Abstract
Coral communities continue to be threatened by chronic and acute stressors and there is a pressing need to understand the mechanisms that maintain their persistence. In this work, we use a model of coral-macroalgae dynamics to explore the effects of different assumptions regarding grazing and coral recruitment seasonality in coral-algae systems. We find that the grazing functional form constrains the potential for alternative stable states and coexistence, highlighting the need to further elucidate herbivore dynamics on reef systems. We also show that coral recruitment from external sources facilitates coral persistence and recovery, both when recruitment is assumed to be constant over time, and when recruitment occurs seasonally. In systems with alternative stable states, the total number of larvae that reaches a patch is the primary driver that dictates whether a system with low coral cover can flip into the coral-dominated basin of attraction. However, in a limited parameter space, the duration and timing of this larval pulse can also determine whether coral can recover in a bistable system. These results highlight the multiple factors that influence whether a coral reef is likely to remain in its present state or not, especially as ocean conditions change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reefs worldwide have experienced transitions from coral-dominated regimes to relatively degraded communities in which macroalgae are the primary benthic taxa (Done 1992; Tanner and Hughes 1994; McManus and Polsenberg 2004; Hughes et al. 2007). Although understanding these shifts remains an active area of research and scientific debate (Dudgeon et al. 2010; Mumby et al. 2013), there is growing consensus that both local and global stressors can fundamentally alter reef systems, increasing the likelihood that reefs will end up in a coral-depauperate state (Mumby and Steneck 2008; Mumby 2009). Unfortunately, there is mounting evidence that these regime shifts will become more common, with higher sea surface temperatures due to climate change predicted to increase the frequencies of mass bleaching, high-intensity storms, and disease outbreaks, all of which will likely translate into widespread coral mortality events (Donner et al. 2007; De’ath et al. 2012; Burge et al. 2014; Heron et al. 2016). Although there is evidence that factors such as effluent discharge, depth, and latitude are predictors of benthic community composition, herbivore abundance is considered a key determinant of the overall reef state (Jouffray et al. 2015). On smaller spatial scales, the removal of herbivores through fishing can confer a competitive advantage to macroalgae, since this effectively decreases a significant source of their mortality (Hughes et al. 2007). These stressors and several others (e.g., sedimentation stress, ocean acidification) have been linked to declines in coral cover that subsequently lead to unfavorable regime shifts on reefs (Richmond 1993; Anthony et al. 2011; Bozec and Mumby 2015); the dynamics that drive the recovery of these systems, particularly with regard to coral recruitment, remain underexplored.
Competition for space between corals and macroalgae is widely regarded as a major structuring force in reef communities (McCook et al. 2001). In recent years, there has been a prevailing yet controversial paradigm that there is “bistability” (potential for two stable states) on reefs, namely coral- and algal-dominated states (Mumby et al. 2007). Theory regarding alternative stable states (May 1977) in coral reef systems predicts that there are two potential stable equilibria or dynamical endpoints, in this case, characterized by dominance of either corals or macroalgae even for a fixed environment delineated by a particular set of parameters. Fundamentally, alternative stable states can arise through the introduction of nonlinearities in the response of different taxa to the presence of each other for any fixed environmental condition. These nonlinearities represent the complex underlying direct and indirect ecological interactions in a system (Scheffer 2009).
On a bistable reef, whether community trajectories, i.e., taxa cover over time, evolve towards one state or another is dependent on initial conditions (i.e., starting cover of coral and macroalgae). Nonetheless, perturbations can alter these trajectories and may be classified as either “pulse” or “press” depending on whether the disturbance to one or several species is instantaneous or sustained, respectively (Bender et al. 1984). In terms of reef systems, these perturbations can either (1) directly affect the cover of each benthic taxon or (2) the underlying conditions on the reef (Petraitis and Dudgeon 2004). The first type of change directly affects trajectories, allowing the system to move between “basins of attraction.” The second alters the parameters that characterize the reef and define those basins, leading to a succession of quasi-stationary states and, potentially, to navigating over regions in parameter space where trajectories lead towards a particular stable state. It is important to note that bistable systems do not necessarily preclude the existence of other reef states such as bare substrate or coexistence of corals and macroalgae. However, although these configurations may be empirically observable, they are theoretically transient and underlying dynamics propel these systems towards either the coral- or macroalgal-dominated stable state (Mumby 2009). These coexistence states may also occur in reality but are not reproduced in models because important underlying dynamics may be excluded from the theoretical framework due to simplifying assumptions (e.g., neglecting interactions with other benthic fauna such as sponges and calcareous algae).
In coral-algae systems, a process that can give rise to alternative stable states is the dependence of the grazing rate on coral cover (Mumby et al. 2007; Blackwood and Hastings 2011). Higher levels of coral cover potentially increase the grazing rate on macroalgae by concentrating grazing effort (Williams et al. 2001; Mumby et al. 2013). Indeed, increasing reef complexity is frequently correlated to higher abundances of herbivores such as parrotfish (Mumby and Wabnitz 2002; Howard et al. 2009; Harborne et al. 2012; Vergés et al. 2011) and sea urchins (Lewis and Wainwright 1985). In turn, structural complexity, in general, is positively correlated with coral cover (Alvarez-Filip et al. 2011), creating an indirect dependency between coral cover and grazing on macroalgae. However, this relationship is nuanced and can be both highly variable among herbivore species (Mumby and Wabnitz 2002; Harborne et al. 2012; Bozec et al. 2013) and non-linear due to the presence of multiple species. Due to uncertainty surrounding the grazing rate, it is important to systematically explore different functional forms in order to assess the effects of underlying assumptions of algal mortality in coral-algal competition models.
Previous modeling work on coral reef regime shifts has highlighted the importance of external coral recruitment, namely that it reduces the grazing level required to maintain a coral-dominated state (Elmhirst et al. 2009). However, larval recruitment in coral-algal competition has typically been modeled as a continuous input of coral larvae (Elmhirst et al. 2009; Fung et al. 2011; Baskett et al. 2014; Fabina et al. 2015), which can approximate corals known as “brooders” that release larvae year-round and are a significant component of coral communities in the Caribbean (Richmond and Hunter 1990). However, this assumption is often highly unrealistic because larval dispersal is affected by turbulent ocean processes which can deliver larvae in discrete “packets” through time (Berkley et al. 2010) and specifically for reefs in the Indo-Pacific and elsewhere that are predominantly characterized by seasonally spawning corals (Richmond and Hunter 1990; Elmhirst et al. 2009). Corals that exhibit seasonal reproduction, known as “broadcast spawners,” typically release gametes once or twice a year in multi-species mass spawning events timed with sea surface temperatures and the lunar cycle (Fadlallah 1983; Harrison et al. 1984; Richmond and Hunter 1990; Baird et al. 2009). Coral larvae that develop from external fertilization can settle after a brief pre-competency period of a few days and can survive for over 100 days in the water (Connolly and Baird 2010). Given this diversity in recruitment strategies, recruitment seasonality could have vastly different implications for the recovery potential of different coral groups, particularly on reefs that may have alternative stable states.
Here, we explore the interaction among grazing form, larval recruitment, bistability, and coral recovery with a mathematical model for coral-algal competition, focusing on the ecological implications of alternative stable states in reef systems. We test three hypotheses: (1) the choice of grazing functional form constrains the potential for alternative stable states; (2) coral recruitment from external sources alters stability and facilitates coral persistence; and (3) the duration and timing of coral recruitment can affect coral recovery in bistable systems.
Methods
Model description
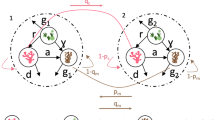
To test the effects of recruitment timing on coral stability, we extend the Mumby et al. (2007) coral-algal competition model by allowing the grazing functional form to vary as well as explicitly accounting for both constant and seasonal larval recruitment. Let C, M, and F represent the fractional area of a reef occupied by coral, macroalgae, and free space, respectively (Fig. 1). Here, free space refers to bare substrate or turf algae, entities that can be colonized by coral and macroalgae. The coral class can increase by growth, at rate r, and by larval recruitment at rate l(C), which is composed of larval input from within the reef patch (local retention) and from outside sources (external recruitment). Coral cover decreases through natural or externally induced mortality, at rate μ, as well as with macroalgal overgrowth, at rate a. Macroalgae grow at rate γ over free space and experience mortality through grazing, at rate g(C).
Model schematic and overview of grazing functional forms and external recruitment. a Corals increase through growth r, intra-patch larval recruitment fδ, external recruitment ω(t), background coral mortality μ, macroalgal growth γ macroalgal overgrowth onto coral a, and the grazing of macroalgae by herbivores g(C). b Grazing can be concave (green), linear (blue), convex (red), or constant (purple). Baseline grazing or the grazing rate at zero coral cover (C = 0) is denoted by g0, while g1 is the maximum grazing rate when C = 1. c The total external recruitment ω(t) depends on the amplitude or maximum larval input rate \( \widehat{\upomega} \) and the duration T (where T is a fraction of a year) over which larvae are received. The average larval input rate per year is denoted by \( \overline{\upomega} \)
In this spatially implicit formulation, the percent cover added to the coral class by recruitment is linearly dependent upon the number of larvae produced within a patch and on external recruitment, which can depend on time t. To approximate the recruitment contribution of brooding species, the total number of larvae that are produced within the patch is f C A, where f is the fecundity per year (e.g., no. of larvae m−2 year−1) and A is the total area of the reef in square meters. To approximate the input of spawning species, ω(t) is the rate at which larvae arrive from external sources (year−1). Finally, the rate at which the coral class increases by recruitment can be written as
where δ is the typical size (e.g., m2) per individual of a newly settled recruit. The full system is then
with F determined by the conservation of available space given by C + M + F = 1. Note that reef area does not appear explicitly and so does not affect subsequent results.
To create a flexible function that can partially account for the uncertainty surrounding the grazing functional form, we allow the shape of the grazing rate on macroalgae, as a function of coral cover, to range from concave to convex (Fig. 1) by setting
where g0 is the baseline grazing rate when there is no coral (C = 0), g1 is the maximum grazing rate when coral cover is 100% (C = 1) and α > 0 dictates its shape. When g1 > g0, g is a smooth, monotonically increasing function of C. Its shape is either (i) linear for α = 1, (ii) concave for 0 < α < 1, or (iii) convex for α > 1. In the linear case, grazing simply increases with coral cover at a constant rate. In the concave and convex scenarios, processes such as inter- and intra-specific competition result in a non-linear response of grazers to coral. While linear and concave relationships with grazing rate as a function of reef complexity have been observed in parrotfish (Bozec et al. 2013), a convex grazing form is derived in the original Mumby et al. model from the assumption that herbivores divide their grazing time between turf and macroalgal benthic states (Mumby et al. 2007).When g0 = g1, grazing is constant across all levels of coral cover. This scenario arises if the dominant grazer on a reef has a large territory relative to the size of the reef such that changes in coral cover on a single patch have a negligible effect on the overall grazing rate (Mumby and Wabnitz 2002).
In addition to different grazing forms, we consider reproduction scenarios in terms of coral larvae that arrive from an external source. To represent brooding species that produce larvae year-round and recruit locally, corals from within the patch produce larvae at a constant rate f, while we explore scenarios where external recruitment is either constant or seasonal in nature. In the constant external reproduction case, corals from external sources produce larvae at constant rate \( \omega (t)=\widehat{\omega} \), respectively. In this scenario, the external supply of larvae can be conceptualized as originating from a patch of coral that is size \( A\ \widehat{\omega}/f \). In contrast to this assumption, most species of coral reproduce annually, frequently during multi-species mass spawning events (Baird et al. 2009). To incorporate pulsed coral spawning, we impose seasonality in the external recruitment ω(t) scenario by assuming that corals reproduce for a fraction of a year (t is time in years). This seasonality is implemented using a square pulse of amplitude or maximum potential larval input rate \( \widehat{\omega} \)and duration T, setting \( \omega (t)=\omega \left(t;\widehat{\omega},T\right) \) with
where mod(a,b) is the remainder of the division of a by b. When T ≥ 1, we recover the constant larval input scenario, with \( \omega \left(t;\widehat{\omega},T\ge 365\right)=\widehat{\omega} \). Note that ω(t) is the “potential” larval input since settlement is constrained by the available free space.
Model analysis: constant larval input
To explore the effects of grazing functional form and external coral recruitment, we create a series of two-parameter bifurcation plots by varying baseline grazing (g0) and coral mortality (μ) rates. This parameter space represents combinations of two potential anthropogenic impacts: (i) herbivore removal through fishing, which leads to changes in grazing and consequent macroalgal mortality and (ii) warming waters due to climate change, which leads to increased coral mortality due to bleaching. Bleaching exhibits high temporal variability with effects deriving from both intra-annual punctuated events to inter-annual and decadal time scales (Heron et al. 2016; Glynn 1993). As a first-order approximation, we aggregate these effects through a constant coral mortality rate for the following reasons. First, there is evidence that pre-bleaching thermal stress leads to declines in zooxanthellae abundance and chlorophyll concentration, trends that are linked to an overall decrease in coral cover (Barron et al. 2010). Second, elevated mortality that lasts several months after a temperature anomaly has been observed in some coral species (Jones 2008), indicating the potential for a delayed response. Mortality due to bleaching is therefore not necessarily confined to the duration over which temperatures exceed a particular threshold and can exert detrimental effects before and after the event.
We solve the system numerically across combinations of coral mortality and baseline grazing under four different grazing functional forms: constant, linear, convex, and concave (see Fig. 1). For the constant reproduction model, we analyze the system using standard techniques to find equilibria and classify their stability (see app. B). We delineate regions in the μ-g0 parameter space that correspond to the following types of stable equilibria: free space only (all-F) where both coral and macroalgae percent covers are less than 0.01; coral-dominated (all-C), with coral percent cover at 0.01 or greater and with macroalgae at less than 0.01; macroalgae-dominated (all-M), with macroalgal percent cover at 0.01 or greater and less than 0.01 coral cover; coral-dominated + macroalgae (mostly-C), where there is more coral than macroalgae; macroalgae-dominated + coral (mostly-M), where macroalgal cover is greater than coral cover; and bistable, where trajectories lead to one of two different states, depending upon initial conditions. We choose to focus on the baseline grazing rate g0 since it naturally follows from the analytical conditions and we are particularly interested in dynamics when coral cover is low in order to address coral recovery potential. In Fig. 2, we present the full stability classification system alongside characteristic phase-plane diagrams of each regime without external coral recruitment.
a Stability regions for convex grazing and no external recruitment delineated by a two-parameter bifurcation plot with coral mortality rate μ against baseline grazing rate g0. Parameter values are in Table 1. b Illustrative simplices are numbered to correspond with each of the regions in (a). The vertices are labeled F for free space, C for coral, and M for macroalgae. Black dots represent stable equilibria while the dashed line in B.3 is the separatrix that separates the coral and macroalgae basins of attraction. The closer an equilibrium point is to a vertex, the higher the percentage of area that can be attributed to that component. Arrows represent the direction of trajectory solutions through time and their colors represent how quickly (warmer colors) or slowly (cooler colors) they evolve towards equilibrium. Simplex B.1 is coral-dominated (all-C), B.2 is free space only (all-F), B.3 is bistable, and B.4 is macroalgal-dominated (all-M). Parameter values are as follows: (B.1) μ = 0.1, g0 = 1.2, (B.2) μ = 1.0, g0 = 1.5, (B.3) μ = 0.1, g0 = 0.7, (B.4) μ = 0.5, g0 = 0.3
Model analysis: seasonal larval input
Compared to the system with constant recruitment, seasonal recruitment trajectories are not differentiable and the fixed points in the former become periodic orbits in the latter. To understand the time evolution of these trajectories, we generate a set of simplex plots when there is no recruitment (ω(t) =0), maximum recruitment (\( \omega (t)=\widehat{\omega} \)) and average recruitment (ω(t)=\( \overline{\omega} \)). Additionally, we provide an exact representation of possible trajectories with a recurrence map where we create vector fields using points from either the beginning or end of the recruitment period every year. These vector fields describe the direction of the system’s evolution across years. For a set duration, amplitude, and periodicity of the larval recruitment pulse, we define a separatrix that separates the initial conditions that lead to one of the alternative stable states.
To test whether seasonal recruitment affects recovery, we restrict our analyses to a parameter space that allows for alternative stable states when there is no external recruitment. The reasons for this are twofold: first, the parameter values that produce bistable dynamics have been thoroughly explored both here and in other studies, and are well within empirically measured ranges (Fung et al. 2011; Fabina et al. 2015), and second, we are interested in whether bistable dynamics are robust to the inclusion of external larval input. We measure the potential for coral population recovery on a patch by considering the scenario in which a bistable reef is presently in the macroalgae-dominated state (initial conditions M(0) = 0.7, C(0) = 0.01). Under these conditions, we quantify equilibrium coral cover as a function of maximum larval input rate, \( \widehat{\omega} \), and recruitment duration, T. Additionally, we explore the effects of duration by holding average external recruitment \( \overline{\omega} \) constant while duration varies, i.e., setting \( \widehat{\omega}=\overline{\omega}/T \).
Results
Effect of grazing functional form
For a given set of parameters (see Table 1), the grazing functional form determines the potential for bistability and coexistence (Fig. 3; app. C, D). We note that under constant grazing, although the values chosen for Fig. 3 do not produce bistability along the coral mortality and baseline grazing axes (g0 = 0 and μ = 0, respectively), bistability is theoretically possible under other parameter sets (app. C, D). Under convex, linear, and concave grazing, we see four states are possible without external recruitment: all-F, all-C, all-M, and bistable, where either coral or macroalgae will dominate, depending upon initial conditions (see app. D for parameter conditions that delineate the full set of stability regions). For all grazing types, the all-F state exists in the region with relatively high grazing and high coral mortality rates. The all-C benthic region persists in the high grazing and low coral mortality space, while low grazing and high coral mortality rates define the all-M region. In the convex, linear, and concave grazing systems, the bistable region separates the all-C and all-M states in g0-μ parameter space.
Stability regions delineated by coral mortality μ and baseline grazing g0 rate for systems with constant (a–c), convex (d–f), linear (g–i), and concave (j–l) grazing forms under zero, low, and high constant coral external recruitment rates (\( \widehat{\omega}=0 \), \( \widehat{\omega}=0.1 \) and \( \widehat{\omega}=1.0 \); T = 1). Colors represent the type of stable equilibrium or equilibria reached when solving the system under different combinations of μ and g0 on the x- and y-axes, respectively. See “Results” for a detailed explanation of each stability region. Parameter values are in Table 1
At any particular reef, the stability classification is evident from the configuration of the zero-growth isoclines for coral and macroalgae. Specifically, representing the reef configurations in a phase-plane diagram with coral cover on the x-axis and macroalgae cover on the y-axis, the coral isocline must cross the macroalgae isocline from above for a fixed point to be stable (app. C).
Effect of constant larval input (T = 1)
The addition of constant external recruitment (ω(t) = ω > 0) alters the set of possible equilibrium benthic community outcomes (Fig. 2) as we show in Fig. 3, where we move from low to high external recruitment. We find an interaction between grazing type and recruitment such that the concave, linear, and convex functional forms are qualitatively similar when there is no external recruitment, but increasing concavity leads to a larger all-C region in parameter space in scenarios with larval input (e.g., Fig. 3, plots e, h, k). Among the varying/non-constant grazing forms, there are significant differences with regard to the size of the coral-dominated, coexistence, and bistable regions. Under low and high recruitment, constant, convex, and linear grazing systems, a relatively smaller all-M region is still present; this region no longer exists in the concave grazing system. Note that, even though the coral-dominated all-C region is the largest across all grazing types, no region is uniform in terms of fractional cover. For example, some all-C states may have a large free-space component at some parameter values, whereas it could signify a reef with 100% coral cover at other parameter combinations. Nonetheless, higher levels of constant coral larval recruitment fundamentally shift the system to favor coexistence of the coral and macroalgae classes, as well as benthic configurations that do not exclude corals.
Effect of recruitment duration (0 < T < 1)
Previously, we have considered a system that is provided with a constant external inflow of larvae, produced from an external source. In this section, we explore rates of larval recruitment that more realistically capture the reproductive timing of broadcast-spawning species, which happens over a limited time period within a year. We model this using a larval recruitment amplitude parameter, \( \widehat{\omega} \), which acts during the spawning time period, T (Fig. 1). With this additional component, the reef system will periodically change its dynamics between no external recruitment and high external recruitment scenarios (Fig. 3e, f; Fig. 4a).
Illustrative simplices showing different and complementary interpretations of the trajectories that occur under seasonal reproduction. For all simplices, the two same trajectories with seasonal recruitment (and T < 1) are represented in solid black lines, characterized by two different initial conditions (the black dots) that lead to either a coral- or macroalgae-dominated state. a The dynamics are exactly characterized by two different simplices: one when there is no external recruitment and another when recruitment is at its maximum value, \( \widehat{\omega} \). The trajectories follow the lines in each of the simplices for a duration T. The sizes of the coral and macroalgae basins of attraction, delineated by the separatrix (dashed line), vary over time based on whether external recruitment is assumed to be maximum or zero. When we set a constant recruitment rate with the same average value of ω over an entire year, i.e., \( \omega (t)=\overline{\omega}\equiv \widehat{\omega}\ T \), the trajectories described by that simplex do not follow the real trajectories but describe, to a high degree of approximation (or exactly for T = 1), the average trajectory of the system. b To create a simplex that exactly describes the trajectories of the system, we create a vector field generated by the yearly recurrence map (red dots on the simplices) to describe the trajectory evolution from year to year. For a set timing of the pulse, we can define a separatrix that separates the initial conditions that lead to either the coral or macroalgae basin of attraction
In Fig. 4, we see that plotting the vector field at the beginning vs. end of each recruitment period changes the position of separatrix. This reveals that the outcome of initial conditions contained somewhere between the band created by the two separatrices cannot be determined unless we know the exact timing of the larval pulse. The width of this band is null at the C-M line, where there is no free space, and increases with the amount of free space. This results from the increase in amplitude of the fluctuations generated by the pulse. In turn, this shows the relative importance of the timing of the pulse for a subset of initial conditions, in particular, those with free space > 0.
In Fig. 5, we consider a system that starts in an all-M configuration and let it experience reproductive seasons across several years with different combinations of maximum larval input rate \( \widehat{\omega} \) and duration T. Under scenarios of both low and high baseline grazing g0, the primary driver of coral recovery (i.e., reaching the high coral cover state) is clearly the average recruitment \( \overline{\omega} \) as it describes the average trajectory (see Fig. 4). When we isolate the effects of recruitment duration, we find that for values of \( \overline{\omega} \) that lie on the transition between the coral- and algae-dominated regions, the duration of the pulse that favors a transition to the coral-dominated basin is dependent on the properties of the reef and the grazers’ response to coral cover. In our system with relatively high baseline grazing (Fig. 5a), a longer duration at the threshold level of recruitment is more favorable, while a shorter duration is able to facilitate coral recovery in our system with low baseline grazing (Fig. 5b). When baseline grazing is high, the macroalgae basin of attraction leads to a fixed point with a significant amount of free space, while this fixed point is almost 100% macroalgae when baseline grazing is low (given by 1 − g0/γ, app. B, D). Overall, different values for the baseline grazing rate g0 can favor different durations when recruitment is at a critical \( \overline{\omega} \) value. In particular, it seems that shorter durations can be more important when macroalgae has experienced a perturbation that drives it to a lower cover value (i.e., more free space) than at equilibrium. In this case, a relatively high number of coral larvae that arrive within a short time period can occupy the free space and lead to recovery failure and a collapse of the macroalgae class.
Equilibrium coral cover as a function of maximum larval input rate \( \widehat{\omega} \) and recruitment duration T. Colored lines represent “recruitment isoclines” where the time average of external recruitment ω(t), \( \overline{\omega} \), is held constant while duration varies, i.e., \( \widehat{\omega}=\overline{\omega}/T \). We show the outcome for different values of the baseline grazing rate g0. Green represents \( \overline{\omega}=0.3 \) and blue \( \overline{\omega}=0.2 \) in both plots, while the black line in (a) represents \( \overline{\omega}=0.9 \) and (b) \( \overline{\omega}=0.95 \). In the inset of each plot, we show the corresponding coral cover as a function of duration for the three recruitment isoclines. The trajectories are computed for a specific set of initial conditions with low coral cover and some free space (C(0) = 0.01 and M(0) = 0.7). (a) g0 = 0.2 and g1 = 5 and (b) g0 = 0.02 and g1 = 15
Discussion
Corals face threats across multiple spatial scales. Local stressors such as excess nutrient input and overfishing can alter coral-algal dynamics such that macroalgae are conferred a competitive advantage over coral. For example, nutrient input can increase macroalgal growth rate while the removal of herbivorous fish can lower the algal mortality rate. On a global scale, ocean temperatures have been linked to higher rates of bleaching and disease transmission that increase coral mortality (Hughes et al. 2017; Bruno et al. 2007), while ocean acidification has been shown to decrease calcium carbonate accretion rates, effectively slowing coral growth (Anthony et al. 2011). Storms can be detrimental to both algae and coral, frequently clearing reefs and creating space for benthic recruits. The effects of these disturbances and recovery potential are contingent on the equilibrium state of a particular reef and though reefs may rarely be at equilibrium, the various types of equilibria determine the trajectory of a reef community through time in response to various stressors. Here, we explored the effects of coral recruitment on a coral-algae system with a modeling framework to show how grazing and larval input affects stability and recovery to a coral-dominated state. To do so, we first established that different grazing functional forms, representing a variety of real-world herbivore community compositions (Steneck 1988), exhibit bistability when the grazing rate is dependent on coral cover. To isolate the influence of recruitment duration, we focused on the bistable region and held total larval input constant while varying the fraction of a year over which larvae are received.
Effect of grazing functional form
Our results imply that the dynamical properties of real-world coral-algae systems will ultimately depend on the type of grazing and the characteristics of recruitment. For instance, the Mumby et al. model (2007) was based on convex grazing and was derived on the assumption that grazers spend their time foraging on macroalgae and turf algae (“free space” in our formulation) in proportion to their relative abundance. In our model, we consider “free space” as any substrate that can be colonized (sensu Baskett et al. 2014), allowing grazing rates to respond to coral cover in different ways based on experimental evidence that artificially increasing coral cover can lower macroalgal cover (Williams et al. 2001). We show that the response of grazers to coral cover can dictate the range of parameter values over which a reef is classified into one of the six benthic community compositions discussed here. For example, a reef dominated by herbivores that do not respond to coral cover, such as those with relatively large foraging ranges, will be unlikely to display bistable dynamics (Fig. 3, plots a–c). When grazers respond positively to increasing coral cover, here represented as convex, linear, and concave relationships, this feedback can promote bistability in the system. Under scenarios with external recruitment, increasing concavity in the grazing functional form (i.e., a faster increase in grazing rate with coral cover) favors coral dominance within the explored parameter space defined by mortality (μ) and baseline grazing rates (g0; Fig. 3, plots d–l).
Effect of recruitment timing
Intuitively, higher rates of constant coral recruitment lead to an increased share of the parameter space defined by coral mortality (μ) and baseline grazing (g0) for the all-C regime and decrease the size of the all-M regime (Fig. 2). This matches previous findings where external coral recruitment lowered the grazing intensity required to maintain coral persistence (Elmhirst et al. 2009). In addition, seasonal coral recruitment also changes underlying dynamics and its overall effects can be best approximated by considering the average external recruitment rate (\( \overline{\omega} \)) (Fig. 4a). In a given system, the larval input duration combined with a specific subset of \( \overline{\omega} \) can have a notable impact on bistable reefs, affecting the probability of switching between coral and algal domains of attraction (Fig. 5). We believe that this scenario is particularly relevant to disturbances such as coral bleaching, disease, or storms that cause declines in coral cover: periodic recruitment with enough larvae can change underlying dynamics to favor coral dominance, or can provide a pulse of coral that “pushes” the trajectory of the system from a macroalgae-dominated to a coral-dominated equilibrium. This is effectively equivalent to setting ideal initial conditions, or the starting fractional covers of coral and algae, that favor trajectories leading to coral dominance on a bistable reef. Indeed, connectivity-based efforts that seek to facilitate a “rescue effect” through coral recruitment could be successful if a sufficient number of larvae are able to settle during a given recruitment period (Cruz and Harrison 2017).
Regardless of the community composition of a patch and the surrounding reef network in terms of the brooder vs. spawner life-history strategies, seasonality can be both a benefit and a detriment to the recovery of corals and the strength of the dependence of algal grazing on coral cover is especially important in understanding the effects of seasonality. In particular, if the baseline grazing is relatively low, it is beneficial to receive larvae quickly. Conversely, when baseline grazing is high, a larval settlement rate that approaches constant reproduction is more effective (Fig. 5). In reality, brooders produce fewer larvae than spawners but at a pace that more closely resembles constant recruitment (Fadlallah 1983; Richmond and Hunter 1990). However, we find that the ability of spawners to potentially produce more viable larvae is a relatively more important factor than the seasonality of their reproduction (usually once or twice a year).
The insights we have gained regarding the importance of recruitment characteristics are particularly useful in the design of spatial metacommunity models that aim to link dispersal patterns to local- and regional-scale processes in order to project coral-algal dynamics into the future (Melbourne-Thomas et al. 2011a; Melbourne-Thomas et al. 2011b). This study suggests that sub-monthly temporal dynamics in recruitment, assuming a threshold amount of larvae is met, are significant to the overall dynamics of coral-algal systems. As a consequence, numerical models of these systems should operate at this timescale when total recruiment lies in the transition region, otherwise using an average recruitment value over larger timescales is sufficient to capture the primary effects of external larval input.
Model assumptions and caveats
Our relatively simple model is analytically tractable and provides clear, biologically interpretable results. However, this modeling framework excludes much of the rich complexity that exists on real coral reef systems. For example, we ignore the diversity of life-history characteristics present across coral and macroalgae species, as well as the intergroup competition that would inevitably occur. In particular, corals exhibit a range of growth rates and susceptibility to mortality and reproductive modes (Darling et al. 2012), while macroalgae have a wide array of chemical and physical adaptations that make them more or less harmful to corals and that affect their digestion by herbivores (Hay 1997). Recent work using a similar model but with two types of coral, a resistant species exhibiting slower growth and lower mortality and a resilient species with faster growth and higher mortality, found that this diversity can lead to higher equilibrium coral cover than with either species in isolation (Baskett et al. 2014). Including this level of detail by adding another state variable to represent a second coral group, as well as differentiating the two types by growth rate (r), macroalgal overgrowth onto coral (a) and mortality (μ), for example, could potentially shift our results to favor coral-dominated states and increase the coral basin of attraction in bistable cases.
We found that a threshold level of average recruitment, whether constant or seasonal, can facilitate a switch from the macroalgae to coral attractor. This goes against previous statements that coral recruitment alone cannot facilitate recovery once a bistable reef trajectory lies within the macroalgae domain of attraction (Mumby and Steneck 2008; Mumby et al. 2014). However, we did not differentiate between coral recruits and coral adults, which have different background mortality rates as well as susceptibility to macroalgal overgrowth (Arnold et al. 2010). Previous work using a simulation model linked reduced grazing rates to an increase in post-settlement coral mortality such that recruits did not survive to adulthood; this bottleneck was the main driver of coral decline (Mumby et al. 2006). This leads us to believe that for both the constant and seasonal reproduction cases, incorporating this ontogenetic mortality shift would likely decrease the effect of recruitment on the ability of the system to reach the coral basin of attraction and decrease the equilibrium coral cover, since a lower proportion of coral larvae would translate into adult coral cover.
Although we addressed the complexity underlying recruitment patterns and subsequent ecological consequences by separately assessing the effects of amplitude and duration, we did not consider the temporal variability in larval release and spatial heterogeneity (i.e., reef patch size, coral cover, fecundity) that occur on realistic reef networks (Kleypas et al. 2016). In particular, we know that stochastic connectivity can facilitate species coexistence (Berkley et al. 2010), while from a single-species perspective, this variability has been shown to reduce population growth and the overall equilibrium abundance in simulations of fish populations in the Southern California Bight (Watson et al. 2012). Furthermore, seasonality in spawning can magnify these negative effects, as illustrated by a damselfish simulation study in the Florida Keys (Snyder et al. 2014), while larval behaviors such as vertical migration can be a mitigating force (Snyder et al. 2014; Kough and Paris 2015). Future work, particularly when considering multiple reef patches across a heterogeneous landscape, must investigate the interactions between stochastic connectivity and seasonal spawning. In general, our modeling framework provides a basis from which additional complexities can be explored and highlights important future research directions, particularly in terms of elucidating site-specific coral reproductive patterns that are crucial in understanding the future of coral-algae systems.
Conclusions
Whether or not the community trajectory of a coral reef will lead to a coral- or macroalgal-dominated state will depend on the arrangement of the basins of attraction on the dynamical landscape, which is partly dictated by the type of grazing present and has direct implications for the efficacy of any ensuing conservation efforts. Additionally, the recruitment of coral larvae from external sources alters the underlying dynamical stability and can facilitate a shift from a macroalgae- to a coral-dominated regime, suggesting that the competitive dynamics on reefs can be critically affected by larval recruitment. Together, these factors determine the recovery potential of corals from perturbations.
References
Alvarez-Filip L et al (2011) Region-wide temporal and spatial variation in Caribbean reef architecture: is coral cover the whole story? Glob Chang Biol 17(7):2470–2477
Anthony KRN et al (2011) Ocean acidification and warming will lower coral reef resilience. Glob Chang Biol 17(5):1798–1808
Arnold SN, Steneck RS, Mumby PJ (2010) Running the gauntlet: inhibitory effects of algal turfs on the processes of coral recruitment. Mar Ecol Prog Ser 414:91–105
Baird AH, Guest JR, Willis BL (2009) Systematic and biogeographical patterns in the reproductive biology of Scleractinian corals. Annu Rev Ecol Evol Syst 40(1):551–571
Barron MG et al (2010) Experimental bleaching of a reef-building coral using a simplified recirculating laboratory exposure system. J Mar Biol 2010:1–8
Baskett ML, Fabina NS, Gross K (2014) Response diversity can increase ecological resilience to disturbance in coral reefs. Am Nat 184(2):E16–E31
Bender E, Case TJ, Gilpin M (1984) Perturbation experiments in community ecology: theory and practice. Ecology 65(1):1–13
Berkley HA et al (2010) Turbulent dispersal promotes species coexistence. Ecol Lett 13(3):360–371
Blackwood JC, Hastings A (2011) The effect of time delays on Caribbean coral-algal interactions. J Theor Biol 273(1):37–43
Bozec Y-M, Mumby PJ (2015) Synergistic impacts of global warming on the resilience of coral reefs. Phil Trans R Soc B 370:20130267
Bozec YM et al (2013) Reciprocal facilitation and non-linearity maintain habitat engineering on coral reefs. Oikos 122(3):428–440
Bruno JF et al (2007) Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol 5(6):1220–1227
Burge CA et al (2014) Climate change influences on marine infectious diseases: implications for management and society. Annu Rev Mar Sci 6:249–277
Connolly SR, Baird AH (2010) Estimating dispersal potential for marine larvae: dynamic models applied to scleractinian corals. Ecology 91(12):3572–3583
Cruz DWD, Harrison PL (2017) Enhanced larval supply and recruitment can replenish reef corals on degraded reefs. Sci Rep 7(1):1–13
Darling ES et al (2012) Evaluating life-history strategies of reef corals from species traits. Ecol Lett 15(12):1378–1386
De’ath G et al (2012) The 27-year decline of coral cover on the great barrier reef and its causes. Proc Natl Acad Sci U S A 109(44):17995–17999
Done TJ (1992) Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247(1–3):121–132
Donner SD, Knutson TR, Oppenheimer M (2007) Model-based assessment of the role of human-induced climate change in the 2005 Caribbean coral bleaching event. Proc Natl Acad Sci 104(13):5483–5488
Dudgeon SR et al (2010) Phase shifts and stable states on coral reefs. Mar Ecol Prog Ser 413:201–216
Elmhirst T, Connolly SR, Hughes TP (2009) Connectivity, regime shifts and the resilience of coral reefs. Coral Reefs 28(4):949–957
Fabina NS, Baskett ML, Gross K (2015) The differential effects of increasing frequency and magnitude of extreme events on coral populations. Ecol Appl 25(6):1534–1545
Fadlallah YH (1983) Sexual reproduction, development and larval biology in scleractinian corals. Coral Reefs 2(3):129–150
Fung T, Seymour RM, Johnson CR (2011) Alternative stable states and phase shifts in coral reefs under anthropogenic stress. Ecology 92(4):967–982
Glynn PW (1993) Coral reef bleaching: ecological perspectives. Coral Reefs 12(1):1–17
Harborne AR, Mumby PJ, Ferrari R (2012) The effectiveness of different meso-scale rugosity metrics for predicting intra-habitat variation in coral-reef fish assemblages. Environ Biol Fish 94(2):431–442
Harrison PL et al (1984) Mass spawning in tropical reef corals. Science 223(4641):1186–1189
Hay ME (1997) The ecology and evolution of seaweed-herbivore interactions on coral reefs. Coral Reefs 16(5):S67–S76
Heron SF et al (2016) Warming trends and bleaching stress of the world’s coral reefs 1985–2012. Sci Rep 6(November):38402
Howard KG, Schumacher BD, Parrish JD (2009) Community structure and habitat associations of parrotfishes on Oahu, Hawaii. Environ Biol Fish 85(2):175–186
Hughes TP et al (2007) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17(4):360–365
Hughes TP et al (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Jones RJ (2008) Coral bleaching, bleaching-induced mortality, and the adaptive significance of the bleaching response. Mar Biol 154(1):65–80
Jouffray J-B et al (2015) Identifying multiple coral reef regimes and their drivers across the Hawaiian archipelago. Phil Trans R Soc B 370:20130268
Kleypas JA et al (2016) Larval connectivity across temperature gradients and its potential effect on heat tolerance in coral populations. Glob Chang Biol 22:3539–3549
Kough AS, Paris CB (2015) The influence of spawning periodicity on population connectivity. Coral Reefs 34(3):753–757
Lewis SM, Wainwright PC (1985) Herbivore abundance and grazing intensity on a Caribbean coral reef. J Exp Mar Biol Ecol 87(3):215–228
May RM (1977) Thresholds and breakpoints in ecosystms with a multiplicity of stable states. Nature 269:471–477
McCook LJ, Jompa J, Diaz-Pulido G (2001) Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 19(4):400–417
McManus JW, Polsenberg JF (2004) Coral-algal phase shifts on coral reefs: ecological and environmental aspects. Prog Oceanogr 60(2–4):263–279
Melbourne-Thomas J et al (2011a) A multi-scale biophysical model to inform regional management of coral reefs in the western Philippines and South China Sea. Environ Model Softw 26(1):66–82
Melbourne-Thomas J et al (2011b) Regional-scale scenario modeling for coral reefs: a decision support tool to inform management of a complex system. Ecol Appl 21(4):1380–1398
Mumby PJ (2009) Phase shifts and the stability of macroalgal communities on Caribbean coral reefs. Coral Reefs 28(3):761–773
Mumby PJ, Steneck RS (2008) Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol Evol 23(10):555–563
Mumby PJ, Wabnitz CCC (2002) Spatial patterns of aggression, territory size, and harem size in five sympatric Caribbean parrotfish species. Environ Biol Fish 63(3):265–279
Mumby PJ et al (2006) Revisiting the catastrophic die-off of the urchin Diadema antillarum on Caribbean coral reefs: fresh insights on resilience from a simulation model. Ecol Model 196(1–2):131–148
Mumby PJ, Hastings A, Edwards HJ (2007) Thresholds and the resilience of Caribbean coral reefs. Nature 450:98–101
Mumby PJ, Steneck RS, Hastings A (2013) Evidence for and against the existence of alternate attractors on coral reefs. Oikos 122(4):481–491
Mumby PJ et al (2014) Operationalizing the resilience of coral reefs in an era of climate change. Conserv Lett 7(3):176–187
Petraitis PS, Dudgeon SR (2004) Detection of alternative stable states in marine communities. J Exp Mar Biol Ecol 300(1–2):343–371
Richmond RH (1993) Coral reefs: present problems and future concerns resulting from anthropogenic disturbance. Am Zool 33(6):524–536
Richmond R, Hunter C (1990) Reproduction and recruitment of corals: comparisons among the Caribbean, the Tropical Pacific, and the Red Sea. Mar Ecol Prog Ser 60:185–203
Scheffer M (2009) Alternative stable states and regime shifts in ecosystems. In: Levin SA (ed) The Princeton Guide to Ecology. Princeton University Press, Princeton, pp 395–406
Snyder RE, Paris CB, Vaz AC (2014) How much do marine connectivity fluctuations matter? Am Nat 184(4):523–530
Steneck RS (1988) Herbivory on coral reefs: a synthesis. Proceedings of the 6th International Coral Reef Symposium, pp. 37–49
Tanner JE, Hughes TP (1994) Species coexistence, keystone species, and succession: a sensitivity analysis. Ecology 75(8):2204–2219
Vergés A et al (2011) Spatial patterns in herbivory on a coral reef are influenced by structural complexity but not by algal traits. PLoS One 6(2):e17115
Watson JR et al (2012) Changing seascapes, stochastic connectivity, and marine metapopulation dynamics. Am Nat 180(1):99–112
Williams ID, Polunin NVC, Hendrick VJ (2001) Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar Ecol Prog Ser 222:187–196
Acknowledgments
We thank Peter Mumby (University of Queensland), Anieke van Leeuwen (Royal Netherlands Institute for Sea Research) and two anonymous reviewers for insightful feedback during the preparation of this manuscript.
Funding
Financial support for this article is from National Science Foundation grants GEO-1211972 (SAL, VVV and JRW) and OCE-1426746 (SAL and JRW), the US Defense Advanced Research Projects Agency grant D17AC00005 (SAL and VVV), the National Defense Science and Engineering Graduate Fellowship (LCM), and the New Jersey Sea Grant with funds from the National Oceanic and Atmospheric Administration (NOAA) Office of Sea Grant, U.S. Department of Commerce, under NOAA grant NA140AR4170203 and the New Jersey Sea Grant Consortium (LCM). The statements, findings, conclusions, and recommendations are those of the authors and do not necessarily reflect the views of New Jersey Sea Grant or the U. S. Department of Commerce. NJSG-18-936.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McManus, L.C., Watson, J.R., Vasconcelos, V.V. et al. Stability and recovery of coral-algae systems: the importance of recruitment seasonality and grazing influence. Theor Ecol 12, 61–72 (2019). https://doi.org/10.1007/s12080-018-0388-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-018-0388-x