Abstract
In this study, the vitamin B12 contents were analyzed in the edible portions of various shellfish (bivalves and snails). High vitamin B12 contents (30.5–53.3 μg/100 g wet weight) were detected in mussels, surf clams, bloody clams, and freshwater clams. However, scallops and abalone had extremely low vitamin B12 contents (0.1–1.1 μg/100 g wet weight) which was attributed to only the muscle portions being edible. These results suggest that high levels of vitamin B12 are accumulated in the viscera of shellfish. Vitamin B12 levels were also significantly higher in bivalves than in snails. The corrinoid compounds purified from all bivalves were identified as “true” vitamin B12 using liquid chromatography–electrospray ionization/tandem mass spectrometry. In edible snails, abalone, and pond snails, however, both vitamin B12 and pseudovitamin B12 (an inactive corrinoid) were observed to be the major and minor corrinoid compounds, respectively. Based on these results, we conclude that the whole bodies of these edible bivalves are excellent sources of vitamin B12 for humans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vitamin B12 (B12) compounds are synthesized only by certain bacteria which are primarily concentrated in the bodies of predatory animals relatively high in the natural food chain [1]. The usual dietary sources of B12 are animal food products (i.e., meat, milk, egg, fish, shellfish) [2]. Japanese people obtain most (approximately 84 %) of their daily B12 intake from fish and shellfish [3]. Shellfish that siphon large quantities of B12-synthesizing bacteria from seawater and freshwater are excellent sources of B12 [4]. However, these B12-synthesizing bacteria can also synthesize other corrinoids with a different base moiety in the lower ligand of the molecule [1, 5]. In our previous study [6], we extracted and purified corrinoid compounds from popular edible shellfish (clams, oysters, mussels) using silica gel thin-layer chromatography (TLC) and reversed-phase high performance liquid chromatography and identified the “true” B12 by NMR spectroscopy. However, our recent study using B12 antibody affinity chromatography and liquid chromatography–electrospray ionization/tandem mass spectrometry (LC/ESI–MS/MS) [7] indicates that the edible portion and viscera of abalone Haliotis discus hannai primarily contains pseudovitamin B12 (pseudo-B12), which is inactive in humans. Moreover, the freshwater clam Corbicula japonica contains only a small amount of pseudo-B12 and unidentified corrinoid compounds [8]. Limited information is available on whether edible shellfish (particularly their edible portions) contain B12 or other corrinoid compounds (such as pseudo-B12) that are inactive in humans.
In this study, we identified B12 compounds from the edible portions of various shellfish (bloody clam, freshwater clam, mussel, scallop, surf clam, abalone, pond snail, and whelk) using LC/ESI–MS/MS. We found that all bivalves tested contained substantial amounts of true B12 but that inactive corrinoid compounds were also present in abalone and pond snail. These results indicate that the whole bodies of these bivalves are excellent sources of B12 for humans.
Materials and methods
Materials
B12 cyanocobalamin was obtained from Sigma (St. Louis, MO). Pseudovitamin B12 7-adenyl cyanocobamide was isolated from Spirulina sp. [5] and used in our study. Silica gel 60 TLC aluminum sheets were obtained from Merck (Darmstadt, Germany). All other reagents were of the highest commercially available purity. Raw shellfish (scallop Mizuhopecten yessoensis, mussel Mytilus galloprovincialis, surf clam Pseudocardium sachalinense, bloody clam Anadara broughtonii, freshwater clam Corbicula japonica, abalone Haliotis diversicolor aquatilis, whelk Buccinum middendorffi, and pond snail Bellamya chinensis mallaeta) were purchased from a local market in Tokyo, Japan (n = 5).
Extraction and assay of B12 compounds from shellfish
The edible portions (2 g) of the shellfish were suspended in 40 ml of distilled water and homogenized using a universal homogenizer (Polytron, Kinematica, Switzerland). The total corrinoid compounds were extracted from each sample by boiling at pH 4.5 and assayed using a microbiological method based on Lactobacillus delbrueckii ATCC7830, as previously described [5]. L. delbrueckii ATCC7830 can utilize deoxyribosides and deoxyribonucleotides (known to be an alkali-resistant factor) as well as B12. The correct B12 values were therefore calculated by subtracting the values for the alkali-resistant factor from the values for the total B12 compounds.
TLC-bioautography assay using B12-dependent Escherichia coli 215
A bioautography assay to detect corrinoid compounds was performed as previously described [9]. The B12 extracts [20-ml samples were prepared as described above and partially purified and concentrated using a Sep-Pak Plus® C18 cartridge (Waters Corp., Milford, MA)] were washed with 5 ml of 75 % (v/v) ethanol and equilibrated with 5 ml of distilled water. The C18 cartridge was washed with 5 ml of distilled water, and B12 compounds were eluted using 2 ml of 75 % (v/v) ethanol. The eluate was evaporated in a centrifugal concentrator (VC960; TAITEC Corp., Saitama, Japan), and the residual fraction was dissolved in 1.0 ml of distilled water. Concentrated B12 extracts (1 μl) and B12 and pseudo-B12 (each 0.1 mg/l) were spotted onto the silica gel 60 TLC plates and developed in the dark using 2-propanol/NH4OH (28 %)/water (7:1:2 v/v) at room temperature (25 °C). The TLC plate was dried and overlaid with agar-containing basal medium and precultured E. coli 215, followed by incubation at 30 °C for 20 h. The gel plate was subsequently sprayed with methanol solution containing 2,3,5-triphenyltetrazolium salt, and B12 compounds were visualized as red, which indicated E. coli growth.
Immunopurification of corrinoid compounds from edible shellfish
Each extract (40 ml) was partially purified and concentrated using a Sep-Pak® Plus C18 cartridge (Waters Corp) as described above. The eluate was evaporated in a centrifugal concentrator (VC960; TAITEC Corp), and the residual fraction was dissolved in 2.0 ml of distilled water. The purified extract was loaded onto an immunoaffinity column [EASI-EXTRACT® Vitamin B12 Immunoaffinity Column (P80); R-Biopharm AG, Darmstadt, Germany], and the corrinoids were purified following the manufacturer’s protocol.
Identification of corrinoid compounds by LC/ESI–MS/MS
The B12 compounds were purified by passage through the immunoaffinity column, dissolved in 0.1 % (v/v) acetic acid, and filtered through a Nanosep MF centrifuge device (0.4 μm; Pall Corp., Tokyo, Japan) to remove any small particles. An aliquot (2 μl) of the filtrate was analyzed using a LC/MS IT-TOF (ion trap–time-of-flight) system coupled to an Ultra-Fast LC system (Shimadzu, Kyoto, Japan). Each purified corrinoid was injected into an InertSustain column (3 μm, 2.0 × 100 mm; GL Science, Tokyo, Japan) and equilibrated with 100 % solvent A [0.1 % (v/v) acetic acid] and 0 % solvent B (100 % methanol) at 40 °C. Corrinoids were eluted using a linear gradient of methanol (15 % solvent B for 0–5 min, 15–90 % solvent B for 5–11 min, and 90–15 % solvent B for 11–15 min). The flow rate was 0.2 ml/min. ESI conditions were determined by injecting the corrinoids into the MS detector, thereby identifying the optimum parameters for detecting parent and daughter ions of B12 compounds. The ESI–MS system was operated in the positive ion mode, and argon was used as the collision gas. Pseudo-B12 (m/z 672.7749) and B12 (m/z 678.2914) as [M + 2H]2+ were confirmed by comparing the observed molecular ions and retention times.
Results
B12 contents of the edible portions of various shellfish
We analyzed the B12 contents of the edible portions of various shellfish that are commonly consumed in Japan using the L. delbrueckii ATCC 7830 microbiological assay method (Table 1). High B12 contents (30.5–53.3 μg/100 g wet weight) were detected in mussels, surf clams, bloody clams, and freshwater clams. Moderate B12 contents (10.5–21.4 μg/100 g wet weight) were observed in whelks and pond snails. However, scallops and abalone had extremely low B12 contents (0.1–1.1 μg/100 g wet weight). The B12 contents determined in our analyses are similar to those described in the Standard Tables of Food Composition in Japan [10].
Identification of corrinoid compounds from edible shellfish using the E. coli 215 bioautography assay
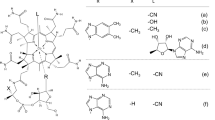
The corrinoids observed in all edible shellfish samples were analyzed using an E. coli 215 bioautogram after separation by silica gel 60 TLC. The corrinoids observed in all bivalve samples and whelks produced clear spots which had an R f value identical to that of B12 (Fig. 1a), but not pseudo-B12. The remaining snail samples yielded two spots, the R f values of which were identical to those of pseudo-B12 and B12 (Fig. 1b).
Vitamin B12 (B12)-dependent Escherichia coli 215 bioautogram analysis of corrinoid compounds from the edible portions of various shellfish. a Bivalves. Lanes: 1 B12 2 pseudo-B12 3 fresh water clam (whole body), 4 bloody clam (whole body), 5 scallop (adductor muscle), 6 surf clam (whole body), 7 mussel (whole body), 8 mixture of B12 and pseudo-B12. b Snails. Lanes: 1, 3, 5 Mixture of B12 and pseudo-B12, 2 whelk (whole body), 4 abalone (without viscera), 6 pond snail. Each sample was spotted on the circle marked in the thin-layer chromatography (TLC) plate and developed. The data represent typical bioautograms from three independent experiments
Identification of corrinoid compounds purified from selected edible shellfish using LC/ESI–MS/MS
The bloody clam was selected from among the edible bivalves that contained high B12 levels for further analysis. A bloody clam extract was purified using a B12 immunoaffinity column and analyzed by LC/ESI–MS/MS. B12 and pseudo-B12 were eluted as peaks with retention times of 7.5 and 7.4 min, respectively (Fig. 2a-1, b-1). The mass spectrum of B12 indicated that a doubly charged ion with an m/z of 678.2889 [M + 2H]2+ was prominent (Fig. 2a-2). The exact mass calculated from its formula (C63H88CoN14O14P) was 1354.5674, and the isotope distribution data showed that B12 was the major divalent ion under our LC/ESI–MS conditions. For pseudo-B12 with an exact mass of 1343.5375 (C59H83CoN17O14P), a doubly charged ion with an m/z of 672.7749 [M + 2H]2+ was prominent (Fig. 2b-2). The MS/MS spectra of B12 and pseudo-B12 indicated that the dominant ions at m/z 359.0983 (Fig. 2a-3) and m/z 348.0674 (Fig. 2b-3), respectively, were attributable to the nucleotide moiety of each corrinoid compound. The corrinoids purified from the bloody clam sample were eluted to yield a single total ion peak with a retention time of 7.5 min, where the mass spectrum showed that the doubly charged B12 ion was formed at m/z 678.2916 (Fig. 3a-2). The MS/MS spectrum of the compound was identical to that of B12 (Fig. 3a-3). These results indicate that B12 is the predominant corrinoid compound in the bloody clam. As shown in Table 1, identical results were obtained with other edible bivalve samples.
Liquid chromatography–electrospray ionization/tandem mass spectrometry (LC/ESI–MS/MS) chromatograms of B12 and pseudo-B12. B12 was analyzed by an LC/MS ion trap–time-of-flight (IT-TOF) (Shimadzu), as described in the text. a-1 Total ion chromatogram (TIC; ×1) and reconstructed chromatogram of m/z 678.2914 (×2) for B12. a-2 Mass spectrum of the ion peak of B12, where the magnified mass spectrum from m/z 678.0 to 680.0 is shown in the insert. a-3 MS/MS spectrum of the B12 peak. b-1 TIC (×1) and the reconstructed chromatogram at m/z 672.7749 (×2) for pseudo-B12. b-2 The mass spectrum of the ion peak of pseudo-B12, where the magnified mass spectrum from m/z 678.0 to 680.0 is shown in the insert. b-3 MS/MS spectrum of the pseudo-B12 peak
LC/ESI–MS/MS chromatograms of the corrinoid compounds purified from the edible portions of bloody clams and abalone. a-1 TIC (×1) and reconstructed chromatograms of the purified corrinoid compounds [m/z 678.2914 (×2)] from a bloody clam sample. a-2 Mass spectrum of the corrinoid compounds purified at 7.5 min (insert magnified spectrum from m/z 678 to 680). a-3 MS/MS spectrum of the m/z 678.2916 peak of the corrinoid compounds. b-1 TIC (×1) and reconstructed chromatograms of the corrinoid compounds [m/z 678.2914 (×2) and m/z 672.7749 (×10)] purified from an abalone sample. b-2, b-4 Mass spectra of the corrinoid compounds purified at 7.4 and 7.5 min, respectively (inserts magnified spectra, respectively, from m/z 678 to 680). b-3, b-5 MS/MS spectra for the m/z 672.7789 and m/z 678.2920 peaks of the corrinoid compounds, respectively
In the compounds purified from abalone, ion peaks were detected at m/z 672.7749 and m/z 678.2914 for pseudo-B12 and B12, respectively, and their retention times were identical to those of pseudo-B12 and B12 (Fig. 3b-1). The mass spectra at the retention times of 7.4 and 7.5 min showed that doubly charged ions were formed at m/z 672.7789 (Fig. 3b-2) and m/z 678.2920 (Fig. 3b-4) in both pseudo-B12 and B12, respectively. The respective MS/MS spectrum of each compound was identical to that of pseudo-B12 (Fig. 3b-3) and B12 (Fig. 3b-5). Similar results were obtained with a pond snail sample (data not shown). Abalone and pond snails contained approximately 89 and 83 % of B12, respectively, based on the respective ion peak areas. The results of our preliminary experiments also suggested that traces of other inactive corrinoid compounds (benzimidazolyl cyanocobamide, 5-hydroxybenzimidazolyl cyanocobamide, etc.) were present in the pond snail extract, but they could not be identified completely. These results indicate that B12 and pseudo-B12 were the major and minor corrinoid compounds, respectively, in abalone and pond snails.
Discussion
Some of the bivalves assayed in our study whose muscle and viscera were both edible contained considerably B12 content (30.5–53.3 μg/100 g wet weight). However, the B12 contents (0.1–1.1 μg/100 g wet weight) of the muscle portions of the scallops and abalone were considerably lower. Regardless of the shellfish species, the B12 content was higher in the viscera than in the muscle. Although bivalves siphon large quantities of B12-synthesizing bacteria from seawater and freshwater, the majority of edible snails are herbivorous, and plants and seaweeds (except purple and green lavers) contain only trace amounts of B12 [2]. Thus, the vitamin B12 contents of shellfish may be attributable to their respective diet, because the production of B12 by intestinal bacteria is very low in shellfish [11]. In general, the bodies of edible bivalves contain much higher B12 levels than those of edible snails.
In our previous study, we showed that a considerable proportion (approximately 65 %) of the corrinoid compounds comprised pseudo-B12 in both the edible portion and viscera of abalone Haliotis discus hannai. In particular, the viscera contained substantial amounts of pseudo-B12 [7]. The muscle portion of abalone Haliotis diversicolor aquatilis contained most of the B12, but the small amount of pseudo-B12 observed in abalone may have been derived from its viscera. These observations suggest that the viscera of certain edible snails (abalone and pond snails) contain substantial amounts of pseudo-B12 and, therefore, that they may not be suitable as B12 sources. However, pseudo-B12 was not detected in another snail, the whelk, in our study. Abalone and pond snails are herbivorous but whelk is carnivorous. The pseudo-B12 found in abalone and pond snails may be derived from their diets because various blue-green algae contain substantial amounts of pseudo-B12 [2].
Corrinoid compounds have been purified from most popular edible shellfish, including oyster, short-necked clams, and mussels (each whole body), which have been identified as B12 [6, 11, 12]. These observations and the results obtained in the present study indicate that edible bivalves generally contain B12, but not pseudo-B12. However, freshwater clams Corbicula japonica caught in Lake Togo (Tottori prefecture, Japan) were found to contain a small amount (10.4 %) of inactive corrinoid compounds (pseudo-B12 and unidentified compounds) [8]. It remains unclear why these inactive corrinoid compounds were present in shellfish, but they may be attributable to the presence of bacteria that synthesize pseudo-B12 (and other inactive corrinoids) inside and/or outside their bodies. Thus, further detailed biochemical studies are required to elucidate the origins of these inactive corrinoid compounds. However, these minor and/or trace inactive corrinoid compounds may not be absorbed; therefore, they may not be available to humans because of their poor binding and uptake through the intrinsic factor (the highly specific B12-binding protein) involved in the gastrointestinal absorption of B12 [13]. The consumption of approximately 5–8 g of edible bivalves, which contain high B12 levels (mean value 30–50 μg/100 g wet weight), could supply the recommended dietary allowance for adults (2.4 μg/day) [14]. Thus, the results obtained in our study indicate that even if certain edible bivalves contain small or trace amounts of various inactive corrinoid compounds, the whole bodies of bivalves, which we found to contain higher amounts of B12 than snails, would still be excellent sources of B12.
References
Watanabe F, Yabuta Y, Tanioka Y, Bito T (2013) Biologically active vitamin B12 compounds in foods for preventing deficiency among vegetarians and elderly subjects. J Agric Food Chem 61:6769–6775
Watanabe F (2007) Vitamin B12 sources and bioavailability. Exp Biol Med 232:1266–1274
Yoshino K, Inagawa M, Oshima M, Yokota K, Umesawa M, Endo M, Yamagishi K, Tanigawa T, Sato S, Shimamoto T, Iso H (2005) Trends in dietary intake of folate, vitamin B6, and vitamin B12 among Japanese adults in two rural communities from 1971 through 2001. J Epidemiol 15:29–37
Herbert V (1996) Vitamin B12. Present knowledge in nutrition, 7th edn. International Life Sciences Institute Press, Washington, DC
Watanabe F, Katsura H, Takenaka S, Fujita T, Abe K, Tamura Y, Nakatsuka T, Nakano Y (1999) Pseudovitamin B12 is the predominant cobamide of algal health food, spirulina tablets. J Agric Food Chem 47:4736–4741
Watanabe F, Katsura H, Takenaka S, Enomoto T, Miyamoto E, Nakatsuka T, Nakano Y (2001) Characterization of vitamin B12 compounds from edible shellfish, clam, oyster, and mussel. Int J Food Sci Nutr 52:263–268
Tanioka Y, Takenaka S, Furusho T, Yabuta Y, Nakano Y, Watanabe F (2012) Characterization of vitamin B12-related compounds isolated from edible portions of abalone. Vitamins 86:390–394 (in Japanese)
Ishihara Y, Ueta K, Bito T, Takenaka S, Yabuta Y, Watanabe F (2013) Characterization of vitamin B12 compounds from the brackish-water bivalve Corbicula japonica. Fish Sci 79:321–326
Tanioka Y, Yabuta Y, Miyamoto E, Inui H, Watanabe F (2008) Analysis of vitamin B12 in food by silica gel 60 TLC and bioautography with vitamin B12-dependent Escherichia coli 215. J Liq Chrom Rel Technol 3:1977–1985
Ministry of Education, Culture, Sports, Science and Technology (2010) Report of the subdivision of resources. In: Standard tables of food composition in Japan—2010. The Council for Science and Technology, Ministry of Education, Culture, Sports, Science and Technology, Tokyo, pp 154–158 (in Japanese)
Sugita H, Mase K, Iwata M, Kato S, Sugiura C, Ueda R, Deguchi Y (1991) Vitamin B12-producing ability of the gut microflora of marine gastropods. Suisanzoshoku 39:363–369 (in Japanese)
Ueta K, Ishihara Y, Yabuta Y, Masuda S, Watanabe F (2011) TLC-analysis of a corrinoid compound from Japanese rock oyster “Iwa-gaki” (Crassostres nippona). J Liq Chrom Rel Tecnol 34:928–935
Ueta K, Takenaka S, Yabuta Y, Watanabe F (2011) Broth from canned clams is suitable for use as an excellent source of free vitamin B12. J Agric Food Chem 59:12054–12058
Stupperich E, Nexo E (1991) Effect of the cobalt-N coordination on the cobamide recognition by the human vitamin B12 binding proteins intrinsic factor, transcobalamin, and haptocorin. Eur J Biochem 199:299–303
Shibata K, Fukuwatari T, Imai E, Hayakawa H, Watanabe F, Takimoto H, Watanabe T, Umegaki K (2013) Dietary reference intakes for Japanese 2010: water-soluble vitamins. J Nutr Sci Vitaminol 59:S67–S82
Acknowledgments
The authors thank Mr. T. Noguchi for his technical assistance. This work was supported partly by a Grant-in-Aid for Young Scientists (B) (No. 24700856) from JSPS KAKENHI (YT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanioka, Y., Takenaka, S., Furusho, T. et al. Identification of vitamin B12 and pseudovitamin B12 from various edible shellfish using liquid chromatography–electrospray ionization/tandem mass spectrometry. Fish Sci 80, 1065–1071 (2014). https://doi.org/10.1007/s12562-014-0787-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-014-0787-x