Abstract
We determined the vitamin B12 content in both the muscles and head innards of various shrimp species [Argis lar (Owen, 1839); Togezako shrimp, Argis toyamaensis (Yokoya, 1933); Pandalopsis japonica, Balss, 1914; Pandalus eous Makarov, 1935] using a microbiological assay based on Lactobacillus delbrueckii subsp. lactis ATCC7830. Approximately 2–4 µg vitamin B12/100 g wet weight—a considerable amount—was detected in shrimp muscles. The shrimp head innards contained significantly higher levels of vitamin B12 (~ 12–33 µg/100 g wet weight). Commercially available shrimp-innard products contained ~ 30 µg vitamin B12/100 g wet weight. We purified vitamin B12 compounds from the extracts of shrimp muscles and head innards using an immunoaffinity column. The muscle extract contained only one corrinoid compound, which was identified as vitamin B12 using liquid chromatography–electrospray ionization/tandem mass spectrometry, whereas the shrimp head innards contained three corrinoid compounds, which included large amounts of vitamin B12 and two smaller amounts of vitamin B12-d-monocarboxylic acid and tentatively identified vitamin B12 dicarboxylic acids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vitamin B12 (B12), also known as cobalamin, is a water-soluble vitamin that is one of a group of compounds containing a corrin ring. The lower ligand of B12 is attached to the cobalt-coordinated corrin ring through the nucleotide loop containing 5,6-dimethylbenzimidazole as a base (Watanabe and Bito 2016). B12 is synthesized by specific archaea and bacteria but not by animals or plants (Watanabe et al. 2014). The synthesized B12 is accumulated mainly in higher predatory animals through the natural food chain; therefore, animal-derived foods contain considerable amounts of B12 (Watanabe and Bito 2018a, b). In particular, fish and shellfish are reported to be important nutritional sources of B12 for humans (Bourre and Paquotte 2008; Scheers et al. 2014). Thaumarchaeota and cyanobacteria have recently been identified as the major authentic B12 and pseudovitamin B12 (pseudo-B12) producers, respectively, in the oceans (Heal et al. 2017). Pseudo-B12 is inactive in humans because it carries adenine in place of 5,6-dimethylbenzimidazole in the nucleotide moiety of the molecule (Watanabe et al. 1999).
To maintain an adequate supply of B12 for the general population, more detailed information on biologically active B12 compounds in seafoods is warranted. Therefore, we have purified and identified corrinoid compounds from various seafood species (Bito et al. 2018). Although most of the meats from the fish and shellfish that we tested contained B12 but not pseudo-B12 (Bito et al. 2018), pseudo-B12 was detected in the edible portion of some sea snails (Teng et al. 2015).
Shrimp is one of the most consumed seafoods worldwide, and both aquaculture-produced and wild-caught shrimp are used to supply seafood products for human consumption (Butcherine et al. 2019). Shrimp is a good source of several types of nutrients, such as amino acids, polyunsaturated fats, and minerals (Kaymaci and Altun 2016); however, the detailed characteristics of B12 have not been elucidated in this edible seafood. Although microbiological assays have determined that there is ~ 2 µg B12/100 g wet weight in the raw muscle of edible shrimp (Ministry of Education, Culture, Sports, Science and Technology 2010), information on the B12 content in the other edible portions, such as the shrimp head innards, are lacking; shrimp head innards are used to make shrimp sauces and other products in Asian countries. Moreover, it is unclear whether both shrimp muscles and head innards contain pseudo-B12.
In the present study, we determined the B12 contents of the shrimp head innards and muscles in four species of shrimp and purified and characterized corrinoid compounds from both of these edible portions using liquid chromatography–electrospray ionization/tandem mass spectrometry (LC/ESI–MS/MS). To the best of our knowledge, this is the first study to demonstrate that shrimp head innards contain substantial amounts of B12 plus two unidentified B12 compounds, which might be inactive for humans.
Materials and methods
Materials
The cyanocobalamin that was used as B12 in this study was purchased from Sigma-Aldrich (St. Louis, MO). B12-b-, -d-, and -e-monocarboxylic acids were prepared and their concentrations were determined on the basis of ε361 = 28.06 × 103 M−1 cm−1 as described previously (Watanabe et al. 1992). The revised designation of these B12 acid compounds were used according to Anton et al. (1980). Lactobacillus delbrueckii subsp. lactis ATCC 7830 was purchased from ATCC (Manassas, VA). A B12 assay medium based on L. delbrueckii subsp. lactis ATCC 7830 was purchased from Nissui (Tokyo). Four types of fresh shrimp (Alaskan pink shrimp, Pandalus eous Makarov, 1935; Morotoge shrimp, Pandalopsis japonica Balss, 1914; Kuro shrimp, Argis lar [Owen, 1839], and Togezako shrimp, Argis toyamaensis [Yokoya, 1933]) were purchased from local markets in Tottori, Japan. Products made from shrimp head innards were purchased from various seafood markets in Japan.
Extraction and assay of B12 in shrimp muscles and head innards
B12 was assayed using a microbiological method with L. delbrueckii subsp. lactis ATCC 7830, which has been adopted by the Standard Tables of Food Composition in Japan (Watanabe and Bito 2018a, b). After removing the shells from the fresh samples, the muscles and head innards were separated. Each muscle (2.0 g) or innard sample (1.0 g) was homogenized using a mortar and pestle and then added to 40 ml distilled water, 10 ml of 0.57 M acetic acid buffer (pH 4.5), and 0.4 ml of 0.05% (w/v) potassium cyanide (KCN). Total B12 was extracted by boiling the solution for 30 min in a draft chamber. After cooling, 0.6 ml of 10% (w/v) metaphosphoric acid was added to the B12 extract and the vessel was filled to 100 ml with distilled water. The prepared extract was filtrated through a 150-mm-diameter Whatman filter paper (GE Healthcare UK, UK). The filtrate was then divided into two portions of 25 ml each, the pH of one portion adjusted to 6.0, and the vessel filled to 50 ml with distilled water to use as extract A for the total B12 assays. The pH of the remaining portion was adjusted to 11 and the portion autoclaved (MC-23; ALP, Tokyo) at 121 °C for 30 min. After cooling, the pH of the solution was readjusted to 6.0, and the vessel was filled to 50 ml with distilled water to use as extract B to determine the alkali-resistant factor.
A 2.5-ml assay mixture was created containing 0.01 ml of extract A, B, or standard B12 solution (0, 0.5, 1.0, 2.0, 3.0, 4.0, or 5.0 µg/l B12) and 1.25 ml B12 basal medium for the assay (Nissui) prepared according to the manufacturer’s protocol, and 1.24 ml distilled water. This mixture was placed in polypropylene tubes (13 × 100 mm; Bio-Rad Laboratories, Hercules, CA), vigorously shaken to mix it, and autoclaved at 121 °C for 5 min. After Lactobacillus delbrueckii subsp. lactis ATCC 7830 were precultured in a B12 inoculum broth (Nissui) for 18 h, the bacterial cells were washed several times and diluted with sterile saline. The diluted bacteria solution was aseptically added to each assay mixture and allowed to stand for 16–18 h at 37 °C. To estimate bacterial growth, the turbidity of each assay mixture was measured at 600 nm using the a ultraviolet–visible (UV-Vis) spectrophotometer (UV-2550; Shimadzu, Kyoto, Japan).
Lactobacillus delbrueckii subsp. lactis ATCC 7830 requires B12 as an essential nutrient, but exhibits nucleotide and deoxyribonucleotide activity, which is known to be an alkali-resistant factor, as well as B12. The correct B12 values were calculated by subtracting the values for the alkali-resistant factor from the total B12 values. The recovery rate of B12 from the extraction was calculated to be 105% after adding a known amount of authentic B12 to a sample.
Analysis of B12 compounds purified from the muscles or head innards of shrimp using LC/ESI–MS/MS
After ~ 30 g muscle or ~ 2 g head innards from Alaskan pink shrimp and Kuro shrimp, respectively, was homogenized using a mortar and pestle, the B12 compounds were extracted by boiling at pH 4.5 in the presence of KCN, as described above. The B12 compounds were partially purified from the extract using Sep-Pak Vac 20 cc (5 g) C18 cartridges (Waters, Milford, MA) that were washed with 20 ml 75% (v/v) ethanol and then equilibrated with 20 ml Milli-Q water (Merck Millipore, Burlington, MA). The extract was filtered through a 150-mm-diameter Whatman filter paper (GE Healthcare) and loaded onto the C18 cartridge, which was washed with 25 ml Milli-Q water (Merck Millipore). The B12 compounds were eluted with 10 ml of 75% (v/v) ethanol. The eluate was evaporated to dryness under reduced pressure using the Integrated SpeedVac System ISS110 centrifugal concentrator (Savant Instruments, Holbrook, NY). The residue was dissolved in 1 ml Milli-Q water (Merck Millipore) and loaded into an EASI-EXTRACT vitamin B12 immunoaffinity column (P80; R-Biopharm, Darmstadt, Germany), and the B12 compounds were purified according to the manufacturer’s instructions. The purified B12 compounds were dissolved in Milli-Q water (Merck Millipore) and filtered through a Millex-LH membrane filter (Merck Millipore). Aliquots (5 µl) of the filtrate were analyzed using an ACQUITY UPLC H-Class Xevo G2-SQT (Waters). Each purified sample was injected into a 3-µm 2.1 × 100-mm InertSustain column (GL Science, Tokyo) equilibrated with 85% solvent A [0.1% (v/v) acetic acid] and 15% solvent B (methanol) at 40 °C. The B12 compounds were eluted using a linear gradient of methanol (15% solvent B for 0–5 min, 15–90% solvent B for 5–11 min, and 90–15% solvent B for 11–15 min) at a flow rate 0.2 ml/min. The identification of authentic B12 (m/z 678.2914 representing [M+2H]2+; retention time 9.55 min) was confirmed by comparing the observed molecular ions and their retention times.
To determine the relative content (percent) of B12, B12-d-monocarboxylic acid, and tentatively identified B12-dicarboxylic acids in the head innards of both shrimp, the absorbance was measured at 361 nm. The relative content of each peak area against total peak area (i.e., the sum of the peak area of B12 and B12-acid compounds) was calculated. The recovery rate of B12 from the extraction and purification was calculated to be 102% after adding a known amount of authentic B12 to a sample.
High-performance liquid chromatography analysis of B12-b-, -d-, and -e-monocarboxylic acids and unidentified B12 compounds found in the head innards of Alaskan pink shrimp P. eous
B12 compounds were purified from the head innard extract from the Alaskan pink shrimp using the B12 immunoaffinity column as described above. The purified compounds were dissolved in 80 μl Milli-Q water (Merck Millipore), filtered through a Millex-LH membrane (Merck Millipore), and loaded into the Shimadzu HPLC system (SPD-10AV UV–Vis detector, SCL-10A VP system controller, DGU-20A3 degasser, LC-10Ai liquid chromatograph, and CTO-20A column oven). A 35-µl aliquot of the purified compounds was loaded into a reversed-phase high-performance liquid chromatography column (φ 4.6 × 150 mm; Wakosil-II 5C18RS; FUJIFILM Wako Pure Chemical, Osaka, Japan), isocratically eluted with 20% (v/v) methanol solution containing 1% (v/v) acetic acid at 40 °C, and monitored by measuring the absorbance at 361 nm. The flow rate was 1 ml/min. Authentic B12 and B12-b-, -d-, and -e-monocarboxylic acids (each 1 μg) were analyzed under the same conditions.
Results
The B12 contents of the muscles and head innards of four species of shrimp were determined using a microbiological assay based on L. delbrueckii subsp. lactis ATCC 7830 (Table 1). The shrimp muscles contained ~ 2.4–4.3 µg B12/100 g wet weight, the values of which were identical to those described in the Standard Tables of Food Composition in Japan (Ministry of Education, Culture, Sports, Science and Technology 2010) but approximately eight times greater in the head innards than in the muscles. Shrimp-innard products A and B also contained ~ 28.5–30.1 µg B12/100 g wet weight. The muscles, head innards, and whole bodies (except the shells) of the shrimp tested contained ~ 0.2, 0.3, and 0.5 µg B12/fresh total body weight (g), respectively. These results suggest that shrimp is a source of B12 for humans.
To evaluate whether the muscles and head innards of Alaskan pink shrimp contain B12 or an inactive corrinoid, such as pseudo-B12, corrinoids were purified using an immunoaffinity column and analyzed using LC/ESI–MS/MS. Authentic B12 eluted as one ion peak with a retention time of 9.6 min (Fig. 1a). The mass spectrum of authentic B12 indicated that a doubly charged ion with an m/z of 678.2894 [M+2H]2+ was prominent (Fig. 1b). The exact mass calculated from its formula of C63H88CoN14O14P was 1354.5674 g/mol, and the isotope-distribution data showed that B12 was the major doubly charged ion under LC/ESI–MS/MS conditions. The MS/MS spectrum of authentic B12 indicated that its singly charged ions at m/z 359.1007 and m/z 997.4782 were attributable to the nucleotide and corrin ring moieties, respectively (Fig. 1c). The compounds purified from the muscles of Alaskan pink shrimp eluted as one ion peak with a retention time of 9.5 min (Fig. 2a), and its mass spectrum primarily comprised a doubly charged ion with m/z 678.2894 [M+2H]2+ (Fig. 2b). The MS/MS spectrum of the purified compounds with singly charged ions at m/z 359.1007 and m/z 997.4782 were identical to those of authentic B12 (Fig. 2c).
Liquid chromatography–electrospray ionization/tandem mass spectrometry (LC/ESI–MS/MS) chromatograms of authentic B12. a Total ion chromatogram (TIC) and mass chromatogram of authentic B12 (m/z 678.29). b Mass spectrum of authentic B12 (insert magnified spectrum from m/z 678 to 680). c MS/MS spectrum of the peak of authentic B12 at m/z 678.2894
LC/ESI–MS/MS chromatograms of corrinoid compounds purified from muscles of Pandalus eous Makarov, 1935. a TIC and mass chromatogram of the corrinoid compounds (m/z 678.29). b Mass spectrum of the corrinoid compounds at 9.49 min (insert magnified spectrum from m/z 678 to 680). c MS/MS spectrum of the peak of the corrinoid compounds at m/z 678.2894. For abbreviations, see Fig. 1
The corrinoid compounds purified from the head innards of Alaskan pink shrimp eluted as three ion peaks with retention times of 9.4, 9.8, and 10.0 min (Fig. 3a), respectively. The MS spectrum of the ion peak with a retention time of 9.4 min showed that the doubly charged B12 ion was formed at m/z 678.2894 [M+2H]2+ (Fig. 3b). The MS/MS spectrum of the latter peak was identical to that of authentic B12 (Fig. 3e). The MS spectra of the ion peaks with a retention time of 9.8 min (compound A) and 10.0 min (compound B) showed that a major doubly charged ion was formed at m/z 678.7849 [M+2H]2+ and 679.2751 [M+2H]2+, respectively (Fig. 3c, d). The MS/MS spectra of compounds A and B with a singly charged ion at m/z 359.1007 were identical to that of authentic B12; however, the MS/MS spectra of compounds A and B with singly charged ions at m/z 998.4603 and m/z 999.4493, respectively, were not identical to that of authentic B12 at m/z 997.4782 (Fig. 3f, g). These results suggest that the head innards of Alaskan pink shrimp contained B12 and two B12 compounds, A and B, which were B12 compounds with different corrin ring moieties that have one and two additional hydrogen atoms, respectively (Figs. 2, 3). Similar results were obtained for the other shrimp tested.
LC/ESI–MS/MS chromatograms of corrinoid compounds purified from the head innards of P. eous Makarov, 1935. a TIC and mass chromatogram of the corrinoid compounds (m/z 678.29). b–d Mass spectra of the corrinoid compounds with retention times of 9.49 min (the magnified spectrum from m/z 678 to 680 is shown as an insert in b), 9.81 min (the magnified spectrum from m/z 678 to 681 is shown as an insert in c), and 10.04 min (the magnified spectrum from m/z 679 to 681 is shown as an insert in d), respectively. e–g MS/MS spectra of the peaks of the corrinoid compounds with retention times of 9.49 min (m/z 678.2894), 9.81 min (m/z 678.7849), and 10.04 min (m/z 679.2751), respectively. For abbreviations, see Fig. 1
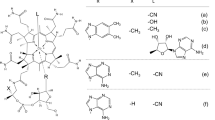
The chemical structure of B12 and the molecular masses of the unidentified B12 compounds were used to predict their identity. Compound A was hypothesized to be B12-monocarboxylic acid while compound B was predicted to be B12-dicarboxylic acid (Fig. 4). Immunoaffinity chromatography was used to purify corrinoids from head innard extract obtained from Alaskan pink shrimp. This was followed by analysis using a C18-reversed phase HPLC column. HPLC studies showed that B12, unidentified compound A and unidentified B compound eluted as three separate peaks with retention times of 9.8, 16.2, and 18.3 min, respectively (Fig. 5a). HPLC analysis of known forms of B12 under the same conditions showed that authentic B12-b-, -d-, and -e-monocarboxylic acids eluted as single peaks with retention times of 9.7, 13.5, 16.2, and 20.0 min, respectively (Fig. 5b–e). The retention time of B12-d-monocarboxylic acid was identical to that of compound A (retention time of 16.2 min). When B12-d-monocarboxylic acid was analyzed using LC/ESI–MS/MS, it eluted as an ion peak with a retention time of 9.6 min (Fig. 6a). This was similar to be the retention time observed for compound A (retention time of 9.8 min) (Fig. 3a). Thus, MS and MS/MS spectra of B12-d-monocarboxylic acid (Fig. 6b, c) were identical to that of compound A (Fig. 3c, f).
Chemical structures of unidentified B12 compounds A and B predicted using LC/ESI–MS/MS analysis. Compounds A and B were identified as B12-monocarboxylic acid and a B12-dicarboxylic acid, respectively. For abbreviations, see Fig. 1
LC/ESI–MS/MS chromatograms of B12-d-monocarboxylic acid. a TIC and mass chromatogram of B12-d-monocarboxylic acid (m/z 678.78), b mass spectrum of the corrinoid compounds with retention times of 9.6 min (insert magnified spectrum from m/z 678 to 681), c MS/MS spectrum showing the peak of the corrinoid compound with a retention time of 9.6 min (m/z 678.78). For abbreviations, see Fig. 1
However, the identity of compound B could not be established as the preparation methods for B12-bd-, -be-, and -de-dicarboxylic acids have not been reported. At present, compound B can be hypothetically identified as one of these B12-dicarboxlyic acids.
To determine the relative B12 content (%), B12-d-monocarboxylic acid and B12-dicarboxlyic acid in the shrimp head innards of Alaskan pink shrimp and Kuro shrimp were selected because of their high B12 contents compared with those of the other shrimp tested. The relative B12 content (%) of each peak area against the total peak area (i.e., the sum of the peak area of B12 and unidentified compounds) was calculated by measuring the absorbance at 361 nm (Table 2). Approximately 65% of the B12 compounds identified in the head innards of Alaskan pink shrimp and 25% in the case of Kuro shrimp were derived from B12-monocarboxylic and -dicarboxylic acids. The total B12 content in shrimp head innard products A and B contained ~ 22% these B12 acid compounds.
The ability of B12-d-monocarboxylic acid to show B12 activity was studied using the standard microbiological assay with L. delbrueckii subsp. lactis ATCC 7830. In particular, the effect of various doses of B12 and B12-d-monocarboxylic acid (0.01–0.05 pmol) on the growth of the lactic acid bacterium was studied (Fig. 7). At a concentration of 0.05 pmol, the activity shown by B12-d-monocarboxylic acid was only 6% of that displayed by the authentic B12. These results suggest that B12-d-monocarboxylic acid hardly functions as B12 in this bacterium.
Discussion
Although shrimp is one of the most consumed seafoods worldwide and is a good source of several nutrients (Kaymaci and Altun 2016), the detailed characteristics of B12 have not been elucidated in this seafood. Thus, we determined the B12 content in both the muscles and head innards of various shrimp species using a microbiological assay based on L. delbrueckii subsp. lactis ATCC7830. As shown in Table 1, approximately 2–4 µg B12/100 g wet weight was detected in shrimp muscles. The shrimp head innards contained significantly higher levels of B12 (~ 12–33 µg/100 g wet weight). Consumption of approximately eight to 14 shrimp muscles, six to seven shrimp head innards, and three to six whole shrimp bodies could supply the recommended dietary allowance of B12 for adults (2.4 µg/days) (Institute of Medicine 1998), which suggests that shrimp is a suitable source of B12 for humans.
We purified B12 compounds from the extracts of shrimp muscles and head innards using an immunoaffinity column, and the purified B12 compounds were identified using LC/ESI–MS/MS. The shrimp muscle extracts were characterized by the presence of only B12 while the extracts obtained from shrimp head innards contained three corrinoid compounds. These head innard extracts contained large amounts of B12 and smaller amounts of two unidentified B12 compounds denoted as compound A and compound B. The HPLC and LC/ESI–MS/MS analyses identified compound A as B12-d-monocarboxylic acid (Figs. 5, 6). The identity of compound B could not be established completely and it was hypothetically identified as one of the B12-dicarboxylic acids because of the presence of additional masses of two hydrogen atoms for B12 compounds having different corrin ring moieties. The content of the B12-mono and -dicarboxylic acids varied from 17 to 65% of the total B12 content in shrimp head innards and their products (Table 2), which suggested that these B12 acid compound levels were dependent on the species sampled.
An intrinsic factor (IF) plays an important role in the gastrointestinal absorption of B12 in humans. This protein is known to have high specific binding for authentic B12; however, it lacks any significant affinity towards B12-b-, -d-, and -e-monocarboxylic acids or B12-bde-tricarboxylic acid (Kolhouse and Allen 1977). The low affinity of this IF protein for B12 mono- and tricarboxylic acids explains the poor absorption of these compounds following their oral administration in rabbits (Kolhouse and Allen 1977). Saido et al. (1993) reported that oral and intravenous administration of B12-b-, -d-, and -e-monocarboxylic acids did not show any improvement in the B12 levels of B12-deficient rats. These studies strongly suggest that both B12-d-monocarboxylic acid and B12-dicarboxylic acids found in shrimp head innards might not be absorbed by the human intestine. However, shrimp head innards can be still considered a source of B12 for humans owing to their contents of B12-mono and -dicarboxylic acids (17–65% of the total B12 content).
Anton et al. (1980) reported the formation of B12-mono- and -dicarboxylic acids on mild acid hydrolysis of B12. These B12 acids are derived from the propionamide side chains b, d, and e, which are more susceptible to hydrolysis than the acetamide side chains a, c, and g (Bernhauer et al. 1966) (Fig. 4). The presence of these B12 acid compounds in shrimp head innards is quite unusual. It is possible that trace elements are absorbed from sea water and accumulate in shrimp head innards (Kaymaci and Altun 2016), and might react with B12 to produce these acid compounds. However, no detailed mechanism has been reported so far to explain this hypothesis.
Euglena gracilis Z, a B12-dependent alga, has been widely utilized in microbiological assays for B12. Supplementation of growth culture medium with B12-d-monocarboxylic acid increased the growth of Euglena to similar levels to those observed in authentic B12-supplemented culture medium (Watanabe et al. 1992). L. delbrueckii subsp. lactis ATCC 7830 is a bacterium commonly used to determine the B12 content of food. As shown in Fig. 7, B12-d-monocarboxylic acid did not show any significant effect on the growth of this bacterium. There are no reports on the effect of B12-dicarboxylic acids on the growth of L. delbrueckii subsp. lactis ATCC 7830. Since the structure of B12 changed more in the case of B12-dicarboxylic acid compared to B12-d-monocarboxylic acid, it is possible that B12-dicarboxylic acids are inactive in this bacterium. The possible inactivity of B12-d-monocarboxylic and B12-dicarboxylic acids in L. delbrueckii subsp. lactis ATCC 7830 suggests that these compounds might not affect the B12 content of shrimp head innards.
References
Anton DL, Hogenkamp HPC, Walker TE, Matwiyoff NA (1980) Carbon-13 nuclear magnetic resonance studies of the monocarboxylic acids of cyanocobalamin. Assignments of the b-, d-, and e-monocarboxylic acids. J Am Chem Soc 102:2215–2219
Bernhauer K, Wagner F, Beisbarth H, Rietz P, Vogelmann H (1966) Zur Chemie und Biochemie der Corrinoide XXVI. Darstellung und Charakterisierung von Corrinoidcarbonsauren. Biochem Z 344:289–309
Bito T, Tanioka Y, Watanabe F (2018) Characterization of vitamin B12 compounds from marine foods. Fish Sci 84:747–755
Bourre JME, Paquotte PM (2008) Contributions (in 2005) of marine and fresh water products (finfish and shellfish, seafood, wild and farmed) to the French dietary intakes of vitamins D and B12, selenium, iodine and docosahexaenoic acid: impact on public health. Int J Food Sci Nutr 59:491–501
Butcherine P, Benkendorff K, Kelaher B, Barkla BJ (2019) The risk of neonicotinoid exposure to shrimp aquaculture. Chemosphere 217:329–348
Heal KR, Qin W, Ribalet F, Bertagnolli AD, Coyote-Maestas W, Hmelo LR, Moffett JW, Devol AH, Armbrust EV, Stahl DA, Ingalls AE (2017) Two distinct pools of B12 analogs reveal community interdependencies in the ocean. Proc Natl Acad Sci USA 114:364–369
Institute of Medicine (1998) Vitamin B12. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Institute of Medicine, National Academies Press, Washington, DC, pp 306–356
Kaymaci S, Altun BE (2016) Seasonal variation in the accumulation of trace elements and contaminants in five shrimp species from Iskenderun Bay and their consumibility as human food. Bull Environ Contam Toxicol 97:237–243
Kolhouse JF, Allen RH (1977) Absorption, plasma transport, and cellular retention of cobalamin analogues in rabbit: evidence for the existence of multiple mechanisms that prevent the absorption and tissue dissemination of naturally occurring cobalamin analogues. J Clin Invest 60:1381–1392
Ministry of Education, Culture, Sports, Science and Technology (2010) In: Standard Tables of Food Composition in Japan-2010. The Council for Science and Technology, Ministry of Education, Culture, Sports, Science and Technology, Tokyo, pp 174–176 (in Japanese)
Saido H, Watanabe F, Tamura Y, Manai Y, Nakano Y (1993) Effects of vitamin B12 analogues with alternations in the side chains of the corrin ring on urinary methylmalonate excretion in vitamin B12-deficient rats. Biosci Biotechnol Biochem 57:607–610
Scheers N, Lindqvist H, Langkilde AM, Undeland I, Sandberg AS (2014) Vitamin B12 as a potential compliance marker for fish intake. Eur J Nutr 53:1327–1333
Teng F, Tanioka Y, Hamaguchi N, Bito T, Takenaka S, Yabuta Y, Watanabe F (2015) Determination and characterization of vitamin B12 compounds in edible sea snails, ivory shell Babylonia japonica and turban shell Turdo Batillus cornutus. Fish Sci 81:1105–1111
Watanabe F, Bito T (2016) Corrinoids in food and biological samples. In: Atta-ur-Rahman X (ed) Frontiers in natural product chemistry, vol 2. Bentham, Sharjah, pp 229–244
Watanabe F, Bito T (2018a) Vitamin B12 sources and microbial interaction. Exp Biol Med 243:148–158
Watanabe F, Bito T (2018b) Determination of cobalamin and related compounds in foods. J AOAC Int 101:1308–1313
Watanabe F, Nakano Y, Stupperich E (1992) Different corrinoid specificities for cell growth and the cobalamin uptake system in Euglena gracilis Z. J Gen Microbiol 138:1807–1813
Watanabe F, Katsura H, Takenaka S, Fujita T, Abe K, Tamura Y, Nakatsuka T, Nakano Y (1999) Pseudovitamin B12 is the predominant cobamide of an algal health food, Spirulina tablets. J Agric Food Chem 47:4736–4741
Watanabe F, Yabuta Y, Bito T, Teng F (2014) Vitamin B12-containing plant food sources for vegetarians. Nutrients 6:1861–1873
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science KAKENHI grant number 16K07736 (to F. W.).
Author information
Authors and Affiliations
Contributions
N. O. and N. H. performed most of the experiments. Y. U. and S. T. analyzed the B12 compounds using LC/ESI–MS/MS and interpreted the results. N. O., T. B., and F. W. designed the experiments, interpreted the results, and wrote the manuscript. All authors reviewed and commented on the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okamoto, N., Hamaguchi, N., Umebayashi, Y. et al. Determination and characterization of vitamin B12 in the muscles and head innards of edible shrimp. Fish Sci 86, 395–406 (2020). https://doi.org/10.1007/s12562-019-01397-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-019-01397-x