyces parvulus Av-R5 exhibited broad-spectrum antimicrobial activity against multidrug-resistant gram-positive bacteria, viz., Bacillus cereus MTCC-430, Staphylococcus aureus MTCC-96, Bacillus subtilis MTCC-441, Staphylococcus epidermidis MTCC-435, Bacillus cereus JNMC-1, Bacillus subtilis JNMC-2, Staphylococcus aureus JNMC-3, and Staphylococcus epidermidis JNMC-4; gram-negative bacteria, viz., Escherichia coli MTCC-1687, Klebsiella pneumoniae MTCC-3384, Proteus vulgaris MTCC-744, Pseudomonas aeruginosa MTCC-741, Escherichia coli JNMC-5, Klebsiella pneumoniae JNMC-6, and Proteus vulgaris JNMC-7; and fungal pathogens, viz., Candida albicans MTCC-183 and Aspergillus niger MTCC-872. Similar findings have been reported in related studies of endophytic Streptomyces parvulus NBRC 13193T, and Streptomyces parvulus 12811T associated with root of Dracaena cochinchinensis showed remarkable antifungal activity against plant pathogens, viz., Fusarium graminearum, Aspergillus carbonarius, and Aspergillus westerdijkiae [29]. On the basis of broad-spectrum antimicrobial activity of Streptomyces parvulus against plant and human pathogens, we can say that they have formed a symbiotic and beneficial association with plants. Actinomycin D has been reported from endophytic Streptomyces sp. Tc022 associated with root of Alpinia galanga exhibiting strong antifungal activity against Colletotrichum musae and Candida albicans [31]. Similarly, other researchers reported actinomycin D from Streptomyces parvulus (KJ200636.1) associated with Codonopsis lanceolata is a potential antivirulence agent against Staphylococcus aureus infection which significantly inhibited biofilm formation by two methicillin-sensitive Staphylococcus aureus ATCC-25923 and ATCC-6538 and one methicillin-resistant Staphylococcus aureus ATCC-33591 [32]. Difference in the antimicrobial activity of Streptomyces parvulus between similar species with different host tissues might be related with the chemical difference of host plants and the physiological condition of the host plant which trigger the production of antibiotics for the survival of Streptomyces parvulus against pathogens.
Culture medium is a key factor for the growth as well as metabolite production by microorganisms. In the present study, maximum synthesis of actinomycin by endophytic Streptomyces parvulus Av-R5 was found by using glucose soybean meal broth medium. Actinomycin synthesis by Streptomyces parvulus Av-R5 began after the 2nd day and reached a final level on the 10th day. In the present study, on the co-variation between cell growth and antibiotic production test, we found that production of actinomycin by Streptomyces parvulus Av-R5 was started at the early logarithmic phase while most of the antibiotics as secondary metabolites usually will be produced at the late exponential or stationary phase. Different investigators used different fermentation media for the production of actinomycin by various Streptomyces strains, viz., actinomycin synthesis by Streptomyces thermocarboxydus 173998 in tryptone soya broth began after 6 days [14]. Similarly, Streptomyces parvulus DAUFPE 3124 began actinomycin synthesis after 6 days in chemically defined medium containing fructose 30 g/L, soy milk 30 g/L, and calcium carbonate 2 g/L [12]. Streptomyces parvulus AB184326 synthesizes actinomycin in Czapek-Dox broth after 10 days [33]. Similarly, actinomycin synthesis by Streptomyces parvulus RSPSN2 began after 10 days in PM-2 medium (w/v) (g/100 mL) containing yeast extract 0.5 g, dextrose 1.0 g, starch 2 g, casein hydrolysate 0.5 g, ammonium sulfate 0.5 g, and calcium carbonate 0.4 g [34]. Streptomyces parvulus KUAP106 began actinomycin synthesis after 5 days in starch casein nitrate broth [35]. Actinomycin synthesis by Streptomyces avermititis in MPG medium began after 11 days [36]. Further, it was observed that major as well as minor changes in the amount of the medium components could be triggering the synthesis of actinomycin by Streptomyces strains at their logarithmic growth phase.
Actinomycin synthesis by different Streptomyces parvulus strains has been strongly influenced by the carbon and nitrogen sources present in the production medium [14]. Thus, the synthesis of actinomycin by endophytic Streptomyces parvulus Av-R5 has been carried out by incorporation of different carbon and nitrogen sources in the production medium (GSB) at a concentration of 1%. In the present study, the incorporation of glucose, starch, and xylose in the production medium significantly influenced the synthesis of actinomycin and also supported abundant cellular growth of endophytic Streptomyces parvulus Av-R5. It was reasoned, therefore, that these sugars might be consumed rapidly for the production of cell material and would be available in sufficient amount for actinomycin synthesis by the isolate Av-R5. However, other researchers reported that d-fructose enhances the actinomycin synthesis by Streptomyces parvulus, and complete inhibition of actinomycin synthesis by Streptomyces parvulus was observed when glucose and galactose were used as a carbon source [12, 13, 37]. It was observed that the incorporation of soybean meal, sodium nitrate, malt extract, and yeast extract as a nitrogen source was a good source for actinomycin production by endophytic Streptomyces parvulus Av-R5, whereas ammonium nitrate, ammonium sulfate, beef extract, potassium nitrate, and urea was unfavorable. However, actinomycin synthesis by endophytic Streptomyces parvulus Av-R5 was found to be completely inhibited in the presence of peptone. In this study, the combination of glucose and soybean meal was found to be excellent carbon and nitrogen sources for cell growth as well as actinomycin synthesis by Streptomyces parvulus Av-R5. Glutamic acid, phosphates, sodium nitrate, and l-threonine have been reported to induce the synthesis of actinomycin by different species of Streptomyces [38]. The nature and the amount of carbon and nitrogen sources have been considered as a direct precursor for actinomycin D biosynthesis by Streptomyces strains. In the present study, actinomycin D biosynthesis by endophytic Streptomyces parvulus Av-R5 has been achieved when the concentration of glucose and soybean meal is < 2%, whereas higher concentration (>2%) has been found to be excellent for cell growth of Streptomyces parvulus Av-R5. Many researchers reported that a higher concentration of glucose (> 1%) in the production medium repressed the synthesis of actinomycin D by Streptomyces parvulus due to the repression of the phenoxazinone synthase enzyme activity which catalyzes the synthesis of phenoxazinone ring of actinomycin [39]. In the present study, the observations revealed that actinomycin biosynthesis by our strain Av-R5 has been completely inhibited in the presence of peptone. Peptones are the most widely used nitrogen source in microbial media and are made by incubating milk or meat with trypsin, pepsin, or other proteolytic enzymes to digest the protein to a mixture of amino acids, peptides, and polypeptides. Hence, the cleavage of peptide bonds of actinomycin by proteolytic enzymes present in the peptone may lead to the loss of synthesis by Streptomyces parvulus Av-R5.
Response surface methodology helps in evaluation of relationship between the dependent (antibiotic production) variable and independent (medium components) variables. The maximum synthesis of actinomycin by endophytic Streptomyces parvulus Av-R5 was obtained with the media containing glucose 11.16 g/L, soybean meal 10.25 g/L, calcium carbonate 1.32 g/L, and sodium chloride 11.18 g/L at pH 7.19 inoculated with 6.04% seed inoculum and incubated at 31.42 °C for 10 days. In the present study, actinomycin production by Streptomyces parvulus Av-R5 increased with increasing the concentration of glucose and sodium chloride from 10.25 to 11.25 g/L, decreasing the concentration of soybean meal from 11.25 to 10.25 g/L, and increasing the concentration of calcium carbonate from 0.97 to 1.47 g/L, respectively. Above these concentrations, the actinomycin production by Streptomyces parvulus Av-R5 was found to decrease gradually. In addition, the above optimized medium yielded a much higher level of actinomycin (400 mg/L), in comparison to the highest level reported (289 mg/L) for the actinomycin production [12] using Streptomyces parvulus and similar medium components. Media and culture conditions optimized for Streptomyces parvulus Av-R5 in static cultures yielded a much higher level of actinomycin than the reported production of actinomycin optimized for Streptomyces sp. in bioreactors [12, 13]. Actinomycin biosynthesis by Streptomyces strains has been greatly influenced by carbon and nitrogen ratio. The higher yield of actinomycin (87.7 g/L) by Streptomyces parvulus DAUFPE 3124 has been achieved at fructose and l-threonine C/N ratio of 20/3.57 g/L [40]. Similarly, a higher yield of actinomycin by Streptomyces thermocarboxydus 173998 has been achieved at 10 g/L carbon source, viz., starch (305 mg/L), maltose (225 mg/L), and mannitol (223 mg/L); lower yield of actinomycin has been reported in the presence of arabinose (185 mg/L), fructose (192 mg/L), glucose (110 mg/L), raffinose (100 mg/L), glycerol (99 mg/L), sucrose (83 mg/L), and xylose (16 mg/L) [14]. Recently, actinomycin D synthesis by agricultural soil bacteria Streptomyces hydrogenans IB310 has been optimized through response surface methodology. Highest yield of actinomycin D (18.912 mg/L) has been obtained at glycerol 1.949 g/L, oatmeal 2.676 g/L, and tween 80 0.524 g/L [4]. In the present study, optimum temperature between 30.25 and 32.5 °C and pH between 7.00 and 7.25 greatly influenced the cell growth and favor the higher yield of actinomycin synthesis by Streptomyces parvulus Av-R5. It was observed that Streptomyces parvulus Av-R5 from root of Aloe vera under one factor at a time optimized culture condition and central composite design optimization approach resulted in 1.20- and 1.41-fold increases of actinomycin production as compared from un-optimized medium. So, the obtained result from optimization is very useful for improving industrial production of actinomycin.
According to the literature, Streptomyces parvulus has the advantage of producing predominantly actinomycin D [41]. In the present study, Streptomyces parvulus Av-R5 from root of Aloe vera also predominantly produced actinomycin D and actinomycin X0β. In addition, thin-layer chromatography of the antimicrobial compound from Streptomyces parvulus Av-R5 showed a single band when using different solvents. Sousa et al. [12] have reported that Streptomyces parvulus culture supernatant had only a single band for actinomycin D, while that of the other species of Streptomyces showed multiple bands in TLC and produce different unknown compounds. In this study, actinomycin D and actinomycin X0β were separated by HPLC with the retention times 10.96 and 6.81 min with the percentages of 64.128 and 6.489, respectively. Actinomycin D was found to be present in large quantity in the extract of Streptomyces parvulus Av-R5 and possesses antimicrobial activity against multidrug-resistant human pathogens. Actinomycin D can be synthesized by different species of Streptomyces as part of a mixture of several actinomycins with a minor amount of actinomycin X0β and actinomycin X2 [14, 36]. New biosynthetic actinomycin mixtures could be produced when different nitrogen sources and amino acids were added to growing cultures of actinomycin-producing Streptomyces strains [38]. It was observed that metabolites treated with proteolytic enzymes, viz., lysozyme, proteinase K, and trypsin, significantly inhibited antibacterial activity suggesting polypeptide nature of the compound. Polypeptide nature of antibiotics has also been reported in various species of Streptomyces, viz., Streptomyces griseoruber [42], Streptomyces parvulus [37], Streptomyces sindenensis [13], Streptomyces avermititis [36], and Streptomyces flavogriseus [43]. In addition, only a few strains of Streptomyces parvulus have been reported to produce relatively large quantities of actinomycin D, including Streptomyces parvulus GQ451836 (180 mg/L) [33], Streptomyces parvulus DAUFPE 3124 (133 mg/L) [12], Streptomyces parvulus (152 mg/L) [34], and by other species such as Streptomyces griseoruber (210 mg/L) [42], Streptomyces thermocarbodoxydus 173998 (305 mg/L) [14], a mutant strain of Streptomyces sindenensis (850 mg/L) [13], and Streptomyces flavogriseus NJ-4 (960 mg/L) [43]. Whereas, our endophytic Streptomyces parvulus Av-R5 from root of Aloe vera exhibited a unique ability to produce large quantities of actinomycin D and actinomycin X0β with a production value of 360 mg/L after 4 days and 400 mg/L after 10 days in glucose soybean meal broth media, as compared to the similar species of Streptomyces parvulus reported earlier from soil and plants [31, 32].
Actinomycin production by various species and strains of Streptomyces has been studied extensively [36, 44]. Commercially, actinomycin D is produced by Streptomyces parvulus [45]. However, production and optimization of actinomycin by endophytic Streptomyces parvulus associated with medicinal plants have not been reported in literature. Actinomycins are chromophoric peptide antibiotics produced through an oxidative condensation of two molecules of the 3-hydroxy-4-methyl-anthranilic peptide and differences found in the components of actinomycin mixtures are due to the number, arrangements, and kinds of amino acids present in the peptides [36, 38, 44]. Hence, the search of actinomycin from Streptomyces species present inside unique habitats like plants has a great deal of research into the new analogues of actinomycin with unique chemical, physical, and biological characteristics. The present results indicated that the actinomycin D from endophytic Streptomyces parvulus Av-R5 from root of Aloe vera showed extensive inhibition to the growth of Aspergillus niger MTCC-872 and Candida albicans MTCC-183. Antifungal activities of actinomycin D biosynthesized by endophytic Streptomyces parvulus against plant pathogens have been reported earlier [29, 31, 32]. Recently, actinomycin D produced by agricultural soil bacteria Streptomyces hydrogenans IB310 exhibited antagonistic activity against bacterial and fungal phytopathogens, viz., Agrobacterium tumefaciens, Pseudomonas syringae, Xanthomonas campestris, Botrytis allii, Fusarium oxysporum, and Ustilago maydis [4]. As stated in earlier reports, endophytic Streptomyces species present inside the host plants protects the plants against pathogenic microorganisms by the production of antibiotics; in return, the plant provides the chemical nutrients for the growth and survival of endophytic Streptomyces species and influences the metabolite production efficiency of endophytic Streptomyces species. Hence, the possibility of the products from endophytes may be used for biocontrol of the related plant disease.
Conclusion
It is concluded from the present study that endophytic Streptomyces sp. Av-R5 associated with root of Aloe vera has antibiotic potential. Cultural characteristics and nucleotide sequence of the isolate suggested that the isolate was Streptomyces parvulus NBRC 13193T (AB184326). Spectroscopic analysis of antimicrobial compound and significant reduction in the residual activity of active metabolites in the presence of proteolytic enzymes suggested that the active compound has polypeptide nature. In this case, a high yield of actinomycin D and actinomycin X0β (400 mg/L) was achieved with Streptomyces parvulus Av-R5 fermented in glucose soybean meal broth media, which can be used in industrial fermentation processes to obtain high yields. Also, glucose and soybean meal is cheaper and an easily available substrate by organic wastes. Therefore, our result of 400 mg/L antibiotic production using glucose soybean meal broth media is encouraging as a value addition of waste and bioprocess economy. In addition, ethyl acetate extract of actinomycins showed broad-spectrum antimicrobial activity against multidrug-resistant gram-positive and gram-negative bacteria and fungi. Different fermentation conditions for the production of actinomycins by Streptomyces parvulus have been reported from soil sources but the production and optimization of actinomycins from endophytic Streptomyces have not been reported yet. In addition, the actinomycin D produced by our isolate Av-R5 showed extensive inhibition to the growth of fungal pathogens which are reported to be found in the agricultural soil and become phytopathogens; hence, the actinomycin D from endophytic Streptomyces parvulus Av-R5 associated with root of Aloe vera may be used for biocontrol of the related plant diseases.
References
Alanis AJ (2005) Resistance to antibiotics: are we in the post antibiotic era? Arch Med Res 36:697–705
Strobel G, Daisy B, Castillo U, Harper J (2004) Natural products from endophytic microorganisms. J Nat Prod 67:257–268
Qin S, Li J, Chen HH, Zhao GZ, Zhu WY, Jiang CL, Xu LH, Li WJ (2009) Isolation, diversity and antimicrobial activity of rare actinobacteria from medicinal plants of tropical rain forests in Xishuangbanna, China. Appl Environ Microbiol 75:6176–6186
Kulkarni M, Gorthi S, Banerjee G, Chattopadhyay P (2017) Production, characterization and optimization of actinomycin D from Streptomyces hydrogenans IB310 (an antagonistic bacterium against phytopathogens). Biocatal Agric Biotechnol 10:69–74
Igarashi Y (2004) Screening of novel bioactive compounds from plant associated actinomycetes. Actinomycetol 18:63–66
Castillo UF, Strobel GA, Mullenberg K, Condron MM, Teplow DB, Folgiano V, Gallo M, Ferracane R, Mannina L, Viel S, Codde M, Robison R, Porter H, Jensen J (2006) Munumbicins E-4 and E-5: novel broad spectrum antibiotics from Streptomyces NRRL 3052. FEMS Microbiol Lett 255:296–300
Hasegawa T, Lechevalier MP, Lechevalier HA (1978) A new genus of the Actinomycetales, Actinosynnema genus novel. Int J Syst Bacteriol 28:304–310
El-Tarabily KA (2003) An endophytic chitinase producing isolate of Actinoplanes missouriensis with potential for biological control of root rot of lupine caused by Plectosporium tabacinum. Aust J Bot 51:257–266
Coombs JT, Michelsen PP, Franco CMM (2004) Evaluation of endophytic actinobacteria as antagonists of Gaeumannomyces graminis var. tritici in wheat. Biol Control 29:359–366
Cao LX, Qiu ZQ, You JL, Tan HM, Zhou S (2005) Isolation and characterization of endophytic Streptomycete antagonists of Fusarium wilt pathogen from surface sterilized banana roots. FEMS Microbiol Lett 247:147–152
El-Tarabily KA, Nassar AH, Sivasithamparam K (2009) Plant growth promotion and biological control of Pythium aphanidermatum a pathogen of cucumber by endophytic actinomycetes. J Appl Microbiol 106:13–26
Sousa MFVQ, Lopes CE, Pereira NJ (2002) Development of a bioprocess for the production of actinomycin D. Braz J Chem Eng 19:277–285
Praveen V, Tripathi CKM, Bihari V, Srivastava SC (2008) Production of actinomycin D by the mutant of a new isolate of Streptomyces sindenensis. Braz J Microbiol 39:689–692
Hamza AA, Ali HA, Clark BR, Murphy CD, Elobaid EA (2013) Isolation and characterization of actinomycin D producing Streptomyces sp. from Sudanese soil. Afr J Biotechnol 12:2624–2632
Zhu CH, Lu FP, He YN, Han ZL, Du LX (2007) Regulation of avilamycin biosynthesis in Streptomyces viridochromogenes: effects of glucose, ammonium ion and inorganic phosphate. Appl Microbiol Biotechnol 73:1031–1038
Chandrakar S, Gupta AK (2015) Antibiotic potential of endophytic actinomycetes of medicinal herbs against human pathogenic bacteria. Proc Natl Acad Sci, India, Sect B Biol Sci 87:905–915
Shirling EB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16:313–340
Kawato M, Shinobu R (1959) Cover slip culture of Streptomyces herbaricolour nov. sp. supplement; a simple technique for the microscopical observation. Mem Osaka Univ Lib Arts and Educ 8:114–119
Pridham TG, Gottlieb D (1948) The utilization of carbon compounds by some Actinomycetales as an aid for species determination. J Bacteriol 56:107–114
Jeon YS, Lee K, Park SC, Kim BS, Cho YJ, Ha SM (2014) EzEditor: a versatile sequence alignment editor for both rRNA-and protein-coding genes. Int J Syst Evol Microbiol 64:689–691
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Balagurunathan R, Subramanian A (1993) Studies on marine Streptomyces nigrifaciens taxonomy and standardization of antibiotic production. Cienc Mar 19:435–443
Munimbazi C, Bullerman LB (1998) Isolation and partial characterization of antifungal metabolites of Bacillus pumilus. J Appl Microbiol 84:959–968
Carvalho T, Sand SVD (2016) Evaluation of antimicrobial activity of the endophytic actinomycetes R18 (6) against multiresistant gram-negative bacteria. An Acad Bras Cienc 88:155–163. https://doi.org/10.1590/0001-3765201620140655
Solanki R, Kundu A, Das P, Khanna M (2015) Characterization of antimicrobial compounds from Streptomyces sp. World J Pharm Res 4:1626–1641
Zhang X, Ye X, Chai W, Lian XY, Zhang Z (2016) New metabolites and bioactive actinomycins from marine derived Streptomyces sp. ZZ338. Mar Drugs 14:181–189
Gos FMWR, Savi DC, Shaaban KA, Thorson JA, Aluizio R, Possiede YM, Rohr J, Glienke C (2017) Antibacterial activity of endophytic actinomycetes isolated from the medicinal plant Vochysia divergens (Pantanal, Brazil). Front Microbiol 8. https://doi.org/10.3389/fmicb.2017.01642
Zhao S, Liu C, Zheng W, Ma Z, Cao T, Zhao J, Yan K, Xiang W, Wang X (2017) Micromonospora parathelypteridis sp. nov., an endophytic actinomycetes with antifungal activity isolated from the root of Parathelypteris beddomei (Bak.) Ching. Int J Syst Evol Microbiol 67:268–274
Salam N, Khieu TN, Liu MJ, Vu TT, Ky SC, Quach NT, Phi QT, Narsing Rao MP, Fontana A, Sarter S, Li WJ (2017) Endophytic actinobacteria associated with Dracaena cochinchinensis Lour: isolation, diversity and their cytotoxic activities. Biomed Res Int 2017:1–11. https://doi.org/10.1155/2017/1308563
Khieu TN, Liu MJ, Nimaichand S, Quach NT, Ky SC, Ph QT, Vu TT, Nguyen TD, Xiong Z, Prabhu DM, Li WJ (2015) Characterization and evaluation of antimicrobial and cytotoxic effects of Streptomyces sp. HUST012 isolated from medicinal plant Dracaena cochinchinensis Lour. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.00574
Taechowisan T, Wanbanjob A, Tuntiwachwuttikul P, Taylor WC (2006) Identification of Streptomyces sp. Tc022, an endophyte in Alpinia galanga, and the isolation of actinomycin D. Ann Microbiol 56:113–117
Lee JH, Kim YG, Lee K, Kim CJ, Park DJ, Ju Y, Lee JC, Wood TK, Lee J (2016) Streptomyces-derived actinomycin D inhibits biofilm formation by Staphylococcus aureus and its hemolytic activity. Biofoul 32:45–56
Rahman MA, Islam MZ, Khondkar P, Islam MA (2010) Characterization and antimicrobial activities of a polypeptide antibiotic isolated from a new strain of Streptomyces parvulus. Bangladesh Pharm J 13:14–17
Shetty PR, Buddana SK, Tatipamula VB, Naga VVV, Ahmad J (2014) Production of polypeptide antibiotic from Streptomyces parvulus and its antibacterial activity. Braz J Microbiol 45:303–312
Usha R, Ananthaselvi P, Venil CK, Palaniswamy M (2010) Antimicrobial and antiangiogenesis activity of Streptomyces parvulus KUAP106 from mangrove soil. Eur J Biol Sci 2:77–83
Chen C, Song F, Wang Q, Abdel-Mageed WM, Guo H, Fu C, Hou W, Dai H, Liu X, Yang N, Xie F, Yu K, Chen R, Zhang L (2012) A marine-derived Streptomyces sp. MS449 produces high yield of actinomycin X2 and actinomycin D with potent anti-tuberculosis activity. Appl Microbiol Biotechnol 95:919–927. https://doi.org/10.1007/s00253-012-4079-z
Williams WK, Katz E (1977) Development of a chemically defined medium for the synthesis of actinomycin-D by Streptomyces parvulus. Antimicrob Agents Chemother 11:281–290
Katz E, Pienta P, Sivak A (1956) The role of nutrition in the synthesis of actinomycin. Appl Microbiol 6:236–241
Gallo M, Katz E (1972) Regulation of secondary metabolite biosynthesis catabolic repression of phenoxazinone synthase and actinomycin formation by glucose. J Bacteriol 109:659–667
Sousa MFVQ, Lopes CE, Junior NP (2001) A chemically defined medium for production of actinomycin D by Streptomyces parvulus. Braz Arch Biol Technol 44:227–231
Meienhofer J, Atherton E (1973) Structure activity relationship in the actinomycins. Adv Appl Microbiol 16:203–300
Praveen V, Tripathi CKM (2009) Studies on the production of actinomycin-D by Streptomyces griseoruber—a novel source. Lett Appl Microbiol 49:450–455
Wei Z, Xu C, Wang J, Lu F, Bie X, Lu Z (2017) Identification and characterization of Streptomyces flavogriseus NJ-4 as a novel producer of actinomycin D and holomycin. Peer J 5:e3601. https://doi.org/10.7717/peerj.3601
Praveen V, Tripathi CKM, Bihari V (2008) Studies on optimum fermentation conditions for actinomycin D production by two new strains of Streptomyces spp. Med Chem Res 17:114–122
Kurosawa K, Bui VP, Van EJL, Willis LB, Lessard PA, Ghiviriga I, Sambandan TG, Rha CK, Sinskey AJ (2005) Characterization of Streptomyces MITKK-103, a newly isolated actinomycin X2 producer. Appl Microbiol Biotechnol 72:145–154
Acknowledgements
Authors are thankful to the Head, SLS, PRSU, Raipur, for providing the necessary facilities for the research. The authors also thank Microbial Type Culture Collection and Gene Bank (MTCC), Chandigarh for providing cultures and Pandit Jawaharlal Nehru Medical College, Raipur, Chhattisgarh for providing clinical cultures for research.
Funding
This study was funded by University Grant Commission, New Delhi, India (F.7-145/2007BSR) and granted financial support from DST, New Delhi under FIST program and UGC, New Delhi for DRS-SAP III.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic Supplementary Material
ESM 1
(DOCX 1891 kb)
Rights and permissions
About this article
Cite this article
Chandrakar, S., Gupta, A.K. Actinomycin-Producing Endophytic Streptomyces parvulus Associated with Root of Aloe vera and Optimization of Conditions for Antibiotic Production. Probiotics & Antimicro. Prot. 11, 1055–1069 (2019). https://doi.org/10.1007/s12602-018-9451-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-018-9451-6