Abstract
Despite their ecological importance as bioeroders and their economic importance in commercial, artisanal, and recreational fisheries, there have been relatively few studies on parrotfish (Scaridae) ecology in Hawaii. Belt transects were conducted around the island of Oahu to survey current parrotfish distributions, size structure, species composition and associated habitats. Scarid communities in this heavily fished region are dominated by smaller species and smaller individuals within all species. Specific habitat characteristics such as rugosity, substrate diversity, and percent live coral cover were positively correlated with scarid numerical abundance. Scarids, however, were patchily distributed and were often absent from preferable habitats, suggesting that intense fishing pressure may be an important factor preventing these fish from fully exploiting available habitats. This study is the first thorough, broad-scale investigation of scarid community structure in Hawaii, and provides important information that has management and conservation implications for parrotfish in Hawaii and throughout tropical coral reef ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parrotfish (Family: Scaridae) play an important role in coral reef ecosystems throughout the tropics, functioning as bioeroders, corallivores, and algal consumers (Choat 1991; Bellwood et al. 2004; Mumby 2006; Rotjan and Lewis 2008). They are also an important component of many small-scale commercial, recreational, artisanal and subsistence coral reef fisheries (Smith 1993; Page 1998; Jennings et al. 1999b). There is growing concern about the status of scarid populations in many parts of their geographic range. Parrotfish may be particularly susceptible to detrimental effects of fishing pressure because of both their life-history and behavioral traits. These fishes are sequential protogynous hermaphrodites, often relatively long-lived reef fish species, and are also easily harvested while resting at night. The widespread use of SCUBA to harvest parrotfishes has also increased catches substantially (Smith 1993; Jennings et al. 1999b). Spearing fish while using SCUBA has been prohibited in some countries, but it remains legal in the U.S.A. and is prominent in Hawaii.
Herbivore abundance may be reduced when the physical structure of the reef deteriorates due to coral mortality or anthropogenic nutrient-driven phase shifts from coral-dominated environments to algal-dominated environments (Done 1992; Williams and Polunin 2001). Numerous studies have documented the importance of topographic structure or cavities in reef environments as refugia from predation, particularly for juvenile fish (Risk 1972; Roberts and Ormond 1987; Williams et al. 2001). Therefore, although they initially increase the food supply, algal dominated reef communities may ultimately impede population growth of herbivorous fish, and there may be an algal threshold above which herbivores cannot control algae (Williams and Polunin 2001; Williams et al. 2001). Reduced herbivore abundance can then exacerbate the problem of algal overgrowth of coral by leaving algal growth unchecked (Conklin and Stimson 2004; Mumby 2006).
Because of their importance as dominant herbivores throughout the tropics, and in order to better manage their populations and maintain overall reef health, it is important to understand the structure of parrotfish communities and their interactions with degraded habitats. Studies investigating habitat associations with scarid community characteristics have often produced conflicting or inconclusive results. Bell and Galzin (1984) and Tolimieri (1998) found significantly higher numerical abundance of scarids with increased coral cover. However, Gust (2002), Hart et al. (1996), and Ohman and Rajasuriya (1998) found no significant correlations between benthic habitat measures and scarid abundance. No consistent, broad-scale pattern of family-level trends in scarid-habitat associations is evident, and patterns found in the reported studies may largely reflect local differences or study methodology.
The present study investigates parrotfish-habitat associations in Hawaii by studying a relatively large geographic area. The primary goal of this study is to describe the community structure of parrotfish on Oahu, Hawaii, and investigate the habitat associations of parrotfish with coarse and fine-scale habitat characteristics. A secondary goal is to explore the potential impact that continued habitat degradation could have on parrotfish communities in Hawaii.
Methods
Study site
Hawaii hosts seven species of scarids, three of which are endemic (Table 1). All species are found throughout the Hawaiian archipelago. Hawaiian parrotfish range in maximum total length (Ledlie et al. 2007) from ~30 cm (Scarus psittacus) to over 70 cm (S. rubroviolaceus). Previous fish assemblage analyses in Hawaii indicate that scarids are the second most abundant fish family by weight in the Main Hawaiian Islands (Friedlander et al. 2003). No prior attempt has been made to thoroughly characterize parrotfish populations around any of the Hawaiian islands, and few studies have been conducted to characterize populations of any Hawaiian nearshore fisheries species.
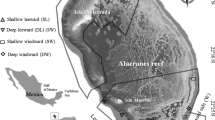
The field portion of this study was conducted around the island of Oahu (Fig. 1) from November 2005 through March 2007. Oahu has the most dense human population of the islands in Hawaii and correspondingly has some of the most heavily fished reefs and most degraded habitat in Hawaii (Smith 1993). Oahu has a few small marine reserves offering various degrees of protection from fishing, and these reserves were included as potential sampling sites in this study.
Sampling design
Sampling followed a hierarchical stratified random design with two levels: island sector and habitat type. Oahu was first categorized into four sectors (north, south, windward and leeward), which roughly correspond to the directions of exposure to seasonal swells. Coarse-scale habitat types were classified by GIS-based benthic habitat maps developed by the National Oceanic and Atmospheric Administration’s National Ocean Service (NOAA/NOS) (Coyne et al. 2003). These maps were developed using orthorectified aerial photographs and hyperspectral imagery, and have been used in other fish community-habitat associations (Friedlander and Brown 2003). Three major bottom types representing potential parrotfish habitat were sampled: hard bottom colonized by coral (CH), uncolonized hard bottom (UH), and macroalgae (MA). Within each of these habitat types, random starting points for underwater visual censuses were generated using the Animal Movement extension to ArcView 3.2 (Hooge and Eichenlaub 1997). For each starting point, habitat maps and bathymetry were used to determine a heading along which the survey would proceed, so that the entire survey would stay at a relatively constant depth and within a contiguous patch of habitat.
A total of 140 transects were conducted, with a minimum of 20 transects for each sector (Samoilys and Carlos 2000) (Fig. 1). The number of transects conducted on each shoreline and within each habitat were representative of the potential area available (Table 2). Only habitat patches larger than the transect size were sampled. In the West sector, for example, there were only a few small areas of CH habitat available to sample, and none of these permitted transects through contiguous CH habitat, so no transects of this kind were conducted in this sector.
Survey methods
Prior to surveys, divers were trained to estimate fish size under water using fish models of known length (Bell et al. 1985; Friedlander et al. 2003). A survey team consisted of two divers. The primary responsibility of one diver was to navigate along the pre-determined heading and deploy a 100 m transect tape, while the other diver censused fish in a 5 m × 100 m swath. Transect height was 5 m above the substrate. During the survey, the diver conducting the census visualized the transect ahead and included any individuals that might leave the census area when disturbed. Such avoidance behavior is common in parrotfish, probably influenced by spearfishing with SCUBA (Friedlander et al. 2007). For each transect, parrotfish data were recorded (number, species, total length (TL) to the nearest cm, and color phase). Biomass for parrotfishes on each transect was computed by converting length to weight using the allometric length-weight conversion: W = aLb, where length (L) is expressed in centimeters, weight (W) is expressed in grams, and a and b are constants of allometric growth, obtained through a related study on scarid life history in Hawaii (Howard 2008).
Benthic habitat characteristics were recorded using a one-meter square quadrat, with 25 point intercepts (Friedlander and Brown 2003). The substrate beneath each quadrat point was identified and recorded. Benthic macroorganisms were identified to the species level. For statistical purposes during analyses, substrate features were grouped into seven live categories (live coral, crustose-coralline algae, sponge, filamentous turf, non-turf macroalgae, tunicate, and non-scleractinian anthozoans), and three other categories (sand, rubble and rock). Rugosity measurements were taken using a 10 m chain made of small links (1.3 cm per link) draped carefully to follow the contours of the substrate. The rugosity index was determined as the ratio of the chain length to the horizontal distance covered by the chain, and can be used to compare habitats at different sites (McCormick 1994; Mumby and Wabnitz 2002). Benthic habitat data and rugosity measurements were made at fixed intervals (10, 30, 50, 70, and 90 m) along the transect tape.
Data analysis
Demographic and habitat-related data were analyzed using one-way Analysis of Variance where appropriate, and nonparametric approaches were used for non-normal distributions. Correlation coefficients were used to evaluate relationships between specific habitat variables and scarid numbers and biomasses. Since multiple correlations were performed, sequential Bonferroni tests were used to attain an overall alpha of 0.05 (Rice 1989).
PRIMER (Plymouth Routines in Multivariate Ecological Research) (Clarke and Gorley 2005) multivariate software was used to identify environmental trends in the demographic characteristics of these species (Ohman and Rajasuriya 1998; Clarke et al. 2006). For multivariate statistics, both habitat and scarid community data were 4th root transformed to adjust for non-normality of the data. Bray-Curtis similarity was used to create a rank similarity matrix on habitat characteristic measures and to construct Multi Dimensional Scaling (MDS) ordinations. An MDS ordination is a visual configuration of the samples in two or three dimensions which can satisfy all conditions imposed by the similarity matrix, with relative distance between samples in the configuration relating to relative similarity between those samples (Clarke and Warwick 2001). To test for significant differences among groups, ANOSIM (Analysis of Similarities) permutation tests were performed. RELATE functions were used to test for specific associations between habitat variables and scarid community structure. This approach uses a modified Spearman rank correlation with statistic Global rho (values −1 to 1).
Results
Habitat characteristics
Benthic habitat data collected confirmed that the a priori habitat delineations indicated by the NOS benthic habitat maps were appropriate. One-way ANOVA indicated that areas designated as CH do have significantly more live coral cover than the other habitat types (p<<0.001); they also contain significantly higher percent cover of tunicates (Kruskal-Wallis p = 0.019) and non-scleractinian anthozoans (zoanthid and octocoral) (Kruskal-Wallis p = 0.026). The habitats designated as MA have significantly more macroalgae than the other habitat types (ANOVA p<< 0.001). Filamentous turf best characterized UH habitats. However, ANOSIM revealed a high degree of overlap in habitat attributes between these coarse-scale habitat types, with a Global R of 0.132 (p<< 0.001), where a measure of 1 is total dissimilarity and 0 is complete overlap. This is probably because benthic features such as depth, sand, rubble and rock were indistinguishable among the three course-scale habitat types (Table 3). In comparing the three major habitat types, CH habitat was significantly more rugose than other habitat types, using one-way ANOVA, p = 0.017. Sector identity, as a proxy for exposure, was not significantly correlated with any habitat characteristics within habitat types.
Scarid community structure
Six of the seven scarid species found in Hawaii were observed during our surveys. The only species not observed was Calotomus zonarchus, which is uncommon in the Main Hawaiian Islands except for Kauai. Fifty-four percent of the surveys revealed no parrotfish of any species. The three main fishery species (Scarus rubroviolaceus, Chlorurus perspicillatus, and Calotomus carolinus) made up only a small part of the overall scarid community. Scarus psittacus occurred on 36%, Scarus dubius on 7%, Chlorurus spilurus on 17%, S. rubroviolaceus on 14%, C. perspicillatus on 1%, and C. carolinus on 6% of all transects.
The stratified random sampling design can be used in conjunction with the area of available habitat based on NOAA/NOS benthic maps, to estimate parrotfish numbers and biomass around Oahu. However, these maps are restricted by water clarity and light penetration to relatively shallow depths. Therefore, they generally do not classify habitat deeper than 30 m. Because these maps formed the a priori basis of our stratification scheme, our survey locations were restricted to habitats included in these maps. Estimates of fish abundance therefore apply only to the habitat included in these maps, and do not include deeper or unclassified habitats. Within classified habitats, the estimated numerical abundance of all scarid species combined is 277.3 scarids/ha ± 82.6 fish·ha−1. Based on the potential reef area for all three habitat types around Oahu (24,127 ha) and the relative abundances and biomasses of scarids for each habitat type, we extrapolate that there are approximately 6,690,417 ± 1,992,890 scarids around Oahu (see also Tables 4 and 5). The biomass of all scarid species combined is estimated at 13.07 ± 4.05 kg/ha. The potential area around Oahu predicts a combined biomass of 315,340 ± 97,714 kg of scarids (Table 5). For estimates of numerical abundance and biomass of the six scarid species quantified within classified habitats, see Table 5.
The Oahu scarid community is numerically dominated by small fish. The most numerically abundant species are two of the smallest species, S. psittacus and C. spilurus, (Fig. 2). Within species, the relative size of individuals is small, and the majority of individuals are reproductively immature (Fig. 2). Initial-terminal phase ratios varied considerably among species: S. psittacus 77:1, S. dubius 22:0, S. rubroviolaceus 7:1, C. carolinus 23:1, C. spilurus 120:1, C. perspicillatus 3:1.
Relative abundance (in numbers of scarids) on Oahu in bars. Dots represent mean total length with ±1 s.d. confidence intervals, and horizontal bars represent the mean total length at sexual maturity (Lm) for that species (obtained from FishBase (Froese and Pauly 2008))
Scarid-habitat association
To investigate multivariate associations between scarid community structure and habitat characteristics, a RELATE test was conducted using PRIMER multivariate statistical software to test the null hypothesis that there is no relationship between the scarid community characteristics and specific habitat variables. This null hypothesis was not rejected in terms of scarid biomass (ρ = −0.01, p = 0.991), but was rejected in terms of scarid numerical abundance (ρ = 0.28, p = 0.01). Since the null hypothesis was not rejected for scarid biomass, only scarid numerical abundance was used in the remaining multivariate analyses. A BIO-ENV computation determined which environmental characteristics (including depth and rugosity) most strongly correlated with scarid numerical abundance: live coral and non-scleractinian anthozoans, with a Spearman rank correlation 0.422. This computation was set to test for each habitat variable and all combinations of 1–5 variables for whatever combination or single variable best predicted the relationship. All multivariate statistical procedures used the ten substrate categories, rugosity, and depth as habitat variables.
For associations between scarid communities and specific habitat characteristics, non-parametric tests were used because of the non-normality of the scarid distributions. Kruskal-Wallis tests indicate some association between coarse-scale habitat type and scarid community measures (Fig. 3). CH habitat is associated with significantly more scarids, greater biomass, greater species richness and greater species diversity than the other habitat types investigated. The majority of scarid biomass seems to be associated with CH habitat on the windward shore exposure of Oahu (Mood’s median χ 2 = 9.78, p = 0.020), but there is considerable variation among species (Fig. 4a, b).
Congruent with the BIO-ENV analysis on scarid numerical abundance, there are weak, positive correlations between scarid numerical abundance and rugosity, live coral cover, substrate diversity and crustose coralline algae. There are weak, negative correlations between scarid numerical abundance and (1) proportion of sand, and (2) non-turf macroalgae. Most of these correlations are heavily influenced by the most abundant species: S. psittacus and C. spilurus. Species-specific analyses were performed with biomass rather than numerical abundance to reflect the importance of size structure in the population and for the fishery. Total scarid biomass is correlated in the same pattern as that for numerical abundance, except that substrate diversity is not deemed significant under serial Bonferroni tests. The biomass of S. dubius, the rarest species, was weakly positively correlated with rubble, and C. spilurus was the only species with biomass significantly positively correlated with non-scleractinian anthozoa (Table 6).
Discussion
This study provides the first thorough, broad-scale investigation of scarid communities in Hawaii and presents important evidence for the role of habitat characteristics in scarid distributions. Many reef fish-habitat association studies that included parrotfish have shown little or no evidence of associations between various habitat variables and scarid community measures (Ohman and Rajasuriya 1998; Tolimieri 1998; Gust 2002), suggesting that ecological characteristics of parrotfish (such as social structure or vagility) may limit their habitat associations. If so, reef fish assemblage-habitat association studies may have reduced power to detect these associations where parrotfish predominate. The current study, however, has shown weak but significant associations between parrotfish communities and habitat characteristics.
Since some other studies have not detected significant habitat associations for scarids in other locations, the differences in results may reflect regional differences or differences in methodology, such as the geographic scale of the study. Spatial variation in abundance and distribution of individual parrotfish species has been noted within regions on reefs (Gust et. al. 2001; Hoey and Bellwood 2007), and may contribute to these inconclusive findings. Likewise, regionally-specific species assemblages may confound overall patterns due to biological characteristics of particular clades. For instance, members of the subfamily Sparisomatinae typically feed on fleshy macroalgae and are often sea grass associated, while Scarinae species tend to prefer algal turf and crustose coralline algae and are reef associated (Choat 1991; Streelman et al. 2002).
The results presented here are opposite to those found in a study conducted in American Samoa (Sabater and Tofaeono 2007) where no significant relationship was found between scarids and coral cover, and a positive relationship was found with fleshy macroalgae. While similar in overall methodology, the study in American Samoa covered an area about 1/7th that of the Oahu study, and benthic habitat data were collected using a video camera. Although differences in food preference among species at these locations may be possible, all non-endemic Hawaiian parrotfish species are common in American Samoa, and both locations are dominated by species in the subfamily Scarinae (Page 1998). Further research is needed so that comparisons of scarid-habitat associations among locations where species assemblages are similar can be made, and the factors influencing disparate results can be determined.
Fishing pressure has been implicated in declines of parrotfish population abundances and biomasses in the Caribbean (Hawkins and Roberts 2003; Hughes et al. 2007), Fiji (Jennings et al. 1999b), Tanzania (McClanahan et al. 1999), Seychelles (Jennings et al. 1996) and elsewhere. In the present study, no scarids were found on most transects surveyed, even though many of these surveys were in locations that appeared to contain relatively healthy reefs. In contrast, Hanauma Bay Nature Preserve is the largest no-take reserve on Oahu, and parrotfish biomass in that reserve is ten times that of healthy coral reefs elsewhere on Oahu, according to our present study. This suggests that heavy fishing pressure on Oahu may be an important factor limiting the ability of coastal scarids to exploit the relatively few patches of favorable habitat around Oahu.
The relative impact of various anthropogenic stressors, such as habitat degradation and fishing pressure, are difficult to determine (Jennings et al. 1996). Our data suggest that increased algal abundance and decreased rugosity resulting from a reduction in live coral cover are detrimental to parrotfish populations. For one fished species, S. rubroviolaceus, there are few individuals in the smallest size classes. This could be a result of habitat degradation, where hiding spaces have been lost, reducing the abundance of smaller and more vulnerable fish (Ledlie et al. 2007). However, expected Poisson distributions were found for S. psittacus and C. spilurus, two unfished species of parrotfish, so it is unlikely that the lack of small S. rubroviolaceus individuals is a result of habitat loss. Shifts in fish communities to a greater relative abundance of smaller species with faster turnover have been demonstrated when these communities are exposed to heavy fishing pressure (Jennings et al. 1999a; Hawkins and Roberts 2003). It seems credible that a similar phenomenon may be occurring on Oahu reefs.
Parrotfishes around Oahu, particularly fished species, continue to be stressed. It is evident that parrotfish communities in Hawaii are structured by both habitat and fisheries influences, and future management and conservation efforts should address both issues. Scarid-habitat associations detected were generally weak; these correlations may have been confounded by effects of fishing activity. Failure of some previous studies to find habitat associations for scarids may either indicate regional differences or different behavior of other species. This study demonstrates that specific habitat characteristics, such as topographic relief, are important to parrotfish to maintain their numerical abundance and biomass.
References
Bell JD, Galzin R (1984) Influence of live coral cover on coral-reef fish communities. Mar Ecol Prog Ser 15:265–274
Bell JD, Craik GJS, Pollard DA, Russell BC (1985) Estimating length frequency distributions of large reef fish underwater. Coral Reefs 4:41–44
Bellwood DR, Hughes TP, Folke C, Nystrom M (2004) Confronting the coral reef crisis. Nature 429:827–833
Choat JH (1991) The biology of herbivorous fishes on coral reefs. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic, San Diego, pp 120–155
Clarke KR, Gorley RN (2005) PRIMER. Primer-E Ltd, Plymouth
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation. PRIMER-E, Plymouth
Clarke KR, Chapman MG, Somerfield PJ, Needham HR (2006) Dispersion-based weighting of species counts in assemblage analyses. Mar Ecol Prog Ser 320:11–27
Conklin EJ, Stimson J (2004) An attempt to increase numbers of herbivorous fishes as a means of controlling populations of fleshy macroalgae on coral reefs in Kaneohe Bay, Hawaii. Pac Sci 58:189–200
Coyne MS, Battista TA, Anderson M, Waddell J, Smith W, Jokiel P, Kendell MS, Monaco ME (2003) NOS NCCOS CCMA 152. Benthic Habitats of the Main Hawaiian Islands. In: NOAA (ed), NOAA.
Done TJ (1992) Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247:121–132
Friedlander AM, Brown E (2003) Fish habitat utilization patterns and evaluation of the efficacy of Marine Protected Areas in Hawaii: integration and evaluation of NOS digital benthic habitat maps and reef fish monitoring studies. pp. 79 Year 1 Report, Department of Land and Natural Resources, Division of Aquatic Resources, Honolulu, Hawaii
Friedlander AM, Brown EK, Jokiel PL, Smith WR, Rodgers KS (2003) Effects of habitat, wave exposure, and marine protected area status on coral reef fish assemblages in the Hawaiian archipelago. Coral Reefs 22:291–305
Friedlander AM, Brown EK, Monaco ME (2007) Coupling ecology and GIS to evaluate efficacy of marine protected areas in Hawaii. Ecol Appl 17:715–730
Froese R, Pauly, D (2008) FishBase International Center for Living Aquatic Resources (ICLARM), International Center for Living Aquatic Resources (ICLARM)
Gust N (2002) Scarid biomass on the northern Great Barrier Reef: the influence of exposure, depth and substrata. Environ Biol Fish 64:353–366
Gust N, Choat JH, McCormick MI (2001) Spatial variability in reef fish distribution, abundance, size and biomass: a multi-scale analysis. Mar Ecol Prog Ser 214:237–251
Hart AM, Klumpp DW, Russ GR (1996) Response of herbivorous fishes to crown-of-thorns starfish Acanthaster planci outbreaks. II. Density and biomass of selected species of herbivorous fish and fish-habitat correlations. Mar Ecol Prog Ser 132:21–30
Hawkins JP, Roberts CM (2003) Effects of fishing on sex-changing Caribbean parrotfishes. Biol Conserv 115:213–226
Hoey AS, Bellwood DR (2007) Cross-shelf variation in the role of parrotfishes on the Great Barrier Reef. Coral Reefs 27:37–47
Hooge PN, Eichenlaub B (1997) Animal movement extension to arcview. In: A.S.C.-B.S. Office (ed), U.S. Geological Survey, Anchorage, AK.
Howard KG (2008) Community structure, life history, and movement patterns of parrotfishes: large protogynous fishery species. University of Hawaii-Manoa, Honolulu, p 120
Hughes TP, Bellwood DR, Folke CS, McCook LJ, Pandolfi JM (2007) No-take areas, herbivory and coral reef resilience. Trends Ecol Evol 22:1–3
Jennings S, Boulle DP, Polunin NVC (1996) Habitat correlates of the distribution and biomass of Seychelles' reef fishes. Environ Biol Fish 46:15–25
Jennings S, Greenstreet SPR, Reynolds JD (1999a) Structural change in an exploited fish community: a consequence of differential fishing effects on species with contrasting life histories. J Anim Ecol 68:617–627
Jennings S, Reynolds JD, Polunin NVC (1999b) Predicting the vulnerability of tropical reef fishes to exploitation with phylogenies and life histories. Conserv Biol 13:1466–1475
Ledlie MH, Graham NAJ, Bythell JC, Wilson SK, Jennings S, Polunin NVC, Hardcastle J (2007) Phase shifts and the role of herbivory in the resilience of coral. Coral Reefs 26:641–653
McClanahan TR, Muthiga NA, Kamukuru AT, Machano H, Kiambo RW (1999) The effects of marine parks and fishing on coral reefs of northern Tanzania. Biol Conserv 89:161–182
McCormick M (1994) Comparison of field methods for measuring surface topography and their associations with a tropical reef fish assemblage. Mar Ecol Prog Ser 112:87–96
Mumby PJ (2006) The impact of exploiting grazers (Scaridae) on the dynamics of Caribbean coral reefs. Ecol Appl 16:747–769
Mumby PJ, Wabnitz CCC (2002) Spatial patterns of aggression, territory size, and harem size in five sympatric Caribbean parrotfish species. Environ Biol Fish 63:265–279
Ohman MC, Rajasuriya A (1998) Relationship between habitat structure and fish communites on coral and sandstone reefs. Environ Biol Fish 53:19–31
Page M (1998) The biology, community structure, growth and artisanal catch of parrotfishes of American Samoa, Department of Marine & Wildlife Resources, American Samoa
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Risk MJ (1972) Fish diversity on a coral reef in the Virgin Islands. Atoll Res Bull 153:1–6
Roberts CM, Ormond RF (1987) Habitat complexity and coral reef fish diversity and abundance on Red Sea fringing reefs. Mar Ecol Prog Ser 41:1–8
Rotjan RD, Lewis SM (2008) Impacts of coral predators on tropical reefs. Mar Ecol Prog Ser 367:73–91
Sabater MG, Tofaeono SP (2007) Scale and benthic composition effects on biomass and trophic group distribution of reef fishes in American Samoa. Pac Sci 61:502–520
Samoilys MA, Carlos G (2000) Determining methods of underwater visual census for estimating the abundance of coral reef fishes. Environ Biol Fish 57:289–304
Smith MK (1993) An ecological perspective on inshore fisheries in the main Hawaiian Islands (Fisheries of Hawaii and US-associated Pacific Islands). Mar Fish Rev 55:31–46
Streelman JT, Alfaro M, Westneat MW, Bellwood DR, Karl SA (2002) Evolutionary history of the parrotfishes: biogeography, ecomorphology, and comparative diversity. Evolution 56:961–971
Tolimieri N (1998) Contrasting effects of microhabitat use on large-scale adult abundance in two families of Caribbean reef fishes. Mar Ecol Prog Ser 167:227–239
Williams ID, Polunin NVC (2001) Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs 19:358–366
Williams ID, Polunin NVC, Hendrick VJ (2001) Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar Ecol Prog Ser 222:187–196
Acknowledgements
This study was supported by a contract with the Western Pacific Regional Fishery Management Council. Logistical support was provided by the Hawaii Cooperative Fishery Research Unit, William Aila, and Paul Sensano. Additional field assistance was provided by Kelly Boyle, Danielle Jayewardene, Nicholas Whitney, and Ling Ong. This work benefited from discussion with Alan Friedlander. Charles Birkeland, Kassie Cole, David Carlon, and Matthew McGranaghan provided useful comments on the draft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Howard, K.G., Schumacher, B.D. & Parrish, J.D. Community structure and habitat associations of parrotfishes on Oahu, Hawaii. Environ Biol Fish 85, 175–186 (2009). https://doi.org/10.1007/s10641-009-9478-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-009-9478-3