Abstract
We evaluated the efficacy and toxicity of T-cell-replete haploidentical stem cell transplantation (TCR-haploSCT) using low-dose antithymocyte globulin (ATG) in children with refractory/relapsed (R/R) acute leukemia. From October 2009 to April 2016, 39 consecutive patients with R/R acute leukemia who underwent TCR-haploSCT were included. At the time of TCR-haploSCT, 17 patients were in complete remission (CR), but 22 had active disease. Thirty-three patients received a myeloablative regimen and six received a reduced-intensity conditioning regimen. Graft-versus-host disease (GvHD) prophylaxis comprised tacrolimus, methotrexate, prednisolone, and low-dose ATG (thymoglobulin 2.5 mg/kg). Neutrophil engraftment (> 0.5 × 109/L) was 95% after a median of 13 days. The median follow-up period was 527 days, with mean 3-year overall and disease-free survival rates of 45.1% [standard deviation (SD), ± 8.5%) and 33.8% (SD, ± 7.9%), respectively. The cumulative incidence of acute GvHD was 73.0%, but that of grade III–IV acute GvHD was 34.1%. The 3-year cumulative incidences of relapse and transplant-related mortality were 50.3 and 15.9%, respectively. Age < 10 years at transplantation was associated with a better overall survival in the multivariate analysis. These data suggest that TCR-haploSCT using a low-dose ATG combined with the GvHD prophylaxis described here has a significant anti-leukemic activity, particularly in younger patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because of recent advances in the treatment of pediatric acute leukemia, the probability of 5-year leukemia-free survival is now as high as 80–90% in patients with acute lymphoblastic leukemia (ALL) [1] and 55% in those with acute myeloid leukemia (AML) [2]. However, some patients with refractory or relapsed disease are difficult to treat; the cure rates for such patients remain dismal. Among pediatric patients with relapsed acute leukemia treated with a combination of chemotherapy and allogeneic stem cell transplantation (SCT), a survival rate of 30–50% is expected [3,4,5]. However, patients who fail to achieve complete remission (CR) after relapse, experience primary induction failure, relapse after SCT, or relapse with an unfavorable cytogenetic risk factor, have an extremely poor prognosis [6, 7]. In those high-risk pediatric acute leukemia patients, allogeneic SCT is the most likely curative option [8, 9].
Historically, bone marrow transplantation from an HLA-matched donor reduces graft-versus-host disease (GvHD) and graft rejection [10]. With the high incidence of severe GvHD, the survival rate post-SCT decreases as the degree of HLA disparity increases [11]. However, the relative risk of relapse is lower in patients with acute or chronic GvHD [12]. Szydlo et al. reported that transplant-related mortality (TRM) was significantly higher after haploidentical relative or one antigen mismatched unrelated donor transplants than after HLA-identical sibling transplants [13]. The difficulties of acute GvHD and graft rejection associated with HLA-haploidentical stem cell transplantation (haploSCT) have been overcome using ex vivo T-cell-depleted CD34-positive (CD34+) peripheral blood stem cells (PBSCs) [14]. However, in high-risk patients, this method is associated with a high incidence of both infectious complications and relapse due to delayed T-cell recovery. With the development of improved immunosuppression/modulation methods including high-dose antithymocyte globulin (ATG) [15], alemtuzumab [16], or high-dose post-transplantation cyclophosphamide (PTCY) [17], haploSCT is becoming more widely used for patients who lack the conventional HLA-identical related donors or matched unrelated donors.
Recently, Ikegame et al. reported the result of a multicenter phase I/II study using reduced-intensity SCT with low-dose ATG [18]. They reported that the 1-year survival rate for patients who had not achieved CR at the time of undergoing transplantation was 42.3% and the cumulative incidence of grade II to IV acute GvHD was 30.7%. We also consider that T-cell-replete (TCR) haploSCT is a form of T-cell therapy with a high degree of efficacy in patients with hematologic malignancies, based on the allogeneic immune reaction.
We aimed to evaluate the efficacy of our TCR-haploSCT protocol to establish whether we were able to achieve an optimal graft-versus-leukemia (GvL) effect on T cells by performing TCR-haploSCT using myeloablative conditioning with intensive GvHD prophylaxis, including low-dose ATG.
Materials and methods
Patients
We retrospectively examined 39 consecutive pediatric patients with relapsed or refractory acute leukemia (R/R AL) who received TCR-haploSCT in our institution from October 2009 to April 2016. We included children with relapsed or refractory disease, defined by the presence of following criteria: (1) relapse after SCT; (2) very early or early relapse in ALL (< 36 months from the initial diagnosis); (3) failure to respond to ≥ 2 induction chemotherapy regimens; and (4) relapse with t(9; 22), monosomy 7, 5q-, FLT3-ITD and mixed lineage leukemia (MLL) gene rearrangement. Children with a Karnofsky performance score < 50 were not eligible for this study. The patients’ characteristics are summarized in Table 1.
This study was approved by the Research Ethics Committee of Fukushima Medical University. Written informed consent was obtained from the children’s guardians.
Donors and stem cell source at TCR-haploSCT
HLA genotyping was conducted using PCR-Luminex (Luminex Corporation, Austin, Texas), based on reverse sequence-specific oligonucleotide (PCR-rSSO) technology (Genosearch HLA, Medical & Biological Laboratories Co., Ltd., Nagoya, Japan) in our hospital. We intentionally selected the one-haploidentical donor even if an HLA-identical sibling donor was available. Family donors who were HLA-identical or had one allele mismatch in the HLA-A, B, C, and DR loci, were excluded. Both PBSCs and bone marrow cells were collected from family donors (15 mothers, 22 fathers, and 2 siblings) using standard mobilization protocols. G-CSF (400 μg/m2/day; Filgrastim, Kyowa Hakko Kirin Pharma Inc., Japan) was administered to the donors for 5 consecutive days to mobilize the stem cells into the peripheral blood. PBSC harvesting was initiated on days 4 and 5 after G-CSF administration. PBSC collection was performed using COBE Spectra or Spectra Optia (Terumo BCT, Tokyo, Japan). Thirty-six patients received G-CSF-mobilized PBSCs alone, while three patients received the graft with G-CSF-mobilized PBSC plus non-mobilized bone marrow.
Conditioning regimen and GvHD prophylaxis used for TCR-haploSCT
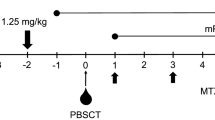
Myeloablative conditioning was administered to 33 patients (TBI-based for 19 and busulfan-based for 14 patients), whereas reduced-intensity conditioning was administered to 6 patients who had organ dysfunction or active infection. Reduced-intensity stem cell transplantation (RIST) was defined as a regimen consisting of 5 Gy or less of total body irradiation in a single fraction, or 8 Gy or less if using multiple fractions, 9 mg/kg or less of busulfan, and 140 mg/m2 or less of melphalan. Our GvHD prophylaxis method has been reported previously [19]. Briefly, GvHD prophylaxis comprised a combination of tacrolimus, methotrexate, and prednisolone for all patients. To prevent GvHD, all patients received ATG (thymoglobulin 1.25 mg/kg/day; Sanofi, Paris, France) intravenously for 2 consecutive days, on days − 2 to − 1. Prednisolone was started at a dose of 1 mg/kg/day in 2 divided doses from day 0. If there were no signs of acute GvHD, prednisolone was tapered every week from day + 29, and was discontinued at least 2–3 months after transplantation. Tacrolimus (0.03 mg/kg/day) was started intravenously on day − 1 with targeted trough levels of 8–15 ng/ml and 5–8 ng/ml before and after neutrophil engraftment, respectively, and was continued for 4 weeks. Thereafter, tacrolimus was given orally twice a day with a targeted trough level of 3–7 ng/ml. Methotrexate was given intravenously at a dose of 10 mg/m2 on day + 1, and at a dose of 7 mg/m2 on days + 3 and + 6. Donor–recipient chimerism was assessed through amplification of the polymorphic short tandem repeat region by either polymerase chain reaction (21 patients) or fluorescence in situ hybridization (FISH) analysis for the identification of sex chromosomes (17 patients) on bone marrow samples at day + 30, + 60, and + 90, after TCR-haploSCT.
Supportive care
Each patient was isolated in a laminar air-flow room and standard decontamination procedures were followed. Intravenous immunoglobulin was administered at a minimum dose of 100 mg/kg every week until day + 100. Trimethoprim/sulfamethoxazole was administered for at least 1 year as prophylaxis against Pneumocystis infections. Acyclovir was administered at 10 mg/kg for 35 post-transplantation to prevent herpes simplex virus infections. Peripheral blood was tested for CMV-pp65 antigen once weekly. After grafting, ganciclovir or foscarnet was initiated in patients when CMV antigen-positive cell numbers increased over 10/150,000 of white blood cells (CMV antigenemia). All patients received G-CSF intravenously from day + 1 until sustained neutrophil recovery was achieved.
Definitions and statistical analysis
Neutrophil engraftment was defined as an absolute neutrophil count of at least 0.5 × 109/L on 3 consecutive days, whereas platelet recovery was defined as a platelet count of at least 20 × 109/L without transfusion support. The diagnosis of acute and chronic GvHD was based on standard clinical criteria, and histopathologic confirmation was ensured, if possible. Overall survival was defined as the length of time from the TCR-haploSCT to death from any cause. Leukemia-free survival was defined as the length of time from the TCR-haploSCT to leukemia relapse and any cause of death. Overall survival and leukemia-free survival were calculated using the Kaplan–Meier method [20]. The cumulative incidences of relapse and TRM were estimated using competing risks by Gray’s method [21]. If no events occurred, patients were censored at day 1825. To evaluate the influence of factors on overall survival, the log-rank test and proportional hazards modelling were used for univariate and multivariate analyses. In the univariate analysis, the impact of acute GvHD was assessed by a landmark analysis that was limited to the patients who developed acute GvHD and survived without relapse for at least 80 days. Factors with a two-sided P value of < 0.10 in the univariate analysis were included in the multivariate analysis. For the multivariate analysis, a Cox proportional hazards model was used to select covariates. P values < 0.05 were considered statistically significant. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [22], which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
The patients’ characteristics are shown in Table 1. The median age of patients was 10.1 (range 0.3–19.4) years. The diagnoses included ALL (n = 25), AML (n = 12), and myeloid natural killer cell precursor leukemia (M/NKL) (n = 2). At the time of undergoing TCR-haploSCT, 17 patients were in CR (including 4 Philadelphia chromosome positive ALL patients who achieved hematologic CR but had positive bcr-abl mRNA by RT-PCR) and 22 had active disease. The following HLA disparities were observed: 2/8 in 3, 3/8 in 11, and 4/8 in 25 patients. Donors comprised 22 fathers, 15 mothers, and 2 siblings. Thirty-three patients received myeloablative conditioning (TBI-based: 19, busulfan-based: 14) and all received a rabbit ATG (thymoglobulin 2.5 mg/kg) containing regimen for in vivo T-cell depletion/modulation. Seventeen patients received allogeneic SCT prior to TCR-haploSCT. Disease status and genetic aberrations of leukemic cells are shown in Table 2. Eighty-three percent of AML patients were active disease at TCR-haplo-SCT. It was significantly high incidence compared to ALL patients (P = 0.03). Among 25 patients of ALL, all seven patients of Philadelphia chromosome positive ALL were in complete remission using tyrosine kinase inhibitor; however, four patients still positive bcr-abl mRNA in peripheral blood.
Engraftment and chimerism
Patients received a median of 9.7 × 106 CD34 + hematopoietic cells/kg (range 1.7–52.3 × 106 cells/kg) and 5.0 × 108 CD3-positive (CD3+) T cells/kg (range 1.8–10.5 × 108 cells/kg). The median durations required to achieve neutrophil engraftment and platelet engraftment were 13 (range 10–15) days and 23.5 (range 7–93) days, respectively. Overall, 37 (95%) patients achieved primary neutrophil engraftment. On day + 30, near full donor chimerism was documented in 34 of 38 evaluable patients. Two patients showed donor-type graft failure.
Acute GvHD and chronic GvHD
Acute GvHD was observed in 28 of 37 patients (grade I: 2, II: 15, III: 10, IV: 1) and chronic GvHD was observed in 22 of 31 patients. Six patients developed severe chronic GvHD. The cumulative incidences of acute GvHD and severe GvHD (grade III to IV) were 73.0 and 34.1%, respectively (Fig. 1a, b). The 1-year cumulative incidence of chronic GvHD was 71%.
Toxicity, relapse, and cause of death
The cumulative incidence of relapse at 3 years was 50.3% (95% CI 33.0–65.4) (Fig. 2a), and the TRM rate was 15.9% (95% CI 6.3–29.5). Of the 39 patients, 6 died because of transplantation-related toxicities. The causes of death were as follows: aspergillosis, based on grade IV acute GvHD (n = 1); EBV-associated lymphoproliferative disease (n = 1); invasive aspergillosis (n = 2); graft failure (n = 1); and, pseudomonas pneumoniae, based on grade III intestinal acute GvHD (n = 1). The 3-year cumulative incidence of relapse was 46% (95% CI 20.8–68.1) in patients < 10 years old and 55.0% (95% CI 29.9–74.4) in those ≥ 10 years old (P = 0.597). However, the 3-year cumulative incidence of TRM was 0% in patients < 10 years old (95% CI 0–0) and 30% (95% CI 11.3–51.5) in those ≥ 10 years old. The difference of the cumulative incidence of TRM between these age groups was statistically significant (P = 0.0128).
Survival and prognostic factors
Of the 16 patients who survived to the end of the study’s follow-up period, 13 achieved leukemia-free survival. As shown in Fig. 2b, over a median follow-up period of 547 days (range 59–1825 days), the probability of leukemia-free survival was 48.7% [standard deviation (SD), 8.0] at 1 year, 33.8% (SD, 7.9) at 2 years and 33.8% (SD, ± 7.9%) at 3 years; the probability of overall survival was 63.9% (SD, 7.7) at 1 year, 54.8% (SD, 8.2) at 2 years, and 45.1% (SD, ± 8.5%) at 3 years. Three of the 4 patients with hematologic CR but who was bcr-abl-positive survived without disease progression.
Several predictive factors for overall survival were evaluated using univariate and multivariate analyses. Donor sex mismatch, disease type, disease status, age at transplantation, and infused CD3+ cell dose were significant prognostic factors in the univariate analysis (Table 3). The 3-year overall survival rate of patients with donor sex mismatch was 70.0% (95% CI 45.1–85.3) vs 23.0% (95% CI 7.2–43.9) among patients without mismatch (P = 0.02). According to disease status, the 3-year overall survival rate was significantly different between patients who underwent TCR-haploSCT while in CR or with active disease [80.2% (95% CI 49.6–93.3) vs. 19.8% (95% CI 6.3–38.9), respectively; P < 0.001]. Low CD3+ cell dose of the graft was also a poor prognostic factor [3-year overall survival CD3+ cell ≥ 5.0 × 108/kg: 66.7% (95% CI 39.6–83.7) vs < 5.0 × 108/kg: 23.4% (95% CI 7.4–44.4), P = 0.01]. Younger age at TCR-haploSCT was associated with a more favorable overall survival rate [3-year overall survival < 10 years old: 77.2% (95% CI 49.5–90.9) vs. ≥ 10 years old: 16.5% (95% CI 4.1–36.1); P < 0.001]. The 3-year leukemia-free survival in the younger age group was also significantly better than that of older age group [3-year leukemia-free survival of patients < 10 years old: 54.0% (95% CI 28.2–74.1) vs. ≥ 10 years old: 15.0% (95% CI 3.7–33.5); P = 0.008]. Other factors, including acute GvHD, chronic GvHD, number of transplantations, disease type (AML vs. ALL), and conditioning regimens were not significantly associated with overall survival. In the multivariate analysis, younger age at TCR-haploSCT (< 10 years old) was significantly associated with better overall survival (Table 4).
Discussion
The GvL effect was elicited mainly by donor lymphocyte targeting for allogeneic antigen based on the HLA differences between donor and recipient. However, these lymphocyte reactions are not specific to leukemia cells and often cause severe GvHD as well as graft rejection. Anasetti et al. reported that the rate of primary graft failure was correlated with the degree of HLA incompatibility—it was 12.3% among bone marrow transplant recipients whose donors had only one identical haplotype, compared with 2.0% among recipients whose donors were HLA-identical siblings [23]. They also reported that the incidence of severe acute GvHD was associated with the degree of recipient HLA incompatibility [11]. The survival rate decreased as the degree of HLA disparity increased, but the relative risk of relapse was significantly lower after SCT from an HLA-mismatched sibling than from an HLA-identical sibling among patients with acute leukemia [11]. With improved immunosuppression achieved using ATG, alemtuzumab, and PTCY, haploSCT has become an acceptable procedure over the past decade [15,16,17]. It has been established that haploSCT provides an opportunity for patients to benefit from SCT through an alternative donor source when an HLA-matched donor is not available. On the other hand, haploSCT is associated with a stronger GvL effect than is transplantation from an HLA-matched donor [24].
In vivo T-cell-depletion by ATG is a popular strategy for preventing GvHD in patients undergoing haploSCT. However, the optimal dose of ATG is controversial. High-dose ATG could reduce the incidence of acute GvHD, but increases the incidence of leukemia relapse and infection. At high concentrations (0.1–1.0 mg/mL), ATG induces cell lysis of both resting and activated T cells via human classic complement pathway activation. In contrast, at low concentrations, ATG induces apoptosis of activated cells (by Fas/Fas-ligand interaction) but not of resting cells [25]. To achieve partial in vivo T-cell-depletion, we employed low-dose ATG (thymoglobulin 2.5 mg/kg). Therefore, our GvHD prophylaxis using low-dose ATG may be effective because of selective elimination of highly activated donor-specific alloreactive T cells, with sparing of non-activated T cells. Liu et al. reported that high-dose ATG (thymoglobulin: 10 mg/kg) increased the risk of EBV reactivation compared with low-dose ATG (6 mg/kg) in haploSCT because of the delay in T-cell recovery [26].
In this study, 6 of the 39 patients died from transplant-related causes and the cumulative incidence of TRM was 15.9%; 1 of these patients died from EBV reactivation and 3 from aspergillosis. These deaths occurred early in the study period. Based on those experiences, we employed intensive surveillance for EBV viral load using PCR to ensure the early detection of viral reactivation. Moreover, we tested for Aspergillus antigen once a week at least, and performed surveillance chest computed tomography. Thereafter, no other patient experienced severe EBV reactivation. We also adopted the use of voriconazole to prevent break-through infection with Aspergillus. Several reports have shown that GvHD is associated with a reduced risk of relapse after SCT for hematologic malignancy due to the GvL effect [11, 12, 27]. Recently, O’Hare et al. reported outcomes of 44 children who underwent SCT for refractory AML in the United Kingdom [28]. Their data demonstrated remarkably good outcomes with 5-year overall and leukemia-free survival rates of 43%. From their data, patients who developed acute GvHD showed significantly superior leukemia-free survival rates than those who did not (56 vs. 30%, respectively, P = 0.043). Moreover, the 6-year leukemia-free survival rate of patients who received myeloablative conditioning and developed acute GvHD was excellent (61%) in comparison with patients who received reduced-intensity conditioning and/or did not develop acute GvHD (26%). In the current study, 33 (85%) patients received TCR-haploSCT by myeloablative conditioning. We would like to note that myeloablative conditioning was well tolerated, especially in the younger age group (< 10 years old), without TRM. However, 2 patients died from TRM and 3 patients experienced leukemia relapse among the 6 total patients who had reduced-intensity conditioning. Therefore, we believe that reduced-intensity conditioning is not appropriate for extremely high-risk patients without any organ dysfunction. Moreover, to maximize the GvL effect and facilitate engraftment, very high doses CD3+ cells (median 5.04 × 108/kg) and CD34+ cells (median 8.9 × 106/kg) were infused in this study. This was associated with early neutrophil engraftment and a high incidence of GvHD. In fact, the cumulative incidence of acute GvHD was 73.0%, while that of severe grade III–IV GvHD was 34.1%. Only 2 patients died as a result of severe acute GvHD. In general, it was possible to manage acute GvHD with an escalating dose of steroids, methotrexate, or additional low-dose ATG. In the current study, the survival of patients who received higher doses of CD3+ cells was excellent, although it is difficult to elucidate the relationship between CD3+ cell count and survival, because the study was retrospective and included a very small number of patients.
In 2013, Liu et al. reported the results of their trial concerning unmanipulated haploSCT using ATG (7.5 mg/kg) in 212 pediatric patients with acute leukemia [29]. They reported that the 5-year leukemia-free survival rates in first CR, second CR, were 68.9 and 56.6%, respectively, for children with for ALL. However, survival rates beyond second CR or in patients with non-remission were dismal (22.2%). Sawada et al. reported dismal outcomes of 8 patients with R/R AL: The researchers used a modified PTCY approach on day + 3 alone [30]. Jaiswal et al. also reported an overall survival rate of 64.3% for high-risk pediatric patients with leukemia after haploSCT based on PTCY. They demonstrated that acute GvHD developed in 80% of patients < 10 years old compared with only 13.3% in those aged 10–20 years (P = 0.001), despite similar graft composition and significantly higher TRM (60 vs. 0%, respectively; P = 0.001) [31]. In the present study, younger (< 10 years old) rather than older children had excellent survival rates. Therefore, we considered that the better survival rate of the younger patient group was related to the low incidence of TRM in these patients. To control alloreactivity of the graft, low-dose ATG might be better for younger patients whereas PTCY may be more suitable for older patients.
In conclusion, our data indicate that TCR-haploSCT following low-dose ATG-containing conditioning combined with our GvHD prophylaxis is well tolerated, facilitates engraftment, and has significant anti-leukemic activity, particularly in younger pediatric patients with R/R AL. However, this was a retrospective study involving a small cohort from a single institution, and additional evaluations are needed in prospective clinical trials.
Abbreviations
- SCT:
-
Stem cell transplantation
- ATG:
-
Antithymocyte globulin
- R/R AL:
-
Refractory/relapsed acute leukemia
- TCR:
-
T-cell-replete
- GvHD:
-
Graft-versus-host disease
- GvL:
-
Graft-versus-leukemia
- PTCY:
-
Post-transplantation cyclophosphamide
- MLL:
-
Mixed lineage leukemia
- CMV:
-
Cytomegalovirus
- G-CSF:
-
Granulocyte-colony stimulating factor
- TRM:
-
Transplant-related mortality
- PBSCs:
-
Peripheral blood stem cells
- CR:
-
Complete remission
- FLT3-ITD:
-
FMS like tyrosine kinase- internal tandem duplication
- M/NKL:
-
Myeloid natural killer cell precursor leukemia
- ALL:
-
Acute lymphoblastic leukemia
- AML:
-
Acute myeloid leukemia
- HLA:
-
Human leukocyte antigen
- EBV:
-
Epstein–Barr virus
- SD:
-
Standard deviation
References
Pui CH, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33:2938–48.
Rubnitz JE, Inaba H. Childhood acute myeloid leukaemia. Br J Haematol. 2012;159:259–76.
Tallen G, Ratei R, Mann G, Kaspers G, Niggli F, Karachunsky A, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28:2339–47.
Parker C, Waters R, Leighton C, Hancock J, Sutton R, Moorman AV, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376:2009–17.
Nakayama H, Tabuchi K, Tawa A, Tsukimoto I, Tsuchida M, Morimoto A, et al. Outcome of children with relapsed acute myeloid leukemia following initial therapy under the AML99 protocol. Int J Hematol. 2014;100:171–9.
Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. 2005;131:579–87.
Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–8.
Balduzzi A, Valsecchi MG, Uderzo C, De Lorenzo P, Klingebiel T, Peters C, et al. Chemotherapy versus allogeneic transplantation for very-high-risk childhood acute lymphoblastic leukaemia in first complete remission: comparison by genetic randomisation in an international prospective study. Lancet. 2005;366:635–42.
Kobayashi S, Ito M, Sano H, Mochizuki K, Akaihata M, Waragai T, et al. T-cell-replete haploidentical stem cell transplantation is highly efficacious for relapsed and refractory childhood acute leukaemia. Transfus Med. 2014;24:305–10.
Armitage JO. Bone marrow transplantation. N Engl J Med. 1994;330:827–38.
Anasetti C, Beatty PG, Storb R, Martin PJ, Mori M, Sanders JE, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990;29:79–91.
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62.
Szydlo R, Goldman JM, Klein JP, Gale RP, Ash RC, Bach FH, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–77.
Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–54.
Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107:3065–73.
Rizzieri DA, Koh LP, Long GD, Gasparetto C, Sullivan KM, Horwitz M, et al. Partially matched, nonmyeloablative allogeneic transplantation: clinical outcomes and immune reconstitution. J Clin Oncol. 2007;25:690–7.
O’Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–86.
Ikegame K, Yoshida T, Yoshihara S, Daimon T, Shimizu H, Maeda Y, et al. Unmanipulated haploidentical reduced-intensity stem cell transplantation using fludarabine, busulfan, low-dose antithymocyte globulin, and steroids for patients in non-complete remission or at high risk of relapse: a prospective multicenter phase I/II study in Japan. Biol Blood Marrow Transplant. 2015;21:1495–505.
Mochizuki K, Kikuta A, Ito M, Sano H, Akaihata M, Kobayashi S, et al. Feasibility of tacrolimus, methotrexate, and prednisolone as a graft-versus-host disease prophylaxis in non-T-cell-depleted haploidentical hematopoietic stem cell transplantation for children. Clin Transplant. 2011;25:892–7.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Anasetti C, Amos D, Beatty PG, Appelbaum FR, Bensinger W, Buckner D, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320:197–204.
Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH, et al. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transpl. 2011;17:821–30.
Genestier L, Fournel S, Flacher M, Assossou O, Revillard JP, Bonnefoy-Berard N. Induction of Fas (Apo-1, CD95)-mediated apoptosis of activated lymphocytes by polyclonal antithymocyte globulins. Blood. 1998;91:2360–8.
Liu J, Xu LP, Bian Z, Chang YJ, Wang Y, Zhang XH, et al. Differential impact of two doses of antithymocyte globulin conditioning on lymphocyte recovery upon haploidentical hematopoietic stem cell transplantation. J Transl Med. 2015;13:391. https://doi.org/10.1186/s12967-015-0748-x.
Weisdorf D, Zhang MJ, Arora M, Horowitz MM, Rizzo JD, Eapen M. Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biol Blood Marrow Transpl. 2012;18:1727–33.
O’Hare P, Lucchini G, Cummins M, Veys P, Potter M, Lawson S, et al. Allogeneic stem cell transplantation for refractory acute myeloid leukemia in pediatric patients: the UK experience. Bone Marrow Transpl. 2017. https://doi.org/10.1038/bmt.2017.3 (e-pub ahead of print 20 February 2017).
Liu DH, Xu LP, Liu KY, Wang Y, Chen H, Han W, et al. Long-term outcomes of unmanipulated haploidentical HSCT for paediatric patients with acute leukaemia. Bone Marrow Transpl. 2013;48:1519–24.
Sawada A, Shimizu M, Isaka K, Higuchi K, Mayumi A, Yoshimoto Y, et al. Feasibility of HLA-haploidentical hematopoietic stem cell transplantation with post-transplantation cyclophosphamide for advanced pediatric malignancies. Pediatr Hematol Oncol. 2014;31:754–64.
Jaiswal SR, Chakrabarti A, Chatterjee S, Ray K, Chakrabarti S. Haploidentical transplantation in children with unmanipulated peripheral blood stem cell graft: the need to look beyond post-transplantation cyclophosphamide in younger children. Pediatr Transplant. 2016;20:675–82.
Acknowledgements
We wish to thank all the clinicians and nurses who helped to support the care of patients in this study. We also thank the technicians at the Department of Blood Transfusion and Transplantation Immunology, Fukushima Medical University Hospital, especially Mr. Satoshi Ono for his help with the HLA typing and Mr. Shunichi Saito for CD34+ and CD3+ cell assays. We would like to thank Editage (http://www.editage.ip) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
About this article
Cite this article
Sano, H., Mochizuki, K., Kobayashi, S. et al. T-cell-replete haploidentical stem cell transplantation using low-dose antithymocyte globulin in children with relapsed or refractory acute leukemia. Int J Hematol 108, 76–84 (2018). https://doi.org/10.1007/s12185-018-2423-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-018-2423-5