Abstract
In this study, we characterized and quantified vitamin B12 compounds in popular edible snails Babylonia japonica and Turdo Batillus cornutus using a microbiological assay based on Lactobacillus delbrueckii subsp. lactis ATCC 7830. The meat and viscera of B. japonica contained 27.2 ± 9.1 and 92.8 ± 25.8 μg of vitamin B12 per 100 g, respectively. However, the meat and viscera of T. cornutus contained extremely low amounts of vitamin B12 (3.0 ± 1.5 and 15.1 ± 8.3 μg of vitamin B12 per 100 g, respectively). We identified the vitamin B12 compounds from the edible portions (meat and viscera) of B. japonica and T. cornutus using liquid chromatography–electrospray ionization/tandem mass spectrometry. We found that B. japonica contained substantial amounts of true vitamin B12, while pseudovitamin B12 was the predominant corrinoid in T. cornutus. These results indicate that the meat and viscera of B. japonica are excellent sources of vitamin B12 for humans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vitamin B12 compounds are synthesized only by certain bacteria and are concentrated mainly in the bodies of higher predators in the natural food chain. The usual dietary sources of vitamin B12 are animal products (i.e., meat, milk, egg, fish, and shellfish) [1]. The Japanese obtain most (approximately 84 %) of their daily vitamin B12 intake from fish and shellfish [2]. Shellfish siphon large quantities of vitamin B12-synthesizing bacteria from seawater and freshwater and are excellent sources of vitamin B12 (>10 μg/100 g wet weight) [1, 3]. However, these vitamin B12-synthesizing bacteria can also synthesize other corrinoids that contain a different base moiety in the lower ligand of the molecule [4].
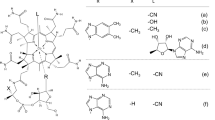
Our previous studies indicated that vitamin B12 levels were significantly higher in edible bivalves (approximately 60 μg/100 g wet weight) than in edible snails (approximately 20 μg/100 g wet weight) [5]. The corrinoid compounds purified from most edible bivalves (clams, oysters, mussels, etc.) have been identified as ‘true’ vitamin B12 [6]. In the edible sea snail abalone, vitamin B12 and pseudovitamin B12 (Coβ-cyano-N7-adeninyl-cobamide, an inactive corrinoid for humans; as shown in Fig. 1) were observed to be the major and minor corrinoid compounds, respectively [5]. However, there is little information available on the vitamin B12 compounds of other edible sea snails. Among the edible sea snails, ivory shell Babylonia japonica and turban shell Turdo Batillus cornutus are the most popular food items in Japan. Their meat and viscera are edible because high levels of vitamin B12 accumulate in the viscera of shellfish [5]. If these popular snails contain a large amount of “true” vitamin B12, they would be good sources of vitamin B12 in humans.
In this study, we identified vitamin B12 compounds from the edible portions (meat and viscera) of B. japonica and T. cornutus using liquid chromatography–electrospray ionization/tandem mass spectrometry (LC/ESI–MS/MS). We found that B. japonica contains substantial amounts of “true” vitamin B12, while pseudovitamin B12 was the predominant corrinoid in T. cornutus. These results indicate that the meat and viscera of B. japonica are excellent sources of vitamin B12 for humans.
Materials and methods
Materials
Vitamin B12 (cyanocobalamin) was obtained from Sigma-Aldrich (St Louis, Missouri, USA). Pseudovitamin B12 that had been purified from Aphanizomenon flos-aquae and identified with proton nuclear magnetic resonance (1H–NMR) spectroscopy [7] was used in this study. A vitamin B12 assay medium based on Lactobacillus delbrueckii (formerly L. leichmannii) ATCC 7830 was obtained from Nissui (Tokyo, Japan). Raw B. japonica and T. cornutus were purchased from local markets in Tottori prefecture, Japan.
Extraction and assay of vitamin B12 from edible snails
After the shells were removed from B. japonica and T. cornutus, their edible portions (meat and viscera) were sampled. Each sample was homogenized using a mixer (TML160; Tescom & Co., Ltd., Tokyo, Japan). An aliquot (2.0 g) of the homogenate was used as the sample for the vitamin B12 assay. Vitamin B12 compounds were extracted from each sample by boiling for 30 min under acidic conditions (pH 4.5) and then assayed using a microbiological method based on L. delbrueckii ATCC 7830, according to a previously described method [4]. The extraction procedures were done in a Dalton (Tokyo, Japan) draft chamber in the dark.
Because L. delbrueckii ATCC 7830 can utilize deoxyribosides and deoxyribonucleotides (known to be an alkali-resistant factor) as well as vitamin B12, the correct vitamin B12 values were calculated by subtracting the values for the alkali-resistant factor from the total vitamin B12 values.
Identification of sea snail vitamin B12 compounds by LC/ESI–MS/MS
Each vitamin B12 extract (40 mL) was partially purified and concentrated using a Sep-Pak® Plus C18 cartridge (Waters Corp., MA, USA), as described previously [4]. The eluate was evaporated to dryness under reduced pressure, and then was dissolved in 3 mL of distilled water and centrifuged at 10,000×g for 10 min to remove insoluble material. The supernatant fraction was loaded onto an immunoaffinity column [EASI-EXTRACT® vitamin B12 immunoaffinity column (P80), R-Biopharm AG, Darmstadt, Germany], and were purified according to the manufacturer’s protocol. The purified vitamin B12 compounds were dissolved in 0.1-% (v/v) acetic acid and filtered through a Nanosep MF centrifuge device (0.4 μm; Pall Corp., Tokyo, Japan) to remove small particles. An aliquot (2 μL) of the filtrate was analyzed using an LC–MS ion trap-time-of-flight (IT−TOF) system coupled to an ultra-fast LC system (Shimadzu, Kyoto, Japan). Each purified corrinoid was injected into an inert-sustain column (3 μm, 2.0 × 100 mm; GL Science, Tokyo, Japan) equilibrated with 85 % of solvent A [0.1-% (v/v) acetic acid] and 15 % of solvent B (100-% methanol) at 40 °C. Corrinoids were eluted using a linear gradient of methanol (15 % of solvent B for 0–5 min, 15–90 % of solvent B for 5–11 min, and 90–15 % of solvent B for 11–15 min). The flow rate was 0.2 mL/min. ESI conditions were determined by injecting the corrinoids into the MS detector, thereby identifying the optimum parameters for detecting parent and daughter ions of vitamin B12 compounds. The ESI–MS system was operated in a positive ion mode, and argon was used as the collision gas. The identities of pseudovitamin B12 (m/z 672.7749) and vitamin B12 (m/z 678.2914) as [M + 2H]2+ were confirmed by comparing the observed molecular ions and retention times.
Results
Vitamin B12 content of edible sea snails
We analyzed the vitamin B12 content of the edible sea snails of the ivory-shelled B. japonica and the turban-shelled T. cornutus, which are commonly consumed in Japan, using the L. delbrueckii ATCC 7830 microbiological assay method (Table 1). The viscera of B. japonica contained a substantial amount of vitamin B12 (approximately 92.8 μg/100 g wet weight); 3.4 times greater than that of the meat (approximately 27.2 μg/100 g wet weight). The meat and viscera of T. cornutus contained significantly lower amounts of vitamin B12 (approximately 3.0 and 15.1 μg/100 g wet weight, respectively). These results indicated that high levels of vitamin B12 accumulate in the viscera of these edible sea snails. The vitamin B12 content (approximately 4.7 μg) per whole body of B. japonica was 2.8 times greater than that of T. cornutus. The vitamin B12 contents determined in our analysis are significantly higher than those [4.3 and 1.3 μg of vitamin B12 per 100 g of edible portion (without shell and viscera) of ivory shell and turban shell, respectively] described in the Standard Tables of Food Composition in Japan 2010 [8].
Identification of corrinoid compounds from edible sea snails using LC/ESI–MS/MS analysis
Edible snail extracts were purified using a vitamin B12 immunoaffinity column and then analyzed using LC/ESI–MS/MS. Authentic pseudovitamin B12 and vitamin B12 were eluted as peaks with retention times of 7.4 and 7.5 min, respectively (Fig. 2a, d, respectively). The mass spectrum of authentic pseudovitamin B12 indicated that a doubly-charged ion with an m/z of 672.7769 [M + 2H]2+ was prominent (Fig. 2b). The exact mass calculated from its formula (C59H83CoN17O14P) was 1343.5375 and the isotope distribution data showed that pseudovitamin B12 was the major doubly-charged ion under the LC/ESI–MS conditions used in our analyses. For authentic vitamin B12, which has an exact mass of 1354.5674 (C63H88CoN14O14P), a doubly-charged ion with an m/z of 678.2883 [M + 2H]2+ was prominent (Fig. 2e). The MS/MS spectra of authentic pseudovitamin B12 and vitamin B12 indicated that their dominant ions at m/z 348.0695 and m/z 359.0984, respectively, were attributable to the nucleotide moiety of each corrinoid compound (Fig. 2c, f). The corrinoids purified from the meat of B. japonica were eluted as an ion peak with m/z 678.2914 at a retention time of 7.5 min. The mass spectrum showed that a doubly-charged ion was formed at m/z 678.2928 (Fig. 3a, b). The MS/MS spectrum of the compound was identical to that of vitamin B12 (Fig. 3c). Identical spectral data were obtained for the corrinoids purified from B. japonica viscera (Fig. 3d–f). These results indicate that vitamin B12 is the predominant corrinoid compound in B. japonica. The compounds purified from T. cornutus meat eluted as several total ion peaks, indicating that impurities remained. The ion peaks of m/z 672.7749 and m/z 678.2914 due to pseudovitamin B12 and vitamin B12, respectively, were also found (Fig. 4a). Their retention times of 7.3 and 7.4 min, respectively, were similar to those of authentic pseudovitamin B12 (retention time of 7.4 min) and vitamin B12 (retention time of 7.5 min). Such slight differences in retention times may be due to the existence of impurities in the purified compounds. The mass spectra of the materials eluting at retention times of 7.3 and 7.4 min showed doubly-charged ions at m/z 672.7764 (Fig. 4b) and m/z 678.2856 (Fig. 4d), respectively. The MS/MS spectra of these compounds were identical to those of pseudovitamin B12 (Fig. 4c) and vitamin B12 (Fig. 4e). Similar results were obtained with the visceral sample, but no vitamin B12 was detected (Fig. 4f–h). These results indicate that pseudovitamin B12 is the predominant corrinoid compound in T. cornutus. Similar results were reported in abalone [5]. This result indicated that T. cornutus would not be a suitable source of vitamin B12.
LC/ESI–MS/MS chromatograms of authentic pseudovitamin B12 and vitamin B12. Pseudovitamin B12 and vitamin B12 were analyzed with LCMS-IT-TOF (Shimadzu) as described in the text. a, d Total ion chromatograms (TICs) of authentic pseudovitamin B12 and vitamin B12, respectively. b, e Mass spectra of the ion peaks from pseudovitamin B12 (inserts magnified mass spectra from m/z 672 to 675) and vitamin B12 (inserts magnified mass spectra from m/z 678 to 680), respectively. c, f MS/MS spectra of the peaks of pseudovitamin B12 and vitamin B12, respectively
LC/ESI–MS/MS chromatograms of the vitamin B12 compounds purified from the meat and viscera of B. japonica. a, d TICs and ion chromatograms for m/z 678.2914 (×10) and 672.7749 (×10) of the vitamin B12 compounds purified from the meat and viscera of B. japonica, respectively. b, e Mass spectra of the ion peaks of the meat and visceral vitamin B12 compounds at retention times of 7.5 min (inserts magnified mass spectrum from m/z 678 to 680), respectively. c, f MS/MS spectra for the peaks of the muscle and visceral vitamin B12 compounds at m/z 678.2928 and at m/z 678.2917, respectively
LC/ESI–MS/MS chromatograms of the vitamin B12 compounds purified from the meat and viscera of T. cornutus. a, f TICs and ion chromatograms for m/z 678.2914 (×10 and ×20) and 672.7749 (×10 and ×20) of the vitamin B12 compounds purified from the meat and viscera of T. cornutus, respectively. b, d Mass spectra of the ion peaks of the meat vitamin B12 compounds at retention times of 7.3 min (inserts magnified mass spectrum from m/z 672 to 675) and 7.4 min (inserts magnified mass spectrum from m/z 678 to 680), respectively. c, e MS/MS spectra for the peaks of the muscle vitamin B12 compounds at m/z 672.7764 and at m/z 678.2856, respectively. g Mass spectrum of the ion peak of the visceral vitamin B12 compounds at a retention time of 7.3 min (inserts magnified mass spectrum from m/z 672 to 675). h MS/MS spectrum for the peak of the visceral vitamin B12 compound at m/z 672.7743
Discussion
The vitamin B12 content of foods were determined using the L. delbrueckii ATCC 7830 bioassay method. Our previous studies showed that the observed correlation rate between the values determined by the L. delbrueckii ATCC 7830 bioassay and intrinsic factor (the most specific vitamin B12-binding protein)-based chemiluminescence method is excellent, except for foods containing substantial amounts of pseudovitamin B12 [9]. These results indicated that L. delbrueckii ATCC 7830 utilizes pseudovitamin B12 as well as vitamin B12. Thus, pseudovitamin B12 found in the edible portions of turban shells was determined as vitamin B12 using the L. delbrueckii ATCC 7830 bioassay.
The differences in content and vitamin B12 compounds between these edible sea snails is dependent on their dietary habitats, because B. japonica and T. cornutus are carnivorous and herbivorous sea snails, respectively. Vitamin B12 is synthesized only by certain bacteria and is concentrated mainly in the bodies of higher predators in the natural food chain. The usual dietary sources of vitamin B12 are animal-derived products but not plant-derived products [1]. The vitamin B12 content of tengusa Gelidium pacificum Okamura and wakame Undaria pinnatifida, the foods of T. cornutus, are very low (0.2 − 0.5 μg/100 g dry weight) [8]. Yamada et al., [10] demonstrated that wakame predominantly contained certain vitamin B12 analogues. Moreover, various blue-green algae (cyanobacteria) contain substantial amounts of pseudovitamin B12 [1].
The consumption of one whole body (meat and viscera, approximately 11 g) of B. japonica, which contains a considerably high vitamin B12 level (approximately 4.7 μg), could supply the entire recommended dietary allowance for an adult (2.4 μg/day) [11]. Our unpublished studies indicated that the edible portions (without shell and viscera) of other carnivorous sea snails, e.g., whelks Buccinum striatissium and Neptunea intersculpta, also contain considerable amounts of vitamin B12 [10.13 ± 3.33 (n = 5) and 28.72 ± 5.48 (n = 5) μg/100 g, respectively]. The results presented here indicate that these edible carnivorous sea snails would be excellent sources of vitamin B12 for humans.
References
Watanabe F (2007) Vitamin B12 sources and bioavailability. Exp Biol Med 232:1266–1274
Yoshino K, Inagawa M, Oshima M, Yokota K, Umesawa M, Endo M, Yamagishi K, Tanigawa T, Sato S, Shimamoto T, Iso H (2005) Trends in dietary intake of folate, vitamin B6, and vitamin B12 among Japanese adults in two rural communities from 1971 through 2001. J Epidemiol 15:29–37
Herbert V (1996) Vitamin B12. Present knowledge in nutrition, 7th edn. International Life Sciences Institute Press, Washington, pp 191–205
Watanabe F, Katsura H, Takenaka S, Fujita T, Abe K, Tamura Y, Nakatsuka T, Nakano Y (1999) Pseudovitamin B12 is the predominant cobamide of an algal health food, Spirulina tablets. J Agric Food Chem 47:4736–4741
Tanioka Y, Takenaka S, Furusho T, Yabuta Y, Nakano Y, Watanabe F (2014) Identification of vitamin B12 and pseudovitamin B12 from various edible shellfish using liquid chromatography–electrospray ionization/tandem mass spectrometry. Fish Sci 80:1065–1071
Watanabe F, Katsura H, Takenaka S, Enomoto T, Miyamoto E, Nakatsuka T, Nakano Y (2001) Characterization of vitamin B12 compounds from edible shellfish, clam, oyster, and mussel. Int J Food Sci Nutr 52:263–268
Miyamoto E, Tanioka Y, Nakao T, Barla F, Inui H, Fujita T, Watanabe F, Nakano Y (2006) Purification and characterization of a corrinoid-compound in an edible cyanobactrium Aphanizomenon flos-aquae as nutritional supplementary food. J Agric Food Chem 54:9604–9607
Ministry of Education, Culture, Sports, Science and Technology (2010) Report of the subdivision of resources. In: Standard tables of food composition in Japan, 2010. The council for Science and Technology, Ministry of Education, Culture, Sports, Science and Technology, Tokyo, pp 170–173 (in Japanese)
Watanabe F, Takenaka S, Abe K, Tamura Y, Nakano Y (1998) Comparison of a microbiological assay and fully automated chemiluminescent system for the determination of vitamin B12 in foods. J Agric Food Chem 46:1433–1436
Yamada S, Shibata Y, Takeyama M, Narita Y, Sugawara K, Fukuda M (1996) Content and characteristics of vitamin B12 in some seaweeds. J Nutr Sci Vitaminol 42:497–505
Shibata K, Fukuwatari T, Imai E, Hayakawa H, Watanabe F, Takimoto H, Watanabe T, Umegaki K (2013) Dietary reference intakes for Japanese 2010: water-soluble vitamins. J Nutr Sci Vitaminol 59:S67–S82
Acknowledgments
This work was supported by JSPS KAKENHI Grant number 25450168 (FW).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teng, F., Tanioka, Y., Hamaguchi, N. et al. Determination and characterization of vitamin B12 compounds in edible sea snails, ivory shell Babylonia japonica and turban shell Turdo Batillus cornutus . Fish Sci 81, 1105–1111 (2015). https://doi.org/10.1007/s12562-015-0920-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-015-0920-5