Abstract
To study the production of secondary metabolites of Maesa lanceolata and Medicago truncatula, hairy root cultures of both plant species were established. Because maintenance of large numbers of cultures is laborious and costly, we developed a cryopreservation protocol and stored different isolated lines over time. Using encapsulation-dehydration, high survival rates were observed for both Maesa and Medicago hairy roots. Root tips were isolated and encapsulated in calcium-alginate beads, containing 0.1 M sucrose. The encapsulated hairy roots were precultured for 3 days using basal medium containing high sucrose concentrations. Medicago root tip growth during the preculturing time lead to unwanted outgrowth which could be tempered by addition of plant growth inhibitors. After preculturing, the beads were dehydrated in the air flow of a laminar flow until 35–40% of the initial bead weight was reached. Dehydrated beads were plunged into liquid nitrogen and after different storage times thawed in a water bath at 40°C. The survival rates were 90% for Maesa and 53% for Medicago, which are sufficient to allow implementation in large storage experimental set-ups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Saponins are glycosides of a steroidal or triterpenoid polycyclic structure. They are produced by plants as a defence mechanism against fungal and insect attack (Papadopoulou et al. 1999; Francis et al. 2002). Several types of saponins have been associated with medicinal activity (Sparg et al. 2004). For further investigations on the production of triterpenoid saponins two unrelated plant species were selected.
Maesa lanceolata (Maesaceae), also known as False assegai, is a medicinal plant, growing in the tropics of Africa and Asia. The plant is used in traditional medicine in many African countries (Sindambiwe et al. 1996, 1998; Maes et al. 2004; Bagalwa and Chifundera 2007). Bioassay guided fractionation of the methanol extract of dried leaves resulted in the isolation of a saponin mixture (Sindambiwe et al. 1996). The other species investigated is Medicago truncatula (Leguminosae) or Barrel clover, which produces insecticidal and antinutritional saponins with unfavourable effects on animal feed. M. truncatula has emerged recently as a model legume for the study of plant functional genomics and represents also an interesting model species for study and modulation of saponin biosynthesis (Kapusta et al. 2005; Urbanczyk-Wochniak and Sumner 2007).
To genetically manipulate and evaluate the production of saponins in M. lanceolata and M. truncatula, hairy roots were established by infecting leaf discs and seedlings with Agrobacterium rhizogenes. Hairy roots are more complex structures compared to cell suspensions, and the presence of differentiated cells and tissue has been proposed to ascertain a higher genetic stability (Hu and Du 2006). Other advantages of hairy roots include rapid biomass accumulation, typically accompanied with a high production of secondary metabolites and the possibility for upscaling in specialized bioreactors (Kim et al. 2002; Georgiev et al. 2007).
The down side of the use of in vitro hairy root cultures is that the maintenance is labour intensive and involves culture handling with a high risk of microbial contamination and subsequent loss of original cultures (Grout 1995). Also, prolonged subculturing may affect quantitative or qualitative aspects of secondary metabolite production due to the accumulation of loss of function mutations. The conservation of original cultures using cryopreservation technology can avoid these problems. Storing samples in liquid nitrogen eliminates the need for periodic subculturing and reduces the risk of accumulation of somaclonal variation (Teoh et al. 1996).
Three main techniques can roughly be distinguished for plant cryopreservation; vitrification, encapsulation-dehydration and controlled rate freezing. Despite the progress that has been made in the last decade concerning cryopreservation of plant material, there are only few reports on cryopreservation of hairy root cultures. Vitrification protocols are described for hairy roots of Atropa belladonna (Touno et al. 2006), Panax ginseng (Yoshimatsu et al. 1996) and Angelica acutiloba (Yoshimatsu 2000), while the encapsulation-dehydration method was used for Vinca minor (Hirata et al. 2002) and Armoracia rusticana (horseradish) hairy roots (Hirata et al. 1998). One single report describes a slow freezing method for Artemisia annua hairy roots (Teoh et al. 1996).
The present research was carried out to establish a robust method for the cryopreservation of hairy roots from saponin producing plants. Vitrification and encapsulation-dehydration procedures were tested and for the latter technique, preculture conditions and dehydration times were optimized.
Materials and methods
Plant material
Maesa lanceolata seeds were collected in Moshi, Tanzania by Frank Mbago (Department of Botany, University of Dar-Es-Salaam). The seeds were rinsed in 70% (v/v) ethanol for 30 s and subsequently surface sterilized with a 70% (v/v) solution of a commercial disinfection product (Haz-tabs; Guest Medical, Kent, UK). After three washes with distilled water, the seeds were placed on Murashige-Skoog (MS) basal medium (Murashige and Skoog 1962) supplemented with 0.8% (w/v) agar (Lab M plant tissue culture agar MC29, Amersham). Seeds were germinated in a 16/8 h light/dark photoperiod at 26°C.
Medicago truncatula plant material of the cultivar Jemalong J5 was obtained from the group of Marcelle Holsters (VIB—department of Plant Systems Biology—Research group on plant microbes). Seeds of the plants were harvested and scarred with concentrated sulphuric acid. Sterilization was performed using 12% (w/v) sodium hypochlorite. After a 3–4 h treatment with 1 mM 6-benzylaminopurine (BAP), germination of the seeds took place on wet and aseptic Whatmann paper in the dark at 25°C.
Production and maintenance of hairy root cultures
Maesa lanceolata and M. truncatula hairy roots were induced using Agrobacterium rhizogenes (strain LBA 9402/12) transformation on leaf discs or seedlings. The Agrobacterium strain was transformed with the pK7WG2D plasmid, in which an eGFP-ER gene was inserted after a 35S promoter sequence. For both species, the bacteria were grown in the same way. Agrobacteria were transferred from a glycerol stock to 3 ml of liquid YEB medium. This medium contained antibiotics (100 mg/l rifampicin, 100 mg/l spectinomycin and 300 mg/l streptomycin) and consisted of 5 g/l beef extract, 1 g/l yeast extract, 5 g/l peptone, 0.15 M sucrose and 2 ml/l 1 M MgSO2. The bacterial cultures were incubated on a rotary shaker (220 rpm) at 28°C. After 48 h 20 μl of this bacterial preculture was brought into 5 ml of fresh YEB medium with the same antibiotic concentrations as mentioned before. The bacterial cultures were again incubated on a rotary shaker (220 rpm) at 28°C. Forty-eight hours later, these cultures can be used for the transformation. The method for transformation of Maesa and Medicago was somewhat different. Both procedures are described.
Maesa lanceolata hairy roots were induced by wounding leaf material. Wounded leaves were dipped into the bacterial culture and afterwards placed on solid Murashige–Skoog (MS) basal medium (Murashige and Skoog 1962) supplemented with 0.8% (w/v) agar, 0.06 M sucrose and 1 g/l BA. Because there were no antibiotics in the culture medium, the bacteria could still grow, which led to a co-culture of the bacteria and the leaves. After 3 days of co-cultivation with the Agrobacteria, the leaves were placed on solid MS basal medium supplemented with 0.8% (w/v) agar, 0.06 M sucrose and 500 mg/l cefotaxime (Duchefa, The Netherlands) to arrest Agrobacteria growth. Hairy roots were isolated from the leaf material after 15–30 days and were placed on solid Schenk & Hildebrandt (SH) basal medium (Schenk and Hildebrandt 1972) supplemented with 0.8% (w/v) agar and 0.09 M sucrose.
Medicago truncatula hairy roots were induced by wounding seedlings. Wounded seedlings were dipped into the bacterial culture and afterwards placed on solid MS basal medium supplemented with 0.8% (w/v) agar, 0.03 M sucrose and 1 g/l BA. After 7–10 days of co-cultivation with the Agrobacteria, the leaves were placed on solid MS basal medium supplemented with 0.8% (w/v) agar, 0.03 M sucrose and 500 mg/l cefotaxime. Hairy roots were isolated from the seedlings after 15–30 days and were placed on MS basal medium supplemented with 0.8% (w/v) agar and 0.03 M sucrose.
Both hairy root cultures were cultured in the dark at 25°C and were subcultured every month. For the selection of the transgenic material, GFP was used as a visible marker. Hairy roots that showed green fluorescence were considered as transgenic.
Vitrification
To test the toxicity of the plant vitrification solution 2 (PVS2: 0.4 M glycerol, 15% (v/v) DMSO, 15% (v/v) ethylene glycol and 0.4 M sucrose) (Sakai et al. 1990), M. lanceolata and M. truncatula hairy root tips (±5 mm in length) were excised from 2-week-old root cultures and precultured for 3 days at 25°C in the dark on SH solid medium (Maesa) or MS solid medium (Medicago) containing 0.3 M sucrose. After the preculture, the samples were transferred to cryovials (10 root tips/cryotube) and incubated at 0°C in 2 ml loading solution (2 M glycerol and 0.4 M sucrose) for 10 min. The loading solution was removed and 2 ml cooled PVS2 solution (0°C) was added to the root tips. Different incubation times (0, 2, 5, 10, 15 and 20 min) with PVS2 solution were tested. After PVS2 incubation, samples were plunged into liquid nitrogen. After storage for 3 days, samples were thawed rapidly in a water bath at 40°C and washed for 5 min with 2 ml of basal medium containing 1 M sucrose. Afterwards the hairy roots were placed on SH or MS solid medium with 0.09 M sucrose.
To assess the toxicity of PVS2, root tips were treated the same way as described above but after treatment with PVS2 solution, the root tips were directly washed for 5 min with basal medium containing 1 M sucrose and were placed on SH or MS solid medium with 0.09 M sucrose. To determine the effect of loading solution on the survival of hairy roots after PVS2 treatment, half of the samples were pretreated with loading solution for 10 min and the other half of the samples were directly incubated in PVS2 solution. Different incubation times with PVS2 were tested (0, 2, 5, 10, 15 and 20 min).
Trypan blue staining was performed for some samples to get an idea about the viability of the hairy roots directly after thawing. For this staining, root tips were put in a drop of trypan blue (Fluka) on a microscopic slide.
However, in all cases the survival rate was recorded as the percentage of hairy roots showing definite elongation or side root formation after cultivation for 6 weeks in the dark at 25°C.
Encapsulation-dehydration
Root tips (2–3 mm in length) were excised from 2-week-old root cultures of M. lanceolata and M. truncatula. The isolated root tips were immediately suspended in 100 ml of 3% (w/v) sodium alginate solution containing 0.1 M sucrose and dropped into 100 ml 0.1 M CaCl2, plus 0.1 M sucrose. The roots were kept in the solution for 10 min to allow solidification of the calcium alginate beads. Next, the beads were transferred to a shake flask containing 25 ml liquid SH medium for Maesa, or MS medium for Medicago, supplemented with 0.5 M sucrose (50 beads/shake flask). After 24 h, the medium was replaced with medium containing 0.75 M sucrose and 24 h later with medium containing 1 M sucrose. Continued growth of M. truncatula caused extrusion of the root tips from the alginate beads that render them unsuitable for freezing. Outgrowth of M. truncatula root tips was reduced by adding 3 mM ABA (abscisic acid) or 0.01 mM paclobutrazol (Bonzi®) to the preculture media. Optimal concentrations of ABA and paclobutrazol for growth reduction of M. truncatula hairy roots were determined in previous experiments (unpublished results).
After 3 days of preculture in medium with increasing sucrose concentrations, the encapsulated root tips were transferred to a Petridish and dehydrated by placing the dish in the air current of a laminar flow hood for 0–5 h, until the bead weight attained ±35–40% of the initial weight. Dehydrated beads were placed in cryovials (30 beads/cryovials) and then directly immersed in liquid nitrogen.
After storage for 3 days, the beads were thawed rapidly in a water bath at 40°C and then transferred without washing onto solid SH medium containing 0.09 M sucrose (Maesa) or onto solid MS medium containing 0.03 M sucrose (Medicago). The survival rate was recorded as the percentage of hairy roots growing out of the beads after cultivation for 6 weeks in the dark at 25°C.
Statistical analysis
Experiments were done in triplicate, each replicate containing a minimum of ten samples. The data represent mean values from the three independent replicates. Data were nonnormal, hence, ranking was calculated using proc rank (SAS 9_1_3 software). Statistical differences between samples were determined by non parametric oneway analysis of variance, also using SAS 9_1_3 software. Graphs were established using SigmaPlot 10 software.
Results
Induction of hairy root cultures of M. lanceolata and M. truncatula
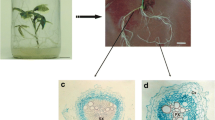
Maesa lanceolata and Medicago truncatula hairy roots appeared from wounded sites 15–30 days after inoculation with Agrobacterium rhizogenes strain LBA 9402/12. Uninfected control explants did not form adventitious roots. GFP was used as a visible marker to select transformed hairy roots. Isolated hairy roots, in contrast to untransformed roots, grew autonomously in hormone free medium. Figure 1a–d represent the hairy root cultures of Maesa and Medicago, grown in the dark at 25°C on solid and in liquid medium. Hairy roots of Medicago grew much faster compared to Maesa hairy roots. Medicago hairy roots also appeared more fragile, thinner and more yellow than those of Maesa. In general, both hairy root cultures grew well on solid and in liquid medium. For the cryopreservation experiments we used hairy roots growing on plates since these were easier to handle.
Morphological features of M. lanceolata and M. truncatula hairy roots before and after freezing. a–b Hairy roots of Maesa and Medicago on solid medium at 25°C in the dark. c–d Hairy roots of Maesa and Medicago on liquid medium at 25°C in the dark, 120 rpm. e–f Hairy roots of Maesa and Medicago growing out of the calcium-alginate beads, 2 weeks after thawing. g–h Hairy roots of Maesa and Medicago after cryopreservation, allowed to recover for 4 weeks
Cytotoxicity of vitrification solution
One of the major disadvantages of the vitrification protocols using PVS2 is its cytotoxicity (Volk et al. 2006; Xue et al. 2008). PVS2 toxicity tests with M. lanceolata and M. truncatula hairy roots were preformed prior to freezing. Treatment of M. lanceolata root tips with PVS2 solution for more than 2 min strongly reduced their survival (Fig. 2a). After 5 min of PVS2 treatment the survival rate was reduced to 16%. Pretreatment of the roots with loading solution (LS) had a clear positive effect on viability of the Maesa hairy roots, maintaining the survival rate at 66% after 5 min of PVS2 treatment. Longer incubation times lead to a strong decrease in viability for both root tips treated with loading solution and without loading solution (Fig. 2a).
The effect of PVS2 treatment on hairy roots of (histogram a) M. lanceolata and (histogram b) M. truncatula, prior to freezing. Different incubation times in PVS2 solution were tested. Also the influence of 10 min pretreatment with loading solution was investigated for both Maesa and Medicago. Each experiment consisted of three repeats with at least 10 hairy roots in each repeat. Different letters indicate significant differences (P < 0.05), ‘*’ significant difference (P < 0.05) between two different treatments on the same time point; according to non parametric ANOVA
Medicago hairy roots also proved to be very sensitive to PVS2 treatment. However, as for Maesa hairy roots, pretreatment with loading solution had a positive effect. When PVS2 was directly applied to the Medicago hairy roots, survival decreased significantly after 2 min. When the roots were pretreated with loading solution, survival was still 53%. However, survival decreased to 0% after 15 min of incubation in PVS2 solution (Fig. 2b).
Microscopic inspection of root tips, using trypan blue staining, showed that after 15 min incubation time there were no more living cells in the Medicago and Maesa root tips. The high toxicity of the PVS2 solution to Maesa and Medicago root tips, disallowed recovery of the material after liquid nitrogen freezing was performed. For the freezing experiments, different PVS2 incubation times were tested (0, 2, 5, 10, 15 and 20 min) and part of the samples was treated with loading solution prior to incubation in PVS2 solution. Neither of these conditions led to regeneration of Maesa or Medicago hairy roots after freezing in liquid nitrogen (results not shown).
Encapsulation-dehydration of hairy roots from M. lanceolata and M. truncatula
The application of the encapsulation-dehydration protocol is considered to be more complicated than vitrification and requires additional testing before it can be implemented for a many species.
Dehydration to very low intracellular moisture levels, so that crystallisation cannot occur, is essential for the survival of cryopreserved hairy roots. An experiment was therefore set up to determine the maximal dehydration time for encapsulated Maesa and Medicago hairy roots. Hairy roots were dehydrated for up to 5 h. Concurrently, they were weighed every half hour and the percentage of the initial bead weight was determined. The weight loss of the beads was similar for Maesa and Medicago hairy root beads (Fig. 3a). The weight of the beads decreased rapidly until 2 h of dehydration. From then on the weight of the beads was approximately constant, reaching a minimum after 5 h of dehydration when 35% of the initial bead weight was achieved for Maesa and 36% for Medicago. Every hour during dehydration, control beads were placed on solid medium to regenerate. After 6 weeks the survival of the hairy roots was evaluated (Fig. 3b). As expected, the survival of the dehydrated controls decreased with increasing dehydration time for both Maesa and Medicago. There were no significant different effects for Maesa and Medicago. The survival before dehydration was 100% for both species (Fig. 3b, time point 0) and was 25 and 30% after 5 h of dehydration for Maesa and Medicago, respectively. For both Maesa and Medicago, there is a continuous decline in survival percentages with no significant drops (Fig. 3b).
Control experiments to determine the optimal dehydration time for M. lanceolata and M. truncatula (without freezing). (Curve a) Encapsulated hairy roots of Maesa and Medicago were dehydrated for 5 h by the air of the laminar flow cabinet. Beads were weighed every 30 min. (Histogram b) Survival of the dehydrated hairy roots of Maesa and Medicago. Beads were put on a plate with solid medium and survival rates were recorded 6 weeks after thawing. Each experiment consisted of three repeats with at least 10 hairy roots in each repeat. Different letters indicate significant differences (P < 0.05), ‘*’ significant difference (P < 0.05) between two different treatments on the same time point; according to non parametric ANOVA
Freezing of 0–4 h dehydrated M. lanceolata hairy root tips was tolerated (Fig. 4). Survival of 90% of the hairy roots was observed after 2.5 h of dehydration, which represents no significant difference to the unfrozen controls. After 4 h of dehydration, survival rate dropped to 20% after freezing. Because of these positive results, no further optimalization of the protocol was attempted for M. lanceolata hairy root conservation.
Survival rates of M. lanceolata hairy roots after cryopreservation. Hairy root beads were dehydrated for different time points before freezing. Survival rates were recorded 6 weeks after thawing. Each experiment consisted of three repeats with at least 10 hairy roots in each repeat. Different letters indicate significant differences (P < 0.05), ‘*’ significant difference (P < 0.05) between two different treatments on the same time point; according to non parametric ANOVA
The survival after encapsulation-dehydration of M. truncatula was much lower compared to M. lanceolata (Table 1). The highest survival rate (16%) was obtained with samples dehydrated for 5 h. However, during the 3 days of preculture, already 24% of the hairy root tips had grown out of the bead. This meant that about 1/4 of all hairy roots were not protected anymore against freezing damage. To prevent this outgrowth, we included the growth inhibitors abscisic acid (ABA) and paclobutrazol. These substances were added solely to the preculture media and not to the alginate or calcium solutions, to avoid negative effects after thawing. Using ABA, still 19% of the hairy roots had grown out of the bead after the preculture. Addition of paclobutrazol lowered this percentage to 7%. The survival rates of the hairy roots after freezing are given Table 1. The highest survival rate using ABA as a growth inhibitor was 30%, while the highest survival rate using paclobutrazol as a growth inhibitor was 55%. Both ABA and paclobutrazol clearly inhibit growth of the hairy roots during preculture and don’t have a negative effect on the viability of the hairy roots which results in higher survival percentages of hairy roots after freezing. Survival percentages were, however, higher than expected so ABA and paclobutrazol have possibly other effects on the hairy roots, which make them more tolerant for dehydration and freezing.
For M. lanceolata as for M. truncatula we observed no morphological abnormalities after cryopreservation of the hairy roots (Fig. 1e–h). The growth rates were also the same for non frozen and frozen samples (results not shown).
Discussion
To evaluate and change the production of pharmaceutical important saponins, hairy root lines were established from M. lanceolata and M. truncatula. Using a standard protocol for Agrobacterium mediated transformation we obtained hairy root lines from both species. In order to maintain the original cultures we have developed a cryopreservation protocol.
Because vitrification involves less handling steps in comparison to other cryopreservation techniques (Engelmann 2004), we first tried this method for the cryopreservation of M. lanceolata and M. truncatula hairy roots. However, the PVS2 solution commonly used for vitrification lead to significant damage of the hairy root tips, also observed for some other tissue and cell structures (Volk et al. 2006). Only very low percentages of the M. lanceolata and M. truncatula hairy roots survived incubation of 5 min and longer in PVS2, without pretreatment with a loading solution. Roots that were first treated with loading solution seemed to be more resistant to PVS2 solution, as survival percentages decrease from 10–15 min of treatment with PVS2. However, none of the Maesa or Medicago hairy roots survived freezing in liquid nitrogen after vitrification. In literature, there are only three studies in which vitrification is successfully applied on hairy roots. There is a vitrification procedure described for Panax ginseng (Yoshimatsu et al. 1996), Angelica acutiloba (Yoshimatsu 2000) and Atropa belladonna (Touno et al. 2006) hairy roots. Survival percentages are respectively, 60, 96, and 93%. On the other hand, there are also reports that confirm the negative effect of PVS2 solution on the viability of plant material of some species (Volk et al. 2006; Xue et al. 2008). Xue et al. (2008) tried to cryopreserve hairy roots of three different species using encapsulation-vitrification. For this technique hairy roots were also encapsulated in calcium-alginate beads but were desiccated through treatment with PVS2 solution (Sakai 2000; Xue et al. 2008). No hairy roots of Gentiana macrophylla and Astragalus membranaceus survived when using the PVS2 solution to vitrify the material. Only 25% of the hairy roots of Eruca sativa survived treatment with PVS2 solution (Xue et al. 2008). In view of our own negative results and the few positive results reported in literature on cryopreservation of hairy roots, we concluded that vitrification was not a suitable method to preserve hairy root cultures.
Encapsulation-dehydration is a cryopreservation method that is more labour intensive than vitrification but does not require treatment with cytotoxic compounds (Niino and Sakai 1992). Hairy roots are encapsulated in calcium-alginate beads, precultured for 3 days in medium with increasing sucrose concentration and then dehydrated by the air of a laminar flow cabinet. For M. lanceolata hairy roots we obtained survival percentages of 90% after encapsulation-dehydration. For M. truncatula we first encountered the problem that the hairy roots grew too fast and grew out of the bead even before freezing. In this way the root tips were not protected anymore against freezing damage and this resulted in very low percentages of survival. Isolation of smaller root tips was technically too difficult for practical use. So to overcome this problem we added growth inhibitors to the preculture medium, namely ABA and paclobutrazol. Both substances are frequently used as growth retardants in cryopreservation and cold preservation of plant material (Hirata et al. 1998; Hirata et al. 2002; Cha-Um et al. 2007). ABA is also generally known as a plant hormone that plays a role in the acquisition of desiccation tolerance and osmotic stress (Ingram and Bartels 1996; Sharp et al. 2004). ABA induces late-embryogenesis-abundant (lea) genes, which encode LEA proteins. These proteins have increased levels during drought and are involved in dehydration tolerance in plants (Ingram and Bartels 1996). Paclobutrazol is a growth retardant that has a negative effect on root growth but it is also an inhibitor of ABA catabolism, so addition of paclobutrazol may increase the endogenous ABA levels in M. truncatula hairy roots during desiccation and as a consequence lea genes may be induced which might make the hairy roots less susceptible to drought stress (Krizan et al. 2006; Cha-Um et al. 2007). Addition of ABA or paclobutrazol to the preculture media resulted in survival percentages of respectively, 30 and 53%. We believe that these higher percentages are partly due to the reduction of outgrowth and partly due to the higher desiccation tolerance, upon addition of plant growth regulators ABA and paclobutrazol.
There are very few reports on encapsulation-dehydration of hairy roots. The first one was published by Hirata et al. (1998), describing an encapsulation-dehydration protocol for Horseradish hairy root cultures. They obtained survival rates of 60% of the cryopreserved hairy roots. Later, the same group published a report on encapsulation-dehydration of Vinca minor hairy roots (Hirata et al. 2002). A survival rate of more than 70% was obtained in this case. Our results show that for M. lanceolata 90% and for M. truncatula 53% of the hairy roots can be regenerated after cryopreservation. The same protocol is potentially useful for other species, with ABA and paclobutrazol as possible cryoprotectants. In the future we will use the established encapsulation-dehydration protocol routinely for the storage of large numbers of different transgenic root lines of M. lanceolata and M. truncatula.
References
Bagalwa M, Chifundera K (2007) Environmental impact evaluation of the stem bark extract of Maesa lanceolata used in Democratic Republic of Congo. J Ethnopharmacol 114:281–284. doi:10.1016/j.jep.2005.11.036
Cha-Um S, Kirdmanee C, Huyen PX, Vathany T (2007) Disease-free production and minimal-growth preservation of in vitro banana (Musa spp.). In: Hummer KE (ed) Proceedings of the second international symposium on plant genetic resources of horticultural crops, Vols. 1 and 2. Acta horticulurae, Seoul, pp 233–240
Engelmann F (2004) Plant cryopreservation: progress and prospects. In Vitro Cell Dev Biol Plant 40:427–433. doi:10.1079/IVP2004541
Francis G, Kerem Z, Makkar HP, Becker K (2002) The biological action of saponins in animal systems: a review. Br J Nutr 88:587–605. doi:10.1079/BJN2002725
Georgiev MI, Pavlov AI, Bley T (2007) Hairy root type plant in vitro systems as sources of bioactive substances. Appl Microbiol Biotechnol 74:1175–1185. doi:10.1007/s00253-007-0856-5
Grout BWW (1995) Introduction to the in vitro preservation of plant cells, tissues and organs. In: Grout BWW (ed) Genetic preservation of plant cells in vitro. Springer, Berlin, pp 1–20
Hirata K, Goda S, Phunchindawan M, Du D, Ishio M, Sakai A, Miyamoto K (1998) Cryopreservation of horseradish hairy root cultures by encapsulation-dehydration. J Ferment Bioeng 86:418–420. doi:10.1016/S0922-338X(99)89017-5
Hirata K, Mukai M, Goda S, Ishio-Kinugasa M, Yoshida K, Sakai A, Miyamoto K (2002) Cryopreservation of hairy root cultures of Vinca minor (L.) by encapsulation-dehydration. Biotechnol Lett 24:371–376. doi:10.1023/A:1014564804048
Hu ZB, Du M (2006) Hairy root and its application in plant genetic engineering. J Integr Plant Biol 48:121–127. doi:10.1111/j.1744-7909.2006.00121.x
Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47:377–403. doi:10.1146/annurev.arplant.47.1.377
Kapusta I, Janda B, Stochmal A, Oleszek W (2005) Determination of saponins in aerial parts of barrel medic (Medicago truncatula) by liquid chromatography-electrospray ionization/mass spectrometry. J Agric Food Chem 53:7654–7660. doi:10.1021/jf051256x
Kim Y, Wyslouzil BE, Weathers PJ (2002) Invited review: secondary metabolism of hairy root cultures in bioreactors. In Vitro Cell Dev Biol Plant 38:1–10. doi:10.1079/IVP2001243
Krizan B, Ondrusikova E, Dradi G, Rocasaglia R (2006) The effect of paclobutrazol on in vitro rooting and growth of GF-677 hybrid peach rootstock. Acta Physiol Plant 28:21–26. doi:10.1007/s11738-006-0064-4
Maes L, Vanden Berghe D, Germonprez N, Quirijnen L, Cos P, De Kimpe N, Van Puyvelde L (2004) In vitro and in vivo activities of a triterpenoid saponin extract (PX-6518) from the plant Maesa balansae against visceral leishmania species. Antimicrob Agents Chemother 48:130–136. doi:10.1128/AAC.48.1.130-136.2004
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Niino T, Sakai A (1992) Cryopreservation of alginate-coated in vitro-grown shoot tips of apple, pear and mulberry. Plant Sci 87:199–206. doi:10.1016/0168-9452(92)90151-B
Papadopoulou K, Melton RE, Leggett M, Daniels MJ, Osbourn AE (1999) Compromised disease resistance in saponin-deficient plants. Proc Natl Acad Sci USA 96:12923–12928. doi:10.1073/pnas.96.22.12923
Sakai A (2000) Development of cryopreservation techniques. In: FaT Engelmann H (ed), Cryopreservation of tropical germplasm. Current research progress and application. JIRCAS, Rome, pp 1–7
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus-Sinensis Osb Var Brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33. doi:10.1007/BF00232130
Schenk RU, Hildebrandt (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant-cell cultures. Can J Bot 50:199–204. doi:10.1139/b72-026
Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT (2004) Root growth maintenance during water deficits: physiology to functional genomics. J Exp Bot 55:2343–2351. doi:10.1093/jxb/erh276
Sindambiwe JB, Balde AM, De Bruyne T, Pieters L, Van den Heuvel H, Claeys M, Van den Berghe DA, Vlietinck AJ (1996) Triterpenoid saponins from Maesa lanceolata. Phytochemistry 41:269–277. doi:10.1016/0031-9422(95)00552-8
Sindambiwe JB, Calomme M, Geerts S, Pieters L, Vlietinck AJ, Vanden Berghe DA (1998) Evaluation of biological activities of triterpenoid saponins from Maesa lanceolata. J Nat Prod 61:585–590. doi:10.1021/np9705165
Sparg SG, Light ME, van Staden J (2004) Biological activities and distribution of plant saponins. J Ethnopharmacol 94:219–243. doi:10.1016/j.jep.2004.05.016
Teoh KH, Weathers PJ, Cheetham RD, Walcerz DB (1996) Cryopreservation of transformed (hairy) roots of Artemisia annua. Cryobiology 33:106–117. doi:10.1006/cryo.1996.0011
Touno K, Yoshimatsu K, Shimomura K (2006) Characteristics of Atropa belladonna hairy roots cryopreserved by vitrification method. Cryo Letters 27:65–72
Urbanczyk-Wochniak E, Sumner LW (2007) MedicCyc: a biochemical pathway database for Medicago truncatula. Bioinformatics 23:1418–1423. doi:10.1093/bioinformatics/btm040
Volk GM, Harris JL, Rotindo KE (2006) Survival of mint shoot tips after exposure to cryoprotectant solution components. Cryobiology 52:305–308. doi:10.1016/j.cryobiol.2005.11.003
Xue SH, Luo XJ, Wu ZH, Zhang HL, Wang XY (2008) Cold storage and cryopreservation of hairy root cultures of medicinal plant Eruca sativa Mill., Astragalus membranaceus and Gentiana macrophylla Pall. Plant Cell Tissue Organ Cult 92:251–260. doi:10.1007/s11240-007-9329-x
Yoshimatsu K (2000) Cryopreservation of medicinal plant resources: retention of biosynthetic capabilities in transformed cultures. In: FaT Engelmann H (ed) Cryopreservation of tropical germplasm. Current research progress and application. JIRCAS, Rome, pp 77–90
Yoshimatsu K, Yamaguchi H, Shimomura K (1996) Traits of Panax ginseng hairy roots after cold storage and cryopreservation. Plant Cell Rep 15:555–560. doi:10.1007/BF00232452
Acknowledgements
This work was funded by IWT-Flanders; SBO Combiplan, Project No. SBO#040093.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lambert, E., Goossens, A., Panis, B. et al. Cryopreservation of hairy root cultures of Maesa lanceolata and Medicago truncatula . Plant Cell Tiss Organ Cult 96, 289–296 (2009). https://doi.org/10.1007/s11240-008-9486-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9486-6