Abstract
In recent years, Dactylopius opuntiae (Cockerell) (Hemiptera: Dactylopiidae) has become an increasing threat to the cultivation of prickly pear crops in Morocco. To control this harmful insect scale pest, biological control is usually accompanied by chemical control applications. In this context, the use of some insecticides can alter the numerical response of beneficial organisms (predators) associated with this cochineal. In this study, we investigated the effect of the residues of some insecticides «(d-limonene (60 g/L) applied at 100 mL/hL, mineral oil (780 g/L) at 2000 mL/hL, potassium salts of fatty acid (500 g/L) at 40 mL/hL, pyriproxyfen (100 g/L) at 25 mL/hL, and potassium salts of fatty acid (C7-C18) (500 g/L) at 300 mL/hL)» on the numerical response of Cryptolameus montrouzieri (Mulsant) (Coleoptera: Coccinellidae) feeding on D. opuntiae females under laboratory conditions. The sublethal concentration residues of all tested insecticides did not have lethal effects on Cryptolaemus montrouzieri females. D-limonene (Efficiency of Conversion of Ingested food (E.C.I) = 1371.85), mineral oil (E.C.I = 1383.06), potassium salts of fatty acid (E.C.I = 1583.24), and pyriproxyfen (E.C.I = 987.13) were the most compatible with the predator C. montrouzieri, as they did not significantly affect the number of eggs laid by the females compared to the untreated control. Potassium salt of fatty acid (C7–C18) (500 g/L) (E.C.I = 905.93) was the least compatible with C. montrouzieri, as it led to a significant reduction in the number of eggs laid by predatory females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, D. opuntiae (Cockerell) (Hemiptera: Dactylopiidae) caused damage to Opuntia spp. cactus crops in Morocco and many countries worldwide. The pest was first detected in Morocco in 2014 during sampling in the cactus crop (Bouharroud et al., 2016) in the Sidi Bennour region (north-west of Marrakech). Today, the cochineal has spread to other regions of the country (e.g. Abda, Doukkala, Chaouia, Rhamna, Youssoufia, Benguerir, Abda, Azilal, Benimellal, Taourirt, Haouz, and Sous-Nord-Meknès-Gharb) where kilometers of cactus are totally destroyed with heavy ecological and economic losses (El Aalaoui et al., 2019).
Natural enemies associated with D. opuntiae and other Dactylopiidae species comprise only predators (Baskaran et al., 1999; Adalma-Aguilera et al., 2005; Vanegas-Rico et al., 2010; Lima et al., 2011; Castro et al., 2012; Barbosa et al., 2014; Giorgi et al., 2017), including Coleoptera (Coccinellidae), Diptera, and Lepidoptera. For the control of D. opuntiae, biological control agents are usually accompanied by chemical control applications (El Aalaoui et al., 2019; Yousef-Yousef & Quesada-Moraga, 2020). The effect of insecticides on biological control agents is considered in integrated pest management (IPM) programs worldwide (Martinou & Stavrinides, 2015). Biological control agents are often more sensitive to insecticides compared to other insects (Khan et al., 2012), and the use of pesticides compatible and safe to natural enemies is recommended. In this context, many studies have reported sublethal effects of some pesticides that can affect biological and reproductive parameters and the behavior of predators (Sahito et al., 2011; Halappa et al., 2013; Planes et al., 2013; Anjitha et al., 2013; Wanumen et al., 2016; Xiao et al., 2016; Nawaz et al., 2017). The predatory potential of a predator can be represented by two components: numerical response and functional response. A functional response is defined by the number of prey attacked per predator as a function of prey density (Solomon, 1949; Holling, 1959; Li et al., 2006; Ambrose et al., 2010; He et al., 2012; El Aalaoui et al., 2020), and a numerical response is defined by the number of progeny in relation to increasing prey density (Solomon, 1949; Ofuya & Akingbohungbe, 1988; Omkar & Pervez, 2004). The study of numerical response under laboratory and field conditions is monitored for conclusive estimation of the biocontrol potential of each predator. It is evident that a part of the energy derived from the prey biomass consumed is converted into egg production, and the rest is lost as the metabolic costs of food conversion and respiration to maintain life (Baumgärtner et al., 1987).
Many studies regarding the numerical response of predators to offered prey have been carried out worldwide. Hippodamia tredecimpunctata tibialis Says showed a greater numerical response than Coleomagilla maculata lengi Timberlake at high densities of Rhopalosiphum maidis (Fitch) due to higher fecundity, with both predators exhibiting a linear response by oviposition (Wright & Laing, 1980). The same trend was obtained for Scymnus levaillanti (Mulsant) at a lower density of prey Aphis gossypii (Glover) (Hemiptera: Aphididae) (Uygun & Atlihan, 2000). However, a curvilinear relationship was also observed between the density of A. gossypii and the number of eggs laid by Propylea dissecta (Omkar & Pervez, 2004).
Many factors can affect egg production, such as prey density, temporary prey isolation (Evans & Dixon, 1986), body size (Agarwala & Bardhanroy, 1999), pesticides (Li et al., 2006; Ambrose et al., 2010; He et al., 2012; Malaquias et al., 2014; Martinou & Stavrinides, 2015), intraguild predation (Martinou et al., 2010), mutual interference, and cannibalism (Chong & Oetting, 2006). However, the effects of pesticides on the numerical responses of many important natural enemies have not been investigated.
Recently, the role of Cryptolameus montrouzieri (Mulsant) (Coleoptera: Coccinellidae) as a biological control agent against D. opuntiae is expected to gain further importance. This lady beetle was introduced into Brazil for the biological control of D. opuntiae and citrus mealybugs (Sanches & Carvalho, 2010). In northern Israel, releases of Cryptolaemus montrouzieri (approximately 100.000 individuals) were not successful in managing the rapid and early outbreak of the cochineal D. opuntiae (Protasov et al., 2017) but according to Mendel et al. (2018), the beetle C. montrouzieri significantly reduced the cochineal populations on cactus along the coast in Israel. More recently, C. montrouzieri was introduced into Morocco to control D. opuntiae, and laboratory studies have shown positive results for the successful development, reproduction, and predation of D. opuntiae (El Aalaoui et al., 2019).
In the present study, we investigated the side effects of some insecticides on the numerical response parameters of C. montrouzieri feeding on D. opuntiae.
Materials and methods
Cryptolameus montrouzieri mature females used in this study were obtained from a colony established with adults imported by the Entomology laboratory of INRA, Morocco (National Institute of Agricultural Research). Adults were placed in entomological cages (80 × 80 × 80 cm) made of a wooden frame covered by a mesh fabric to allow ventilation under laboratory conditions at 26 ± 2 °C, 60 ± 10% RH, and a photoperiod of 12:12 (Light:Dark) h and were provided with Opuntia ficus-indica (L.) (Miller 1768) cladodes infested by D. opuntiae collected from the fields of Zemamra, Morocco (32°37′48“ N, 8°42’0” W) as a source of nutrition and oviposition support (El Aalaoui et al., 2019). Access to water was provided via a cotton wick inserted into a 25 ml glass vial filled with water. The tested insecticides were d-limonene (60 g/L) at 100 mL/hL, mineral oil (780 g/L) at 2000 mL/hL, potassium salts of fatty acid (500 g/L) at 40 mL/hL, pyriproxyfen (100 g/L) at 25 mL/hL, and potassium salts of fatty acid (500 g/L) (C7–C18) at 300 mL/hL; all tested insecticides are authorized for use by the National Plant Protection Organization (NPPO) named ONSSA in Morocco for the control of D. opuntiae. The insecticides had different modes of action and exhibited a high mortality against D. opuntiae nymphs, and d-limonene (60 g/L) and mineral oil (780 g/L) had high mortality against both nymphs and adult females of the scale pest (D. opuntiae) under field conditions (El Aalaoui et al., 2019; El Aalaoui & Sbaghi, 2022). Table 1 shows the characteristics of the insecticides used. The rates of each insecticide used in the current study were sublethal, as they did not cause short-term mortality in C. montrouzieri females (El Aalaoui et al., 2019; El Aalaoui & Sbaghi, 2022). A determined amount of each treatment was poured in a liter of water, and sprayed as a mist over Petri dishes (9.5 cm in diameter), and their lids cover using a Potter spray tower (Burkard Scientifc Ltd., Uxbridge, UK) at a rate of 1 ml of pesticide solution, which resulted in a spray deposit of 2.55 mg cm−2 similar to that recommended for bioassays according to the IOBC Working Group “Pesticides and Beneficial Organisms” (Candolfi et al., 2001; Martinou & Stavrinides, 2015). We used Petri dishes in this study as a support because the use of living plants (cladodes) could affect prey consumption and act as a confounding factor on numerical response modeling (Martinou & Stavrinides, 2015). Control Petri dishes were sprayed with tap water. The predator C. montrouzieri and D. opuntiae females were not sprayed. After spraying, the Petri dishes and their lids were allowed to dry out for 24 h under laboratory conditions, and then a fixed number of D. opuntiae adult females (5 days old) were introduced into each Petri dish at the following densities: 1, 5, 10, 15, 20, and 25 with a paint brush. An individual C. montrouzieri female (13 days old) was transferred to each Petri dish after mating with sexually mature male ladybeetles and was allowed to forage for 24 h, after which it was removed. The consumed D. opuntiae adult females and the number of eggs laid by each female were recorded. Ten replicates of each prey density for each insecticide treatment tested were used, and all experiments were repeated three times. The Efficiency of Conversion of Ingested food (E.C.I) (in number) into egg biomass (in number) was calculated as the number of eggs laid × 100 / number of prey consumed at different prey densities (Omkar, 2004). The data on oviposition, number of prey consumed, and E.C.I at different prey densities were subjected to analysis using Tukey’s LSD test (p ≤ 0.0001) with the software package SPSS ver. 18.0 (Carver & Nash, 2011).
Results
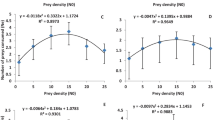
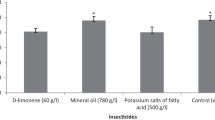
For all insecticides and controls tested in this study, the number of eggs laid by C. montrouzieri females (Fig. 1) was significantly different among treatments (F = 20.824, df = 5, p < 0.0001). The number of eggs laid was significantly higher in the case of the control (21.96), d-limonene (21.26), mineral oil (22.22), potassium salts of fatty acid (22.30), and pyriproxyfen (21.04) treatments and lower in the case of potassium salts of fatty acid (C7–C18) (14.81) treatment. The predatory C. montrouzieri females laid a maximum number of eggs at the highest prey density (25), and a minimum at the lowest prey density (1). The insecticide treatments tested did not alter the shape of the numerical response curve compared to the control treatment (tap water). Additionally, the number of prey consumed by C. montrouzieri females at different prey densities varied significantly with insecticide treatments (F = 23.454, df = 5, p < 0.0001). The number of prey consumed was significantly higher in the case of pyriproxyfen treatment (2.30) compared to the control and the other insecticide treatments tested (Table 2). The E.C.I value was significantly higher (F = 21.714, df = 5, p ≤ 0.0001) in the case of potassium salts of fatty acid (1583.24), d-limonene (1371.85), and mineral oil (1383.06) treatments and lowest in the case of potassium salts of fatty acid (C7–C18) (905.93) and pyriproxyfen (987.13) treatments (Table 2). During the observation period (24 hours), no predator female mortality was recorded in any tested treatment.
Numerical response models of Cryptolaemus montrouzieri for the five insecticide treatments and the control. A D-limonene (60 g/L), D pyriproxyfen (100 g/L), B mineral oil (780 g/L), E Potassium salts of fatty acid (C7-C18) (500 g/L), C potassium salts of fatty acid (500 g/L), F control (tap water). The error bar represents the standard deviation
Discussion
For all insecticide treatments tested, the number of eggs laid by C. montrouzieri females increased significantly with increasing prey density (P < 0.0001). There was a quick and significant increase in oviposition between densities 1 and 15 compared to that between densities 15 and 25, probably because of the waxy cotton produced by D. opuntiae females that makes C. montrouzieri females attractive for oviposition. Vanegas-Rico et al. (2016) reported that wax from D. opuntiae adults dried at 50 °C for three days rendered Hyperaspis trifurcata Schaeffer (Coleoptera: Coccinellidae) females attractive for oviposition. The insecticide treatments tested did not alter the curvilinear shape of the numerical response curve compared to the control treatment (tap water). In the same context, a curvilinear relationship was reported between the density of A. gossypii and the number of eggs laid by Propylea dissecta (Mulsant) (Coleoptera: Coccinellidae) (Omkar & Pervez, 2004) and between the density of Aphis fabae (Scopoli) (Hemiptera: Aphididae) and the number of eggs laid by Scymnus syriacus (Marseul) (Coleoptera: Coccinellidae) (Sabaghi et al., 2011). Additionally, the prey density-dependent fecundity curve was sigmoidal for Cheilomenes lunata (Fabricius) (Coleoptera: Coccinellidae) (Ofuya & Akingbohungbe, 1988), curvilinear for Cheilomenes sexmaculata (Fabricius) (Coleoptera: Coccinellidae) feeding on the black bean aphid, A. fabae (Agarwala & Bardhanroy, 1997) and even for Lysiphlebus fabarum (Marshall) (Hymenoptera: Aphidiidae) parasitizing the same pest (A. fabae) (Mahmoudi et al., 2010), linear for Hippodamia tredecimpunctata and C. maculate feeding on R. maidis (Wright & Laing, 1980), and even for S. levaillanti feeding on A. gossypii (Uygun & Atlihan, 2000). D-limonene, mineral oil, potassium salts of fatty acid, and pyriproxyfen was the most compatible with the predator C. montrouzieri in comparison to potassium salts of fatty acid (C7–C18), as they did not significantly affect the number of eggs laid by the females compared to the control treatment (tap water). The toxic effect of d-limonene on pests was initiated by Taylor and Vickery (1974) as a plant insecticide that acts as a reproductive inhibitor and growth regulator for many insect species (Karr, 1989); however, the work of Brennan et al. (2013) reported that d-limonene causes chitin degradation in pests. Mineral oils are known for their use in agriculture against several pests on a range of crops. They block the spiracles of pest adults and nymphs while preventing gas exchange in the eggs, which causes their asphyxiation and death (Cranshaw & Baxendale, 2011; Helmy et al., 2012). The potassium salts of fatty acids, which are contact agents, act by penetrating the integument of arthropods, obstructing cell membranes, and causing dehydration and death (Tsolakis & Ragusa, 2008). Additionally, the use of pyriproxyfen, which is an analog of the insect juvenile hormone, results in the marked suppression of insect metamorphosis, embryogenesis, and adult development (Rimoldi et al., 2017). However, pyriproxyfen’s mode of action is more specific and depends on the presence of insecticide-specific endocrine receptors in embryos developed within eggs (Sullivan & Goh, 2008). All these insecticides do not have lethal effects on C. montrouzieri and do not significantly affect its predation potential (consumption) (El Aalaoui & Sbaghi, 2022).
The results of the number of prey consumed by C. montrouzieri females in relation to insecticide treatments tested were not similar to those of the numerical response study since the number of prey consumed was significantly higher for pyriproxyfen and lower for potassium salts of fatty acid, d-limonene, and potassium salts of fatty acid (C7-C18) (Table 2). We can explain these variations by the possibility that the hungry ladybird could entirely consume the first few preys they encounter and use the following prey with progressively reduced voracity (Hodek & Honek, 1996). Additionally, it is evident that a part of the energy derived from the prey biomass consumed was converted into egg production, and the rest was lost as the metabolic costs of food conversion and respiration to maintain life (Baumgärtner et al., 1987). In addition, at the time of oviposition, we observed a depression in C. montrouzieri females, possibly due to the high concentration of carminic acid in the bodies of consumed D. opuntiae females, which makes C.montrouzieri females suffer and spend a lot of time laying eggs. In addition to pesticides, many other factors can affect egg production, such as prey density, temporary prey isolation (Evans & Dixon, 1986), body size (Agarwala & Bardhanroy, 1999), intraguild predation, mutual interference, and cannibalism (Chong & Oetting, 2006; Martinou et al., 2010).
E.C.I values were significantly higher for potassium salts of fatty acid, mineral oil, and d-limonene treatments and lower for potassium salts of fatty acid (C7–C18), pyriproxyfen, and control treatments. The lower E.C.I. values recorded for some insecticide treatments may be explained by the fact that the predatory C. montrouzieri females survive under these treatments, spending much of the energy derived from consumed prey biomass on maintenance and metabolic costs, which may have a negative effect on the number of eggs laid (Omkar & Pervez, 2004). Our results indicate that d-limonene, mineral oil, potassium salts of fatty acid, pyriproxyfen, and potassium salts of fatty acid (C7–C18) residues do not have lethal effects on C. montrouzieri females.
Conclusion
The results of the effect of the residues of some insecticides on the numerical response of C. montrouzieri in the current study showed that the predator was compatible with some and least compatible with other insecticide treatments. D-limonene (60 g/L), mineral oil (780 g/L), potassium salts of fatty acid (500 g/L), and pyriproxyfen (100 g/L) were the most compatible with the predator C. montrouzieri, in contrast to potassium salt of fatty acid (C7–C18) (500 g/L), as they do not significantly affect the number of eggs laid by females. Further open-field studies are needed to confirm the results obtained in the present study under controlled laboratory conditions. Indeed, many biotic and abiotic factors, as well as pesticides and other factors, can affect the establishment and reproduction of predators under field conditions.
Data availability
Not applicable.
References
Adalma-Aguilera, C., Llanderal-Cãzares, C., Soto-Hernández, M., & Castillo-Márquez, L. E. (2005). Producción de granacochinilla (Dactylopius coccus Costa) em plantas de nopal a la intempérie y em microtúneles. Agrociencia, 39, 161–171.
Agarwala, B. K., & Bardhanroy, P. (1997). Oviposition behavior and reproduction efficiency in ladybeetles (Coccinellidae: Coleoptera). A case study of Menochilus sexmaculata (Fabricus). Journal of Aphidology, 11, 49–59.
Agarwala, B. K., & Bardhanroy, P. (1999). Numerical response of ladybird beetles (Coccinellidae: Coleoptera) to aphid prey (Homoptera: Aphididae) in a field bean in Northeast India. Journal of Applied Entomology, 123, 401–405.
Ambrose, D. P., Rajan, S. J., & Raja, J. M. (2010). Impacts of Synergy-505 on the functional response and behavior of the reduviid bug, Rhynocoris marginatus. Journal of Insect Science, 10(1), 187. https://doi.org/10.1673/031.010.18701
Anjitha, A., Krishnamoorthy, S. V., & Kuttalam, S. (2013). Toxicity of insecticides to the coccinellid predators, Cryptolaemus montrouzieri Mulsant and Scymnus coccivora Ayyar of papaya mealybug, Paracoccus marginatus Williams and Granara de Willink. Journal of Biological Control, 27(1), 18–23.
Barbosa, P. R. R., Oliveira, M. D., Giorgi, J. A., Silva-Torres, C. S. A., & Torres, J. B. (2014). Predatory behavior and life history of Tenuisvalvae notata (Coleoptera: Coccinellidae) under variable prey availability conditions. The Fla Entomol, 97, 1026–1034.
Baskaran, R. K. M., Lakshmi, L. G., & Uthamasamy, S. (1999). Comparative biology and predatory potential of Australian ladybird beetle (Cryptolaemus montrouzieri) on Planococcus citri and Dactylopius tomentosus. Indian Journal of Agricultural Sciences, 69, 605–606.
Baumgärtner, J., Bieri, M., & Delucchi, V. (1987). Growth and development of immature life stages of Propylea 14 punctata L. and Coccinella 7-punctata L. (Coleoptera: Coccinellidae) simulated by the metabolic pool model. Entomophaga, 32, 415–423.
Bouharroud, R., Amarraque, A., & Qessaoui, R. (2016). First report of the Opuntia Cochineal scale Dactylopius opuntiae (Hemiptera : Dactylopiidae) in Morocco. EPPO Bull, 46(2), 308–310.
Brennan, T. C. R., Kromer, J. O., & Nielsen, L. K. (2013). Physiological and transcriptional responses of Saccharomyces cerevisiae to d-limonene show changes to the cell wall but not to the plasma membrane. Appl Environ Microbiol, 79, 3590–3600.
Candolfi, M. P., Barrett, K. L., Campbell, P., Forster, R., Grandy, N., & Huet, M. C. (2001). Guidance document on regulatory testing and risk assessment procedures for plant protection products with non-target arthropods. European standard characteristics of regulatory testing (ESCORT 2) workshop (pp. 21–23). SETAC Europe.
Carver, R. H., & Nash, J. G. (2011). Doing data analysis with SPSS: Version 18.0. Cengage Learning.
Castro, R. M., Barros, R., Paranhos, B. A., Gava, C. A., Fernandes, M. H., Garziera, L., Siqueira, M. C. (2012). Exigências térmicas de Zagreus bimaculosus (Mulsant) (Coleoptera : Coccinellidae). In Embrapa Semiárido-Resumo em anais de congresso (ALICE). In: CONGRESSO BRASILEIRO DE ENTOMOLOGIA, 14., 2012, Curitiba. SEB-40 anos de avanços da Ciência Entomológica Brasileira. Curitiba: SEB, 2012.
Chong, J. H., & Oetting, R. D. (2006). Functional response and progeny production of the Madeira mealybug parasitoid, Anagyrus sp. nov. nr. sinope: The effects of host and parasitoid densities. Biological Control, 39, 320–328.
Cranshaw, W. S., Baxendale, B. (2011). Insect Control: Horticultural Oils. Colorado State Univirsity, http://www.ext.colostate.edu/pubs/insect/05569.html. Accessed 3 May 2017.
El Aalaoui, M., & Sbaghi, M. (2022). Effects of sublethal concentrations of some biorational insecticides in predation potential of Cryptolaemus montrouzieri on Dactylopius opuntiae. International Journal of Tropical Insect Science, 42(1), 519–526.
El Aalaoui, M., Bouharroud, R., Sbaghi, M., El Bouhssini, M., Hilali, L., & Dari, K. (2019). Comparative toxicity of different chemical and biological insecticides against the scale insect Dactylopius opuntiae and their side effects on the predator Cryptolaemus montrouzieri. Archives of Phytopathology and Plant Protection, 52(1-2), 155–169.
El Aalaoui, M., Bouharroud, R., Sbaghi, M., El Bouhssini, M., & Hilali, L. (2020). Functional response and predation potential of Hyperaspis campestris (Herbst 1783)(Coleoptera: Coccinellidae) on Opuntiae Cochineal Dactylopius opuntiae (Hemiptera: Dactylopiidae) in Morocco. Test Engineering & Management, 82, 5976–5985.
Evans, E. W., & Dixon, A. F. G. (1986). Cues for oviposition by ladybird beetles (Coccinellidae): Response to aphids. The Journal of Animal Ecology, 55, 1027–1034.
Giorgi, J. A., Barbosa, P. R. R., Oliveira, J. E. M., & Torres, J. B. (2017). Prodiloides bipunctata Weise (Coccinellidae : Cephaloscymnini): New research on native natural predators of the carmine cochineal, Dactylopius opuntiae (cockerel) (Hemiptera: Dactylopiidae) in the Brazilian semi-arid. The Coleopterists Bulletin, 72(3), 562–564.
Halappa, B., Awaknavar, J. S., Archana, D., Bandi, S., & Kumar, G. A. (2013). Laboratory evaluation of insecticides against australian beetle, Cryptolaemus montrouzieri Mulsant (Coccinellidae: Coleoptera). Current Biotica, 7(3), 196–201.
He, Y., Zhao, J., Zheng, Y., Desneux, N., & Wu, K. (2012). Lethal effect of imidacloprid on the coccinellid predator Serangium japonicum and sublethal effects on predator voracity and on functional response to the whitefly Bemisia tabaci. Ecotoxicol, 21(5), 1291–1300.
Helmy, E. I., Kwaiz, F. A., & El-Sahn, O. M. N. (2012). The usage of mineral oils to control insects. Egyptian Academic Journal of Biological Sciences (A. Entomology), 5(3), 167–174.
Hodek, I., & Honek, A. (1996). Ecology of Coccinellidae (p. 464). Kluwer Academic Publishers.
Holling, C. S. (1959). Some characteristics of simple types of predation and parasitism. Canadian Entomologist, 91, 385–398.
Karr L. L. (1989). Toxic properties of d-limonene in insects and the earthworm Eisenia fetida. Dissertations, University of Iowa, Ames, IA (US).
Khan, H. A. A., Sayyed, A. H., Akram, W., Raza, S., & Ali, M. (2012). The predatory potential of Chrysoperla carnea and Cryptolaemus montrouzieri larvae on different stages of the mealybug, Phenacoccus solenopsis: A threat to cotton in South Asia. Journal of Insect Science, 12, 147.
Li, D. X., Tian, J., & Shen, Z. R. (2006). Effects of pesticides on the functional response of predatory thrips, Scolothrips takahashii to Tetranychus viennensis. Journal of Applied Entomology, 130(5), 314–322.
Lima, M. S., Silva, M. P., Ferreira, W. M., Silva, L. D., & Paranhos, B. A. J. (2011). Predadores associados a Dactylopius opuntiae (Hemiptera: Dactylopiidae) em palma forrageira no estado de Pernambuco, Brasil. Revista Chilena de Entomologia, 36, 51–54.
Mahmoudi, M., Sahragard, A., & Jalali Sendi, J. (2010). Foraging efficiency of Lysiphlebus fabarum Marshall (Hymenoptera: Aphidiidae) parasitizing the black bean aphid, Aphis fabae Scopoli (Hemiptera: Aphididae), under laboratory conditions. Journal of Asia-Pacific Entomology, 13, 111–116.
Malaquias, J. B., Ramalho, F. S., Omoto, C., Godoy, W. A. C., & Silveira, R. F. (2014). Imidacloprid affects the functional response of predator Podisus nigrispinus (Dallas) (Heteroptera: Pentatomidae) to strains of Spodoptera frugiperda (J.E. Smith) on Bt cotton. Ecotoxicol, 23, 192–200.
Martinou, A. F., & Stavrinides, M. C. (2015). Effects of sublethal concentrations of insecticides on the functional response of two Mirid generalist predators. PLoS One, 10(12), e0144413.
Martinou, A. F., Raymond, B., Milonas, P. G., & Wright, D. J. (2010). Impact of intraguild predation on parasitoid foraging behaviour. Ecological Entomology, 35, 183–189.
Mendel, Z., Protasov, A., Carvalho, C.J., Vanegas, J.M., Refugio Lomeli, F., Refugio Leyva, E. (2018). Biological control possibilities of an invasive scale insect in Israel: opuntia cochineal scale insect Dactylopius opuntiae. In XI European Congress of Entomology, Book of Abstracts (p. 40). 2–6 July 2018, Napoli, Italy.
Nawaz, M., Cai, W., Jing, Z., Zhou, X., Mabubu, J. I., & Hua, H. (2017). Toxicity and sublethal effects of chlorantraniliprole on the development and fecundity of a non-specific predator, the multicolored Asian lady beetle, Harmonia axyridis (Pallas). Chemosphere, 178, 496–503.
Ofuya, T. I., & Akingbohungbe, A. E. (1988). Functional and numerical responses of Cheilomenes lunata (Fabricius) (Coleoptera: Coccinellidae) feeding on cowpea aphid, Aphis craccivora Koch (Homoptera: Aphididae). International Journal of Tropical Insect Science, 9(4), 543–546.
Omkar, & Pervez, A. (2004). Functional and numerical responses of Propylea dissecta (Mulsant) (Col., Coccinellidae). Journal of Applied Entomology, 128, 140–146.
Planes, L., Catalán, J., Tena, A., Porcuna, J. L., Jacas, J. A., Izquierdo, J., & Urbaneja, A. (2013). Lethal and sublethal effects of spirotetramat on the mealybug destroyer, Cryptolaemus montrouzieri. Journal of Pest Science, 86(2), 321–327.
Protasov, A., Mendel, Z., Spodek, M., & Carvalho, C. J. (2017). Management of the Opuntia cochineal scale insect, Dactylopius opuntiae (Cockerell) in Israel. Alon Hanotea, 71, 48–51.
Rimoldi, F., Fogel, M. N., Ronco, A. E., & Schneider, M. I. (2017). Comparative susceptibility of two Neotropical predators, Eriopis connexa and Chrysoperla externa, to acetamiprid and pyriproxyfen: Short and long-term effects after egg exposure. Environmental Pollution, 231, 1042–1050.
Sabaghi, R., Hosseini, R., & Sahragard, A. (2011). Functional and numerical responses of Scymnus Syriacus Marseul (Coleoptera: Coccinellidae) to the black bean aphid, Aphis Fabae Scopoli (Hemiptera: Aphididae) under laboratory conditions. Journal of Plant Protection Research, 51, 423–428.
Sahito, H. A., Abro, G. H., Syed, T. S., Memon, S. A., Mal, B., & Kaleri, S. (2011). Screening of pesticides against cotton mealybug Phenacoccus solenopsis Tinsley and its natural enemies on cotton crop. International Research Journal of Biochemistry and Bioinformatics, 19, 232–236.
Sanches N. F., & Carvalho R. S. (2010). Procedimentos para manejo da criação e multiplicação do predador exótico Cryptolaemus montrouzieri. Cruz dasAlmas: BrasilEmbrapa Mandioca e Fruticultura (Circular Técnica, 99).
Solomon, M. E. (1949). The natural control of animal populations. The Journal of Animal Ecology, 18, 1–35.
Sullivan, J. J., & Goh, K. S. (2008). Environmental fate and properties of pyriproxyfen. Journal of Pesticide Science, 33(4), 339–350.
Taylor, W. E., & Vickery, B. (1974). Insecticidal properties of limonene, a constituent of citrus oil. Ghana Journal of Agricultural Science, 7, 61–62.
Tsolakis, H., & Ragusa, S. (2008). Effects of a mixture of vegetable and essential oils and fatty acid potassium salts on Tetranychus urticae and Phytoseiulus persimilis. Ecotoxicology and Environmental Safety, 70(2), 276–282.
Uygun, N., & Atlihan, R. (2000). The effect of temperature on development and fecundity of Scymnus levaillanti. BioControl, 45, 453–462.
Vanegas-Rico, J. M., Lomeli-Flores, J. R., Rodríguez-Leyva, E., Mora-Aguilera, G., & Valdez, J. M. (2010). Natural enemies of Dactylopius opuntiae (Cockerell) on Opuntia ficus-indica (L.) Miller in Central Mexico. Acta Zoológica Mexicana, 26, 415–433.
Vanegas-Rico, J. M., Rodríguez-Leyva, E., Lomeli-Flores, J. R., González-Hernández, H., Pérez-Panduro, A., & MoraAguilera, G. (2016). Biology and life history of Hyperaspis trifurcate feeding on Dactylopius opuntiae. BioControl, 61, 691–701.
Wanumen, A. C., Sánchez-Ramos, I., Viñuela, E., Medina, P., & Adán, Á. (2016). Impact of feeding on contaminated prey on the life parameters of Nesidiocoris tenuis (Hemiptera: Miridae) adults. Journal of Insect Science, 16(1), 103. https://doi.org/10.1093/jisesa/iew084
Wright, E. J., & Laing, J. E. (1980). Numerical response of Coccinellids to aphids in corn in southern Ontario. Canadian Entomologist, 112, 977–988.
Xiao, D., Zhao, J., Guo, X., Chen, H., Qu, M., Zhai, W., Desneux, N., Biondi, A., Zhang, F., & Wang, S. (2016). Sublethal effects of imidacloprid on the predatory seven-spot ladybird beetle Coccinella septempunctata. Ecotoxicol, 25(10), 1782–1793.
Yousef-Yousef, M., & Quesada-Moraga, E. (2020). Towards Dactylopius opuntiae (Cockerell) (Hemiptera : Dactylopiidae) biological and integrated management at field conditions in Cadiz province (Spain). Biocontrol Science and Technology, 30(9), 951–961.
Funding
The research of this study was supported by the National Institute of Agricultural Research (INRA), Morocco.
Author information
Authors and Affiliations
Contributions
Mohamed El Aalaoui. conceptualization, methodology, formal analysis, prepared figures, and wrote the main manuscript text; Mohamed Sbaghi. supervision, visualization, and review of the article. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El Aalaoui, M., Sbaghi, M. Side effects of some insecticides on numerical response of Cryptolameus montrouzieri to Dactylopius opuntiae. Phytoparasitica 51, 513–520 (2023). https://doi.org/10.1007/s12600-023-01073-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-023-01073-y