Abstract
Panax ginseng Meyer is an important medicinal plant producing bioactive compounds. A droplet-vitrification method was developed for cryopreserving adventitious root cultures of mountain ginseng, and variations of this procedure were tested to determine their effects on regrowth rates and osmotic stress responses. Root regrowth rates were examined after exposing root segments to two pre-culture treatments, three loading solutions, and two vitrification solutions with different exposure periods. Pre-culturing excised segments of adventitious roots with 0.3 M sucrose produced the highest rate of regrowth after cryopreservation. Loading for 20 min with 17.5% (w/v) sucrose and 17.5% (w/v) glycerol at room temperature increased the regrowth rate of cryopreserved adventitious roots fourfold, compared with non-loaded samples. Treatments involving different vitrification solutions and exposure periods were compared, and the highest rate of regrowth (15%) after cryopreservation was achieved by incubating adventitious roots in modified plant vitrification solution 3 containing 40% (w/v) glycerol and 40% (w/v) sucrose for 10 min at room temperature, suggesting that ginseng adventitious root tips were sensitive to osmotic stress. Further study is necessary to develop optimal vitrification solutions that enhance the survival rate of cryopreserved adventitious roots of mountain ginseng.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Panax ginseng Meyer, commonly known as ginseng, is an important medicinal plant (Lee 1998). It is used in traditional medicines in many Asian countries because of its multiple pharmacological activities, which include anti-tumor, anti-diabetic, anti-aging, and anti-stress effects (Xie et al. 2006; Lü et al. 2009). More than 150 ginsenosides, the principal bioactive compounds produced by this plant, have been isolated from ginseng. These compounds are classified into the protopanaxadiol, protopanaxatriol, and oleanolic acid groups, based on their structures (Christensen 2008).
There have been successful pilot-scale trials for the commercial production of ginsenosides from adventitious root cultures (Hahn et al. 2003). Numerous recent studies have focused on developing novel adventitious root lines that enhance ginsenoside production (Kim et al. 2013). Maintaining the adventitious root cultures and novel root lines developed in pilot-scale trials may be difficult, however, and presents the risk of losing the cultures to contamination; moreover, long-term culture may alter the production of bioactive compounds, leading to changes in the quality and quantity of metabolites (Kiselev et al. 2011; Fu et al. 2012; Turgut-Kara and Kahraman 2015). Cryopreservation to conserve biological materials in liquid nitrogen at ultra-low temperature (− 196 °C) is therefore recommended, although it is costly for long-term storage (Engelmann and Dussert 2013; Kulus and Zalewska 2014). In recent decades, various types of medicinal hairy root cultures have been cryopreserved successfully by techniques including encapsulation-dehydration of Vinca minor (Hirata et al. 2002), Maesa lanceolata, and Medicago truncatula (Lambert and Geelen 2008; Lambert et al. 2009); encapsulation-vitrification of Eruca sativa, Astragalus membranaceus, and Gentiana macrophylla (Xue et al. 2008); and vitrification of Angelica acutiloba (Yoshimatsu et al. 2000), Atropa belladonna (Touno et al. 2006), and Rubia akane (Park et al. 2014).
There have been few previous studies of cryopreservation of adventitious root cultures (Jung et al. 2001; da Silva Cordeiro et al. 2015). Oh et al. (2009) used vitrification methods to cryopreserve adventitious roots and found that 12% of roots survived immersion in liquid nitrogen. The droplet-vitrification method (Panis et al. 2005) is one of several cryopreservation techniques that have been developed. It increases the likelihood of survival of many plant materials following long-term storage at ultra-low temperature (Kim et al. 2010; Yi et al. 2012).
The droplet-vitrification procedure involves modifying the vitrification method in several ways. Explants are pre-treated in a sucrose-enriched solution, dehydrated in a loading solution and a vitrification solution, placed on aluminum foils strips with a drop of vitrification solution, and directly immersed in liquid nitrogen. The high thermal conductivity of the aluminum foil enhances the freezing and thawing rate; use of the foil strip also minimizes the damage done to samples during insertion or extraction from cryotubes. Nevertheless, the droplet-vitrification method is not in widespread use as it requires a high level of technical skill (Kulus and Zalewska 2014). In this study, the droplet-vitrification method was used to improve cryopreservation of rare and valuable adventitious root lines of ginseng to enhance long-term storage.
2 Materials and methods
2.1 Adventitious root culture of mountain ginseng
Adventitious root formation of wild ginseng (P. ginseng Meyer) was induced through callus culture. The proliferating roots were maintained in 0.75× MS medium, supplemented with 0.25 M indole-3-butyric acid (IBA) and 50 g L−1 sucrose, at 24 ± 1 °C in the dark. The pH of the medium was adjusted to 5.8 before autoclaving at 121 °C for 20 min. Adventitious roots were sub-cultured every 40 days, and 3-week-old root tips were used as material in cryopreservation treatments.
2.2 Droplet-vitrification technique
Root tips, 2–3 mm in length, were detached from 3-week-old adventitious roots of P. ginseng. The root tips (20 segments, four replicates) were immediately immersed in 15 mL liquid 0.75× MS medium supplemented with 0.3 M sucrose and pre-cultured for 24 h on a rotary shaker at 100 rpm. The pre-cultured segments were transferred to C4 loading solution, which contained 17.5% sucrose and 17.5% glycerol (w/vw/v) (Table 1), for 20 min at room temperature. After cryoprotection by C4, the root segments were dehydrated in B5 vitrification solution, containing 40% sucrose and 40% glycerol (w/v) (Table 1), for 10 min with continuous shaking at 100 rpm at room temperature. Before the end of the dehydration process, the root tips were arranged on a foil strip (6 × 30 mm; 10 root segments per strip). The root tips were covered with a drop of B5 vitrification solution and immersed immediately in liquid nitrogen for at least 30 min.
Root tips were rewarmed by placing the foil strips in 15 mL unloading solution, pre-heated to 40 °C, and shaking them gently for approximately 30 s to remove the root tips from the foil. Next, 15 mL fresh unloading solution containing 0.8 M sucrose was added and root tips were incubated at room temperature for 20 min. The segments were blotted dry with filter paper and placed on solid 0.75× MS medium, supplemented with 0.25 M IBA, 50 g L−1 sucrose, and 0.2% Gelrite, at 24 ± 1 °C in the dark.
At each step in the cryopreservation and rewarming process, half of the root segments were transferred to a fresh tube containing 15 mL unloading solution and incubated at room temperature for 20 min. The root tips were then blotted dry and grown on solid MS medium to determine the principal stage determining survival of ginseng roots after cryopreservation.
2.3 Pre-culture treatments
To study the effect of pre-culture on the survival rate after cryopreservation, root tips were not pre-cultured, pre-cultured on 0.3 M sucrose for 24 h, or pre-cultured on 0.3 M sucrose for 24 h and then transferred to 0.5 M sucrose for 5 h. Subsequent cryopreservation and rewarming treatments followed the methodology described above.
2.4 Loading solution treatments
Three different loading solutions containing different concentrations of sucrose and glycerol were used to determine the most appropriate cryoprotection solution for root tips of P. ginseng. The loading solutions tested were C3 (15% sucrose and 15% glycerol, w/v), C4 (17.5% sucrose and 17.5% glycerol, w/v), and C6 (20% sucrose and 20% glycerol, w/v) (Table 1). Root tips were immersed in loading solutions for 20 min at room temperature.
2.5 Vitrification solution treatments
The excised root tips were dehydrated in either modification plant vitrification solutions (PVS) 2 as B5 vitrification solution (40% sucrose and 40% glycerol, w/v) for 10 or 20 min, or PVS3 as A3 solution [22.5% sucrose, 37.5% glycerol, 15.0% dimethylsulfoxide (DMSO), and 15.0% ethylene glycol (EG)] for 10 min at either room temperature or 0 °C (Table 1). The vitrification solutions were sterilized by filtration using a 0.2 µm syringe filter before use.
2.6 Malondialdehyde (MDA) assay
Root tissue was collected, and 0.1 g samples were disrupted under liquid nitrogen and homogenized in 0.1% trichloroacetic acid (TCA) using a TissueLyser II (Qiagen, Hilden, Germany). The homogenate was centrifuged at 5000 rpm for 5 min. The supernatant was mixed with 0.6% TBA and placed in a water bath at 90 °C for 30 min. Absorbance was measured at 532 and 600 nm using a spectrophotometer (Optizen POP, Mecasys Co., Ltd, Korea).
2.7 Antioxidant enzyme activity assays
Samples consisting of 1.0 g fresh adventitious root tissue were disrupted in liquid nitrogen and homogenized in 4 mL extraction buffer (50 mM potassium phosphate buffer, 2.0% polyvinylpolypyrrolidone, and 1.0 mM phenylmethylsulfonyl fluoride). The extracted samples were centrifuged at 13,000 rpm for 10 min at 4 °C (Smart R17, Hanil Science Co., Ltd, Korea). The supernatant was stored at 2 °C and used for enzyme assays within 4 h.
Catalase (CAT, EC 1.11.1.6) activity was determined in reactions containing 500 µmol H2O2 in 10 mL 100 mM phosphate buffer (pH 7.0). CAT activity was determined by monitoring H2O2 consumption at 240 nm for 3 min. The results were expressed as CAT U mg−1 protein (1 mM H2O2 reduction min−1 mg−1 protein).
Peroxidase (POD, EC 1.11.1.7) activity was measured using a modification of the method described by Bisht et al. (1989). The reaction mixture consisted of 1.5 mL phosphate buffer (pH 7.0), 1% guaiacol, and 1% H2O2. POD activity was assessed as the change in absorption at 470 nm as a result of guaiacol oxidation. The results were expressed as U min−1 mg−1 protein. Three different loading solutions with different concentrations of sucrose and glycerol were used to determine the most appropriate cryoprotective solution for root tips of P. ginseng; the solutions tested were C3 (15% sucrose and 15% glycerol, w/v); C4 (17.5% sucrose and 17.5% glycerol, w/v); and C6 (20% sucrose and 20% glycerol, w/v). The segments were immersed in loading solutions for 20 min at room temperature.
2.8 Statistical analysis
All data were analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT) using SPSS statistical 16.0 software (SPSS Inc., Chicago, IL, USA). Data are presented as means and standard errors; different letters on the figures indicate significant differences between results (p < 0.05).
3 Results
3.1 Effect of sucrose concentration and duration of exposure during pre-culture on survival rates of adventitious roots after cryopreservation
The survival rates of cryopreserved root tips exposed to different concentrations (0, 0.3, and 0.5 M) of sucrose for different periods were measured (Fig. 1a). The highest survival rate was obtained after 24 h of pre-culture with 0.3 M sucrose. Root segments that were not pre-cultured with enriched sucrose solution also recovered well after storage in liquid nitrogen (Fig. 1a). Root tips treated by pre-culture in 0.3 M sucrose followed by transfer to 0.5 M sucrose showed the lowest survival rate (Fig. 1a).
Regeneration rates of adventitious root tips of Panax ginseng after 30 days’ regrowth on fresh medium after cryopreservation. a Effect of pre-culture treatment on the regeneration rate. b Effect of loading solution on the regeneration rate. c Effect of vitrification treatment on the regeneration rate. Data are mean ± SE of three replicates; different letters on a column indicate significant differences (p < 0.05) according to Duncan’s multiple range test. LN liquid nitrogen
3.2 Effect of loading solutions on survival rates of adventitious roots after cryopreservation
Of the different loading treatments tested, the balanced weight of glycerol and sucrose in loading solution C4 produced the highest regeneration rate of ginseng root tips (Fig. 1b). Root segments that were not treated with a loading solution also recovered well after cryopreservation, but treatment with solutions C3 (15% glycerol and 15% sucrose, w/v) and C6 (20% glycerol and 20% sucrose, w/v) resulted in low recovery of root tips after treatment with liquid nitrogen.
3.3 Effect of vitrification solutions, and duration and temperature of exposure, on survival rates of adventitious roots after cryopreservation
Ginseng root tips, pre-dehydrated in loading solution, were dehydrated further in highly concentrated cryoprotective solutions. To measure the effects of the vitrification solution composition, duration of exposure, and temperature of treatment, root tips were vitrified with PVS3 for 10 or 20 min or PVS2 at room temperature or on ice. Ginseng root tips exposed to B5 solution, a dilution of PVS3 (80%), for 10 min produced a root recovery rate of 15% after liquid nitrogen treatment; however, prolonged exposure to B5 for 20 min resulted in death of almost all the root segments (Fig. 1c). The recovery rate of root tips after treatment at room temperature with A3 solution, a dilution of PVS2 (90%), was only 5%. The injurious effects of A3 solution were compounded by conducting the treatment on ice, as no explants treated under those conditions survived cryopreservation (Fig. 1c). These results imply that ginseng root tips are extremely sensitive to both chemical toxicity and osmotic stress.
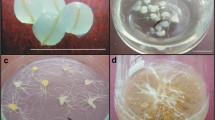
The morphology of root tips was observed following dehydration with vitrification solutions (Fig. 2). Surviving roots initially swelled at the root tips after roughly 2 weeks of regrowth on fresh medium (Fig. 2a). Next, callus formed and developed from the root tips (Fig. 2b). Adventitious roots were induced from callus after 3 weeks of regrowth by the vitrified roots (Fig. 2c), and lateral roots formed on the adventitious roots after 4 weeks of culture (Fig. 2d). This study established a procedure for the long-term culture of adventitious roots of P. ginseng; however, as root tips were sensitive to osmotic stress, the next step was to identify the critical stage in the cryopreservation process during which the greatest damage to tissue occurred.
3.4 Effects of pre-culture, loading solution, vitrification solution, and liquid nitrogen treatment on survival rates of adventitious roots
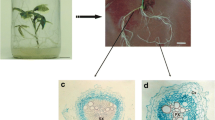
To identify the most critical stage of the cryopreservation process, adventitious roots were collected after each step of the protocol, washed with unloading solution, and cultured on fresh solid medium; control plants were collected before pre-culture. The survival rate and the extent of lateral root formation were measured after 4 weeks of culture (Fig. 3a, b). A regeneration rate of nearly 100% was observed in ginseng root tips after pre-culture and treatment with C4 loading solution (Fig. 3a). After exposure to B5 vitrification solution for 10 min, however, approximately 50% of the root segments became necrotic, resulting in a low survival rate after cryopreservation treatment (Fig. 3a). Formation of lateral roots was also gradually reduced after each successive step in the protocol (Fig. 3b). Images of lateral roots formed after control treatments and after exposure to sucrose-enriched medium in pre-culture, loading solution, vitrification solution, and liquid nitrogen are shown in Fig. 3c–g, respectively.
Recovery and regrowth after each stage of the cryopreservation process. a Explant responses after control treatment and pre-culture, loading solution, and vitrification solution treatments. b Number of lateral roots produced after control treatment and after pre-culture, loading solution, and vitrification solution treatments. Data in (a) and (b) are mean ± SE from three replicates; different letters on a column indicate significant differences (p < 0.05) according to Duncan’s multiple range test. c Morphology of adventitious roots before cryopreservation (controls). d Regrowth of adventitious roots after pre-culture on 0.3 M sucrose for 24 h. e Regrowth of adventitious roots after loading with C4 solution. f Regrowth of adventitious roots after vitrification with B5 solution. g Recovery of adventitious roots after storage in liquid nitrogen. Scale bar: 1 cm
3.5 Malondialdehyde (MDA) levels and antioxidant enzyme activities in adventitious roots during the cryopreservation process
To determine the effects of osmotic stress on post-cryopreservation survival and regeneration of ginseng adventitious roots, malondialdehyde (MDA) levels and antioxidant enzyme (CAT and POD) activities were analyzed after each step of the cryopreservation process (Fig. 4). MDA content increased after the pre-culture, loading, and cryopreservation treatments, and peaked after treatment with B5 vitrification solution (Fig. 4a). By contrast, CAT activity was at its lowest level when root tips were exposed to solution B5 for 10 min (Fig. 4b). CAT activity after the pre-culture, loading, and cryopreservation treatments did not differ significantly from activity in control roots (Fig. 4b). POD activity in root tips showed no significant differences between successive stages of the cryopreservation process, or between treated and control samples (Fig. 4c).
Effect of each stage of the cryopreservation process on malondialdehyde level and antioxidant enzyme activity in adventitious roots of Panax ginseng. a Malondialdehyde (MDA) content measured in adventitious roots after pre-culture, and treatment with loading solution, vitrification solution, and liquid nitrogen. b Level of CAT activity in adventitious roots after each treatment. c Level of POD activity in adventitious roots after each treatment. Data are mean ± SE from three replicates; different letters on a column indicate significant differences (p < 0.05) according to Duncan’s multiple range test
4 Discussion
To enhance the percentage of plant tissue that survives cryopreservation by droplet-vitrification and is able subsequently to regenerate, it is necessary to determine the most appropriate solution and optimum duration of exposure for each stage of the procedure. Unlike in nature, where thermal and photoperiodic factors enable plants to withstand winter cold, tolerance of explants grown in vitro must be induced by modifying culture conditions such as temperature, components of the growth media, and light intensity (Kulus and Zalewska 2014). Pre-culture with an enriching solution containing various sucrose concentrations is an important factor for increasing tolerance of explants during freezing stress (Suzuki et al. 2006). Xue et al. (2008) showed that pre-culture treatment is essential for cryopreservation of hairy roots of E. sativa and A. membranaceus but ineffective for G. macrophylla hairy roots. Pre-culture with sucrose reduces the size of the cell vacuoles and decreases the free water content, demonstrating that dehydration enhances the capacity of the cell membrane to maintain a stable structure (Ibrahim and Normah 2013; Kulus and Zalewska 2014). The current study found that either increasing the sucrose concentration to 0.3 M for 24 h or omitting the pre-culture step produced better post-cryopreservation regrowth of P. ginseng adventitious roots than a step-wise increase in sucrose concentration from 0.3 M for 24 h to 0.5 M for 5 h. This implied that adventitious roots were sensitive to excess osmotic stress.
Engelman and Takagi (2000) showed that a pre-culture stage is insufficient to enhance stress tolerance of explants after cryopreservation, and direct exposure to a high concentration of vitrification solution is toxic. A loading treatment that exposes explants to a moderate amount of cryoprotectant for 20 to 60 min is an important stage before dehydration with highly concentrated vitrification solutions (Nishizawa et al. 1993). The current study showed that the composition of loading solution was an important factor affecting the recovery rate of ginseng explants after cryopreservation. Of the three loading solutions tested, C4, a mixture of 17.5% sucrose and 17.5% glycerol (w/v), was most effective at enhancing tolerance to dehydration by the vitrification solution. This loading solution is also the most effective for cryopreserving Chrysanthemum shoot tips, which are sensitive to osmotic stress (Kim et al. 2009a), and produces the highest level of post-cryopreservation regeneration of R. akane, shown by Kim et al. (2010). Prolonged exposure of ginseng root tips to loading solutions enabled effective root formation after vitrification and cooling to − 196 °C.
The key to successful cryopreservation of plant materials by the vitrification method is overcoming the major difficulties posed by the high chemical toxicity and excess osmotic stress of concentrated vitrification solutions (Fábián et al. 2008; San José et al. 2014). Although a highly concentrated vitrification mixture is required to produce the level of cytoplasmic dehydration essential for prevention of intracellular ice crystal formation, prolonged exposure to such a solution may be cytotoxic (Sakai et al. 1990; San José et al. 2014). Damage caused by vitrification mixture toxicity results from distinct changes to the cell ultra-structure, particularly to the plasma membrane (Fujikawa and Steponkus 1991; Steponkus et al. 1992). For this reason, the duration of dehydration treatment and the composition of the vitrification solution, as well as other experimental conditions, must be determined precisely. The composition of vitrification solutions has been developed over time, and PVS2 and PVS3 are those used most regularly (Sakai and Engelmann 2007). Both are glycerol-based solutions, but PVS2 also contains EG and DMSO, cryoprotectants that perforate the cell wall and membranes. As high concentrations of DMSO cause destruction of the membrane bilayer (Hughes and Mancera 2014), PVS3 is used for explants sensitive to chemical toxicity (Kim et al. 2009b). Although PVS2 has been used for successful cryopreservation of Hyoscyamus niger adventitious roots (Jung et al. 2001), A. acutiloba hairy roots (Yoshimatsu et al. 2000), and A. belladonna hairy roots (Touno et al. 2006), explants exposed to PVS2 are often susceptible to its chemical toxicity. Lambert et al. (2009) found that hairy roots of M. lanceolata and M. truncatula are significantly damaged after incubation with PVS2 for 5 min; in addition, Xue et al. (2008) showed that PVS2 is ineffective at preserving hairy roots of G. macrophylla and A. membranaceus. Oh et al. (2009), however, found PVS2 to be a promising cryopreservative for adventitious roots of P. ginseng, although regeneration rates are low. Kim et al. (2010) reported a post-cryopreservation regeneration rate of 79.5% in R. akane hairy roots after treatment with 80% PVS3 solution (B5).
The present study tested the effect of two vitrification solutions, B5 (an 80% solution of PVS3) and A3 (a 90% solution of PVS2), on the post-cryopreservation regeneration rate of ginseng adventitious roots. The regeneration rate was higher after treatment with B5 than with A3 solution. In addition, treatment of ginseng root tips with B5 solution for a short (10 min) period was more effective than a longer (20 min) treatment. The balance between glycerol and sucrose in B5 solution may affect the successful recovery of cryopreserved materials, as a similar response was reported by Makowska et al. (1999) and Kim et al. (2010). Rapid exposure to PVS2 on ice is essential for reducing damage caused by the vitrification solution, resulting in improved regeneration rates in many plant species (Nishizawa et al. 1993; Kuranuki and Sakai 1995; Thinh 1997; Yoon et al. 2006; Sakai and Engelmann 2007). In the current study, however, recovery of root segments treated with cooled A3 solution was lower than after treatment with room temperature solution. This is consistent with previous studies of many medicinal plants, which found that the optimal temperature for exposure to PVS2 was 25 °C (Matsumoto et al. 1994; Takagi et al. 1997; Sakai and Engelmann 2007). The vitrification solution was a major factor in the cryopreservation process, determining the survival rates of ginseng root segments. Optimizing the composition of the cryoprotectant mixture is thus of key importance for improving the post-cryopreservation regeneration of ginseng adventitious roots.
Many previous studies have shown that plant cells are damaged by cryopreservation treatments (Wesley-Smith et al. 2013). We found that, when post-cryopreservation root segments were regrown on fresh medium, swelling of primordia occurred first, followed by callus formation. Almost all the root tissue became necrotic, and the only surviving zone appeared to be the meristematic region of the root tip. Quain et al. (2009) also reported only callus development from Dioscorea rotundata tissue exposed to liquid nitrogen. Previous studies indicate that, infrequently, larger and more differentiated cells than meristem cells may survive storage in liquid nitrogen (Kulus et al. 2018). In Chrysanthemum, however, only three layers of meristem cells were observed to be viable after cryopreservation (Wang et al. 2014). Injury to the meristem may cause indirect regeneration through callus (Kulus et al. 2018).
Even though the vitrification solution is a major factor determining cell survival, cryopreservation exposes tissue to multiplex stresses, including excision, osmotic damage, dehydration, and temperature fluctuations (Uchendu et al. 2010; Ren et al. 2013). Recent studies have demonstrated that oxidative stresses caused by cryopreservation activate the formation of reactive oxygen species (ROS). This results in cryoinjury to cells, caused by protein denaturation, nucleic acid decomposition, and cell membrane destruction (Poobathy et al. 2013; Chen et al. 2015; Martinez-Montero and Harding 2015; Zhang et al. 2015). The plasma membrane is a primary target of cellular cryodamage (Wen et al. 2010). MDA, a breakdown product of lipid peroxidation, acts adversely on proteins and DNA, leading to DNA mutation and protein deactivation (Jia et al. 2017). Kaczmarczyk et al. (2012) reported that production of MDA is a major sign of lipid peroxidation and an indicator of oxidative stress during cryopreservation. In this study, MDA gradually accumulated in root segments during the pre-culture and loading stages, and levels peaked after exposure to the vitrification solution. Previous studies also show increases in MDA content during cryopreservation of Oryza sativa, Azadirachta indica, Zea mays, Dendrobium wardianum, and Arabidopsis thaliana (Benson et al. 1992; Varghese and Naithani 2008; Wen et al. 2010; Wu and Shen 2011; Ren et al. 2013).
In addition, plants show complex antioxidant responses to oxidative stress that involve a careful balance between enzymatic antioxidants, such as CAT and POD, and many non-enzymatic pathways involving flavones, anthocyanin, vitamin C, and carotenoids (Apel and Hirt 2004; Halliwell 2006; Pandhair and Sekhon 2006). An analysis of CAT activity in ginseng root segments during the cryopreservation process found that it was lowest during the vitrification stage, indicating that the highest levels of oxidative stress accumulated after cryopreservation. CAT is an enzyme that converts hydrogen peroxide to water and oxygen, and is thus essential for detoxifying ROS during stress (Ahmad et al. 2008). Levels of CAT also decreased during the post-cryopreservation stage in Dendrobium protocorm-like bodies (PLBs), leading to a reduced regeneration percentage of cryopreserved PLBs (Poobathy et al. 2013). Jia et al. (2017) reported that exogenous CAT reduced oxidative injury during cryopreservation of Paeonia and Magnolia pollen. We found that ROS-induced oxidative stress was closely related to reductions in the regeneration rate of ginseng root tips after cryopreservation. The optimal composition of the vitrification solution and the application of exogenous antioxidants should therefore be considered in further studies to provide protection from oxidative stress and improve regeneration of cryopreserved ginseng roots.
5 Conclusions
This study established a droplet-vitrification protocol for cryopreserving root tips of ginseng (P. ginseng). Pre-culturing adventitious roots with 0.3 M sucrose produced the highest level of regrowth after cryopreservation in liquid nitrogen for 30 min. Regrowth of cryopreserved adventitious roots was improved fourfold over untreated controls by treatment with C4 loading solution for 20 min at room temperature. The highest rate of regrowth (15% of roots surviving after cryopreservation) was obtained by incubating adventitious roots in modified B5 solution for 10 min at room temperature. Adventitious root tips of ginseng were sensitive to osmotic stress. Future research will therefore focus on improving the post-cryopreservation regeneration rate by optimizing the composition of the vitrification solution and the application of exogenous antioxidative enzymes.
References
Ahmad P, Sarwat M, Sharma S (2008) Reactive oxygen species, antioxidants and signaling in plants. J Plant Biol 51:167–173
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Benson EE, Lynch PT, Jones J (1992) The detection of lipid peroxidation products in cryoprotected and frozen rice cells: consequences for post-thaw survival. Plant Sci 85:107–114
Bisht SS, Sharma A, Chaturvedi K (1989) Certain metabolic lesions of chromium toxicity in radish. Indian J Agric Biochem 2:109–115
Chen G, Ren L, Zhang J, Reed BM, Zhang D, Shen XH (2015) Cryopreservation affects ROS-induced oxidative stress and antioxidant response in Arabidopsis seedlings. Cryobiology 70:38–47
Christensen LP (2008) Ginsenosides: chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res 55:1–99
Da Silva Cordeiro L, Simões-Gurgel C, Albarello N (2015) Multiplication and cryopreservation of adventitious roots of Cleome rosea Vahl. Vitro Cell Dev Biol 51:249–257
Engelman F, Takagi H (2000) Cryopreservation of tropical plant germplasm current research progress and application. Japan International Research Center for Agriculture Sciences, Tsukuba
Engelmann F, Dussert S (2013) Cryopreservation. In: Normah MN, Chin HF, Reed BM (eds) Conservation of tropical plant species. Springer, Berlin, pp 107–119
Fábián A, Jäger K, Darkó É, Barnabás B (2008) Cryopreservation of wheat (Triticum aestivum L.) egg cells by vitrification. Acta Physiol Plant 30:737–744
Fu C, Li L, Wu W, Li M, Yu X, Yu L (2012) Assessment of genetic and epigenetic variation during long-term Taxus cell culture. Plant Cell Rep 31:1321–1331
Fujikawa S, Steponkus PL (1991) Plasma membrane ultrastructural changes by vitrification procedures. Jpn J Free Dry 37:25–29
Hahn EJ, Yu KW, Paek KY (2003) Adventitious root cultures of Panax ginseng CV Meyer and ginsenoside production through large-scale bioreactor system. J Plant Biotechnol 5:1–6
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Hirata K, Mukai M, Goda S, Ishio-Kinugasa M, Yoshida K, Sakai A, Miyamoto K (2002) Cryopreservation of hairy root cultures of Vinca minor (L.) by encapsulation-dehydration. Biotechnol Lett 24:371–376
Hughes ZE, Mancera RL (2014) Molecular mechanism of the synergistic effects of vitrification solutions on the stability of phospholipid bilayers. Biophys J 106:2617–2624
Ibrahim S, Normah MN (2013) The survival of in vitro shoot tips of Garcinia mangostana L. after cryopreservation by vitrification. Plant Growth Regul 70:237–246
Jia MX, Shi Y, Di W, Jiang XR, Xu J, Liu Y (2017) ROS-induced oxidative stress is closely related to pollen deterioration following cryopreservation. Vitro Cell Dev Biol 53:433–439
Jung DW, Sung CK, Touno K, Yoshimatsu K, Shimomura K (2001) Cryopreservation of Hyoscyamus niger adventitious roots by vitrification. J Plant Physiol 158:801–805
Kaczmarczyk A, Funnekotter B, Menon A, Phang PY, Al-Hanbali A, Bunn E, Mancera RL (2012) Current issues in plant cryopreservation. In: Katov II (ed) Current frontiers in cryobiology. Croatia, InTech, pp 417–438
Kim HH, Lee YG, Park SU, Lee SC, Baek HJ, Cho EG, Engelmann F (2009a) Development of alternative loading solutions in droplet-vitrification procedures. CryoLetters 30:291–299
Kim HH, Lee YG, Shin DJ, Ko HC, Gwag JG, Cho EG, Engelmann F (2009b) Development of alternative plant vitrification solutions and loading solutions in droplet-vitrification procedures. Cryobiology 59:320–334
Kim HH, Popova EV, Yi JY, Cho GT, Park SU, Lee SC, Engelmann F (2010) Cryopreservation of hairy roots of Rubia akane (Nakai) using a droplet-vitrification procedure. CryoLetters 31:473–484
Kim DS, Song M, Kim SH, Jang DS, Kim JB, Ha BK, Kim SH, Lee KJ, Kang SY, Jeong IY (2013) The improvement of ginsenoside accumulation in Panax ginseng as a result of γ-irradiation. J Ginseng Res 37:332–340
Kiselev KV, Shumakova OA, Tchernoded GK (2011) Mutation of Panax ginseng genes during long-term cultivation of ginseng cell cultures. J Plant Physiol 168:1280–1285
Kulus D, Zalewska M (2014) Cryopreservation as a tool used in long-term storage of ornamental species–a review. Sci Hortic (Amsterdam) 168:88–107
Kulus D, Abratowska A, Mikuła A (2018) Morphogenetic response of shoot tips to cryopreservation by encapsulation-dehydration in a solid mutant and periclinal chimeras of Chrysanthemum × grandiflorum/Ramat./Kitam. Acta Physiol Plant 40:18
Kuranuki Y, Sakai A (1995) Cryopreservation of in vitro-grown shoot tips of tea (Camellia sinensis) by vitrification. CryoLetters 16:345–352
Lambert E, Geelen D (2008) Cryopreservation of hairy root cultures from Maesa lanceolata. In: Laamanen J, Uosukainen M, Häggman H, Rantala ANS (eds) Cryopreservation of Cropspecies in Europe CRYOPLANET COST Action 871, 20th–23rd Feb 2008, Oulu, Finland
Lambert E, Goossens A, Panis B, van Labeke MC, Geelen D (2009) Cryopreservation of hairy root cultures of Maesa lanceolata and Medicago truncatula. Plant Cell, Tissue Organ Cult 96:289–296
Lee KH (1998) The pharmacology of Chinese herbs. J Nat Prod 61:1575–1576
Lü JM, Yao Q, Chen C (2009) Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol 7:293–302
Makowska Z, Keller J, Engelmann F (1999) Cryopreservation of apices isolated from garlic (Allium sativum L.) bulbils and cloves. CryoLetters 20:175–182
Martinez-Montero ME, Harding K (2015) Cryobionomics: evaluating the concept in plant cryopreservation. In: Barh D, Khan MS, Davies E (eds) Plant omics: the omics of plant science. Springer, Berlin, pp 655–682
Matsumoto T, Sakai A, Yamada K (1994) Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep 13:442–446
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73
Oh SY, Wu CH, Popova E, Hahn EJ, Paek KY (2009) Cryopreservation of Panax ginseng adventitious roots. J Plant Biol 52:348–354
Pandhair V, Sekhon BS (2006) Reactive oxygen species and antioxidants in plants: an overview. J Plant Biochem Biotechnol 15:71–78
Panis B, Piette B, Swennen R (2005) Droplet vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Sci 168:45–55
Park SU, Kong H, Shin DJ, Bae CH, Lee SC, Bae CH, Rha ES, Kim HH (2014) Development of vitrification protocol in Rubia akane (Nakai) hairy roots using a systematic approach. CryoLetters 35:138–144
Poobathy R, Sinniah UR, Xavier R, Subramaniam S (2013) Catalase and superoxide dismutase activities and the total protein content of protocorm-like bodies of Dendrobium Sonia-28 subjected to vitrification. Appl Biochem Biotechnol 170:1066–1079
Quain MD, Berjak P, Acheampong E, Kioko JI (2009) Sucrose treatment and explant water content: critical factors to consider in development of successful cryopreservation protocols for shoot tip explants of the tropical species Dioscorea rotundata (yam). CryoLetters 30:212–223
Ren L, Zhang D, Jiang XN, Gai Y, Wang WM, Reed BM, Shen XH (2013) Peroxidation due to cryoprotectant treatment is a vital factor for cell survival in Arabidopsis cryopreservation. Plant Sci 212:37–47
Sakai A, Engelmann F (2007) Vitrification, encapsulation-vitrification and droplet-vitrification: a review. CryoLetters 28:151–172
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. Brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
San José MC, Valladares S, Janeiro LV, Corredoira E (2014) Cryopreservation of in vitro-grown shoot tips of Alnus glutinosa (L.) Gaertn. Acta Physiol Plant 36:109–116
Steponkus PL, Langis R, Fujikawa S (1992) Cryopreservation of plant tissues by vitrification. In: Steponkus PL (ed) Advances in low-temperature biology. JAI Press, London, pp 1–61
Suzuki M, Ishikawa M, Okuda H, Noda K, Kishimoto T, Nakamura T, Ogiwara I, Shimura I, Akihama T (2006) Physiological changes in gentian axillary buds during two-step preculturing with sucrose that conferred high levels of tolerance to desiccation and cryopreservation. Ann Bot 97:1073–1081
Takagi H, Thinh NT, Islam OM, Senboku T, Sakai A (1997) Cryopreservation of invitro-grown shoot tips of taro (Colocasia esculenta (L.) Schott) by vitrification. 1. Investigation of basic conditions of the vitrification procedure. Plant Cell Rep 16:594–599
Thinh NT (1997) Cryopreservation of germplasm of vegetatively propagated tropical monocots by vitrification. Dr Pap Fac Agric Kobe Univ Japan
Touno K, Yoshimatsu K, Shimomura K (2006) Characteristics of Atropa belladonna hairy roots cryopreserved by vitrification method. CryoLetters 27:65–72
Turgut-Kara N, Kahraman BÜ (2015) Effects of long-term culture of Astragalus chrysochlorus callus onmorphology, genetic structure, gene expression and metabolism. Plant Biosyst 149:329–336
Uchendu EE, Muminova M, Gupta S, Reed BM (2010) Antioxidant and anti-stress compounds improve regrowth of cryopreserved Rubus shoot tips. Vitro Cell Dev Biol 46:386–393
Varghese B, Naithani SC (2008) Oxidative metabolism-related changes in cryogenically stored neem (Azadirachta indica A. Juss.) seeds. J Plant Physiol 165:755–765
Wang RR, Gao XX, Chen L, Huo LQ, Li MF, Wang QC (2014) Shoot recovery and genetic integrity of Chrysanthemum morifolium shoot tips following cryopreservation by droplet-vitrification. Sci Hortic (Amsterdam) 176:330–339
Wen B, Wang R, Cheng H, Song S (2010) Cytological and physiological changes in orthodox maize embryos during cryopreservation. Protoplasma 239:57–67
Wesley-Smith J, Berjak P, Pammenter NW, Walters C (2013) Intracellular ice and cell survival in cryo-exposed embryonic axes of recalcitrant seeds of Acer saccharinum: an ultrastructural study of factors affecting cell and ice structures. Ann Bot 113:695–709
Wu YL, Shen XH (2011) Cryopreservation of Dendrobium wardianum Warner. protocorms by vitrification. Chin J Cell Bio 33:279–287
Xie JT, Attele AS, Yuan CS (2006) Ginseng: beneficial and potential adverse effect. In: Yuan CS, Beiber E, Bauer BA (eds) A textbook of complementary and alternative therapies. CRC Press Company, Boca Raton, London, New York, Washington, DC, pp 71–89
Xue SH, Luo XJ, Wu ZH, Zhang HL, Wang XY (2008) Cold storage and cryopreservation of hairy root cultures of medicinal plant Eruca sativa Mill., Astragalus membranaceus and Gentiana macrophylla Pall. Plant Cell, Tissue Organ Cult 92:251–260
Yi JY, Sylvestre I, Colin M, Salma M, Lee SY, Kim HH, Park HJ, Engelmann F (2012) Improved cryopreservation using droplet-vitrification and histological changes associated with cryopreservation of Madder (Rubia akane Nakai). Korean J Hortic Sci Technol 30:79–84
Yoon JW, Kim HH, Ko HC, Hwang HS, Hong ES, Cho EG, Engelmann F (2006) Cryopreservation of cultivated and wild potato varieties by droplet vitrification: effect of subculture of mother-plants and of preculture of shoot tips. CryoLetters 27:211–222
Yoshimatsu K, Touno K, Shimomura K (2000) Cryopreservation of medicinal plant resources: retention of biosynthetic capabilities in transformed cultures. In: Cryopreservation of tropical plant germplasm: current research progress and application. Proceedings of an international workshop, Tsukuba, Japan, October 1998. International Plant Genetic Resources Institute (IPGRI), pp 77–88
Zhang D, Ren L, Gq Chen ZJ, Reed BM (2015) ROS-induced oxidative stress and apoptosis-like event directly affect the cell viability of cryopreserved embryogenic callus in Agapanthus praecox. Plant Cell Rep 34:1499–1513
Acknowledgements
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Advanced Production Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (Grant No. 315013-4).
Author information
Authors and Affiliations
Contributions
K-CL contributed to data acquisition and wrote the manuscript. H-HK and K-YP participated in interpreted data and revising for intellectual content. S-YP made substantial contributions to the conception and design of the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Communicated by Inhwa Yeam.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Le, KC., Kim, HH. & Park, SY. Modification of the droplet-vitrification method of cryopreservation to enhance survival rates of adventitious roots of Panax ginseng. Hortic. Environ. Biotechnol. 60, 501–510 (2019). https://doi.org/10.1007/s13580-019-00150-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-019-00150-8