Abstract
The prognosis of relapsed/refractory (R/R) pediatric acute leukemia is extremely poor. We retrospectively reviewed 20 consecutive pediatric patients with R/R acute leukemia who underwent a first HLA-haploidentical peripheral blood stem cell transplantation following reduced-intensity conditioning (haplo-RIC-PBSCT) with very low-dose antithymocyte globulin (ATG) between 2012 and 2019. Of these 20 patients, 7 patients had acute lymphoblastic leukemia, and 13 had acute myeloid leukemia. At the time of haplo-RIC-PBSCT, 15 patients had active disease. The median follow-up duration for survivors was 56 months (range 22–108 months). Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus, short-term methotrexate, methylprednisolone, and ATG 1.25 mg/kg on day-2. The 2-year cumulative incidence of transplant-related mortality and relapse were 5.0% [95% confidence interval (CI) 0.7–30.5%)] and 57.8% (95% CI 37.4–79.6%), respectively. Among the 20 patients, 16 (80.0%) developed grade III–IV acute GVHD, and 2 developed severe chronic GVHD. The 2-year event-free survival and overall survival rates were 40.0% (95% CI 19.3–60.0%) and 50.0% (95% CI 27.1–69.2%), respectively. Although the sample size is small, the survival outcomes of the present study are encouraging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prognosis for pediatric acute leukemia has recently been improving, with a high overall survival rate up of to approximately 90% in acute lymphoblastic leukemia (ALL) [1,2,3] and approximately 70% in acute myeloid leukemia (AML) [4]. However, the prognosis of relapsed/refractory (R/R) pediatric acute leukemia, such as primary induction failure or relapse after allogeneic hematopoietic stem cell transplantation (allo-SCT), is extremely poor. One of the most curable options for R/R disease is allo-SCT, especially HLA-haploidentical hematopoietic stem cell transplantation (haplo-SCT) with intension to induce graft-versus-leukemia (GVL) effect. However, a high potential for the GVL effect also means having a high potential for graft-versus-host disease (GVHD), which is a leading cause of morbidity and mortality in allo-SCT. This dilemma makes it difficult to determine the optimal GVHD prophylaxis that preserves the proper GVL effect. GVHD prophylaxis of haplo-SCT is generally performed by T-cell depletion using ex vivo procedures, such as CD34 positive selection [5], CD3/CD19 negative selection [6,7,8], αβTCR/CD19 negative selection [9, 10] and so on, or in vivo procedures, such as post-transplant cyclophosphamide (PTCy) [11], antithymocyte globulin (ATG) [12,13,14], and alemtuzumab [15].
For R/R acute leukemia in pediatric patients, we conducted HLA-haploidentical peripheral blood stem cell transplantation following reduced-intensity conditioning (haplo-RIC-PBSCT), in which GVHD prophylaxis was performed with very low-dose ATG.
Patients and methods
Study design and inclusion criteria
We retrospectively reviewed 20 consecutive pediatric (under 18 years of age) patients with R/R acute leukemia who underwent a first haplo-RIC-PBSCT with very low-dose ATG between 2012 and 2019. In this analysis, ‘relapsed’ is defined as relapsed following previous allo-SCT, which is non-haplo-RIC-PBSCT; and ‘refractory’ is defined as resistant to chemotherapy and not in hematological complete remission (CR) without prior allo-SCT. Unless they did not have haploidentical donor, all patients who meet above inclusion criteria received haplo-RIC-PBSCT, even if they had related/unrelated HLA-matched suitable donor. This study was approved by the institutional review board at Osaka Women’s and Children’s Hospital. All patients or their guardians gave written informed consent.
haplo-RIC-PBSCT procedure
We use reduced-intensity conditioning instead of myeloablative conditioning for all patients regardless of their performance status or organ conditions, emphasizing on preventing conditioning-associated complications. The conditioning regimen mainly consisted of fludarabine (180 mg/m2)/clofarabine (200 mg/m2), melphalan (140 or 210 mg/m2), and etoposide (200 mg/m2). We had used fludarabine-containing regimen until 2013. Since 2014, we have been using clofarabine-containing regimen. Almost all patients received about 1–2 weeks of low-dose etoposide (30 mg/m2/day, civ) and cytarabine (20 mg/m2/day, civ) (LDEC) [16] and/or high-dose cytarabine (6–12 g/m2) (HDCA) just prior to the conditioning regimen to stabilize or reduce the leukemic burden. Initially we started using LDEC regardless of disease status. Since August 2018, in principle, we have been using LDEC for those in CR and HDCA for those with active disease, with some exceptions at the discretion of attending physician. All patients received HLA-haploidentical peripheral blood stem cell (PBSC) grafts from related donors. Starting on day-4, each donor was given granulocyte colony-stimulating factor (G-CSF) (Filgrastim 400 μg/m2/day or Lenograstim 10 μg/kg/day) for 4 or 5 consecutive days and received apheresis with the goal of collecting at least 2 × 106 CD34+/kg of recipient body weight. Nineteen donors received single apheresis on day 0; one donor received twice apheresis on days 0 and 1. As shown in Fig. 1, GVHD prophylaxis consisted of tacrolimus (TAC) (except 1 patient who received cyclosporine) from day-1, short-term methotrexate (7.5 mg/m2/dose on days 1, 3, and 6), methylprednisolone (mPSL) (1 mg/kg/day) from day1, and ATG (thymoglobuline, 1.25 mg/kg) on day-2. To prevent the anaphylaxis caused by ATG, mPSL was administered at 500 mg/m2 on day-2 and at 250 mg/m2 on day-1. The target trough level of TAC was 5–10 ng/mL until around day 30; thereafter, TAC and mPSL were tapered.
Confirmation of donor engraftment
Donor engraftment was mainly confirmed by polymerase chain reaction amplification of the polymorphic short tandem repeat region. For sex-mismatched transplantation, donor engraftment was also confirmed by XY fluorescence in situ hybridization.
Supportive care
During the neutropenic period, each patient was isolated in a laminar air-flow room, prophylactic antibiotics and antifungal agents were administered, and standard decontamination procedures were followed. All patients received trimethoprim/sulfamethoxazole and acyclovir to prevent Pneumocystis jirovecii pneumonia and human herpes simplex infection, respectively. Cytomegalovirus (CMV) antigenemia was monitored every week after engraftment, and patients were administered ganciclovir when the antigen-positive cell number was ≥ 10/150,000 white blood cells. G-CSF was administered from day 5 to when the absolute neutrophil count exceeded 5000/µL.
Definitions and statistical analysis
Neutrophil engraftment was defined as an absolute neutrophil count of 500/µL or more on 3 consecutive days. Full donor chimerism was defined as ≥ 95% of donor-type white blood cells in peripheral blood. Acute GVHD (aGVHD) was graded according to standard criteria [17], while chronic GVHD (cGVHD) was graded according to National Institutes of Health (NIH) criteria [18].
The cumulative incidence of relapse and transplant-related mortality were estimated using Gray’s method. The event-free survival (EFS) and overall survival (OS) were estimated using the Kaplan–Meier method. EFS was defined as the duration from haplo-RIC-PBSCT to any relapse or transplantation-related mortality. OS was defined as the duration from haplo-RIC-PBSCT to death. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics [19].
Results
Patient characteristics
Patient characteristics are shown in Table 1. The median age at the time of haplo-RIC-PBSCT was 9.5 years (range 1–17 years). Thirteen patients were male, and 7 were female. All 20 patients had good performance status, having Eastern Cooperative Oncology Group performance status score of 0 or 1. The median follow-up duration was 16 months (range 1–108 months), and the median duration of survivor follow-up was 56 months (range 22–108 months). Of the 20 patients, 7 patients had ALL (B lineage: n = 5, T lineage: n = 2), and 13 had AML, including M0 (n = 2), M1 (n = 1), M2 (n = 2), M4 (n = 1), M5 (n = 3), and M7 (n = 3) in French–American–British classification. At the time of haplo-RIC-PBSCT, 2 patients were in second CR (CR2), 3 patients were in third CR (CR3), and the remaining 15 patients had active disease (primary induction failure: n = 4, relapse after allo-SCT: n = 11). Serological HLA disparities (in HLA-A, -B, -C, and -DR loci) in graft-versus-host (GVH) direction were observed at 2/8 loci (n = 1), 3/8 loci (n = 7), and 4/8 loci (n = 12). Other details of the patient characteristics are shown in Table 2. LDEC/HDCA, which were administered as pre-conditioning regimen, actually stabilized or reduced the leukemic burden, and there were no major complications due to this regimen observed.
Engraftment
PBSC grafts contained a median of 8.48 (range 2.62–73.70) × 106 CD34+ cells/kg, a median of 4.93 (range 1.63–19.69) × 108 CD3+ cells/kg, and a median of 1.32 (range 0.33–5.35) × 108 CD3+8+ cells/kg (Table 3). All 20 patients achieved neutrophil engraftment and full donor chimerism. The median duration of the neutrophil engraftment was 12 days (range 9–16 days).
Graft-versus-host disease
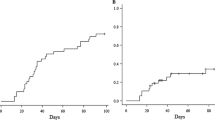
In this series, all patients except one developed aGVHD. Of the 20 patients, 16 (80.0%) developed severe (grade III–IV) aGVHD (Table 3). Management for aGVHD was conducted with the dose up of mPSL and the addition of immunosuppressants including ATG, MTX, dexamethasone palmitate, mycophenolate mofetil, mesenchymal stem cells, infliximab, and etoposide. Although a high incidence of severe aGVHD was observed, there was no death related to aGVHD.
Regarding cGVHD, 4 patients developed mild cGVHD, 2 patients developed moderate, and 2 more developed severe cGVHD (Table 3). The 2-year cumulative incidence of overall and moderate/severe cGVHD were 41.8% (95% confidence interval (CI), 22.4–68.6%) and 16.9% (95% CI 5.7–44.1%), respectively.
Viral reactivation
Episodes of viral reactivation were observed in 16 patients and included CMV (n = 11), Epstein-Barr virus (n = 3), human herpesvirus-6 (HHV-6) (n = 1), BK virus (n = 4), and JC virus (n = 1).
Transplant-related mortality, and relapse
The 2-year cumulative incidence of transplant-related mortality and relapse were 5.0% (95% CI 0.7–30.5%) and 57.8% (95% CI 37.4–79.6%), respectively (Fig. 2). There was one case of transplant-related mortality, whose cause of death was thrombotic microangiopathy following HHV-6 encephalitis. Other major transplant-related complications, including idiopathic pneumonia syndrome (IPS) and hepatic sinusoidal obstruction syndrome (SOS)/veno-occlusive disease (VOD), were not observed in this series.
Survival
As shown in Fig. 3, the 2-year EFS and OS rates were 40.0% (95% CI 19.3–60.0%) and 50.0% (95% CI 27.1–69.2%), respectively.
Discussion
For 20 patients of R/R pediatric acute leukemia, we performed HLA-haploidentical RIC-PBSCT with very low-dose (1.25 mg/kg) ATG. Although the survival outcome was encouraging, almost all cases developed aGVHD, furthermore, of which 85% developed severe aGVHD.
Lu et al. reported the use of ATG in a haplo-SCT setting [13]. They used 10 mg/kg ATG (thymoglobuline) for T-cell depletion and demonstrated that the outcome of haplo-SCT with ATG for leukemia is comparable to that of HLA-identical sibling SCT. Lee et al. reported the use of 12 mg/kg ATG (thymoglobuline) and showed favorable outcomes of patients of acute leukemia and myelodysplastic syndrome [14]. These reports demonstrate the feasibility of haplo-SCT with relatively high-dose ATG as alternative transplantation, namely without strong alloreactivity.
On the other hand, other reports found strong alloreactivity to haploidentical grafts. Ikegame et al. designed a prospective study of haplo-RIST with low-dose ATG (Fresenius, 8 mg/kg) for advanced hematologic malignancies [20]. GVHD prophylaxis in their study consisted of tacrolimus and methylprednisolone (1 mg/kg). They reported that the cumulative incidence of grade II to IV aGVHD was 30.7% and the survival rates at 1 year for patients with CR/chronic phase and non-CR status before transplantation were 62.5% and 42.3%, respectively. They concluded that their protocol is safe and feasible. Sano et al. reported the efficacy and toxicity of T-cell-replete haplo-SCT using low-dose ATG (thymoglobulin, 2.5 mg/kg) in children with R/R acute leukemia [21]. In addition to low-dose ATG, GVHD prophylaxis in their study consisted of tacrolimus, methotrexate, and prednisolone. They reported that the cumulative incidence of aGVHD was 73.0%, but that of grade III-IV aGVHD was 34.1% and the probabilities of EFS and OS at 2 years were 33.8% and 54.8%, respectively.
From our institute, Sawada et al. reported the feasibility of PTCy for advanced pediatric malignancies [22]. Giving emphasis to the GVL or graft-versus tumor effect, the dose of cyclophosphamide was reduced to 50 mg/kg, which is half the normally used dose. They concluded that PTCy was feasible; however, the survival outcome was poor. In the present study, we attempted to control R/R leukemia using stronger alloreactivity of haplo-SCT. While the lower the amount of ATG, the more likely it is that severe GVHD will occur, a stronger GVL effect may also be expected. Therefore, we set the dose of ATG to be low. As mentioned above, a high incidence rate of aGVHD, especially severe aGVHD, was observed. However, we could manage aGVHD by adding immunosuppressants. It is true that increasing the dose of ATG or the addition of other prophylactic agents should be considered to reduce GVHD, but the present procedure might be acceptable in terms of the relatively good survival outcome, low rate of transplant-related mortality, and especially in terms of no GVHD-related mortality.
In conclusion, although the sample size is small and severe aGVHD occurred at a high rate, the 2-year EFS and OS of patients involved in this study, including those who relapsed after allo-SCT, was encouraging. For further improvement of outcomes, it is necessary to use a combination therapy to enhance the anti-leukemic effect, such as molecular target therapeutic agents.
References
Hunger SP, Loh ML, Whitlock JA, Winick NJ, Carroll WL, Devidas M, et al. COG Acute Lymphoblastic Leukemia Committee. Children’s Oncology Group’s 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60(6):957–63.
Schrappe M, Möricke A, Reiter A, Henze G, Welte K, Gadner H, et al. Key treatment questions in childhood acute lymphoblastic leukemia: results in 5 consecutive trials performed by the ALL-BFM study group from 1981 to 2000. Klin Padiatr. 2013;225(Suppl 1):S62-72.
Kato M, Manabe A. Treatment and biology of pediatric acute lymphoblastic leukemia. Pediatr Int. 2018;60(1):4–12.
Gamis AS, Alonzo TA, Perentesis JP, Meshinchi S. COG Acute Myeloid Leukemia Committee. Children’s Oncology Group’s 2013 blueprint for research: acute myeloid leukemia. Pediatr Blood Cancer. 2013;60(6):964–71.
Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–54.
Handgretinger R. New approaches to graft engineering for haploidentical bone marrow transplantation. Semin Oncol. 2012;39:664–73.
Bethge WA, Faul C, Bornhäuser M, Stuhler G, Beelen DW, Lang P, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: an update. Blood Cells Mol Dis. 2008;40(1):13–9.
Lang P, Teltschik HM, Feuchtinger T, Müller I, Pfeiffer M, Schumm M, et al. Transplantation of CD3/CD19 depleted allografts from haploidentical family donors in paediatric leukaemia. Br J Haematol. 2014;165(5):688–98.
Schumm M, Lang P, Bethge W, Faul C, Feuchtinger T, Pfeiffer M, et al. Depletion of T-cell receptor alpha/beta and CD19 positive cells from apheresis products with the CliniMACS device. Cytotherapy. 2013;15:1253–8.
Bertaina A, Merli P, Rutella S, Pagliara D, Bernardo ME, Masetti R, et al. HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood. 2014;124:822–6.
O’Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–86.
Ji SQ, Chen HR, Wang HX, Yan HM, Zhu L, Liu J, et al. G-CSF-primed haploidentical marrow transplantation without ex vivo T cell depletion: an excellent alternative for high-risk leukemia. Bone Marrow Transplant. 2002;30:861–6.
Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107:3065–73.
Lee KH, Lee JH, Lee JH, Kim DY, Seol M, Lee YS, et al. Reduced-intensity conditioning therapy with busulfan, fludarabine, and antithymocyte globulin for HLA-haploidentical hematopoietic cell transplantation in acute leukemia and myelodysplastic syndrome. Blood. 2011;118:2609–17.
Kanda Y, Oshima K, Kako S, Fukuda T, Uchida N, Miyamura K, et al. In vivo T-cell depletion with alemtuzumab in allogeneic hematopoietic stem cell transplantation: combined results of two studies on aplastic anemia and HLA-mismatched haploidentical transplantation. Am J Hematol. 2013;88:294–300.
Ishihara T, Yasui M, Nakayama K, Kondo O, Koyama M, Sawada A, et al. Efficacy of continuous chemotherapy consisting of low dose etoposide and cytarabine in children. Jpn J Pediatr Hematol. 2008;22(5/6):347–53.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Ikegame K, Yoshida T, Yoshihara S, Daimon T, Shimizu H, Maeda Y, et al. Unmanipulated haploidentical reduced-intensity stem cell transplantation using fludarabine, busulfan, low-dose antithymocyte globulin, and steroids for patients in non-complete remission or at high risk of relapse: a prospective multicenter phase I/II study in Japan. Biol Blood Marrow Transplant. 2015;21:1495–505.
Sano H, Mochizuki K, Kobayashi S, Ohara Y, Ito M, Waragai T, et al. T-cell-replete haploidentical stem cell transplantation using low-dose antithymocyte globulin in children with relapsed or refractory acute leukemia. Int J Hematol. 2018;108:76–84.
Sawada A, Shimizu M, Isaka K, Higuchi K, Mayumi A, Yoshimoto Y, et al. Feasibility of HLA-haploidentical hematopoietic stem cell transplantation with post-transplantation cyclophosphamide for advanced pediatric malignancies. Pediatr Hematol Oncol. 2014;31:754–64.
Author information
Authors and Affiliations
Contributions
KH designed and performed the research, analyzed the data, and wrote the manuscript. OK designed and performed the research and treated the patients. YO, HT, AI, AM, MS, MS, KG, SI, MY, and MS treated the patients. AS and MI supervised the research and manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Higuchi, K., Sawada, A., Kondo, O. et al. HLA-haploidentical peripheral blood stem cell transplantation following reduced-intensity conditioning with very low-dose antithymocyte globulin for relapsed/refractory acute leukemia in pediatric patients: a single-institution retrospective analysis. Int J Hematol 115, 406–413 (2022). https://doi.org/10.1007/s12185-021-03270-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03270-z