Abstract
We tested desiccation and/or vitrification procedures to cryopreserve the adventitious roots of Panax ginseng, the source of commercially produced ginsenosides. When only desiccation was applied, the post-freeze survival of 3- to 4-mm root tips was <14% regardless of the composition of the preculture medium or the explant origin. Callus formation was frequently observed after cryopreservation. In contrast, 90% survival and 32.5% root formation efficiency were achieved after cryopreservation when a vitrification protocol was followed. Adventitious root cultures in flasks and bioreactors were reestablished from root tips cryopreserved by vitrification. A prolonged lag-phase and lower biomass production were recorded in post-freeze-regenerated cultures compared with control roots that were subcultured four times in flasks. However, biomass accumulations did not differ between control and regenerated roots at the end of the sixth subculturing period. After 40 days of culture in bioreactors, a mean value of 12.5 g dw L−1 was recorded for post-freeze-regenerated cultures versus 9.1 g dw L−1 for the control roots. Production of triol and diol ginsenosides in our bioreactor cultures also was enhanced after cryopreservation, by 41.0% and 89.8%, respectively. These results suggest that the vitrification method is successful for cryopreservation of P. ginseng adventitious roots.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Panax ginseng C.A. Meyer is an important Asian medicinal plant with numerous pharmacological activities, including anticancer, anti-aging, and antioxidant effects (Choi et al. 2007; Chen et al. 2008). Its major bioactive ingredients are ginseng saponins (ginsenosides). Based on their structural differences, these ginsenosides can be classified into two groups: 20(S)-protopanaxatriols and 20(S)-protopanaxadiols (Chen et al. 2008). Large-scale bioreactor cultures of P. ginseng adventitious roots are a safe and effective alternative to field-grown plants or hairy root cultures (Kim et al. 2007) and allow for the commercial production of ginsenosides (Hahn et al. 2003). This technology requires that the reserve stock of adventitious roots be maintained in 20-L bioreactors and subcultured manually. Such collections, however, are cost- and labor-intensive, and they encumber risks of contamination and operator mistakes. Therefore, cryopreservation is the most reliable and economically effective method for long-term maintenance of clonally propagated plant germ plasm (Sakai and Engelmann 2007). This approach, then, necessitates an efficient cryopreservation protocol that enables one to regenerate adventitious root cultures and also retain their growth and biosynthetic characteristics.
To date, cryopreservation has been successful for the hairy root cultures of numerous medicinal plants (Benson and Hamill 1991; Teoh et al. 1996; Hirata et al. 1998, 2002; Xue et al. 2008; Lambert et al. 2009). However, only a few reports are available for its use with adventitious roots (Jung et al. 2001). A vitrification method based on the consecutive dehydration of plant material with highly concentrated cryoprotector solutions has proven effective for the cryopreservation of Hyoscyamus niger and Atropa belladonna root cultures (Jung et al. 2001; Touno et al. 2006). Nevertheless, that method has led to poor results from Maesa lanceolata hairy roots due to the toxicity of the cryoprotector solution (Lambert and Geelen 2007). In P. ginseng hairy roots, a 54% post-freeze recovery rate has been achieved with a vitrification protocol, but lateral root formation has been suppressed after cryopreservation (Yoshimatsu et al. 1996). In contrast to transformed roots, adventitious roots require auxins for their induction, proliferation, and maintenance (Kim et al. 2007). Thus, the possibility of applying a vitrification protocol to P. ginseng adventitious roots should be thoroughly investigated. A desiccation method is routinely utilized for embryogenic cultures. Its advantages are its simplicity and the omission of a toxic cryoprotector treatment (Bomal and Tremblay 2000; Danso and Ford-Lloyd 2004). However, this method has not previously been tested with cultured roots. Preliminary culturing of plant material on a medium supplemented with high sucrose concentrations to induce desiccation and chilling tolerance is considered essential to the success of this method (Grenier-de March et al. 2005).
Here, we examined desiccation and vitrification methods to cryopreserve the adventitious roots of P. ginseng. The growth and biosynthetic characteristics of regenerated root cultures were assayed and compared with those of untreated roots to monitor the possible effects of cryopreservation.
Materials and Methods
Adventitious Root Culture in Bioreactors
Adventitious roots of P. ginseng C.A. Meyer were induced and proliferated as described by Kim et al. (2007). Cultures were maintained in 5-L bioreactors containing 4 L of a modified MS liquid medium supplemented with 5 mg L−1 of IBA and 50 g L−1 of sucrose (basal medium (BM)) at 22 ± 1°C under darkness (Kim et al. 2007). Air was supplied at 0.1 vvm. Subculturing was performed every 40 days by transferring 5 g L-1 fresh roots to bioreactors with fresh BM.
Desiccation Procedure
Two types of explants were used in our desiccation experiments: (1) root tips (3 to 4 mm long) were excised and incubated for 9 days on a Gelrite-solidified basal medium then transferred to a preculture medium (see below) or (2) 8- to 10-mm roots were excised and incubated for 9 days on a Gelrite-solidified basal medium before 3- to 4-mm root tips were excised and immediately transferred to the preculture medium. Preculturing was done for 7 or 16 days under darkness on a modified MS medium (Kim et al. 2007) that was supplemented with 10% (w/v) sucrose and 2.2 g L−1 Gelrite without growth regulators. Precultured root tips were placed on 5 × 25-mm strips of sterile filter paper (Whatman No. 4; Whatman Ltd., England) in open 90-mm Petri dishes. Desiccation was performed under the air current from a laminar airflow clean bench for 0, 20, 40, or 60 min.
To estimate survival, root tips desiccated for certain periods were transferred to a basal medium and kept at 21 ± 1°C in the dark. Survival was defined by the number of root tips that showed callus induction or elongation 35 days after treatment. Three replications were performed, with 10 to 15 root tips per replication.
Vitrification Procedure
Root tips (3 to 4 mm long) were excised from our bioreactor-cultured adventitious roots after a routine 40-day subculture period. They were incubated for 12 days in 100-ml flasks containing 20 ml of liquid BM at 22 ± 1°C in the dark (rotated at 90 rpm). Afterward, 12 to 15 root tips were suspended for 15 min in 2 ml of loading solution (2 M glycerol and 0.4 M sucrose; Nishizawa et al. 1993) in 2-ml cryotubes (Nalgene, USA). They were then placed for various periods into 2 ml of modified Plant Vitrification Solution 2 (PVS2, Sakai et al. 1990) containing 30% (w/v) glycerol, 15% PEG6000, 15% dimethyl sulfoxide, and 0.4 M sucrose. One milliliter of the PVS2 was then discarded, and the cryotubes were sealed and plunged directly into liquid nitrogen. After at least 1 h, the samples were rewarmed rapidly in a water bath at 41°C for 60 s. The root tips were retrieved from their cryotubes and unloaded with 2 ml of liquid BM containing 1.2 M sucrose without growth regulators for 15 min. For reculturing, the root tips were transferred to Gelrite-solidified BM and incubated at 21 ± 1°C under darkness. Survival (i.e., elongation with or without root formation), root formation, and the number of lateral roots per explant were recorded after 35 days and expressed as percentages. Three replications were performed, with 12 to 15 root tips per replication.
Reestablishment of Flask and Bioreactor Cultures
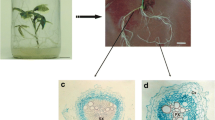
New adventitious roots that had developed from samples cryopreserved by vitrification were further proliferated as shown in Fig. 1. Roots were subcultured twice on Gelrite-solidified BM at 30-day intervals, followed by propagation in 100- and 300-ml flasks. The bioreactors were inoculated from 300-ml flasks at the end of the fifth subculture period. Inoculum sizes were 5 g L−1 fresh weight (fw). Both flask and bioreactor cultures were performed in Basal media at 21 ± 1°C in the dark. Flasks were rotated at 90 rpm, and air in the bioreactors was supplied at 0.1 vvm.
Reestablishment of Panax ginseng adventitious root cultures from cryo-preserved root tips. All cultures were performed in modified MS medium (Kim et al. 2007) supplemented with 5 mg IBA L-1 and 50 g sucrose L-1 at 22 ± 1°C in darkness
Determination of Growth Curve and Root Weights
In flask cultures, growth profiles were recorded twice at the fourth and sixth subculture intervals. Three to four flasks were harvested every 3 to 5 days for each point on our growth curve. Biomass production in the bioreactors was determined at day 40 after inoculation. Three bioreactors were harvested for each treatment. Roots were washed with running tap water for 2 min followed by distilled water. After filtering under vacuum, root fresh weights were recorded, and their dry weights were determined after drying at 60°C for 3 days.
Extraction and Analysis of Ginsenosides
Extraction and analysis of ginsenosides in bioreactor-cultured adventitious roots were performed as described by Kim et al. (2007), using an HPLC system equipped with an Altec Platinum C18 column (33 × 7 mm; particle size 1.5 µm). Ginsenosides were eluted with water/acetonitrile (3:1, v/v) for 10 min, then at 63:37 for 25 min, with a flow rate of 1.2 ml min−1. Ginsenosides were detected at 203 nm. The ginsenoside standards were purchased from Chromadex (USA). Triol fractions were calculated as the sum of ginsenosides Rg1, Re, Rf, and Rh1. The diol fraction was equal to the sum of ginsenosides Rb1, Rb2, Rb3, Rc, Rd, Rg3, and Rh2. The total ginsenoside content was calculated as the sum of those triol and diol ginsenoside fractions. Three bioreactors were harvested for each set of experimental conditions, and analyses were performed for triplicate extracts.
Statistical Analysis
All data were analyzed by SAS Version 9.1 (SAS, Raleigh, NC). The significance of differences between treatments was assessed using a Duncan′s multiple range test at P ≤ 0.05.
Results and Discussion
In all experiments, the first sign of survival was evidenced by swelling of the root tips followed by callus formation or root elongation. New lateral roots developed 10 to 12 days after treatment but only from elongated root tips.
Effect of Cryopreservation Procedure
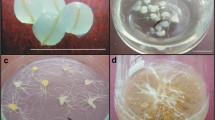
To avoid lethal intracellular ice formation, freezable water should be removed from samples prior to cryopreservation (Sakai 2000). The simplest desiccation procedure involves sufficient desiccation of plant material in the air current of a laminar airflow cabinet followed by rapid freezing (Engelmann 2004). In our study, root tips of P. ginseng could not survive desiccation of >40 min without first being pre-cultured on a sucrose-enriched medium (data not shown). Incubation of root tips in the presence of 10% (w/v) sucrose for 7 or 16 d improved survival to 18 and 100%, respectively, after 40 min of desiccation (Table 1). That desiccation period could be extended to 60 min, with 67% survival, if root tips were first pre-cultured on a sucrose-enriched medium for 16 d. Incubation of excised root tips on the basal medium for 9 d before sucrose preculture (Explant type I) was slightly beneficial when desiccation was performed for 40 min. If desiccation was followed by cryopreservation, survival did not exceed 14% for any of our treatments (Table 1). Moreover, survival of both desiccated and cryo-preserved samples was mostly via callus formation (Fig. 2a). Lateral roots developed occasionally but exhibited slow growth and low capacity to produce new roots (Fig. 2b). Another important disadvantage of this method was that the exact water content of desiccated root tips could not be measured because of their tiny size. Thus it was difficult to determine the proper desiccation time for the explants.
In vitrification method, freezable water is osmotically removed from samples by incubating them in highly concentrated cryoprotector solutions (Sakai and Engelmann 2007). Plant Vitrification Solution 2 (PVS2), designed by Sakai et al. (1990), has been successfully applied to diverse plant materials, including shoot tips, cell suspensions, and embryogenic tissues (Sakai and Engelmann 2007). This solution has been found promising when used in the cryopreservation of H. niger and A. belladonna root cultures (Jung et al. 2001; Touno et al. 2006). However, explants usually can be exposed to PVS2 for only a very short time because of the toxicity and severe osmotic stress provoked by its high cryoprotectant concentration. Therefore, we investigated its negative effect on P. ginseng root tips before performing cryopreservation. Based on results presented in Fig. 3, we assumed that root formation was more susceptible to PVS2 than was survival. Treatment for more than 15 min was detrimental to lateral root development. However, in contrast to the desiccation protocol, callus formation was sometimes observed after PVS2 exposure and cryopreservation regardless of the duration of treatment. We recorded a maximum survival rate of 90% and a root formation efficiency of 32.5% when root tips were cryopreserved after incubation with PVS2 for 15 min (data not shown). Thus, we demonstrated that Panax adventitious roots are more sensitive to cryopreservation than are hairy roots, which have 60% recovery after being cryopreserved by vitrification method (Yoshimatsu et al. 1996). The number of new roots that developed after cryopreservation was significantly lower (P < 0.05) compared with tissues that had been PVS2-treated but not cryopreserved, and the length of exposure to the cryoprotector solution had no apparent effect (Fig. 4). Yoshimatsu et al. (1996) also have found that cryopreservation of P. ginseng hairy root tips significantly suppresses the formation of lateral roots. However, we observed that the amount of newly regenerated roots was sufficient for their further proliferation and the reestablishment of flask and bioreactor cultures (see Fig. 1).
Toxicity assay of Plant Vitrification Solution 2 (PVS2) for adventitious roots. Tips (3-4 mm long) were incubated in basal medium for 12 d, then treated with loading solution (LS) for 15 min followed by PVS2 for 0-20 min. Tips were unloaded with basal medium containing 1.2 M sucrose for 15 min, and regenerated on Gelrite-solidified BM in darkness for 35 d
Effect of cryopreservation on number of new roots developed from P. ginseng root tips that had been pre-treated as described for Fig. 3
Post-Freeze Characteristics of Adventitious Root Cultures
Growth profiles of post-freeze-regenerated root cultures were recorded twice, at the fourth and sixth subcultures in 300-ml flasks. These were compared with a standard growth curve for the untreated roots (Fig. 5). The long lag phase in those two subcultures could have been a direct reflection of freezing injury (Kartha and Engelmann 1994). Lower biomass accumulations in the regenerated culture (compared with the control) were recorded at the fourth flask subculture. However, at the sixth subculture, the final amount of biomass produced by the regenerated culture was equal to that by the untreated control. This culture, therefore, retained its growth capability after cryopreservation as confirmed by root dry weight measurements in the bioreactors. At day 40 of bioreactor-culturing, the dw mean root was 12.5 g L−1 for tissues regenerated after cryopreservation compared with 9.1 g L−1 for the control roots (Table 2). A similar enhancement of growth and biomass productivity has been detected in P. ginseng cell cultures after cryopreservation (Joshi and Teng 2000), and an increase in fresh weight following cryopreservation has also been reported for H. niger adventitious roots (Jung et al. 2001). Finally, no differences in growth rates have been found for either Astragalus membranaceus or Gentiana macrophylla hairy roots after cryopreservation when compared with their noncryopreserved counterparts (Xue et al. 2008).
Contents of both diol and triol ginsenoside groups increased after cryopreservation (Table 2), with the total ginsenoside content (i.e., the sum of triol and diol fractions) rising to 3.27 versus 1.98 mg g−1 dw for the control culture. To our knowledge, this is the first report on post-freeze enhancement of metabolite production in root cultures. P. ginseng hairy roots regenerated after cryopreservation produce five major ginsenosides at a basal level (Yoshimatsu et al. 1996). Production of betacyanin, betaxanthin, and alkaloids in Beta vulgaris and Nicotiana rustica hairy roots also is retained after cryopreservation (Benson and Hamill 1991). Furthermore, the content of tropane alkaloids in post-freeze-recovered adventitious roots of H. niger is comparable to those in the control roots (Jung et al. 2001), and vincamine content remains stable after Vinca minor hairy roots are cryopreserved (Hirata et al. 2002). It is possible that cryopreservation induces a stress response in P. ginseng adventitious roots which, in turn, stimulates the production of secondary metabolites (Kim et al. 2007; Wu et al. 2007).
Our results suggest that the desiccation method is inappropriate for the cryopreservation of P. ginseng adventitious roots because of their extreme sensitivity to such treatment. In contrast, root tips can be successfully cryopreserved through vitrification. Here, adventitious root cultures after cryopreservation resumed their usual growth and biosynthetic characteristics at the same or greater level than the untreated control. Further studies will be conducted to improve post-freeze root formation and to achieve rapid post-freeze regeneration of cultures.
References
Benson EE, Hamill JD (1991) Cryopreservation and post freeze molecular and biosynthetic stability in transformed roots of Beta vulgaris and Nicotiana rustica. Plant Cell Tiss Organ Cult 24:163–172
Bomal C, Tremblay FM (2000) Dried cryopreserved somatic embryos of two Picea species provide suitable material for direct plantlet regeneration and germplasm storage. Ann Bot 86:177–183
Chen CF, Chiou WF, Zhang JT (2008) Comparison of the pharmacological affects of Panax ginseng and Panax quinquefolium. Acta Pharmacol Sin 29:1103–1108
Choi YE, Kim YS, Yi MJ, Park WG, Yi JS, Chun SR, Han SS, Lee SJ (2007) Physiological and chemical characteristics of field- and mountain-cultivated ginseng roots. J Plant Biol 50:198–205
Danso KE, Ford-Lloyd BV (2004) Cryopreservation of embryogenic calli of cassava using sucrose cryoprotection and air desiccation. Plant Cell Rep 22:623–631
Engelmann F (2004) Plant cryopreservation: progress and prospects. In Vitro Cell Dev. Biol Plant 40:427–433
Grenier-de March G, de Boucaud MT, Chmielarz P (2005) Cryopreservation of Prunus avium L. embryogenic tissues. Cryo-Lett 26:341–348
Hahn EJ, Kim YS, Yu KW, Jeong CS, Paek KY (2003) Adventitious root cultures of Panax ginseng C.A. Meyer and ginsenoside production through large-scale bioreactor system. J Plant Biotechnol 5:1–6
Hirata K, Goda S, Phunchindawan M, Du D, Ishio M, Sakai A, Miyamoto K (1998) Cryopreservation of horseradish hairy root cultures by encapsulation-dehydration. J Ferment Bioeng 86:418–420
Hirata K, Mukai M, Goda S, Ishio-Kinugasa M, Yoshida K, Sakai A, Miyamoto K (2002) Cryopreservation of hairy root cultures of Vinca minor (L.) by encapsulation-dehydration. Biotechnol Lett 24:371–376
Joshi A, Teng WL (2000) Cryopreservation of Panax ginseng cells. Plant Cell Rep 19:971–977
Jung DW, Sung CK, Touno K, Yoshimatsu K, Shimomura K (2001) Cryopreservation of Hyoscyamus niger adventitious roots by vitrification. J Plant Physiol 158:801–805
Kartha KK, Engelmann F (1994) Cryopreservation and germplasm storage. In: Indra KV, Thorpe TA (eds) Plant Cell and Tissue Culture. Kluwer, Dordrecht, pp 195–230
Kim YS, Yeung EC, Hahn EJ, Paek KY (2007) Combined effects of phytohormone, indole-3-butyric acid, and methyl jasmonate on root growth and ginsenoside production in adventitious root cultures of Panax ginseng C.A. Meyer. Biotechnol Lett 29:1789–1792
Lambert E, Geelen D (2007) Cryopreservation of hairy root cultures from Maesa lanceolata. Commun Agric Appl Biol Sci 72:225–228
Lambert E, Goossens A, Panis B, van Labeke MC, Geelen D (2009) Cryopreservation of hairy root cultures of Maesa lanceolata and Medicago truncatula. Plant Cell Tiss Organ Cult 96:289–296
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73
Sakai A (2000) Development of cryopreservation techniques. In: Engelmann F, Takagi H (eds) Cryopreservation of Tropical Plant Germplasm: Current Research Progress and Applications. JIRCAS, IPGRI, Rome, pp 1–7
Sakai A, Engelmann F (2007) Vitrification, encapsulation-vitrification and droplet-vitrification: a review. Cryo-Lett 28:151–172
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Teoh KH, Weathers PJ, Cheetham RD, Walcerz DB (1996) Cryopreservation of transformed (hairy) roots of Artemisia annua. Cryobiology 33:106–117
Touno K, Yoshimatsu K, Shimomura K (2006) Characteristics of Atropa belladonna hairy roots cryopreserved by vitrification method. Cryo-Lett 27:65–72
Wu CH, Tewari RK, Hahn EJ, Paek KY (2007) Nitric oxide elicitation induces the accumulation of secondary metabolites and antioxidant defense in adventitious roots of Echinacea purpurea. J Plant Biol 50:636–643
Xue SH, Luo XJ, Wu ZH, Zhang HL, Wang XY (2008) Cold storage and cryopreservation of hairy root cultures of medicinal plant Eruca sativa Mill., Astragalus membranaceus and Gentiana macrophylla Pall. Plant Cell Tiss Organ Cult 92:251–260
Yoshimatsu K, Yamaguchi H, Shimomura K (1996) Traits of Panax ginseng hairy roots after cold storage and cryopreservation. Plant Cell Rep 15:555–560
Acknowledgements
This work was supported by a Korea Science and Engineering Foundation grant funded by the Korean Government (MOST; R01-2007-000-10543-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, S.Y., Wu, C.H., Popova, E. et al. Cryopreservation of Panax ginseng Adventitious Roots. J. Plant Biol. 52, 348–354 (2009). https://doi.org/10.1007/s12374-009-9045-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-009-9045-7