Abstract
Adventitious root cultures of Tarenaya rosea were successfully cryopreserved using the encapsulation-vitrification technique. Histological analysis revealed useful information on the successive steps of cryopreservation. Coupled with complementary histochemical approaches, these studies provided cellular and tissue descriptions of T. rosea root cultures during cryopreservation and contributed to an understanding of cellular stress responses, as well as characterization of the anatomical pattern of root regeneration. The effects of exposure duration to PVS3 solution (0–120 min), unloading treatment (direct and gradual), and recovery medium (liquid and solid) on recovery of cryopreserved roots were investigated. The highest recovery (91%) after cooling in liquid nitrogen (LN) was reached with PVS3 treatment for 90 min, gradual rehydration in unloading solution, and recovery on solid MS medium. The cryopreserved roots showed high multiplication capacity, which was maintained for up to four subcultures. The effect of cryopreservation on root structure was investigated by histological and histochemical studies. Plasmolysis intensified during exposure to loading and PVS3 solutions, but decreased after unloading treatment. The proportion of intercellular spaces increased progressively throughout the cryopreservation protocol, culminating in root cortex disruption. Histochemical analyses revealed polysaccharides, proteins, and both lipidic and pectic substances in intercellular spaces. The vascular cylinder remained intact, ensuring the formation of new roots from the pericycle, showing that proliferative capacity of cryopreserved roots had not diminished.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tarenaya rosea (Vahl ex DC.) Soares Neto & Roalson is an endemic Brazilian species found in coastal plains (restingas) of the Atlantic Forest, which are ecosystems threatened as a result of harmful anthropic actions (Scarano 2009). This species, previously named Cleome rosea Vahl ex DC (Soares Neto et al. 2018), presents medicinal potential, including anti-inflammatory and antigenotoxic (Simões et al. 2006), antiviral (Simões et al. 2010b), and antibacterial activity (Simões-Gurgel et al. 2012). Several biotechnological studies have been developed with T. rosea, producing micropropagated plants, callus, and cell suspension cultures aimed at the synthesis of secondary metabolites (Rocha et al. 2015; Simões et al. 2004, 2009a, b, 2010a; Simões-Gurgel et al. 2011). Adventitious root cultures of this species were also successfully established from root segments of in vitro-propagated plants cultivated in auxin-containing liquid culture medium (Cordeiro et al. 2015; Simões et al. 2009a). In addition, T. rosea root explants sampled from plants, as well as from root cultures, multiplied in liquid medium, demonstrating the capacity for shoot regeneration (Simões et al. 2009a).
Considering the high regenerative potential of roots, their use for metabolite production and as an alternative source of explants for clonal propagation and germplasm conservation, in vitro root cultures have been established for several species (Murthy et al. 2016; Singh et al. 2018; Ślesak et al. 2015; Yang et al. 2019). Such materials have been the subject of cryopreservation studies aimed at ensuring their long-term conservation. Cryopreservation (liquid nitrogen [LN], − 196 °C) allows the storage of biological samples for extended time spans in a small volume, protected from contamination and operational errors, and requiring minor maintenance (Engelmann 2011; Cruz-Cruz et al. 2013; Engelmann and Gonzalez-Arnao 2013; Harding et al. 2013).
Hairy roots, or genetically transformed root cultures, of numerous medicinal plants have been cryopreserved (Hirata et al. 2002; Teoh et al. 1996; Xue et al. 2008; Yi et al. 2012). However, few studies have applied cryopreservation techniques to untransformed, or adventitious, root cultures, such as those of Hyoscyamus niger (Jung et al. 2001) and Panax ginseng (Oh et al. 2009; Le et al. 2019).
Vitrification and encapsulation-vitrification are the most widely used techniques for plant cryopreservation and are based on the treatment of explants with solutions containing cryoprotectants that remove the freezable water from cells and tissues. This osmotic dehydration contributes to the vitrification process, during cooling in LN, avoiding the formation of ice crystals (Sakai and Engelmann 2007). Since explants are covered by a calcium alginate matrix, the encapsulation-vitrification technique may have some advantages over vitrification, such as the easy handling of explants, protection of the most fragile materials, higher and faster recovery, and applicability for different types of propagules (Shin et al. 2014).
During cryopreservation, the materials are submitted to several steps, including explant excision, dehydration/osmoprotection with highly concentrated cryoprotective solutions and/or physical dehydration, LN cooling, rewarming, and recovery (Gonzalez-Arnao et al. 2008). These steps can be very deleterious to materials and result in different levels of injury, those being membrane destabilization, changes in cell structure, and activity patterns of antioxidant enzymes, as well as damage induced by reactive oxygen species (Antony et al. 2014; Benson 1990; Wen et al. 2012). These injuries, when very extensive, can lead to loss of tissue viability during post-cryopreservation recovery (Benson and Bremner 2004). On the other hand, less damaged tissues can recover and return to their normal growth (Sershen et al. 2012). Thus, an important condition for cryopreservation success depends on sufficient preservation of explant structure to ensure regrowth after LN exposure.
Histological analysis can foster an understanding of the effects of successive cryopreservation steps on the structural integrity of cryopreserved materials, revealing the critical steps of protocols and contributing to future strategies to optimize the technique for each specific explant. In this sense, many studies have been performed using microscopic observation to develop methodologies to cryopreserve shoot tips, embryos, cell cultures, and hairy root cultures (Barraco et al. 2014; Fang and Wetten 2011; Fraga et al. 2016; Mathew et al. 2018; Salma et al. 2014).
In our previous cryopreservation studies, adventitious root segments formed from in vitro-propagated plants of T. rosea achieved high recovery when cryopreserved by vitrification (Cordeiro et al. 2015, 2016). Adventitious root cultures of T. rosea display rapid growth, high biomass accumulation, capacity to regenerate shoots, and potential for in vitro metabolite production (Cordeiro et al. 2015; Simões et al. 2009a). Therefore, the use of this system as a source of explants for cryopreservation may be more productive than roots from in vitro-propagated plants. Thus, the present study aimed at establishing a methodology for cryopreservation of T. rosea roots multiplied in liquid medium and performing qualitative and quantitative structural evaluations of the roots during the cryopreservation through histological and histochemical analysis.
Materials and methods
Plant material and culture conditions
In vitro-propagated plants of T. rosea (Simões et al. 2009a) cultured for 30 days on solidified (8 g L−1 agar, Merck, Germany) MS medium (Murashige and Skoog 1962), without plant growth regulators, were used as source of root explants. Cultures were initiated from root segments (0.4–0.5 cm in length) without lateral roots, which were excised from proximal (the first 5–6 cm from the stem) regions. Five root segments were cultured in 50-mL Erlenmeyer flasks containing 20 mL of liquid MS medium supplemented with 0.25 mg L−1 naphthaleneacetic acid (NAA) and 0.1 M sucrose (Merck) (Cordeiro et al. 2015). The pH of all media was adjusted to 5.8 prior to autoclaving at 121 °C for 15 min. The Erlenmeyer flasks were closed with a double-blade aluminum cap and maintained on a gyratory shaker (100 rpm) at 26 ± 2 °C in the dark.
Cryopreservation by encapsulation-vitrification
Root segments obtained from six-week-old adventitious root cultures (Cordeiro et al. 2015) were cryopreserved by applying the encapsulation-vitrification technique. Root segments (0.3–0.4 cm in length) were suspended in 3% (w/v) sodium alginate (Sigma-Aldrich, USA) solution in calcium-free MS liquid medium supplemented with 0.1 M sucrose. By using a pipette with a cut-off tip (with 0.4 cm opening diameter), the roots were individually transferred drop-by-drop to the calcium chloride solution (100 mM calcium chloride in MS medium) containing 0.1 M sucrose and left for 20 min to form beads about 0.4 cm in diameter, each bead containing one root (Fig. 1a). The beads (encapsulated roots) were pretreated in 50-mL Erlenmeyer flasks containing 20 mL liquid MS medium supplemented with 0.25 mg L−1 NAA, with increasing concentrations of sucrose (0.2 M and 0.4 M), remaining at each concentration for 2 days. The flasks were maintained on a gyratory shaker (100 rpm) at 26 ± 2 °C in the dark.
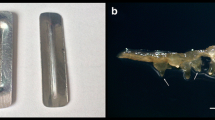
Cryopreservation of root segments obtained from adventitious root cultures of T. rosea using the encapsulation-vitrification technique. a Root segments encapsulated in alginate matrix. b Root multiplication from explants not immersed in LN and maintained in liquid recovery medium after 3 weeks of culture. The arrow shows a fragmented root. c Root multiplication from cryopreserved explants maintained on solid recovery medium after 5 weeks of culture. The arrow shows orange-colored pigment production. d Root cultures obtained from cryopreserved roots after 6 weeks of culture. Root segments were encapsulated in alginate matrix pretreated with increasing concentrations of sucrose (0.2 M and 0.4 M) and exposed to loading solution for 20 min and PVS3 for 90 min prior to immersion in LN. After rewarming, the roots were treated with unloading solution using a gradual procedure and then transferred to liquid or solid recovery medium supplemented with NAA. Bars = 1.0 cm
After pretreatment, the beads were transferred into 2-mL cryogenic tubes (five beads/tube) and treated with 0.8 mL loading solution (0.4 M sucrose and 2.0 M glycerol in MS medium; Nishizawa et al. 1993) for 20 min, followed by exposure for 0, 30, 60, 90, and 120 min to 0.8 mL of PVS3 vitrification solution (50% [w/v] glycerol and 50% [w/v] sucrose in MS medium; Nishizawa et al. 1993). Before cooling, the vitrification solution was replaced by 0.8 mL fresh PVS3 solution. The cryogenic tubes were closed and immersed directly in LN for 2 h. For rewarming, the cryogenic tubes were dipped in a water bath at 38 °C for 2–3 min, and the cryopreserved roots were treated with unloading solution (liquid MS medium containing 1.2 M sucrose). Exposure to the unloading solution was performed using two different procedures: (i) direct unloading, which involves the replacement of total volume (0.8 mL) of PVS3 by the same volume of unloading solution and incubation for 30 min, and (ii) gradual unloading, which involves the replacement of increasing volumes of PVS3 (0.2, 0.4 and 0.8 mL) by the same volumes of unloading solution, followed by incubation for 15 min at each change.
During the recovery step, the encapsulated roots were cultured in liquid or on solid (8 g L−1 agar, Merck, Germany) MS medium supplemented with 0.25 mg L−1 NAA. When recovery was performed in liquid medium, the root segments were exposed to liquid MS medium with decreasing concentrations of sucrose (0.4 M and 0.2 M), remaining at each concentration for 2 days in the dark on a gyratory shaker (100 rpm). After this period, the roots were transferred to 20 mL liquid recovery medium (five beads/flask) added with 0.1 M sucrose. On the other hand, when the recovery step was performed on solid culture medium, the root segments were transferred directly to Petri dishes (9.0 × 1.0 cm) (10 beads/dish), containing 25 mL solid MS medium added with 0.1 M sucrose. The cultures were maintained for 6 weeks in a growth chamber at 26 ± 2 °C in the dark. As the control group, root segments were encapsulated, treated as described previously, but without immersion in LN, and then transferred to recovery culture condition (liquid or solid medium). The recovery (number of explants with lateral root formation) was recorded after 4 weeks and expressed as the percentage of root segments with newly formed lateral roots based on the total number of root segments used per treatment.
To determine the chemical nature of an orange pigment produced by the cryopreserved roots, samples (1.0 g fresh weight) were extracted for 24 h in acetone PA (10 mL) at 4 °C. The solution was filtered through Whatman® filter paper no.1 and analyzed with a spectrophotometer (Shimadzu UV–160, Japan) in the range of 300 to 600 nm.
The newly formed roots were subcultured at 6-week intervals for 24 weeks (during 4 subcultures) in liquid MS medium. Five root segments (0.4–0.5 cm in length, with ~ 0.6 mg fresh weight) were transferred to 50-mL Erlenmeyer flasks containing 20 mL MS medium with 0.25 mg L−1 NAA. The multiplication capacity was evaluated by measuring the fresh (FW) and dry (DW) weight of root cultures.
Histological studies
Sample preparation for microscopy
For histological analysis, slides of root segments (three roots per treatment) were prepared after each of the following steps of the cryopreservation protocol and recovery:
-
1.
Control: Roots excised from 6-week-old root cultures (untreated control);
-
2.
Pretreatment (0.2 M → 0.4 M sucrose): Root segments after pretreatment in solution containing 0.2 M sucrose (2 days), followed by 0.4 M sucrose (2 days);
-
3.
Loading: Root segments after pretreatment and exposure to the loading solution (20 min);
-
4.
PVS3: Root segments after pretreatment, exposure to loading solution and PVS3 solution (90 min), immersed (+LN) or not in LN (−LN);
-
5.
Unloading: Root segments after pretreatment, exposure to loading solution and PVS3, immersion (+LN) or not in LN (−LN), followed by gradual unloading treatment;
-
6.
1 day recovery: Root segments after pretreatment, exposure to loading solution and PVS3, immersion (+LN) or not in LN (−LN), followed by gradual unloading treatment and culture on recovery solid medium supplemented with NAA for 1 day;
-
7.
7 days recovery: Root segments after pretreatment, exposure to loading solution and PVS3, immersion (+LN) or not in LN (−LN), followed by gradual unloading treatment and culture on recovery solid medium supplemented with NAA for 7 days.
The samples described above were immersed in fixative solution prepared with phosphate buffer (0.2 M, pH 7.2) containing paraformaldehyde (2%, w/v), glutaraldehyde (1%, v/v), and caffeine (1%, w/v), where they remained for 48 h at 4 °C. The osmolarity of the fixative solution was adjusted by adding sucrose according to the osmolarity of the last solution in which the materials were treated at each step of the cryopreservation protocol.
Fixed roots were transferred to 70% ethanol, where they remained for 24 h at 4 °C. Afterwards, the samples were dehydrated in successive ethanol baths of increasing concentrations (70, 90, 95, and 100%, v/v, each for 2 h at 4 °C) and stored in 100% ethanol for 24 h at 4 °C. Dehydrated samples were transferred to 50% ethanol and 50% butanol mixture (v/v) for 24 h and then to 100% butanol for 4 days at 4 °C.
For resin embedding (2-hydroxyethyl methacrylate, Technovit® 7100, Heraeus Kulzer, Germany), the roots were transferred to a mixture containing 50% butanol and 50% resin (v/v). After 48 h, they were immersed in 100% resin, where they remained for 72 h at 4 °C. Samples were transferred to fresh resin in embedding molds, and the resin was polymerized at 4 °C for 24 h.
Cross sections (3 μm thickness) from the roots were produced using a rotary microtome (Microm HM 355S, Thermo Scientific, Germany), mounted on glass slides, and double-stained with periodic acid-Schiff’s reagent (PAS, Sigma-Aldrich, France) combined with Naphthol blue-black (NBB; Sigma-Aldrich, France) (Buffard-Morel et al. 1992; Fisher 1968). This treatment stained polysaccharides, such as starch reserves and walls in dark pink (by PAS) and soluble or reserve proteins in blue-black (by NBB).
Qualitative and quantitative analyses
Qualitative and quantitative evaluations of the effects of successive steps of cryopreservation on the cellular and tissue organization of roots were performed. For quantitative analysis, the following parameters were considered:
-
(a)
Proportion of intercellular spaces in the cortex expressed as the ratio between the surface of intercellular spaces and the total cortex surface. This parameter was determined using three roots per treatment and three sections per root (total = 9), as (surface of intercellular spaces / total cortex surface) × 100.
-
(b)
Plasmolysis level expressed as the ratio between the cytoplasm surface and the total cell surface delimited by the cell wall. This parameter was evaluated for cells of the epidermis, cortical parenchyma, endodermis, and vascular cylinder, using three roots per treatment, three sections per root, and five cells per section (total = 45), as 100 − [(cytoplasmic surface / total cell surface) × 100] (Barraco et al. 2014).
The histological slides were observed at × 20 and × 40 magnification under a light microscope (DM-6000, Leica, Germany) equipped with a Retiga 2000R camera, using the QCapture pro 5.1 image capture system (QImaging, Canada). Measurements (μm2) were obtained using Image-Pro Plus 4.5 software (Media Cybernetics, Rockville, USA).
Histochemical analyses
For histochemical analysis, cross sections (10 μm thickness) were obtained with a rotary microtome (Leica RM2125 RTS, Leica Biosystems, Germany). To evaluate the chemical nature of the content present in the intercellular spaces of the root cortex, the sections were submitted to different histochemical tests: Sudan IV for lipid detection (Gerlach 1984), ruthenium red for pectic substances (Johansen 1940), Lugol to detect starch (Jensen 1962), and ferric chloride for general phenolic substances (Johansen 1940). In addition, PAS and NBB double staining, as used in histological analysis, also served as a histochemical test, evidencing polysaccharides and proteins, respectively. As controls, sections without staining were used. These histological slides were analyzed under the Olympus BX 41 light microscope (Olympus Corporation, Japan) equipped with a Q-Color3 camera, using the Image-Pro Express (Media Cybernetics, Rockville, USA) image capture system.
Statistical analysis
Each treatment in the cryopreservation experiments was replicated three times using 20 roots per replicate. For quantitative histological analysis, three roots per treatment and three sections per root were used. Results were presented as means with standard deviation. Data were analyzed using one-way analysis of variance (ANOVA), and the differences among means were tested by Tukey’s range test at p ≤ 0.05. Analyses were carried out with the statistical software GraphPad Prism 5 (GraphPad Software Inc., San Diego, USA).
Results
Cryopreservation
The encapsulation process did not affect root development since root growth was observed after alginate encapsulation, showing the feasibility of applying the technique for root cryopreservation. After pretreatment with high concentrations of sucrose and exposure to loading solution, the encapsulated roots presented recovery capacity in the first week of culture in liquid medium. The roots submitted to these treatments, but not immersed in LN, displayed 100% recovery. However, they did not show any regeneration capacity after LN exposure (Table 1).
After direct unloading and during the recovery in liquid medium, fragmentation of some encapsulated roots was observed (Fig. 1b), mainly for samples immersed in LN. However, despite the fragmentation, noncryopreserved roots reached high recovery (90–100%), while those that were cryopreserved did not recover after LN exposure (Table 1). Considering these results, a new methodology was tested, namely, gradual exposure of the material to the unloading solution (gradual unloading) before culture in liquid medium. This new methodology reduced explant fragmentation and resulted in recovery from 93 to 100% for noncryopreserved roots, but still no recovery was achieved with the cryopreserved roots (Table 1).
Based on these results, the use of solid culture medium during the recovery step was investigated. Solid medium allowed roots to maintain their physical integrity, immersed or not in LN, after direct or gradual unloading treatment, allowing up to 91% recovery. The newly formed roots appeared between the second and third week after cooling. Longer exposure times to PVS3 associated with gradual unloading solution treatment and recovery on solid medium resulted in the highest recovery after cooling, 85 and 91% for 120 and 90 min PVS3 treatments, respectively (Table 1). On the other hand, direct unloading only enabled recovery up to 55% (Table 1).
Cryopreserved roots transferred to solid medium produced an orange pigment on their surface after the third week in culture (Fig. 1c). This pigment production occurred in all cryopreserved explants, but not in newly formed roots (Fig. 1c). The absorption spectrum of this pigment showed peaks in the range of 400 nm (453 nm and 478 nm), a characteristic profile of substances from the carotenoid group (Fig. 2).
The roots developed after cryopreservation showed high multiplication capacity over time in culture (Fig. 3).
Histological and histochemical analyses
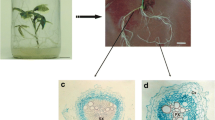
The cross sections of untreated (control) roots showed intercellular spaces in the cortical parenchyma and the presence of starch grains in epidermis and cortical cells, as indicated by red spots. In the vascular cylinder, the vascular tissues were at the primary stage of development (Fig. 4a). The intercellular spaces in cortical parenchyma corresponded to 20% of total cortex surface (Table 2). Plasmolysis was observed in all cell types evaluated, mainly in epidermal and cortical parenchyma cells (Table 2).
Histological sections of T. rosea roots after successive steps of cryopreservation protocol by the encapsulation-vitrification technique. a Excision from root culture (untreated control). b Pretreatment at increasing sucrose concentrations (0.2 M → 0.4 M sucrose). c Treatment with loading solution. d Treatment with PVS3 for 90 min and immersion in LN (arrows: endoderm rupture). e Immersion in LN and treatment with unloading solution (arrows: endoderm rupture). f One day on solid recovery medium without immersion in LN. LN liquid nitrogen, Ep epidermis, CP cortical parenchyma, En endodermis, VC vascular cylinder, Pe pericycle, X xylem; P phloem, SG starch grain. Bars = 50 μm
After the pretreatment (0.2 M → 0.4 M sucrose), the presence of starch grains in cells of vascular cylinder, cortex, and epidermis was detected (Fig. 4b). Although the proportion of intercellular spaces did not show any significant increase, cells of cortical parenchyma and endodermis exhibited a high level of plasmolysis when compared with control roots (Table 2).
After exposure to loading solution, the presence of a dense intercellular content in cortical parenchyma was observed (Fig. 4c). The endoderm cells were narrower and irregularly shaped, and cells of the vascular cylinder had a densely stained cytoplasm, while starch grain accumulation increased in all cell types (Fig. 4c). In addition, the plasmolysis level increased in cortical parenchyma, endodermis, and vascular cylinder cells, but it was lower in the epidermis when compared with the histological aspect of cells at the previous steps of cryopreservation protocol (Table 2).
Treatment with PVS3, followed by immersion (+LN), or not (−LN), increased intercellular spaces to over 31% of total cortex surface, resulting in the increase of intercellular content concentration (Table 2). In addition, exposure to vitrification solution induced cell lysis in endoderm cells and increased plasmolysis level in vascular cylinder cells (Fig. 4d) (Table 2).
The root cells observed after gradual unloading treatment showed an aspect similar to that described after exposure to PVS3 (Fig. 4e). However, the plasmolysis level in all cell types was lower when compared with the explants submitted to previous cryopreservation steps (Table 2).
After one day on recovery medium, the cellular spaces in the cortex were completely filled by the dense content (Fig. 4f) (Table 2). Moreover, clusters of cells with intense blue-stained cytoplasm, similar to meristematic cells in division, were detected in the vascular cylinder, suggesting the beginning of lateral root formation. The presence of starch grains in all cell types, mainly in the vascular cylinder cells under multiplication (Fig. 4f), and the extension of intercellular spaces increased significantly compared with the initial steps of cryopreservation (Table 2). On the other hand, while plasmolysis decreased substantially in cells of the epidermis and central cylinder, it increased in cortical cells (Table 2).
Culture for 7 days on recovery medium was the last step evaluated. Roots immersed in LN presented an unstructured cortex completely filled by a dense content (Fig. 5a). The intercellular spaces represented 74% of the cortex (Table 2). In addition, the development of new roots, characterized by groups of cells with intensely blue-stained cytoplasm, was observed in the vascular cylinder (Fig. 5a) and directly from the proliferation of pericycle cells (Fig. 5b), resulting in the formation of root primordia in regions opposite to the xylem (Fig. 5c). Because of the disruption of cortex tissues, the amount of plasmolysis was quantified only in cells of the epidermis and central cylinder, showing reduction after 7 days post-cryopreservation (Table 2). Similar histological observations were made in roots not immersed in LN (Fig. 5d).
Histological sections of T. rosea roots maintained on solid recovery medium 7 days after cryopreservation. a Root that was immersed in LN presents clusters of cells with intense blue staining of cytoplasm (arrow) in the vascular cylinder, suggesting the initiation of secondary roots. b Periclinal cell divisions of the pericycle (arrows). c Projection of root primordium (arrow) from the vascular cylinder. d Root that was not immersed in LN, presenting secondary roots (arrows) originating from the vascular cylinder. LN liquid nitrogen, CP cortical parenchyma, En endodermis, VC vascular cylinder, Pe pericycle, X xylem, P phloem. Bars = 50 μm
Histochemical analyses were performed in order to characterize the dense content produced in intercellular spaces (Fig. 6a). The Sudan IV test revealed the presence of lipids, as characterized by red staining (Fig. 6b). The presence of pectic substances was detected through the slight reddish staining after applying the ruthenium red test (Fig. 6c). In addition, the associated use of PAS and NBB reagents resulted in purple staining, suggesting the presence of polysaccharides and proteins (Fig. 6d).
Histochemical analysis of T. rosea roots maintained on solid recovery medium 24 h after LN exposure. a Histological section without staining (control). b Staining with Sudan IV. c Staining with ruthenium red. d Staining with PAS (periodic acid-Schiff’s reagent) and NBB (Naphthol blue-black). Bars = 50 μm
Discussion
In the present study, the cryopreservation of in vitro multiplied adventitious roots of T. rosea was achieved using the encapsulation-vitrification technique. In previous studies, adventitious roots directly excised from in vitro-propagated plants were cryopreserved using the vitrification technique (Cordeiro et al. 2015, 2016). However, as roots multiplied in liquid medium are thinner and more fragile, encapsulation with calcium alginate is more suitable since the material can be more easily manipulated and protected from osmotic damage caused by direct contact with the vitrification solutions (Popova et al. 2011; Sakai and Engelmann 2007).
For T. rosea root cultures, longer periods of exposure to PVS3 favored recovery. On the other hand, roots of in vitro-propagated plants did not tolerate long periods in the presence of PVS3, reaching the highest recovery after only 15 min on this solution (Cordeiro et al. 2016). The differences in cryopreservation tolerance observed in these studies may be related to the distinct origins and culture conditions of roots. The roots used in the present work originated from root cultures multiplied in liquid medium, while those of in vitro-propagated plants originated from stems grown on solid medium. Pasqua et al. (2005) reported that Camptotheca acuminata roots maintained in liquid medium have an anatomically altered structure, as indicated by the increase of exoderm cells and the presence of large aerenchymal-like spaces in the cortex. Although the aerenchyma functions as air storage in aquatic plants, under in vitro conditions, these intercellular spaces can act as reservoirs of moisture, which may result in higher water content of roots cultivated in liquid medium when compared with roots growing on solid medium. Therefore, the root cultures established in liquid medium would require a higher level of dehydration before LN exposure.
In the present study, after rewarming, encapsulated roots appeared fragmented after the unloading treatment and recovery in liquid medium. To avoid this fragmentation, the exchange between PVS3 and unloading solution was carried out gradually. The use of a gradual mixing of the two solutions was also efficiently applied in studies with Cymbidium eburneum (Gogoi et al. 2012) and Rubia akane (Kim et al. 2010; Park et al. 2014). However, T. rosea roots submitted to gradual unloading, but then transferred to liquid medium, continued to become fragmented and did not show recovery after immersion in LN. These results suggested that gradual unloading and transfer to solid culture medium should be combined during recovery. This procedure allowed recovery of 85 and 91% for roots exposed to PVS3 for 120 and 90 min, respectively.
Benson and Hamill (1991) obtained better results in the cryopreservation of Beta vulgaris roots when recovery was performed on solid medium compared with liquid medium. After cooling, these authors suggested that cells become more stressed and susceptible to osmotic pressure during cultivation in liquid medium and under agitation. Higher recovery was also reached on solid medium for genetically transformed roots of Artemisia annua (Teoh et al. 1996) and adventitious roots of Panax ginseng (Popova et al. 2011).
The cryopreserved root segments of T. rosea produced an orange pigment, which showed an absorption spectrum characteristic of most carotenoids. Carotenoid production was previously observed in root cultures of T. rosea in the presence of indole-3-butyric acid (IBA) (Cordeiro et al. 2015) and in callus cultures established on medium containing 2,4-dichlorophenoxyacetic acid (2,4-D) (Rocha et al. 2015). In another histological study on the Cleomaceae family, carotenoid was detected in callus of Cleome spinosa, in the form of chromoplasts in the cells and lipid bodies in the apoplast (Albarello et al. 2007). After one month, the orange pigment was observed on the outside surface of calli. Carotenoid production was indicated in callus of C. spinosa as result of in vitro culture conditions. The response was related to stress situations and changes in the ultrastructure of chloroplasts, as well as increase in the number and size of plastoglobuli (Albarello et al. 2007).
In vitro culture conditions, as well as cryopreservation procedures, may lead to increased production of reactive oxygen species, resulting in lipid peroxidation, membrane rupture, or protein and molecular changes (Benson 1990; Martín et al. 2015; Poobathy et al. 2013). Skyba et al. (2010) characterized the recovery of Hypericum perforatum shoot tips at a biochemical level, evaluating the oxidative stress resulting from the cryogenic process. The authors highlighted the level of carotenoids as one of the essential components for an effective antioxidant system since they react with free radicals, playing an important role in the protection of cell membranes and lipoproteins. In this context, the accumulation of carotenoids in the cryopreserved roots of T. rosea may be related to oxidative stress resulting from cryopreservation.
The cryopreserved roots of T. rosea recovered on solid medium and demonstrated their multiplication capacity through four subcultures when transferred to liquid medium. This result is in agreement with our previous studies, which show that roots formed from in vitro-propagated plants of T. rosea were able to maintain their multiplication for up to four subcultures after cryopreservation by vitrification technique (Cordeiro et al. 2015). Root segments of other species, such as Beta vulgaris, Hyoscyamus niger, Atropa belladonna, and Panax ginseng, after recovery in solid medium, were also efficiently transferred and maintained in liquid culture (Benson and Hamill 1991; Jung et al. 2001; Oh et al. 2009; Touno et al. 2006).
The histological and histochemical evaluations performed in the present study provided cellular and tissue descriptions of T. rosea adventitious root cultures during the cryopreservation steps, contributing to an understanding of cellular stress responses, as well as characterization of the anatomical pattern of root regeneration.
Changes in the structure and cellular ultrastructure of cryopreserved materials have been reported in several histological studies (Alla-N’nan et al. 2014; Ding et al. 2008; Fang and Wetten 2011; Fraga et al. 2016). In addition to qualitative evaluations, techniques that allow quantification of some cellular alterations have been employed in cryopreservation studies (Barraco et al. 2014; Salma et al. 2014; Simão et al. 2018; Volk and Caspersen 2007; Yi et al. 2012).
An increase in the accumulation of starch grains was observed in the cells of T. rosea roots after pretreatment with high sucrose concentrations. The pretreatment is aimed at initiating the decrease of water content through an osmotic process, acting on membrane stability. Another effect of sucrose pretreatment is the accumulation of storage compounds such as starch (Fang and Wetten 2011). Histological studies during plant cryopreservation also relate the increased production of starch grains to cell division processes and differentiation (Stewart et al. 2001; Yi et al. 2012).
In the presence of cryoprotectants, an early osmotic response is cell contraction owing to the mobility of water molecules across the membrane. As cryoprotectants penetrate the cell, the dehydration process decreases, and the cytoplasm becomes more viscous (Rubinsky 2003). However, if the exposure time to vitrification solution is too long, dehydration becomes excessive, resulting in cell damage (Wolfe and Bryant 2001). Plasmolysis is a common physiological response in cells that are under such stress (Wen et al. 2012), and its level has been calculated considering the ratio between the surface of cytoplasm and the total surface of the cell (Barraco et al. 2014; Salma et al. 2014; Simão et al. 2018; Yi et al. 2012). In T. rosea root cells, it was possible to observe and quantify the increase in plasmolysis after pretreatment. It intensified during exposure to loading and PVS3 solutions and decreased after treatment with unloading. While the cortex cells showed substantial plasmolysis early in the cryopreservation, the vascular cylinder cells showed a gradual increase of plasmolysis, being more significant after exposure to PVS3. The cryopreservation step that promotes a higher level of plasmolysis depends on localization, cell size, and degree of cell differentiation (Volk and Caspersen 2007). In roots of Rubia akane, cell plasmolysis was induced after pretreatment and remained during the loading and PVS2 treatments, mainly in cells of the endodermis and vascular cylinder (Salma et al. 2014). In shoot tips of Mentha x piperita, Volk and Caspersen (2007) observed that the most vacuolized cells showed evidence of plasmolysis soon after pretreatment. On the other hand, cells with meristematic characteristics, which are smaller, less vacuolated, and with denser cytoplasm, were only plasmolyzed after exposure to vitrification solution.
A progressive increase in the proportion of intercellular spaces filled by dense content was observed in T. rosea root cortex throughout the cryopreservation steps, culminating in cortex disruption. Barraco et al. (2014) described these intercellular spaces as lacunar areas in cryopreserved shoot tips of Dioscorea alata, and pointed out their occurrence during the recovery stage. The histochemical tests performed to evaluate the dense content that filled the intercellular spaces in T. rosea root cortex gave evidence of the presence of polysaccharides and proteins, lipids, and pectic substances. These histological changes described in the cryopreserved roots of T. rosea may be related to the production and extrusion of carotenoids during the third week post-cryopreservation since the presence of lipidic substances was detected inside the roots besides other substances, as confirmed by histochemical evaluation.
No significant change was observed in T. rosea roots after immersion in LN and rewarming, when compared with the previous step of PVS3 treatment, indicating that the cooling/rewarming process did not cause structural damage in the material. Similar results were observed in Rubia akane roots (Salma et al. 2014), Cocos nucifera embryos (Alla-N’Nan et al. 2014), and Dioscorea alata shoot tips (Barraco et al. 2014). On the other hand, in Citrus spp. shoot tips, the plasmolysis level was even higher after LN (Ding et al. 2008). Plasmolysis decreased in all T. rosea cell roots after unloading treatment since this solution, which is based on standard culture medium supplemented with 1.2 M sucrose, aims to minimize possible osmotic damage, resulting from the difference between osmotic potential of the vitrification solution and that of the culture medium (Gonzalez-Arnao et al. 2008; Sakai et al. 2008).
Although the root cortex became unstructured during the cryopreservation process, secondary root formation from pericycle in central cylinder was observed within a few days post-cryopreservation. Therefore, the maintenance of tissue integrity in the central cylinder allowed the regeneration and formation of new secondary roots from T. rosea explants exposed to different steps of the encapsulation-vitrification protocol, thereby showing the capacity of these cells to tolerate osmotic stress caused by dehydration.
The present study allowed the establishment of a protocol for cryopreservation of adventitious root cultures of T. rosea using the encapsulation-vitrification technique. Recovery above 90% and the maintenance of the multiplication capacity of the cryopreserved roots along subcultures demonstrated the efficiency of the protocol. In addition, histological observations throughout all cryopreservation steps and histochemical analysis revealed details of structural and metabolic changes in T. rosea roots that may lead to a better understanding of the changes plants undergo during the cryopreservation process.
References
Albarello N, Ribeiro IG, Simões C, Castro TC, Gianfaldoni MG, Callado C, Kuster RM, Coelho MGP, Mansur E (2007) Histological analysis of calluses from in vitro propagated plants of Cleome spinosa Jacq. Rev Bras Biocienc 5:699–701
Alla-N’nan O, Tiécoura K, Bi SG, Verdeil J-L, Malaurie B (2014) Ultrastructural changes during cryopreservation of plumules and embryos of coconut (Cocos nucifera L.). Int J Agro Agric Res 5(6):103–115
Antony JJJ, Mubbarakh SA, Mahmood M, Subramaniam S (2014) Effect of plasmolysis on protocorm-like bodies of Dendrobium Bobby Messina orchid following cryopreservation with encapsulation–dehydration method. Appl Biochem Biotechnol 172:1433–1444
Barraco G, Sylvestre I, Collin M, Escoute J, Lartaud M, Verdeil JL, Engelmann F (2014) Histocytological analysis of yam (Dioscorea alata) shoot tips cryopreserved by encapsulation–dehydration. Protoplasma 251:177–189
Benson EE (1990) Free radical damage in stored plant germplasm. International Board for Plant Genetic Resources, Rome
Benson EE, Bremner D (2004) Oxidative stress in the frozen plant: a free radical point of view. In: Fuller BJ, Lane N, Benson EE (eds) Life in the frozen state. CRC Press LLC, United States of America, pp 205–241
Benson EE, Hamill JD (1991) Cryopreservation and post freezing molecular and biosynthetic stability in transformed roots of Beta vulgaris and Nicotiana rustica. Plant Cell Tissue Organ Cult 24:163–172
Buffard-Morel J, Verdeil JL, Pannetier C (1992) Embryogenèse somatique du cocotier (Cocos nucifera L.) à partir d’explants foliaires: étude histologique. Can J Bot 70:735–741
Cordeiro LS, Simões-Gurgel C, Albarello N (2015) Multiplication and cryopreservation of adventitious roots of Cleome rosea Vahl. In Vitro Cell Dev Biol Plant 51:249–257
Cordeiro LS, Simões-Gurgel C, Albarello N (2016) Cryopreservation of adventitious roots of Cleome rosea Vahl (Cleomaceae) using a vitrification technique and assessment of genetic stability. CryoLetters 37(4):231–242
Cruz-Cruz CA, González-Arnao MT, Engelmann F (2013) Biotechnology and conservation of plant biodiversity. Resources 2:73–95
Ding F, Jin S, Hong N, Zhong Y, Cao Q, Yi G, Wang G (2008) Vitrification–cryopreservation, an efficient method for eliminating Candidatus Liberobacter asiaticus, the citrus Huanglongbing pathogen, from in vitro adult shoot tips. Plant Cell Rep 27:241–250
Engelmann F (2011) Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell Dev Biol Plant 47:5–16
Engelmann F, Gonzalez-Arnao MT (2013) Introducción a la conservación ex situ de los recursos genéticos vegetales. In: Gonzalez-Arnao MT, Engelmann F (eds) Crioconservación de plantas en América Latina y el Caribe. Instituto Interamericano de Cooperación para la Agricultura (IICA), San José, pp 25–35
Fang JY, Wetten A (2011) Importance of structural integrity of somatic embryos for long-term cryopreservation of cocoa (Theobroma cacao L.) germplasm. Afr J Agric Res 6(17):3954–3961
Fisher DB (1968) Protein staining of ribboned epon sections for light microscopy. Histochem 16:92–96
Fraga HPF, Vieira LN, Puttkammer CC, Silva JM, Anjos KG, Oliveira EM, Guerra MP (2016) High-efficiency cryopreservation of Araucaria angustifolia (Bertol.) Kuntze embryogenic cultures: ultrastructural characterization and morpho-physiological features. Plant Cell Tissue Organ Cult 124:307–318
Gerlach D (1984) Botanische mikrotechnik. Thieme Verlag, Stuttgart
Gogoi K, Kumaria S, Tandon P (2012) A comparative study of vitrification and encapsulation-vitrification for cryopreservation of protocorms of Cymbidium eburneum L., a threatened and vulnerable orchid of India. Cryoletters 33:443–452
Gonzalez-Arnao MT, Panta A, Roca WM, Escobar RH, Engelmann F (2008) Development and large scale application of cryopreservation techniques for shoot and somatic embryo cultures of tropical crops. Plant Cell Tissue Organ Cult 92:1–13
Harding K, Benson EE, Nunes EC, Pilatti FK, Lemos J, Viana AM (2013) Can biospecimen science expedite the ex situ conservation of plants in megadiverse countries? A focus on the flora of Brazil. Crc Cr Rev Plant Sci 32(6):411–444
Hirata K, Mukai M, Goda S, Ishio-Kinugasa M, Yoshida K, Sakai A, Miyamoto K (2002) Cryopreservation of hairy root cultures of Vinca minor (L.) by encapsulation-dehydration. Biotechnol Lett 24:371–376
Jensen WA (1962) Botanical histochemistry: principle and practice. W. H. Freeman, San Francisco
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Company, New York
Jung DW, Sung CK, Touno K, Yoshimatsu K, Shimomura K (2001) Cryopreservation of Hyoscyamus niger adventitious roots by vitrification. J Plant Physiol 158:801–805
Kim HH, Popova EV, Yi JY, Cho GT, Park SU, Lee SC, Engelmann F (2010) Cryopreservation of hairy roots of Rubia akane (Nakai) using a droplet-vitrification procedure. CryoLetters 31:473–484
Le KC, Kim HH, Park SY (2019) Modification of the droplet-vitrification method of cryopreservation to enhance survival rates of adventitious roots of Panax ginseng. Hortic Environ Biotechnol 60:501–510
Martín C, Kremer C, González I, González-Benito ME (2015) Influence of the cryopreservation technique, recovery medium and genotype on genetic stability of mint cryopreserved shoot tips. Plant Cell Tissue Organ Cult 122(1):185–195
Mathew L, McLachlan A, Jibran R, Burritt DJ, Pathirana R (2018) Cold, antioxidant and osmotic pre-treatments maintain the structural integrity of meristematic cells and improve plant regeneration in cryopreserved kiwifruit shoot tips. Protoplasma 255(4):1065–1077
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy HN, Dandin VS, Paek KY (2016) Tools for biotechnological production of useful phytochemicals from adventitious root cultures. Phytochem Rev 15:129–145
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73
Oh SY, Wu CH, Popova E, Hahn EJ, Paek KY (2009) Cryopreservation of Panax ginseng adventitious roots. J Plant Biol 52:348–354
Park SU, Kong H, Shin DJ, Bae CH, Lee SC, Bae CH, Rha ES, Kim HH (2014) Development of vitrification protocol in Rubia akane (Nakai) hairy roots using a systematic approach. CryoLetters 35(2):138–144
Pasqua G, Monacelli B, Valletta A, Santamaria AR, Fiorillo F (2005) Synthesis and/or accumulation of bioactive molecules in the in vivo and in vitro root. Plant Biosyst 139(2):180–188
Poobathy R, Sinniah UR, Xavier R, Subramaniam S (2013) Catalase and superoxide dismutase activities and the total protein content of protocorm-like bodies of Dendrobium sonia-28 subjected to vitrification. Appl Biochem Biotechnol 170:1066–1079
Popova E, Paek KY, Kim HH (2011) Cryopreservation of medicinal plants: the case of in vitro cultures. In: Kumar A, Roy S (eds) Plant tissue culture and applied plant biotechnology. Aavishkar Publishers, Jaipur, pp 153–196
Rocha AS, Rocha EK, Alves LM, Moraes BA, Castro TC, Albarello N, Simões-Gurgel C (2015) Production and optimization through elicitation of carotenoid pigments in the in vitro cultures of Cleome rosea Vahl (Cleomaceae). J Plant Biochem Biotechnol 24:105–113
Rubinsky B (2003) Principles of low temperature cell preservation. Heart Fail Rev 8:277–284
Sakai A, Engelmann F (2007) Vitrification, encapsulation-vitrification and droplet-vitrification: a review. CryoLetters 28(3):151–172
Sakai A, Hirai D, Niino T (2008) Development of PVS-based vitrification protocols. In: Reed BM (ed) Plant cryopreservation–a practical guide. Springer, New York, pp 33–57
Salma M, Engelmann-Sylvestre I, Collin M, Escoute J, Lartaud M, Yi JY, Kim HH, Verdeil JL, Engelmann F (2014) Effect of the successive steps of a cryopreservation protocol on the structural integrity of Rubia akane Nakai hairy roots. Protoplasma 251(3):649–659
Scarano FR (2009) Plant communities at the periphery of the Atlantic rain forest: rare-species bias and its risks for conservation. Biol Conserv 142:1201–1208
Sershen BP, Pammenter NW, Wesley-Smith J (2012) The effects of various parameters during processing for cryopreservation on the ultrastructure and viability of recalcitrant zygotic embryos of Amaryllis belladonna. Protoplasma 249:155–169
Shin DJ, Lee HE, Bae CH, Park SU, Kang HN, Kim HH (2014) Development of an encapsulation-vitrification protocol for Rubia akane (Nakai) hairy roots: a comparison with non-encapsulation. CryoLetters 35(5):377–384
Simão MJ, Collin M, Garcia RO, Mansur E, Pacheco G, Engelmann F (2018) Histological characterization of Passiflora pohlii Mast. root tips cryopreserved using the V-Cryo-plate technique. Protoplasma 255(3):741–750
Simões C, Santos AS, Albarello N, Figueiredo SFL (2004) Shoot organogenesis and plantlet regeneration from stem explants of Cleome rosea Vahl (Capparaceae). J Plant Biotechnol 6:199–204
Simões C, Mattos JCP, Sabino KCC, Caldeira-De-Araújo A, Coelho MGP, Albarello N, Figueiredo SFL (2006) Medicinal potential from in vivo and acclimatized plants of Cleome rosea. Fitoterapia 77:94–99
Simões C, Albarello N, Callado CH, Castro TC, Mansur E (2009a) New approaches for shoot production and establishment of in vitro root cultures of Cleome rosea Vahl. Plant Cell Tissue Organ Cult 98:79–86
Simões C, Bizarri CHB, Cordeiro LS, Castro TC, Coutada LM, Silva AJR, Albarello N, Mansur E (2009b) Anthocyanin production in callus cultures of Cleome rosea: modulation by culture conditions and characterization of pigments by means of HPLC-DAD/ESIMS. Plant Physiol Biochem 47:895–903
Simões C, Albarello N, Callado CH, Castro TC, Mansur E (2010a) Somatic embryogenesis and plant regeneration from callus cultures of Cleome rosea Vahl. Braz Arch Biol Technol 53:679–686
Simões C, Castro TC, Cordeiro LS, Albarello N, Mansur E, Romanos MTV (2010b) Antiviral activity of Cleome rosea extracts from field-grown plants and tissue culture-derived materials against acyclovir-resistant Herpes simplex viruses type 1 (ACVr-HSV-1) and type 2 (ACVr-HSV-2). World J Microbiol Biotechnol 26:93–99
Simões-Gurgel C, Cordeiro LS, Castro TC, Callado CH, Albarello N, Mansur E (2011) Establishment of anthocyanin-producing cell suspension cultures of Cleome rosea Vahl ex DC. (Capparaceae). Plant Cell Tissue Organ Cult 106:537–545
Simões-Gurgel C, Rocha AS, Cordeiro LS, Gayer CRM, Castro TC, Coelho MGP, Albarello N, Mansur E, Rosa ACP (2012) Antibacterial activity of field-grown plants, in vitro propagated plants, callus and cell suspension cultures of Cleome rosea Vahl. J Pharm Res 5:3304–3308
Singh S, Pandey P, Ghosh S, Banerjee S (2018) Anti-cancer labdane diterpenoids from adventitious roots of Andrographis paniculata: augmentation of production prospect endowed with pathway gene expression. Protoplasma 255(5):1387–1400
Skyba M, Urbanová M, Kapchina-Toteva V, Kosuth J, Harding K, Cellárová E (2010) Physiological, biochemical and molecular characteristics of cryopreserved Hypericum perforatum L. shoot tips. CryoLetters 31(3):249–260
Ślesak H, Góralski G, Kwolek D, Dziedzic K, Grabowska-Joachimiak A (2015) Male adventitious roots of Rumex thyrsiflorus Fingerh. as a source of genetically stable micropropagated plantlets. Plant Cell Tissue Organ Cult 123:193–203
Soares Neto RL, Thomas WW, Barbosa MRV, Roalson EH (2018) New combinations and taxonomic notes for Tarenaya (Cleomaceae). Acta Bot Bras 32:540–545
Stewart P, Taylor M, Mycock D (2001) The sequence of the preparative procedures affects the success of cryostorage of cassava somatic embryos. CryoLetters 22:35–42
Teoh KH, Weathers PJ, Cheetham RD, Walcerz DB (1996) Cryopreservation of transformed (hairy) roots of Artemisia annua. Cryobiology 33:106–117
Touno K, Yoshimatsu K, Shimomura K (2006) Characteristics of Atropa belladonna hairy roots cryopreserved by vitrification method. CryoLetters 27:65–72
Volk GM, Caspersen AM (2007) Plasmolysis and recovery of different cell types in cryoprotected shoot tips of Mentha x piperita. Protoplasma 231:215–226
Wen B, Cai C, Wang R, Song S, Song J (2012) Cytological and physiological changes in recalcitrant Chinese fan palm (Livistona chinensis) embryos during cryopreservation. Protoplasma 249(2):323–335
Wolfe J, Bryant G (2001) Cellular cryobiology: thermodynamic and mechanical effects. Int J Refrig 24:438–450
Xue SH, Luo XJ, Wu ZH, Zhang HL, Wang XY (2008) Cold storage and cryopreservation of hairy root cultures of medicinal plant Eruca sativa Mill., Astragalus membranaceus and Gentiana macrophylla Pall. Plant Cell Tissue Organ Cult 92:251–260
Yang X, Popova E, Shukla MR, Saxena PK (2019) Root cryopreservation to biobank medicinal plants: a case study for Hypericum perforatum L. In Vitro Cell Dev Biol Plant 55(4):392–402
Yi JY, Sylvestre I, Collin M, Salma M, Lee SY, Kim HH, Park HJ, Engelmann F (2012) Improved cryopreservation using droplet-vitrification and histological changes associated with cryopreservation of madder (Rubia akane Nakai). Kor J Hort Sci Technol 30(1):79–84
Acknowledgments
The authors are grateful to Jeanne A. T. Glória for the valuable technical assistance with histochemical analyses, to Thaís J. Vasconcellos for help in using the Image-Pro Plus software, and Adriana M. Lanziotti for lab assistance.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - (CAPES/Brazil) - Finance Code 001 - and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) through an international collaborative research project (Program Science without Borders - Project no. A054/2013) between the Núcleo de Biotecnologia Vegetal of the Universidade do Estado do Rio de Janeiro (UERJ/Brazil) and the Institut de Recherche pour le Développement (IRD/Montpellier, France). The work was also supported by the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ/Brazil).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva Cordeiro, L., Collin, M., Callado, C.H. et al. Long-term conservation of Tarenaya rosea (Cleomaceae) root cultures: histological and histochemical analyses during cryopreservation using the encapsulation-vitrification technique. Protoplasma 257, 1021–1033 (2020). https://doi.org/10.1007/s00709-020-01486-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01486-0