Abstract
Background
Currently, the effectiveness of TACE, Lenvatinib, and PD-1/L1 inhibitors used alone or in combination has been thoroughly reported. However, the differences in effectiveness between these treatment protocols require further verification. To this end, this study employs a Bayesian network meta-analysis to compare the efficacy and safety of TACE, Lenvatinib, and PD-1/L1 inhibitors, whether administered by monotherapy or in combination, providing evidence-based medicine for the treatment of unresectable HCC.
Purpose
This study employed a network meta-analysis to evaluate the efficacy and safety of trans-arterial chemoembolization (TACE), Programmed Cell Death Protein/Ligand 1 (PD-1/L1) inhibitors, and Lenvatinib in the treatment of advanced HCC.
Methods
Literature on the treatment of advanced HCC with TACE, PD-1/L1 inhibitors, and Lenvatinib was searched for in both Chinese and English databases, including PubMed, EMBASE, ClinicalTrials.gov, Cochrane Library, CNKI, and Wanfang. Two researchers conducted independent screening and data extraction, and the meta-analysis was performed using R language with the gemtc package.
Results
After retrieval and screening, a total of 21 articles were included, involving 2052 participants and six treatment modalities: Lenvatinib (L), TACE (T), TACE + Lenvatinib (TL), Lenvatinib + PD-1/L1 inhibitors (LP), TACE + Lenvatinib + PD-1/L1 inhibitors (TLP), and TACE + PD-1/L1 inhibitors (TP). In terms of objective response rate (ORR), the TLP regimen provided the optimal effect. In predicting the best ORR, TLP had the highest (75.5%) probability. In terms of disease control rate (DCR), the TLP regimen showed the best effect. In predicting the best DCR, the TLP again offered the highest (76.1%) probability. In terms of overall survival (OS), the best outcome was observed in the TLP protocol. In predicting the best OS, the TLP holds the highest (86.00%) probability. Furthermore, the best outcome in progression-free survival (PFS) was found in the TLP regimen. In predicting the best PFS, the TLP still holds the highest (97.0%) result.
Conclusion
The combination of TACE, Lenvatinib, and PD-1/L1 inhibitors appears to provide the maximum benefit for inoperable HCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary liver cancer is currently the fourth most common malignant tumor and the second leading cause of cancer-related deaths in China, posing a significant threat to the lives and health of the Chinese population [1]. This paper uses the GLOBOCAN 2020 cancer incidence rate and mortality estimates prepared by the International Agency for Research on Cancer to provide an update on the global burden of liver cancer. It is estimated that cancer will remain the main cause of cancer death in 2020, with hepatocellular carcinoma (HCC) estimating a mortality rate of 8.3%; in 2020, new cases and deaths for liver cancer were 905,677 (4.7%), and liver cancer among new deaths was 830,180 (8.3%) [2]. Early stage liver cancer is primarily managed through surgical resection. However, due to its insidious onset and high malignancy, patients are often diagnosed at an advanced stage. In such cases, surgical resection may not be sufficient to achieve a favorable outcome, leading to the recommendation of non-surgical treatments [3]. The combination of multiple treatment modalities is the preferred choice for patients with advanced or unresectable HCC, and currently available treatment measures include trans-arterial chemoembolization (TACE), Lenvatinib, and ICI inhibitors [4,5,6]. In recent years, the application of immune checkpoint inhibitors such as PD-1 and PD-L1 targeting immune regulatory checkpoints on immune and tumor cells has made significant advancements in the treatment of solid tumors. However, due to its relatively low objective response rate, a large proportion of patients do not respond to immunotherapy alone, which has driven exploration of treatment strategies to improve the effectiveness of immunotherapy on HCC.
Trans-arterial chemoembolization is a significant achievement in the development of interventional radiology and has become the recognized first-line non-surgical treatment for liver cancer. It involves catheterization through the femoral artery, selective insertion into the arterial supply of the liver cancer, embolization to lock tumor blood supply and induce ischemic necrosis, and perfusion of chemotherapeutic agents. Moreover, it can establish an environment of insufficient blood supply within the HCC tumor tissue for subsequent treatment [7]. For HCC patients who are unable or unwilling to undergo surgical resection or experienced disease recurrence after surgical treatment, TACE is a primary treatment option [8]. Lenvatinib is a receptor tyrosine kinase (RTK) inhibitor that suppresses other RTKs involved in tumor proliferation, as well as angiogenesis and oncogenic signaling pathways. It also selectively inhibits the kinase activity of vascular endothelial growth factor receptors and fibroblast growth factor receptors [9]. Lenvatinib is an FDA-approved drug and has been adopted in first-line treatment of liver cancer, resulting in an increasing use in clinical practice, especially in combination therapy regimens [10]. Programmed Cell Death Protein 1 (PD-1) or Programmed Cell Death Ligand 1 (PD-L1) inhibitors have been extensively used in the treatment of various malignancies and have become first- or second-line options for systemic treatment of advanced HCC due to promising efficacy for liver cancer substantiated by multiple clinical studies [11,12,13,14]. Advanced HCC is treated with TACE, TKI, or immunotherapy, but these methods do not significantly prolong lifespan as treatment resistance and disease recurrence develop.
Currently, the effectiveness of TACE, Lenvatinib, and PD-1/L1 inhibitors used alone or in combination has been thoroughly reported. However, the differences in effectiveness between these treatment protocols require further verification [13,14,15]. To this end, this study employs a Bayesian network meta-analysis to compare the efficacy and safety of TACE, Lenvatinib, and PD-1/L1 inhibitors, whether administered by monotherapy or in combination, providing evidence-based medicine for the treatment of unresectable HCC.
Materials and methods
Search strategy
A computer-based search was conducted in both Chinese and English databases, including PubMed, EMBASE, ClinicalTrials.gov, Cochrane Library, CNKI, and Wanfang, for literature on the treatment of advanced Hepatocellular Carcinoma (HCC) using TACE, PD-1/L1 inhibitors, and Lenvatinib. Chinese search terms included: 肝癌 (Hepatocellular carcinoma), 肝细胞癌 (HCC), 仑伐替尼 (Lenvatinib), 经导管肝动脉化疗栓塞术 (Transarterial Chemoembolization TACE), 程序性死亡受体 (Programmed Death Receptor), PD-1, PD-L1. The search was performed using a combination of subject headings and free-text terms. The language was restricted to Chinese and English, and the search was conducted up to September 30, 2023.
Inclusion criteria
This study employed the criteria of study population, intervention measures, control measures, outcomes, and study design as the process for literature selection.
Study population Patients with advanced hepatocellular carcinoma who were considered unsuitable for curative surgery; Eastern Cooperative Oncology Group (ECOG) score of 0–2; expected survival time of over 3 months; no prior systemic treatment.
Intervention and control measures At least two treatment modalities, including either TACE, Lenvatinib, or PD-1/L1 inhibitors used as single treatments or in combination.
Outcomes Objective response rate (ORR) and disease control rate (DCR) based on modified response evaluation criteria in solid tumors (mRECIST); overall survival (OS); progression-free survival (PFS). According to WHO standards, it is classified as levels 0–IV, with level 0 being normal and no response. Level IV is the most severe adverse reaction that can endanger life, such as gastrointestinal reactions, neurological reactions, cardiac reactions, skin reactions, hair loss reactions, body temperature, etc., all ranging from levels 0 to IV. Clinicians should closely observe adverse reactions to chemotherapy, and if moderate-to-severe reactions occur, they should be promptly and actively treated. Adverse events (AEs) of grade 3 or higher
ORR refers to the proportion of patients whose tumor volume has shrunk to a predetermined value and can maintain the minimum required time limit. The remission period usually refers to the period from the onset of therapeutic effects to the confirmation of tumor progression. The general definition of objective remission rate is the sum of complete remission and partial remission (CR + PR).
DCR refers to the proportion of patients whose tumors have shrunk or stabilized for a certain period of time, including cases of CR, PR, and SD.
OS is defined as the time between randomization and patient death due to various reasons and is calculated based on the intended treatment population. There will be no bias in the end-point evaluation. OS is the most reliable clinical trial endpoint for evaluating anti-tumor drugs to date and is usually the preferred endpoint when the study can fully evaluate survival.
PFS defines the period between the onset of treatment and the observation of disease progression or death from any cause in a patient.
Study design Prospective or retrospective clinical studies.
Exclusion criteria Reviews, case reports, conference abstracts, and duplicate studies; single-arm studies without a control group; studies with incomplete data or where original data could not be obtained.
Data extraction
Two researchers independently extracted data from each study using a pre-specified Excel sheet (Microsoft Excel 2013, USA). A third investigator cross-checked the data, and any discrepancies were resolved through discussion. The extracted information included first author, publication year, study location (country and region), population type, sample size for each study group, sex, age, control interventions, intervention measures for each study group, and outcome indicators. In cases where specific data could not be extracted or were not clearly reported in the paper, the corresponding authors of the respective studies were contacted.
Quality assessment
The quality of the included literature was assessed using the Cochrane recommended Cochrane Risk of Bias Assessment Tool, discussing bias sources based on seven dimensions, namely, selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias. The risk was classified as high, uncertain, or low. The quality of included retrospective studies was assessed using the Newcastle–Ottawa Scale (NOS). Scores were assigned based on selection, comparability, and outcome, with a maximum score of 9, and studies with a score above 5 were considered high quality.
Statistical analysis
The meta-analysis was conducted using the R language package gemtc. Bayesian mesh meta-analysis using R language package gemtc. Hazard Ratios (HR) and 95% confidence intervals (CI) were analyzed for OS, PFS, and time to progression (TTP). Odds Ratios (OR) and their 95% CI were employed for ORR, DCR, and AEs. P < 0.05 was considered statistically significant. A total of 50,000 iterations were performed, with the initial 20,000 used for algorithm annealing to eliminate the influence of initial values. Forest plots were generated for result comparison, and surface under the cumulative ranking (SUCRA) values were predicted for the efficacy ranking of each intervention. The SUCRA value ranges from 0% to 100%, with higher values indicating better intervention efficacy and higher ranking. The I2 test was used to explore heterogeneity, where I2.pair (Tauared) represents the degree of heterogeneity between adjacent study results, measuring the variance of bias between two study results, and I2.cons (I Squared) represents the degree of overall study result heterogeneity, measuring the variance of bias in all study results. In the network meta-analysis results, each point on the evidence network graph represents an intervention measure, and lines connecting points indicate direct comparisons between two intervention measures. The thickness of the line indicates the number of studies between the two intervention measures, and the size of the circle represents the total sample size of the intervention measure.
This meta-analysis has been registered on the INPLASY, registration number: INPLASY202410065.
Results
Literature selection
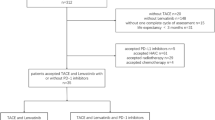
After conducting a computer-based literature search and importing into Endnote, a total of 1352 articles were retrieved. 1352 relevant studies in initial search: PubMed (n = 225), EMBASE (n = 234), Clinical Trials gov (n = 101), Cochrane Libraryand (n = 211), CNKI (n = 268), and Wanfang (n = 313). After removing duplicates, 1126 articles remained. Following the inclusion and exclusion criteria, 1049 articles were excluded based on abstract readings, including 514 being non-clinical studies, 393 lacking specified intervention methods, and 142 being reviews or case reports. Full-text reading was conducted on the remaining 77 articles. Among them, 36 did not include specified outcome indicators and 20 had unclear diagnoses. After screening, 21 meta-analyses were included. The flowchart of the literature selection process is depicted in Fig. 1.
Included literature information
Among the 21 included studies, a total of 2052 participants were involved. There were six treatment modalities considered, namely, Lenvatinib (L), transarterial chemoembolization (TACE), TACE combined with Lenvatinib (TL), Lenvatinib combined with PD-1/L1 inhibitors (LP), TACE combined with Lenvatinib and PD-1/L1 inhibitors (TLP), and TACE combined with PD-1/L1 inhibitors (TP). All included studies were retrospective in nature, and the quality of the literature was assessed using NOS, with all studies scoring above 5, indicating high quality. The basic information of the included studies is presented in Table 1.
ORR network meta-analysis
Network diagram of evidence
Among the included studies, 19 reported outcomes related to ORR, comprising 18 two-arm studies and 1 three-arm study. The three-arm study was divided into three two-arm studies, resulting in 21 sets of ORR data. These included 4 sets of data for L vs. TL, 1 set for LP vs. TLP, 4 sets for T vs. TL, 3 sets for T vs. TLP, 8 sets for TL vs. TLP, and 1 set for TL vs. TLP. The evidence network is illustrated in Fig. 2A.
Forest plot comparing ORR between different treatment modalities
In the pairwise comparisons of the six included intervention measures, Bayesian network meta-analysis found that in improving ORR, combination therapy was superior to monotherapy of L or T, and triple therapy outperformed combination therapy. Specifically, the data for L vs. TLP (OR = 0.15, 95% CI 0.06–0.36), T vs. TLP (OR = 0.17, 95% CI 0.09–0.30), TL vs. TLP (OR = 0.31, 95% CI 0.19–0.49), L vs. TL (OR = 0.49, 95% CI 0.22–1.00), and T vs. TL (OR = 0.55, 95% CI 0.31–0.99) showed statistically significant results. No statistically significant difference was observed between monotherapy of L and T, as well as between different combinations of dual therapies. The forest plot comparing different treatment modalities is shown in Fig. 3A.
Forest plot comparing ORR, DCR, OS and PFS between different treatment modalities; A forest plot comparing ORR between different treatment modalities; B forest plot comparing DCR between different treatment modalities; C forest plot comparing OS between different treatment modalities; D forest plot comparing PFS between different treatment modalities
Ranking of ORR efficacy for different treatment modalities
Using SUCRA to predict the ranking of the efficacy of each intervention measure, it was found that in improving ORR, the TLP protocol was the most effective approach, while the L regimen was the least effective, as presented in Table 2. When predicting the best ORR intervention measure, TLP yielded the highest probability (75.5%), followed by TP (7.9%) and LP (7.6%). The probabilities for other intervention measures were less than 1%, as shown in Fig. 4A.
Ranking chart of best ORR, DCR, OS and PFS probabilities for different intervention measures. A Ranking chart of best ORR probabilities for different intervention measures; B ranking chart of the best DCR probabilities for different intervention measures; C ranking of the best OS probabilities for different methods; D ranking of the best PFS probabilities for different methods
Local consistency and heterogeneity test
Among the included studies on ORR, there is one closed loop in the literature, where T, TL, and TLP form a closed loop. The node-splitting method was employed for local consistency test to evaluate the consistency between direct evidence and indirect evidence. The results indicated consistency between direct and indirect results, and thus, the consistency model was applied (P > 0.05). The forest plot for local consistency test of studies on ORR is shown in Fig. 5A. The heterogeneity test results indicated that I2.pair was 40.81% and I2.cons was 40.03%, suggesting no significant heterogeneity.
Forest plot for local consistency test of studies on ORR, DCR, OS and PFS. A Forest plot for local consistency test of studies on ORR; B forest plot for local consistency testing of studies included for DCR; C forest plot of local consistency test for OS in included studies; D forest plot of local consistency test for PFS in included studies
DCR network meta-analysis
Network diagram of evidence
Among the included studies, 18 reported outcomes related to DCR, comprising 17 two-arm studies and 1 three-arm study. The three-arm study was categorized into three two-arm studies, resulting in 20 sets of DCR data, encompassing 3 sets of data for L vs. TL, 1 set for LP vs. TLP, 4 sets for T vs. TL, 3 sets for T vs. TLP, 8 sets for TL vs. TLP, and 1 set for TL vs. TLP. The evidence network is illustrated in Fig. 2B.
Forest plot comparing DCR between different treatment modalities
In the pairwise comparisons of the six included intervention measures, Bayesian network meta-analysis revealed that triple therapy provided the higher benefit in improving DCR, followed by combination therapy, and then monotherapy of e L or single T. Specifically, the data for L vs. TLP (OR = 0.05, 95% CI 0.01–0.18), T vs. TLP (OR = 0.10, 95% CI 0.04–0.23), TL vs. TLP (OR = 0.32, 95% CI 0.16–0.59), L vs. TL (OR = 0.17, 95% CI 0.03–0.47), and T vs. TL (OR = 0.32, 95% CI 0.14–0.70) exhibited statistically significant results. No statistically significant difference was observed between single L and single T, as well as between different combinations of dual therapies. The forest plot comparing different treatment modalities is shown in Fig. 3B.
Ranking of DCR efficacy for different treatment modalities
SUCRA was employed to predict the ranking of the efficacy of each intervention measure and identified the TLP regimen as the most effective measure and the L monotherapy as the least effective one in improving DCR, as outlined in Table 2. When predicting the best DCR intervention measure, TLP had the highest probability (76.1%), followed by LP (15.8%) and TP (8.1%). The probabilities for other intervention measures were all less than 1%. The ranking chart for the best DCR probabilities for different intervention measures is shown in Fig. 4B.
Local consistency and heterogeneity testing
Within the literature included for DCR, there was one closed loop involving T, TL, and TLP. A node-splitting analysis was performed for local consistency testing to compare whether direct evidence and indirect evidence were consistent. Consistent direct and indirect results were identified, indicating the use of a consistency model (P > 0.05). The forest plot for local consistency testing of studies included for DCR is shown in Fig. 5B. The results of the heterogeneity test indicated that I2.pair was 27.75% and I2.cons was 49.78%. Overall, no significant heterogeneity was found.
OS network meta-analysis
Network diagram of evidence
In this study, 16 studies reported OS outcomes, involving 4 sets of data for L vs. TL, 2 sets for LP vs. TLP, 2 sets for T vs. TL, 2 sets for T vs. TLP, 5 sets for TL vs. TLP, and 1 set for TL vs. TLP. The network diagram of evidence is shown in Fig. 2C.
Forest plot comparing OS between different treatment methods
In the results of pairwise comparisons of the six intervention methods, a network meta-analysis based on Bayesian statistics was employed. It was found that triple therapy provided the best OS results, followed by dual therapy, and then monotherapy using L or T alone. Specifically, statistical significance was identified in comparing TLP vs. T (HR = 0.27, 95% CI 0.16–0.44), TLP vs. L (HR = 0.20, 95% CI 0.09–0.38), TLP vs. LP (HR = 0.42, 95% CI 0.21–0.84), TL vs. T (HR = 0.56, 95% CI 0.32–0.92), and TL vs. L (HR = 0.40, 95% CI 0.20–0.70). No statistically significant differences were observed between using L alone and T alone, as well as between different dual therapies. The forest plot comparing OS between different treatment methods is presented in Fig. 3C.
Ranking of OS efficacy for different treatment methods
The SUCRA method was used to predict the effectiveness ranking of various interventions in improving overall survival (OS). Among them, the TLP intervention demonstrated the highest efficacy, while the L intervention showed the lowest, as shown in Table 2. When predicting the best OS intervention, the probability was highest for TLP (86.00%), followed by TP (13.08%), while the probabilities for other interventions were all less than 1%. The ranking of the best OS probabilities for different interventions is shown in Fig. 4C.
Local consistency and heterogeneity test
In the included OS studies, there is a closed loop involving T, TL, and TLP. A node-splitting method was used for the local consistency test to identify consistency between direct and indirect evidence. The results indicate that direct and indirect results were consistent, leading to the use of a consistency model (all P > 0.05). The forest plot for the local consistency test of OS studies is shown in Fig. 5C. Heterogeneity tests reveal an I2.pair of 18.39% and an I2.cons of 44.59%, suggesting an overall lack of significant heterogeneity.
PFS network meta-analysis
Network diagram of evidence
In this study, 15 articles reported the outcome of PFS. The data includes 4 groups of L vs. TL, 2 groups of LP vs. TLP, 1 group of T vs. TL, 1 group of T vs. TLP, 6 groups of TL vs. TLP, and 1 group of TL vs. TLP, as depicted in Fig. 2D.
Forest plot comparing PFS between different treatment methods
In the pairwise comparisons of the six intervention measures, the network meta-analysis based on Bayesian statistics revealed that triple therapy offered the best outcomes in improving PFS, followed by combination therapy, and then the single use of L or T. The comparisons TLP vs. T (HR = 0.17, 95%CI 0.11–0.25), TLP vs. L (HR = 0.23, 95%CI 0.17–0.31), TLP vs. TL (HR = 0.45, 95%CI 0.36–0.55), TLP vs. LP (HR = 0.55, 95%CI 0.38–0.78), TLP vs. TP (HR = 0.54, 95%CI 0.29–1.00), TL vs. T (HR = 0.37, 95%CI 0.24–0.56), TL vs. L (HR = 0.23, 95%CI 0.17–0.31), LP vs. T (HR = 0.30, 95%CI 0.18–0.52), LP vs. L (HR = 0.42, 95%CI 0.26–0.66), TP vs. T (HR = 0.31, 95%CI 0.15–0.63), and TP vs. L (HR = 0.42, 95%CI 0.22–0.80) all showed statistical significance. No statistical significance was found in the comparison between using L alone and using T alone, as well as in the comparisons between different combination therapies. The forest plot comparing different treatment modalities for PFS is shown in Fig. 3D.
Ranking of PFS efficacy for different treatment methods
Using SUCRA, the effectiveness of each intervention measure in improving PFS was predicted. Among them, the intervention measure TLP showed the best efficacy, while the intervention measure T showed the poorest (Table 2). In predicting the intervention measure that achieves the optimal PFS, the probability of TLP was the highest (97.0%), followed by TP (2.9%), and the probabilities of other intervention measures were less than 1%. The rank probability graph for the best PFS with different intervention measures is illustrated in Fig. 4D.
Local consistency and heterogeneity test
In the literature included for PFS, there is a closed loop consisting of T, TL, and TLP. The node-splitting method was used for the local consistency test to compare whether there is consistency between direct and indirect evidence. The results showed consistent direct and indirect results, and a consistency model was employed (P > 0.05), Table 2. The forest plot for the local consistency test of the included studies on PFS is shown in Fig. 5D. The results of the heterogeneity test indicate that I2.pair is 0% and I2.cons is 0%, indicating no significant heterogeneity overall.
Discussion
Hepatocellular carcinoma (HCC) is the sixth most common cancer globally and ranks as the third leading cause of cancer-related deaths worldwide, following lung and colorectal cancers. HCC constitutes the most prevalent histological subtype, accounting for approximately 80–85% of cases [37]. More than 50% of HCC patients are diagnosed at an advanced stage or exhibit features that exclude them from surgery or local treatment, highlighting the crucial role of systemic therapy in achieving favorable survival outcomes [38]. With advancements in targeted therapy, immunotherapy, and interventional radiology, treatment options of first-line systemic treatments for HCC have been increasingly on the rise [39, 40]. The current study assessed the effects of Lenvatinib in combination with current alternative treatment modalities, including immunotherapy and TACE.
This study employs a Bayesian network meta-analysis to assess the effectiveness of six different treatment approaches involving TACE, Lenvatinib, and PD-1/L1 inhibitors. The research findings demonstrated that the combined use of TACE, Lenvatinib, and PD-1/L1 inhibitors is associated with the most favorable ORR, DCR, OS, and PFS benefits. The REFLECT study, published in the Lancet in 2018, corroborated that Lenvatinib is non-inferior to Sorafenib in terms of efficacy for HCC, providing a survival benefit for advanced HCC patients. Consequently, Lenvatinib has been approved for the treatment of liver cancer [41]. However, Yu et al. [42] found that lenvatinib is a first-line oral multikinase inhibitor approved by the US Food and Drug Administration for the treatment of advanced hepatocellular carcinoma (aHCC) and has shown promising therapeutic potential. However, the results of the Leap-002 study indicate that adding anti-angiogenic drugs to lenvatinib may not significantly improve survival rates. This may be attributed to the lack of representativeness of HBV-related aHCC cases and Asian populations in the study, as well as the increased availability of second-line treatment options for advanced cancer, which may affect the observed efficacy of immunotherapy.
Apart from its anti-vascular effects, Lenvatinib also exerts a regulatory influence on the immune microenvironment of liver cancer. In contrast to conventional choices like Sorafenib, Lenvatinib demonstrates a noteworthy anti-tumor impact in immunocompromised mice. This implies that Lenvatinib could potentially boost immune function by reducing the presence of tumor-associated macrophages, elevating the ratio of activated CD8+ cells, and intensifying the activation and infiltration of natural killer cells. Transarterial chemoembolization involves embolizing the tumor-feeding arteries, causing tumor necrosis. Lenvatinib, being a tyrosine kinase inhibitor, plays a crucial role in suppressing tumor growth by acting on the VEGF pathway. The concurrent application of these two treatment modalities provides synergistic effects. Nevertheless, recurrent TACE procedures entail the potential risk of treatment ineffectiveness [42]. In recent years, numerous clinical reports on the combined treatment of advanced HCC using TACE and Lenvatinib have been published, multiple single arm studies have confirmed the safety and efficacy of unresectable HCC patients, but three RCT studies have not confirmed the benefits of combined treatment.
In the current research, compared to a single treatment method of TACE or Lenvatinib, the combination of TACE and Lenvatinib can improve the ORR, DCR, PFS, and OS in patients with advanced HCC. Furthermore, when comparing the combination of TACE and Lenvatinib with other combinations such as TACE with PD-1/L1 inhibitors, or the concurrent use of Lenvatinib with PD-1/L1 inhibitors, the triple therapy of TACE, Lenvatinib, and PD-1/L1 inhibitors significantly improves PFS and OS. However, no significant improvement was observed in ORR and DCR benefits.
At present, PD-1 inhibitors and PD-L1 inhibitors are authorized immunotherapy medications with demonstrated effectiveness in the treatment of patients with hepatocellular carcinoma [43, 44]. PD-1 can produce inhibitory signals when it binds with PD-L1, leading to the suppression of immune cell activation and providing protection to tumor cells against attacks. The incorporation of PD-1 inhibitors with tyrosine kinase inhibitors is one of the commonly used treatment strategies for unresectable hepatocellular carcinoma, and the combination of PD-1 inhibitors with Lenvatinib has provided significant survival benefits compared to PD-1 inhibitor monotherapy [45, 46]. In a meta-analysis where PD-1 inhibitors and tyrosine kinase inhibitors were used as intervention measures for indirect comparison, it was demonstrated that the combination of PD-1/PD-L1 inhibitors with tyrosine kinase inhibitors can reduce the risk of disease progression and death, which is consistent with our results [47].
This network meta-analysis has several limitations. First, the studies included in the analysis were all retrospective studies, which leads to a lower level of evidence. Second, there was heterogeneity among the patient populations included in the analysis, with participants from different geographical regions and different ethnicities, with varying proportions of HBV infections. Furthermore, since the analysis involved various intervention measures, data for certain interventions were derived from a solitary study. This may have led to a relatively limited sample size, potentially introducing a source of bias in the findings.
Conclusion
Furthermore, the best outcome in PFS was found in the TLP regimen. In predicting the best PFS, the TLP still holds the highest result. The combination of TACE, Lenvatinib, and PD-1/L1 inhibitors appears to provide the maximum benefit for inoperable HCC patients. The combination of TACE, Lenvatinib, and PD-1/L1 inhibitors appears to provide the maximum benefit for inoperable HCC patients.
Availability of data and materials
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
An L, Zheng R, Zhang S, Chen R, Wang S, Sun K, et al. Hepatocellular carcinoma and intrahepatic cholangiocarcinoma incidence between 2006 and 2015 in China: estimates based on data from 188 population-based cancer registries. Hepatobiliary Surg Nutr. 2023;12:45–55.
World Health Organization (WHO). Global Health Estimates. Deaths by cause, age, sex, by country and by region, 2000–2019. WHO. 2020;2020(11):2020.
Nuño Solinís R, Arratibel Ugarte P, Rojo A, Sanchez GY. Value of treating all stages of chronic hepatitis C: a comprehensive review of clinical and economic evidence. Infect Dis Ther. 2016;5:491–508.
Lin H, Luo B, Peng F, Fang C, Gan Y, Yang X, et al. The efficacy of transarterial chemoembolization in downstaging unresectable hepatocellular carcinoma to curative therapy: a predicted regression model. Invest New Drugs. 2022;40:1146–52.
Chakraborty E, Sarkar D. Emerging therapies for hepatocellular carcinoma (HCC). Cancers (Basel). 2022. https://doi.org/10.3390/cancers14112798.
Cerreto M, Cardone F, Cerrito L, Stella L, Santopaolo F, Pallozzi M, et al. The new era of systemic treatment for hepatocellular carcinoma: from the first line to the optimal sequence. Curr Oncol. 2023;30:8774–92.
Wang Y, Li M, Zhang Z, Gao M, Zhao L. Application of radiomics in the efficacy evaluation of transarterial chemoembolization for hepatocellular carcinoma: a systematic review and meta-analysis. Acad Radiol. 2023. https://doi.org/10.1016/j.acra.2023.08.001.
Tan ZB, Zhang J. Recent advances in treatment strategies for hepatocellular carcinoma with portal vein cancer thrombus. Eur Rev Med Pharmacol Sci. 2023;27:8119–34.
Borriello R, Cerrito L, Gasbarrini A, Ponziani FR. Pharmacokinetic considerations for angiogenesis inhibitors used to treat hepatocellular carcinoma: an overview. Expert Opin Drug Metab Toxicol. 2023. https://doi.org/10.1080/17425255.2023.2272598.
Xie D, Shi J, Zhou J, Fan J, Gao Q. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: a Chinese perspective. Clin Mol Hepatol. 2023;29:206–16.
Pasqualotto E, Moraes FCA, Chavez MP, Souza MEC, Rodrigues ALSO, Ferreira ROM, et al. PD-1/PD-L1 Inhibitors plus chemotherapy versus chemotherapy alone for resectable non-small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Cancers (Basel). 2023;15:5143.
Mahdiabadi S, Momtazmanesh S, Karimi A, Rezaei N. Immune checkpoint inhibitors in advanced cutaneous melanoma: a systematic review and meta-analysis of efficacy and review of characteristics. Expert Rev Anticancer Ther. 2023;23:1281–93.
Zhang C, Yang Q. Predictive values of programmed cell death-ligand 1 expression for prognosis, clinicopathological factors, and response to programmed cell death-1/programmed cell death-ligand 1 inhibitors in patients with gynecological cancers: a meta-analysis. Front Oncol. 2021;10: 572203.
Huang D, Zhang J, Zheng X, Gao M. Efficacy and safety of lenvatinib in anaplastic thyroid carcinoma: a meta-analysis. Front Endocrinol (Lausanne). 2022;13: 920857.
Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45.
Shimose S, Iwamoto H, Niizeki T, Tanaka M, Shirono T, Moriyama E, et al. Efficacy of lenvatinib combined with transcatheter intra-arterial therapies for patients with advanced-stage of hepatocellular carcinoma: a propensity score matching. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms241813715.
Yang H, Yang T, Qiu G, Liu J. Efficacy and safety of TACE combined with lenvatinib and PD-(L)1 inhibitor in the treatment of unresectable hepatocellular carcinoma: a retrospective study. J Hepatocell Carcinoma. 2023;10:1435–43.
Zhao C, Xiang Z, Li M, Wang H, Liu H, Yan H, et al. Transarterial chemoembolization combined with atezolizumab plus bevacizumab or lenvatinib for unresectable hepatocellular carcinoma: a propensity score matched study. J Hepatocell Carcinoma. 2023;10:1195–206.
Lang M, Gan L, Ren S, Han R, Ma X, Li G, et al. Lenvatinib plus sintilimab with or without transarterial chemoembolization for intermediate or advanced stage hepatocellular carcinoma: a propensity score-matching cohort study. Am J Cancer Res. 2023;13:2540–53.
Wang J, Zhao M, Han G, Han X, Shi J, Mi L, et al. Transarterial chemoembolization combined with PD-1 inhibitors plus lenvatinib showed improved efficacy for treatment of unresectable hepatocellular carcinoma compared with PD-1 inhibitors plus lenvatinib. Technol Cancer Res Treat. 2023;22:15330338231166764.
Xie QY, Huang LP, Gao FW, Liu DQ, Wang X, Jiang KY, et al. Efficacy of lenvatinib combined with sequential transarterial chemoembolization for primary hepatocellular carcinoma and the effects on serum basic fibroblast growth factor and vascular endothelial growth factor. Front Pharmacol. 2022;13: 965770.
Guo P, Pi X, Gao F, Li Q, Li D, Feng W, et al. Transarterial chemoembolization plus lenvatinib with or without programmed death-1 inhibitors for patients with unresectable hepatocellular carcinoma: a propensity score matching study. Front Oncol. 2022;12: 945915.
Sun B, Zhang L, Sun T, Ren Y, Cao Y, Zhang W, et al. Safety and efficacy of lenvatinib combined with camrelizumab plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a two-center retrospective study. Front Oncol. 2022;12: 982948.
Chen YX, Zhang JX, Zhou CG, Liu J, Liu S, Shi HB, et al. Comparison of the efficacy and safety of transarterial chemoembolization with or without lenvatinib for unresectable hepatocellular carcinoma: a retrospective propensity score-matched analysis. J Hepatocell Carcinoma. 2022;9:685–94.
Xia D, Bai W, Wang E, Li J, Chen X, Wang Z, et al. Lenvatinib with or without concurrent drug-eluting beads transarterial chemoembolization in patients with unresectable, advanced hepatocellular carcinoma: a real-world, multicentre retrospective study. Liver Cancer. 2022;11:368–82.
Qu S, Zhang X, Wu Y, Meng Y, Pan H, Fang Q, et al. Efficacy and safety of TACE combined with lenvatinib plus PD-1 inhibitors compared with tace alone for unresectable hepatocellular carcinoma patients: a prospective cohort study. Front Oncol. 2022;12: 874473.
Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13: 848387.
Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15:663–75.
Ando Y, Kawaoka T, Amioka K, Naruto K, Ogawa Y, Yoshikawa Y, et al. Efficacy and safety of lenvatinib–transcatheter arterial chemoembolization sequential therapy for patients with intermediate-stage hepatocellular carcinoma. Oncology. 2021;99:507–17.
Wang YY, Yang X, Wang YC, Long JY, Sun HS, Li YR, et al. Clinical outcomes of lenvatinib plus transarterial chemoembolization with or without programmed death receptor-1 inhibitors in unresectable hepatocellular carcinoma. World J Gastroenterol. 2023;29:1614–26.
Fan W, Zhu B, Yue S, Zheng X, Zou X, Li F, et al. Idarubicin-loaded DEB-TACE plus Lenvatinib versus lenvatinib for patients with advanced hepatocellular carcinoma: a propensity score-matching analysis. Cancer Med. 2023;12:61–72.
Kuroda H, Oikawa T, Ninomiya M, Fujita M, Abe K, Okumoto K, et al. Objective response by mRECIST to initial lenvatinib therapy is an independent factor contributing to deep response in hepatocellular carcinoma treated with lenvatinib–transcatheter arterial chemoembolization sequential therapy. Liv Cancer. 2022;11:383–96.
Liu J, Liao HJ, Yuan GS, Liang MF, Huang W, Gao XP, et al. Clinical efficacy and safety analysis of hepatic artery chemoembolisation combined with lenvatinib in the treatment of intermediate and advanced hepatocellular hepatocellular carcinoma. J Pract Hosp Clin. 2022;19:55–9.
Zhu F, Wei J, Zhao H, Jiang W, Zhu CD. Clinical efficacy of TACE with lenvatinib and karelizumab in advanced or unresectable hepatocellular carcinoma. 2022;41:557–61.
Liu JY, Hu B, Zhao L, Qiu HM, Zou JN, Yang B, et al. Efficacy of TACE with or without lenvatinib and PD-1 inhibitor in the treatment of unresectable hepatocellular carcinoma and its effect on blood supplying arteries. J Clin Radiol. 2023;42:665–71.
Liu YH, Lin YS, Yang RH, Zhong SB. Effect of chemoembolisation of hepatic artery with lenvatinib on recent efficacy T-lymphocyte immune function and prognosis in patients with intermediate and advanced primary hepatocellular carcinoma. J Clin Misdiagn Mistreat. 2022;35:60–3.
Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387:251–72.
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14.
Chan LL, Chan SL. The evolving role of lenvatinib at the new era of first-line hepatocellular carcinoma treatment. Clin Mol Hepatol. 2023;29:909–23.
Girardi DM, Sousa LP, Miranda TA, Haum FNC, Pereira GCB, Pereira AAL. Systemic therapy for advanced hepatocellular carcinoma: current stand and perspectives. Cancers (Basel). 2023. https://doi.org/10.3390/cancers15061680.
Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73.
Yu X, Wei C, Cui R, Jiang O. Lenvatinib plus Immune checkpoint inhibitors versus lenvatinib monotherapy as treatment for advanced hepatocellular carcinoma: a meta-analysis. Int J Clin Exp Pathol. 2023;16(11):321–31.
Carloni R, Sabbioni S, Rizzo A, Ricci AD, Palloni A, Petrarota C, et al. Immune-based combination therapies for advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2023;10:1445–63.
Rimassa L, Finn RS, Sangro B. Combination immunotherapy for hepatocellular carcinoma. J Hepatol. 2023;79:506–15.
Liu Q, Li R, Li L, Wang G, Ji S, Zheng X, et al. Efficacy and safety of anti-PD-1 monotherapy versus anti-PD-1 antibodies plus lenvatinib in patients with advanced hepatocellular carcinoma: a real-world experience. Ther Adv Med Oncol. 2023;15:17588359231206274.
Stefanini B, Ielasi L, Chen R, Abbati C, Tonnini M, Tovoli F, et al. TKIs in combination with immunotherapy for hepatocellular carcinoma. Expert Rev Anticancer Ther. 2023;23:279–91.
Liu J, Wang P, Shang L, Zhang Z, Tian Y, Chen X, et al. TACE plus tyrosine kinase inhibitors and immune checkpoint inhibitors versus TACE plus tyrosine kinase inhibitors for the treatment of patients with hepatocellular carcinoma: a meta-analysis and trial sequential analysis. Hepatol Int. 2023. https://doi.org/10.1007/s12072-023-10591-0.
Acknowledgements
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
No external funding received to conduct this study.
Author information
Authors and Affiliations
Contributions
Liang Y: writing—reviewing and editing. Liang Y, Gan L: writing—original draft preparation. Zeng D: searching articles. Lin L, Liao F, Wang A: making charts.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study is a literature review and does not require the informed consent of patients, and does not require ethical approval.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Y., Gan, L., Zeng, D. et al. Clinical efficacy of lenvatinib, trans-arterial chemoembolization, and PD-1/L1 inhibitors in advanced hepatocellular carcinoma: a systematic review and network meta-analysis. Clin Transl Oncol 26, 2652–2664 (2024). https://doi.org/10.1007/s12094-024-03458-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-024-03458-9