Abstract
Introduction
Programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors have been increasingly employed for the treatment of various cancers in clinical practice. This study aimed to systematically evaluate the efficacy and safety of PD-1/PD-L1 inhibitors for advanced hepatocellular carcinoma (HCC).
Methods
PubMed, EMBASE, Cochrane library, Web of Science, and Abstracts of American Society of Clinical Oncology proceedings databases were searched. Objective response rate (ORR), disease control rate (DCR), median progression-free survival (PFS), median overall survival (OS), and incidence of adverse events (AEs) and drug withdrawal were pooled. Odds ratio (OR) and hazard ratio (HR) were calculated to analyze the difference in the ORR, DCR, PFS, and OS between groups.
Results
Among the 14,902 initially identified papers, 98 studies regarding use of PD-1/PD-L1 inhibitors in advanced HCC were included. Based on different criteria of response in solid tumors, the pooled ORR, DCR, and median PFS was 16–36%, 54–74%, and 4.5–6.8 months, respectively. The pooled median OS was 11.9 months. Compared to multitarget tyrosine kinase inhibitors (TKIs), PD-1/PD-L1 inhibitors monotherapy significantly increased ORR (OR 2.73, P < 0.00001) and OS (HR 0.97, P = 0.05), and PD-1/PD-L1 inhibitors combined with TKIs significantly increased ORR (OR 3.17, P < 0.00001), DCR (OR 2.44, P < 0.00001), PFS (HR 0.58, P < 0.00001), and OS (HR 0.58, P < 0.00001). The pooled incidence of all-grade AEs, grade ≥ 3 AEs, and drug withdrawal was 71%, 25%, and 7%, respectively.
Conclusion
On the basis of the present systematic review and meta-analysis, PD-1/PD-L1 inhibitors should be the preferred treatment choice for advanced HCC owing to their higher antitumor effect and improved outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Evidence regarding use of programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors for advanced hepatocellular carcinoma (HCC) has been rapidly growing during recent years. |
Therefore, it is necessary to conduct a meta-analysis to examine the efficacy and safety of PD-1/PD-L1 inhibitors in advanced HCC by integrating the currently available data. |
What was the hypothesis of the study? |
The use of PD-1/PD-L1 might be considered as the first-line choice of treatment for advanced HCC. |
What was learned from the study? |
Among the patients with advanced HCC treated with PD-1/PD-L1 inhibitors, the disease control rate could be beyond 50%, and the median overall survival time exceeded 1 year, but the incidence of severe adverse events was approximately 25%. |
Additionally, PD-1/PD-L1 inhibitor monotherapy and in combination with TKIs were more effective than multitarget TKIs monotherapy for the treatment of advanced HCC. |
Introduction
Primary liver cancer is a major public health burden in the world. According to the global cancer data, primary liver cancer is the sixth most common cancer and the third most common cause of cancer-related death [1]. Hepatocellular carcinoma (HCC) is the dominant subtype of primary liver cancer, accounting for 75–90% [1, 2]. Early and intermediate stage HCC can be effectively treated by liver transplantation, surgical resection, and local ablation [3,4,5]. Molecular targeted drugs have been successively approved as the first- or second-line choice of therapy for advanced HCC [6,7,8,9,10,11], but have only a low tumor response rate with a high incidence of adverse events [12, 13].
Since 2015, programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors have been explored for the management of advanced HCC [14]. PD-L1 is one of the PD-1 ligands [15]. PD-1 binds to PD-L1, thereby inhibiting the proliferation of T cells [16, 17]. Therefore, PD-1/PD-L1 inhibitors can achieve anticancer effects by inhibiting tumor growth and promoting cancer cell death [18]. Until now, several phase 2 and 3 randomized trials regarding PD-1/PD-L1 inhibitors for the treatment of advanced HCC have been completed with encouraging results [19,20,21]. Nivolumab and pembrolizumab, which are two major PD-1 inhibitors, have been approved by the US Food and Drug Administration as the second-line treatment options for advanced HCC after the failure of sorafenib in 2017 and 2018, respectively [3,4,5]. Additionally, atezolizumab, a PD-L1 inhibitor, combined with bevacizumab, a vascular endothelial growth factor receptor monoclonal antibody (anti-VEGFR), has been recommended by the National Comprehensive Cancer Network and American Society of Clinical Oncology (ASCO) guidelines as the first-line treatment for most patients with advanced HCC in 2020 [22, 23]. At present, there is rapidly growing evidence regarding use of PD-1/PD-L1 inhibitors for advanced HCC. Thus, an updated systematic review and meta-analysis is very necessary to integrate all currently available data and further clarify their efficacy and safety.
Methods
This work was conducted on the basis of the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guideline. The PRISMA checklist is shown in Supplementary Table 1. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Registration
The PROSPERO registration number is CRD42021264686.
Literature Search
PubMed, EMBASE, Cochrane, Web of Science, and Abstracts of ASCO proceedings databases were searched. Search items were as follows: (“nivolumab” OR “pembrolizumab” OR “atezolizumab” OR “avelumab” OR “cemiplimab” OR “camrelizumab” OR “PD-1/PD-L1” OR “programmed death ligand 1” OR “programmed cell death ligand 1” OR “Opdivo” OR “ONO-4538” OR “MDX-1106” OR “BVMS-936558” OR “Keytruda” OR “MK-3475” OR “MPDL3280A” OR “Tecentriq” OR “RG-7446” OR “MEDI-4736” OR “Mfinzi” OR “IBI-308” OR “SHR-1210”) AND (“hepatocellular carcinoma” OR “HCC” OR “liver cell carcinoma” OR “liver cancer” OR “hepatoma” OR “hepatic malignancy” OR “hepatic malignant tumors”). The last search was performed on August 1, 2021.
Study Selection Criteria
Studies regarding use of PD-1/PD-L1 inhibitors in HCC were potentially eligible. Exclusion criteria were as follows: (1) duplicated papers; (2) case reports; (3) reviews and meta-analyses; (4) guidelines and consensus; (5) comments, letters, notes, reports, and editorials; (6) experimental studies; (7) clinical trial registration alone; (8) patients without HCC; (9) patients did not receive PD-1/PD-L1 inhibitors; (10) the sample size was less than 10; (11) overlapping data; and (12) outcomes of interests were neither relevant nor evaluated.
Data Extraction
The data were extracted as follows: first author, publication year, type of publication, study design, region, enrollment period, sample size, PD-1/PD-L1 inhibitors used, dosage of PD-1/PD-L1 inhibitors used, type of drugs combined, follow-up duration, objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), 6-month and 1-year PFS, 6-month and 1-year OS, and number of patients who developed all-grade, grade ≥ 3 adverse events (AEs), and drug withdrawal secondary to AEs. Notably, among the included studies, ORR, DCR, and PFS were assessed by the independent review committee (IRC) or investigator according to various versions of Response Evaluation Criteria in Solid Tumors (RECIST), such as RECIST version 1.1 (RECIST 1.1), modified RECIST (mRECIST), modified RECIST for immune-based therapeutics (iRECIST), and immune-related RECIST (irRECIST). If a study did not specify whether IRC or investigator assessed the tumor response, it would be considered as the investigator-assessed tumor response.
Quality Assessment
The Cochrane Collaboration’s risk of bias tool was used to assess the quality of included randomized controlled trials. Quality assessment items include random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The risk of bias is graded as low, high, or uncertain.
The Newcastle–Ottawa Scale (NOS) was used to assess the quality of included cohort studies. Quality assessment items include selection, comparability, exposure, and outcomes. A NOS score of 0–3, 4–6, and 7–9 represents low, moderate, and high quality, respectively.
Data Analyses
The meta-analysis was performed by STATA version 14.2 (STATA Corp, College Station, TX, USA) and Review Manager version 5.3 software (Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen). Only a random-effects model was used. First, the ORR, DCR, PFS, and OS were pooled with their 95% confidence intervals (CIs). Cochrane Q test and the I2 statistic were used to test the heterogeneity. P < 0.1 or I2 > 50% represented statistically significant heterogeneity among studies. Meta-regression analyses were employed to explore the source of heterogeneity, where type of publication (full-text vs abstract), study design (prospective vs retrospective), PD-1/PD-L1 inhibitors used (nivolumab vs pembrolizumab vs atezolizumab vs camrelizumab vs durvalumab), median follow-up duration (≥ 10 months vs < 10 months), study quality (high vs moderate and low), sample size (≥ 100 vs < 100), type of PD-1/PD-L1 inhibitors used (PD-1 inhibitors vs PD-L1 inhibitors), type of choice of treatment (monotherapy vs combination therapy), type of drugs combined (anti-VEGFR vs multitarget TKIs vs cytotoxic T lymphocyte-associated antigen 4 [CTLA-4] inhibitors), and region (Asia vs America vs Europe vs multiple countries) were used as covariates. Subgroup analyses were also performed in terms of the covariates aforementioned. Egger’s test was performed to evaluate the publication bias, and P < 0.1 was considered as statistically significant publication bias. The meta-regression and publication bias analyses were performed when the number of studies included was at least 3. Second, the odds ratio (OR) with 95% CI was pooled to compare ORR and DCR between groups; and the hazard ratio (HR) with 95% CI was pooled to compare PFS and OS between groups. P < 0.05 represented statistical significance. Third, the values of incidence of AEs and drug withdrawal with their 95% CIs were pooled.
Results
Study Selection and Characteristics
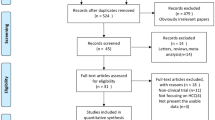
A total of 14,902 papers were initially identified. Finally, 98 studies were included (Fig. 1). The characteristics of included studies are summarized in Table 1. Among them, 44 studies used PD-1/PD-L1 inhibitor monotherapy [12, 14, 19, 21, 24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63], 60 used combination therapy [20, 39, 45,46,47, 57, 63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116], and six used both monotherapy and combination therapy [39, 45,46,47, 57, 63]. Fifty-one studies were published as full-texts [12, 19,20,21, 24, 26, 30, 34, 35, 37, 39,40,41,42, 44,45,46,47,48, 50,51,52, 54, 56,57,58,59,60,61, 67,68,69,70, 72,73,74, 76, 78, 80,81,82,83, 85, 86, 90, 92, 101, 105,106,107, 111] and 47 as abstracts [14, 25, 27,28,29, 31,32,33, 36, 38, 43, 49, 53, 55, 62,63,64,65,66, 71, 75, 77, 79, 84, 87,88,89, 91, 93,94,95,96,97,98,99,100, 102,103,104, 108,109,110, 112,113,114,115,116]; 58 studies were conducted in Asia [12, 24, 28,29,30,31, 36, 39, 40, 44,45,46,47, 50, 52, 53, 59, 60, 63,64,65,66,67,68,69,70,71,72,73,74,75,76, 78, 80,81,82,83,84,85,86, 88,89,90, 93, 95,96,97,98,99,100, 103,104,105,106, 108, 111, 114, 115], 8 in America [26, 27, 43, 49, 56, 77, 79, 110], 9 in Europe [32,33,34,35, 48, 54, 61, 62, 94], and 27 in multiple countries [14, 19,20,21, 25, 37, 38, 41, 42, 51, 55, 57, 58, 87, 91, 92, 101, 102, 107, 109, 112, 113, 116]; 81 studies employed PD-1 inhibitors, including pembrolizumab, nivolumab, cemiplimab, camrelizumab, tiselizumab, toripalimab, sintilimab, penpulimab, and CS1003 [12, 14, 19, 21, 24, 26, 27, 29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44, 46,47,48,49,50,51,52,53,54,55,56, 58, 59, 61,62,63,64,65,66,67,68,69,70,71, 73,74,75, 77,78,79, 81, 83, 85, 87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106, 108, 109, 111, 113, 115], and 17 employed PD-L1 inhibitors, including durvalumab, avelumab, and atezolizumab [20, 25, 28, 45, 57, 60, 72, 76, 80, 82, 84, 86, 107, 110, 112, 114, 116].
Study Quality
Study quality assessment was summarized in Supplementary Fig. 1 and Supplementary Table 2.
Efficacy of PD-1/PD-L1 Inhibitors Based on Single-Arm Studies
ORR
The pooled ORR was 21% (95% CI 17–24%), 22% (95% CI 19–25%), 29% (95% CI 24–35%), 36% (95% CI 30–42%), and 16% (95% CI 12–20%) according to the IRC-assessed RECIST 1.1, investigator-assessed RECIST 1.1, IRC-assessed mRECIST, investigator-assessed mRECIST, and investigator-assessed iRECIST/irRECIST, respectively (Table 2). The heterogeneity was statistically significant in most of these meta-analyses. The heterogeneity might be related to the choice of treatment (Supplementary Table 3). The interaction according to the choice of treatment was statistically significant in most of the subgroup analyses, suggesting that PD-1/PD-L1 inhibitor combination therapy should have a higher ORR than PD-1/PD-L1 inhibitor monotherapy (Supplementary Table 7). The publication bias was not statistically significant in all of these meta-analyses (Table 2).
DCR
The pooled DCR was 60% (95% CI 52–68%), 66% (95% CI 62–71%), 68% (95% CI 58–78%), 74% (95% CI 68–80%), and 54% (95% CI 43–66%) according to the IRC-assessed RECIST 1.1, investigator-assessed RECIST 1.1, IRC-assessed mRECIST, investigator-assessed mRECIST, and investigator-assessed iRECIST/irRECIST, respectively (Table 2). The heterogeneity was statistically significant in all of these meta-analyses (Table 2). The heterogeneity might be related to the choice of treatment (Supplementary Table 4). The interaction according to the choice of treatment was statistically significant in all of subgroup analyses, suggesting that PD-1/PD-L1 inhibitor combination therapy should have a higher DCR than PD-1/PD-L1 inhibitor monotherapy (Supplementary Table 7). The publication bias was not statistically significant in most of these meta-analyses.
PFS
The pooled median PFS was 4.5 months (95% CI 3.6–5.4), 5.6 months (95% CI 4.6–6.6), 6.3 months (95% CI 4.0–8.6), 6.8 months (95% CI 4.6–9.0), and 5.7 months (95% CI 3.8–7.5) according to the IRC-assessed RECIST 1.1, investigator-assessed RECIST 1.1, IRC-assessed mRECIST, investigator-assessed mRECIST, and investigator-assessed iRECIST/irRECIST, respectively (Table 2). The pooled 6-month PFS rate was 60% (95% CI 54–67%), 51% (95% CI 42–60%), 60% (95% CI 50–0.70%), and 52% (95% CI 41–63%) according to the IRC-assessed RECIST 1.1, investigator-assessed RECIST 1.1, IRC-assessed mRECIST, and investigator-assessed mRECIST, respectively. The pooled 1-year PFS rate was 27% (95% CI 20–37%), 24% (95% CI 14–36%), 28% (95% CI 22–34%), and 34% (95% CI 24–41%) according to the IRC-assessed RECIST 1.1, investigator-assessed RECIST 1.1, IRC-assessed mRECIST, and investigator-assessed mRECIST, respectively. The heterogeneity was statistically significant in most of these meta-analyses (Table 2). The heterogeneity might be related to the choice of treatment (Supplementary Table 5). The interaction according to the choice of treatment was statistically significant in most of the subgroup analyses, suggesting that PD-1/PD-L1 inhibitor combination therapy should have a higher PFS than PD-1/PD-L1 inhibitor monotherapy (Supplementary Table 7). The publication bias was not statistically significant in all of these meta-analyses (Table 2).
OS
The pooled median OS was 11.9 months (95% CI 10.6–13.2). The pooled 6-month and 1-year OS rates were 82% (95% CI 76–88%) and 58% (95% CI 52–64%), respectively. The heterogeneity was statistically significant in all of these meta-analyses (Table 2). The heterogeneity might be related to the choice of treatment in most of the meta-regression analyses (Supplementary Table 6). The interaction according to the choice of treatment was statistically significant in all of subgroup analyses, suggesting that PD-1/PD-L1 inhibitor combination therapy should have a higher OS than PD-1/PD-L1 inhibitor monotherapy (Supplementary Table 7). The publication bias was statistically significant in the meta-analyses regarding median OS, but not those regarding 6-month OS and 1-year OS rates (Table 2).
Efficacy of PD-1/PD-L1 Inhibitor Monotherapy Versus Multitarget TKIs Monotherapy
Four studies compared the efficacy of PD-1/PD-L1 inhibitor monotherapy versus multitarget TKIs monotherapy (Table 3). Nivolumab was the only PD-1/PD-L1 inhibitors drug used among these studies. Meta-analyses showed that nivolumab monotherapy significantly increased ORR (OR 2.73, 95% CI 1.87–3.98, P < 0.00001) and OS (HR 0.72, 95% CI 0.52–1.00, P = 0.05). The heterogeneity was statistically significant in the meta-analysis regarding OS, but not that regarding ORR (Fig. 2).
Efficacy of PD-1/PD-L1 Inhibitors Combined with TKIs Versus Multitarget TKIs Monotherapy
Five studies compared the efficacy of PD-1/PD-L1 inhibitors combined with TKIs versus multitarget TKIs monotherapy (Table 3). Meta-analyses showed that PD-1/PD-L1 inhibitors combined with TKIs significantly increased ORR (OR 3.17, 95% CI 2.21–4.54, P < 0.00001), DCR (OR 2.44, 95% CI 1.74–3.44, P < 0.00001), PFS (HR 0.58, 95% CI 0.50–0.68, P < 0.00001), and OS (HR 0.58, 95% CI 0.49–0.70, P < 0.00001). The heterogeneity was not statistically significant in all of these meta-analyses (Fig. 3).
Safety
All-Grade AEs
The pooled rate of all-grade AEs was 71% (95% CI 64–77%) with significant heterogeneity (I2 = 94.0%, P < 0.01) (Supplementary Table 8). The most common all-grade AEs was hypertension (23%) and hand-foot syndrome (23%), followed by fatigue (20%), proteinuria (20%), and reactive cutaneous capillary endothelial proliferation (RCCEP) (19%) (Supplementary Table 9).
Grade ≥ 3 AEs
The pooled grade ≥ 3 AEs rate was 25% (95% CI 21–30%) with significant heterogeneity (I2 = 91.3%, P < 0.01) (Supplementary Table 8). The most common grade ≥ 3 AEs was hypertension (7%), followed by increased aspartate aminotransferase (AST) level (6%), hepatitis (6%), increased gamma-glutamyltransferase level (4%), and increased lipase level (4%) (Supplementary Table 9).
AE-Related Drug Withdrawal
The pooled incidence of drug withdrawal due to AEs was 7% (95% CI 6–9%) with significant heterogeneity (I2 = 69.6%, P < 0.01) (Supplementary Table 8).
Discussion
To the best of our knowledge, this is the most comprehensive systematic review and meta-analysis to verify the efficacy and safety of PD-1/PD-L1 inhibitors for advanced HCC. Major findings are as follows: (1) PD-1/PD-L1 inhibitors can achieve an ORR of 16–36%, DCR of 54–74%, median PFS of 4.5–6.8 months, and median OS of 11.9 months in patients with advanced HCC; (2) PD-1/PD-L1 inhibitor monotherapy and in combination with TKIs therapy outperform multitarget TKIs monotherapy in terms of ORR, PFS, and OS; (3) one in four patients with advanced HCC treated with PD-1/PD-L1 inhibitors develop severe AEs, but only 7% of them discontinue therapy because of severe AEs.
It should be acknowledged that two previous systematic reviews and meta-analyses explored the efficacy and safety of PD-1/PD-L1 inhibitors for advanced HCC [117, 118]. By comparison, our present meta-analysis had some advantages. First, the most important was to compare the efficacy of PD-1/PD-L1 inhibitors versus multitarget TKIs monotherapy for the treatment of advanced HCC, which had not been performed by previous meta-analyses [117, 118]. Second, one previous meta-analysis searched literature until January 2020 and included 23 studies [118]. Another previous meta-analysis searched literature until October 2020 and included only 12 studies [117]; therefore, some eligible studies were missing [27,28,29, 31, 32, 36, 38, 110, 113, 115, 116]. By comparison, our present meta-analysis extended the date of literature search until August, 2021 and finally included 98 studies. Third, two previous meta-analyses extracted the data based on only one criterion of response evaluation in solid tumors [117, 118]. By comparison, our present meta-analysis pooled the data according to five different criteria of response evaluation in solid tumors. Notably, we found that the pooled ORR, DCR, and PFS assessed by investigators according to the mRECIST were higher than those according to other criteria. This may be because mRECIST is more specific for evaluation of HCC, and can objectively and accurately evaluate the ORR, DCR, and PFS in trials of non-cytotoxic drugs for HCC [119, 120]. Fourth, the heterogeneity was statistically significant in both previous and present meta-analyses. The source of heterogeneity was not explored in two previous meta-analyses [117, 118]. By comparison, we explored the source of heterogeneity by subgroup and meta-regression analyses, and found that the heterogeneity might be related to the choice of treatment. More specifically, our subgroup analyses indicated that PD-1/PD-L1 inhibitor combination therapy had a higher tumor response rate and longer survival time than PD-1/PD-L1 inhibitor monotherapy. This is because PD-1/PD-L1 inhibitors combined with other treatment approaches, such as anti-VEGFR, multitarget TKIs, CTLA-4 inhibitors, and transarterial radioembolization, can produce a synergic effect to achieve antitumor activity as compared to PD-1/PD-L1 inhibitor monotherapy [20, 92, 94, 102]. Fifth, only a combination therapy of PD-1/PD-L1 inhibitors and VEGFR-TKIs was analyzed in a previous meta-analysis [118]. By comparison, PD-1/PD-L1 inhibitors combined with VEGFR-TKIs, multitarget TKIs, or CTLA-4 inhibitors were analyzed in our present meta-analysis. We further found that PD-1/PD-L1 inhibitors combined with multitarget TKIs may have a better antitumor effect on advanced HCC than PD-1/PD-L1 inhibitors combined with VEGFR-TKIs or CTLA-4 inhibitors. Sixth, only a few AEs, such as fatigue, rash, pruritus, and increased AST level, were described in two previous meta-analyses [117, 118]. By comparison, all AEs were reviewed, and the most common AEs, including hypertension, hand-foot syndrome, fatigue, proteinuria, and RCCEP, were quantitatively analyzed in our meta-analysis. Lastly, in a previous meta-analysis, patients receiving PD-1/PD-L1 inhibitor combination therapy might have a lower probability of drug withdrawal due to AEs than those receiving PD-1/PD-L1 inhibitor monotherapy [118]. However, on the basis of the data from a larger number of patients and PD-1/PD-L1 inhibitors included, we found a similar probability of drug withdrawal between the two groups.
Of course, our meta-analysis had several limitations. First, most of the included studies were single-arm studies, suggesting that the quality of evidence is relatively poor. Second, the dosage of PD-1/PD-L1 inhibitors was heterogeneous among the included studies, which compromises further subgroup analyses. Third, the characteristics of the study population, such as Child–Pugh class and Eastern Cooperative Oncology Group performance status, may influence the efficacy and safety of PD-1/PD-L1 inhibitors for advanced HCC, but cannot be sufficiently extracted, which fails to perform further subgroup analysis. Fourth, because the use of PD-1/PD-L1 inhibitors was the major intervention evaluated in our study, the type of TKIs combined was not specified. However, it should be noted that TKIs differed vastly in terms of their targets and efficacy.
Conclusion
PD-1/PD-L1 inhibitors increase tumor response and prolong survival of patients with advanced HCC as compared to multitarget TKIs. Additionally, PD-1/PD-L1 inhibitor combination therapy should be superior to PD-1/PD-L1 inhibitor monotherapy in terms of efficacy. Therefore, PD-1/PD-L1 inhibitor monotherapy and combination therapy should be considered as the first-line option for the treatment of advanced HCC. Certainly, more high-quality prospective studies are needed to validate these findings in future.
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604.
Galle PRFA, Llovet JM, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–50.
Zhou JSH, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition). Zhonghua Gan Zang Bing Za Zhi. 2020;28(2):112–28.
Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63.
Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66.
Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34.
Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73.
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90.
Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–96.
Choi WM, Choi J, Shim JH, Lim YS, Lee HC, Kim KM. Regorafenib versus nivolumab after sorafenib failure: real-world data in patients with hepatocellular carcinoma. J Hepatol. 2020;73:S39.
Rimassa L, Danesi R, Pressiani T, Merle P. Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev. 2019;77:20–8.
El-Khoueiry AB, Melero I, Crocenzi TS, et al. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. J Clin Oncol. 2015;33(18_suppl):LBA101.
Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375(18):1767–78.
Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5(230):46.
Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229(1):114–25.
Granier C, De Guillebon E, Blanc C, et al. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open. 2017;2(2):e000213.
El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502.
Finn RS, Qin SK, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905.
Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193.
Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary cancers, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2021;19(5):541–65.
Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38(36):4317–45.
Feng D, Hui X, Shi-Chun L, et al. Initial experience of anti-PD1 therapy with nivolumab in advanced hepatocellular carcinoma. Oncotarget. 2017;8(57):96649–55.
Wainberg ZA. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC). David Geffen School of Medicine at University of California Los Angeles LACA, Department of Medicine: American Society of Clinical Oncology Annual Meeting; 2017.
Feun LG. Phase II study of pembrolizumab in advanced, unresectable hepatocellular carcinoma. University of Miami Sylvester Comprehensive Cancer Center, American Society of Clinical Oncology Annual Meeting; 2018.
He AR, Weiss GJ, Falchook G, et al. Cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with advanced or metastatic hepatocellular carcinoma (HCC): data from an expansion cohort (EC) in a phase I study. Ann Oncol. 2018;29: x34.
Shen L, Zhang L, Hu X, et al. Atezolizumab monotherapy in Chinese patients with locally advanced or metastatic solid tumours. Ann Oncol. 2018;29:ix49.
Yoon SE, Hur JY, Lee KK, et al. Real-world data on nivolumab treatment in Asian patients with advanced hepatocellular carcinoma. Ann Oncol. 2018;29:viii235.
Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–52.
Arora V, Pande A, Singh A, Kumar G, Sarin SK. Nivolumab in the management of hepatocellular carcinoma in patients with advanced cirrhosis: a real life experience from India. Hepatology. 2019;70:539A.
Cedillo M, Lewis S, Lee KM, et al. Assessment of advanced HCC response to nivolumab using standard and immune response criteria. Abdom Radiol. 2019;44(9):3213–4.
Dharmapuri S, Özbek U, Lin JY, Schwartz M, Branch A, Ang C. Outcomes of hepatocellular carcinoma (HCC) patients treated with nivolumab: The Mount Sinai Hospital experience. Ann Oncol. 2019;30:v294.
Finkelmeier F, Czauderna C, Perkhofer L, et al. Feasibility and safety of nivolumab in advanced hepatocellular carcinoma: real-life experience from three German centers. J Cancer Res Clin Oncol. 2019;145(1):253–9.
Kambhampati S, Bauer KE, Bracci PM, et al. Nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh class B cirrhosis: safety and clinical outcomes in a retrospective case series. Cancer. 2019;125(18):3234–41.
Lin CC, Lin SM, Hou MM, et al. Real-world experience of nivolumab therapy for advanced hepatocellular carcinoma in Taiwan: early reduction of serum alpha-fetoprotein associated with therapeutic response and overall survival. Hepatology. 2019;70:538A-A539.
Scheiner B, Kirstein MM, Hucke F, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentrereal-world cohort. Aliment Pharmacol Ther. 2019;49(10):1323–33.
Yau T, Park JW, Finn RS, et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30:v874–5.
Chen S, Huang Z, Jia W, et al. Association of the pretreatment lung immune prognostic index with survival outcomes in advanced hepatocellular carcinoma patients treated with PD-1 inhibitors. J Hepatocell Carcinoma. 2020;7:289–99.
Cui HZ, Dai GH, Guan JZ. Programmed cell death protein-1 (PD-1)-targeted immunotherapy for advanced hepatocellular carcinoma in real world. Oncotargets Ther. 2020;13:143–9.
Desai J, Deva S, Lee JS, et al. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors. J Immunother Cancer. 2020;8(1):e000453.
Fessas P, Kaseb AO, Wang Y, et al. Post-registration experience of nivolumab (nivo) therapy in patients with advanced hepatocellular carcinoma (HCC): an international study. J Clin Oncol. 2020;38(15):e16677.
Kim HS, Hong JY, Cheon J, et al. Different organ-specific response to nivolumab to determine the survival outcome of patients with advanced hepatocellular carcinoma (aHCC). J Clin Oncol. 2020;38(15):4584.
Lee CH, Lee YB, Kim MA, et al. Effectiveness of nivolumab versus regorafenib in hepatocellular carcinoma patients who failed sorafenib treatment. Clin Mol Hepatol. 2020;26(3):328–39.
Lee MS, Ryoo BY, Hsu CH, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21(6):808–20.
Lee PC, Chao Y, Chen MH, et al. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers. 2020;12(1):182.
Lyu N, Kong Y, Li X, et al. Ablation reboots the response in advanced hepatocellular carcinoma with stable or atypical response during PD-1 therapy: a proof-of-concept study. Front Oncol. 2020;10:580241.
Mahn R, Vogt A, Kupczyk P, et al. Programmed cell death protein 1 (PD-1)-inhibition in hepatocellular carcinoma (HCC): a single center experience. Scand J Gastroenterol. 2020;55(9):1057–62.
Ostios-Garcia L, Ramiro-Cortijo D, Peters MLB, Bullock AJ. Association of immune related adverse events with superior outcomes in patients with hepatocellular carcinoma (HCC) treated with nivolumab. J Clin Oncol. 2020;38(15):e16630.
Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–80.
Spahn S, Roessler D, Pompilia R, et al. Clinical and genetic tumor characteristics of responding and non-responding patients to PD-1 inhibition in hepatocellular carcinoma. Cancers. 2020;12(12):1–17.
Sung PS, Jang JW, Lee J, et al. Real-world outcomes of nivolumab in patients with unresectable hepatocellular carcinoma in an endemic area of hepatitis B virus infection. Front Oncol. 2020;10:1043.
Wu L, Cui W, Gou Q, Zhou Z. Efficacy and safety of programmed cell death protein 1 inhibitor and the associated prognostic factors in patients with hepatitis B virus-related advanced hepatocellular carcinoma. Gut. 2020;69(2_suppl):A80–1.
Aljarroudi O, Chaabouni H, Ulusakarya A, et al. Anti-programmed death-1 therapy in advanced hepatocellular carcinoma: a real-world experience. Clin Case Rep. 2021;9(4):2162–7.
Ducreux M, Abou-Alfa G, Ren Z, et al. O-1 Results from a global phase 2 study of tislelizumab, an investigational PD-1 antibody, in patients with unresectable hepatocellular carcinoma. Ann Oncol. 2021;32:S217.
Gaudel P, Mohyuddin GR, Fields-Meehan J. Nivolumab use for first-line management of hepatocellular carcinoma: results of a real-world cohort of patients. Fed Pract. 2021;38(2):89–91.
Kelley RK, Sangro B, Harris W, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol. 2021;39:2991–3001.
Kudo M, Matilla A, Santoro A, et al. CheckMate 040 cohort 5: a phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol. 2021;75(3):600–9.
Kuo YH, Yen YH, Chen YY, et al. Nivolumab versus regorafenib in patients with hepatocellular carcinoma after sorafenib failure. Front Oncol. 2021;11:1988.
Lee DW, Cho EJ, Lee JH, et al. Phase II study of avelumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Clin Cancer Res. 2021;27(3):713–8.
Rimola J, Fonseca LG, Sapena V, et al. Radiological response to nivolumab in patients with hepatocellular carcinoma: a multicenter analysis of real-life practice. Eur J Radiol. 2021;135:109484.
Sardinha M, Simão D, Reis A, et al. P-87 Real-world data of nivolumab in advanced hepatocellular carcinoma: a multi-centric and retrospective study. Ann Oncol. 2021;32:S127.
Shi L, Zhou C, Long X, et al. Thermal ablation plus toripalimab in patients with advanced hepatocellular carcinoma: phase I results from a multicenter, open-label, controlled phase I/II trial (IR11330). Ann Oncol. 2021;32:S826.
Zhang T, Zhang J, Zhang X, Mu H, Yu G, Xing W. Triple combination therapy comprising angiogenesis inhibitors, anti-PD-1 antibodies, and hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2021;39(15_suppl):e16124.
Zeng Z, Zhang L, Wu T, Cheng J, Chen Y, Lu Y. A retrospective study on the efficacy and safety of sorafenib or lenvatinib combined with sintilimab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2021;39(15_suppl):e16127.
Zeng YY, Guo WH, Zhang Z, et al. A real-world study of camrelizumab in the treatment of hepatocellular carcinoma. J Clin Oncol. 2021;39(15_suppl):e16121.
Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27(4):1003–11.
Xie D, Sun Q, Wang X, et al. Immune checkpoint inhibitor plus tyrosine kinase inhibitor for unresectable hepatocellular carcinoma in the real world. Ann Transl Med. 2021;9(8):652.
Wong JSL, Kwok GGW, Tang V, et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J Immunother Cancer. 2021;9(2):e001945.
Wei F, Huang Q, He J, Luo L, Zeng Y. Lenvatinib plus camrelizumab versus lenvatinib monotherapy as post-progression treatment for advanced hepatocellular carcinoma: a short-term prognostic study. Cancer Manag Res. 2021;13:4233–40.
Sum J, Wong L, Kwok GW, et al. Ipilimumab andnivolumab/pembrolizumab inadvanced hepatocellularcarcinoma refractory to priorimmune checkpoint inhibitors. J Clin Oncol. 2021;39(3_suppl):330.
Ohki T, Kondo M, Sato K, et al. Atezolizumab plus bevacizumab as a second-line treatment after lenvatinib failure. Acta Hepatol Jpn. 2021;62(9):585–7.
Mei J, Tang YH, Wei W, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. 2021;11:618206.
Mei J, Li SH, Li QJ, et al. Anti-pd-1 immunotherapy improves the efficacy of hepatic artery infusion chemotherapy in advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:167–76.
Lee IC, Wu CJ, Chen SC, Chao Y, Huang YH. Lenvatinib plus pembrolizumab versus lenvatinib in patients with unresectable hepatocellular carcinoma: a real world study. J Clin Oncol. 2021;39(15 SUPPL):e16138.
Kudo M, Motomura K, Wada Y, et al. Avelumab in combination with axitinib as first-line treatment in patients with advanced hepatocellular carcinoma: results from the phase 1b VEGF liver 100 trial. Liver Cancer. 2021;10(3):249–59.
Kim RD, Harris WP, Sung MW, et al. Results of a phase Ib study of regorafenib (REG) 80 mg/day plus pembrolizumab (PEMBRO) for first-line treatment of advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2021;39(3_suppl):323.
Huang Y, Zhang Z, Liao W, Hu K, Wang Z. Combination of sorafenib, camrelizumab, transcatheter arterial chemoembolization, and stereotactic body radiation therapy as a novel downstaging strategy in advanced hepatocellular carcinoma with portal vein tumor thrombus: a case series study. Front Oncol. 2021;11:650394.
Hsiehchen D, Kainthla R, Zhu H, Jones A, Beg MS. Phase II study of pembrolizumab (pembro) and bavituximab (bavi) in advanced hepatocellular carcinoma (HCC). Ann Oncol. 2021;32:S822–3.
Hiraoka A, Kumada T, Tada T, et al. Atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma: early clinical experience. Cancer Rep. 2021;5:e1464.
He MK, Liang RB, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:1–14.
Hayakawa Y, Tsuchiya K, Kurosaki M, et al. Early experience of atezolizumab plus bevacizumab therapy in Japanese patients with unresectable hepatocellular carcinoma in real-world practice. Investig New Drugs. 2021;40:392–402.
Han C, Ye S, Hu C, et al. Clinical activity and safety of penpulimab (Anti-PD-1) with anlotinib as first-line therapy for unresectable hepatocellular carcinoma: an open-label, multicenter, phase Ib/II trial (AK105–203). Front Oncol. 2021;11:2650.
Cheon J, Yoo C, Hong JY, et al. Prognostic factor analysis of atezolizumab-bevacizumab in unresectable hepatocellular carcinoma: Korean Cancer Study Group (KCSG) study. Ann Oncol. 2021;32:S828.
Chen S, Wu ZQ, Shi F, et al. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol. 2021;148:2115–25.
Ando Y, Kawaoka T, Kosaka M, et al. Early tumor response and safety of atezolizumab plus bevacizumab for patients with unresectable hepatocellular carcinoma in real-world practice. Cancers. 2021;13(16):3958.
Zhu AX, Finn RS, Ikeda M, et al. A phase Ib study of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC). J Clin Oncol. 2020;38(15):4519.
Zhang W, Hu B, Han J, et al. A real-world study of PD-1 inhibitors combined with TKIs for HCC with major vascular invasion as the conversion therapy: a prospective, non-randomized, open-label cohort study. Ann Oncol. 2020;31:S1307.
Zhang W, Bi X, Sun Y, et al. Preliminary results of sintilimab plus different dose of IBI305 (anti-VEGF monoclonal antibody) in patients with advanced hepatocellular carcinoma: a phase Ib study. J Clin Oncol. 2020;38(15_suppl):3079.
Yuan G, Cheng X, Li Q, et al. Safety and efficacy of camrelizumab combined with apatinib for advanced hepatocellular carcinoma with portal vein tumor thrombus: a multicenter retrospective study. Onco Targets Ther. 2020;13:12683–93.
Yau T, Zagonel V, Santoro A, et al. Nivolumab (NIVO) + ipilimumab (IPI) + cabozantinib (CABO) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol. 2020;38(4):478.
Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11):e204564.
Wu CJ, Hung YW, Lee PC, Lee CJ, Hou MC, Huang YH. Safety and efficacy of lenvatinib plus reduced dose pembrolizumab in unresectable hepatocellular carcinoma. Hepatology. 2020;72(1_suppl):705A.
Tai WMD, Loke KSH, Gogna A, et al. A phase II open-label, single-center, nonrandomized trial of Y90-radioembolization in combination with nivolumab in Asian patients with advanced hepatocellular carcinoma: CA 209–678. J Clin Oncol. 2020;38(15_suppl):4590.
Sun H-C, Zhu XD, Huang C, et al. Combination therapy with lenvatinib and anti-PD-1 antibodies for unresectable or advanced hepatocellular carcinoma: a real-world study. J Clin Oncol. 2020;38(15_suppl):e16610.
Shen L, Zhang Y, Guo Y, et al. A phase Ib study of the PD-1 antagonist CS1003 plus lenvatinib (LEN) in Chinese patients (pts) with the first-line (1L) unresectable hepatocellular carcinoma (uHCC). Ann Oncol. 2020;31:S690–1.
Ren Z, Fan J, Xu J, et al. LBA2 Sintilimab plus bevacizumab biosimilar vs sorafenib as first-line treatment for advanced hepatocellular carcinoma (ORIENT-32)2. Ann Oncol. 2020;31:S1287.
Li Q, Chen M, Cao M, et al. Lenvatinib (LEN) plus anti-PD-1 antibodies vs LEN alone for advanced hepatocellular carcinoma (HCC): a real-world study. Ann Oncol. 2020;31:S1310.
Kudo M, Ikeda M, Motomura K, et al. A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): Study 117. J Clin Oncol 2020;38(4).
Jia F, Ren Z, Xu J, et al. Sintilimab plus IBI305 as first-line treatment for advanced hepatocellular carcinoma. Ann Oncol. 2020;31:S692.
Finn RS, Ikeda M, Zhu ANX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960.
El-Khoueiry AB, Kim RD, Harris WP, et al. Phase Ib study of regorafenib (REG) plus pembrolizumab (PEMBRO) for first-line treatment of advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2020;38(4):564.
Chen Y, Zhang L, Ge N, Wang Y, Ren Z. Clinical effect of sequential therapy of LEN combination with anti-PD1 antibody in uHCC patients who progressed on LEN treatment: a real-world data in China. J Clin Oncol. 2020;38(15_suppl):e16654–e1665.
Chen X, Li W, Wu X, et al. Sintilimab plus anlotinib as first-line therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2020;31:S1305.
Chen JZ, Hu XY, Li Q, et al. Effectiveness and safety of toripalimab, camrelizumab, and sintilimab in a real-world cohort of hepatitis B virus associated hepatocellular carcinoma patients. Ann Transl Med. 2020;8(18):1187.
Chen C, An L, Cheng Y, Luo XW, Li ZX, Liu XF. Clinical outcomes and prognosis factors of nivolumab plus chemotherapy or multitarget tyrosine kinase inhibitor in multi-line therapy for recurrent hepatitis B virus-related hepatocellular carcinoma: a retrospective analysis. Front Oncol. 2020;10:1404.
Bang YJ, Golan T, Dahan L, et al. Ramucirumab and durvalumab for previously treated, advanced non-small-cell lung cancer, gastric/gastro-oesophageal junction adenocarcinoma, or hepatocellular carcinoma: an open-label, phase Ia/b study (JVDJ). Eur J Cancer. 2020;137:272–84.
Qin S, Chen Z, Liu Y, et al. A phase II study of anti-PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer. J Clin Oncol. 2019;37(15_suppl):4074.
Llovet J, Shepard KV, Finn RS, et al. A phase Ib trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC): updated results. Ann Oncol. 2019;30:v286–7.
Floudas CS, Xie C, Brar G, et al. Combined immune checkpoint inhibition (ICI) with tremelimumab and durvalumab in patients with advanced hepatocellular carcinoma (HCC) or biliary tract carcinomas (BTC). J Clin Oncol. 2019;37:336.
Xu JM, Zhang Y, Jia R, et al. Anti-programmed death-1 antibody SHR-1210 (S) combined with apatinib (A) for advanced hepatocellular carcinoma (HCC), gastric cancer (GC) or esophagogastric junction (EGJ) cancer refractory to standard therapy: a phase 1 trial. J Clin Oncol. 2018;36(15):4075.
Pishvaian MJ, Lee MS, Ryoo BY, et al. Updated safety and clinical activity results from a phase Ib study of atezolizumab 1 bevacizumab in hepatocellular carcinoma (HCC). Ann Oncol. 2018;29:viii718–9.
Ikeda M, Sung MW, Kudo M, et al. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients (pts) with unresectable hepatocellular carcinoma (uHCC). J Clin Oncol. 2018;36(15):4076.
Cheng AL, Yen CJ, Okusaka T, et al. A phase I, open-label, multi-center, dose-escalation study of codrituzumab, an anti-glypican-3 monoclonal antibody, in combination with atezolizumab in patients with locally advanced or metastatic hepatocellular carcinoma. Ann Oncol. 2018;29:viii234–5.
Chen SC, Yang MH, Chao Y. Combination of sorafenib and anti-PD-1 for advanced hepatocellular carcinoma-real world experience. J Immunother Cancer. 2018;6(1_suppl):P191
Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): phase I safety and efficacy analyses. J Clin Oncol. 2017;35(15_suppl):4073.
He S, Jiang W, Fan K, Wang X. The efficacy and safety of programmed death-1 and programmed death ligand 1 inhibitors for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Front Oncol. 2021;11: 626984.
Rao Q, Li M, Xu W, et al. Clinical benefits of PD-1/PD-L1 inhibitors in advanced hepatocellular carcinoma: a systematic review and meta-analysis. Hepatol Int. 2020;14(5):765–75.
Lencioni R. New data supporting modified RECIST (mRECIST) for Hepatocellular Carcinoma. Clin Cancer Res. 2013;19(6):1312–4.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Conception and design: XQ. Acquisition of data: YL, JP, FG, WX, and XQ. Analysis and interpretation of data: YL, JP, HL, and XQ. Drafting the manuscript: YL, JP, HL, and XQ. Revising the manuscript critically for important intellectual content: YL, JP, HL, and XQ. Final approval of the version to be published: YL, JP, FG, WX, HL, and XQ.
Disclosures
Yuwei Liu, Jiahui Pan, Fangbo Gao, Wentao Xu, Hongyu Li and Xingshun Qi have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Pan, J., Gao, F. et al. Efficacy and Safety of PD-1/PD-L1 Inhibitors in Advanced Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Adv Ther 40, 521–549 (2023). https://doi.org/10.1007/s12325-022-02371-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02371-3