Abstract
Theory and empirical work suggest that coral reefs may exhibit alternative stable states of coral versus macroalgal dominance. However, it is unclear how dispersal of coral and macroalgae among reefs might impact this bistability and the resilience of the coral-dominated state. We develop a mathematical model to investigate how reef cover dynamics are affected by (1) coral and macroalgal dispersal between two reefs and (2) heterogeneous grazer abundances. We find that at low coral and macroalgal dispersal levels, a new type of stable state emerges with both coral and macroalgae present. Furthermore, we show that a reef abundant with coral and grazers can support a coral-dominated stable state in a second reef depauperate of grazers by dispersal of coral larvae. These results help explain previous empirical findings on reefs once thought to be incongruent with traditional coral-macroalgal alternative stable states theory—such as intermediate coral and macroalgal cover stable states and high coral-low grazing scenarios. Our findings indicate that changing dispersal levels (e.g., due to climate change, reef degradation) between reefs changes the possible stable states and the grazing rate at which the coral-dominated state is predicted to be stable. This work demonstrates the relevance of accounting for the level of dispersal among coral reefs or other bistable ecosystems when designing conservation management plans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many ecosystems are thought to have multiple stable states (Scheffer et al. 2001; Petraitis and Dudgeon 2004), such as arctic tundra ecosystems (vegetated versus exposed soil—McLaren and Jefferies 2004), temperate marine rocky reefs (urchin barrens versus kelp forests—Filbee-Dexter and Scheibling 2014), savannah grasslands (grass-dominated versus sparse vegetation—Belsky 1986; Staver et al. 2011), coral reefs (coral-dominated versus macroalgal-dominated—Knowlton 1992; Scheffer et al. 2001; Mumby et al. 2007a), and freshwater lakes (turbid bream-dominated versus clear pike-dominated—Scheffer 1989). Abrupt transitions between stable states can occur via small changes to environmental conditions (e.g. different grazing rates; Filbee-Dexter and Scheibling 2014; Schmitt et al. 2019) and often occur under different conditions in forward and reverse directions (i.e. hysteresis; Petraitis and Dudgeon 2004; Schröder et al. 2005), making recovery of the system to a prior stable state challenging. Consequently, models of these systems are needed to understand and predict the conditions under which they ‘tip’ to an alternative state (Petraitis 2013). While some mechanisms are understood (e.g. grazing rates on coral reefs; Mumby et al. 2007a), other putative mechanisms are less well understood, such as dispersal among ecosystem patches, which can influence ecosystem states and ultimately recovery trajectories. Understanding the role of dispersal in tipping point dynamics will help predict the resilience of different ecosystem states, where resilience is defined as the capacity of the system to absorb (resist or return from) disturbance without transitioning to an alternative state (Holling 1973; Carpenter et al. 2001; Ives and Carpenter 2007; Côté and Darling 2010). The greater the resilience of the favourable ecosystem state of a bistable ecosystem, the easier it is to ensure the continued persistence of that favourable ecosystem state through management efforts.
Dispersal among habitat patches may increase species persistence by allowing species to track a changing climate and have access to resources (Bailey 2007; Krosby et al. 2010), as well as allow for increased gene flow among populations of the same species—potentially enabling faster adaptation to climate change (Manel et al. 2003; Balkenhol et al. 2015). For marine systems, dispersal relies on ocean hydrodynamics that transport propagules among patches (Harrison and Bjorndal 2006; Cowen and Sponaugle 2009), such as coral reef communities whose composition and functioning depend on dispersal of larvae (coral, reef fish; Jones et al. 2009) and propagules (macroalgae, turf algae) among reefs (Veron 1995; Jones et al. 2009; Paris-Limouzy 2011). Marine species dispersal is changing due to anthropogenic drivers like climate change causing habitat loss (bleaching, rising water levels, etc.; Munday et al. 2009) and shortening larval duration times (O’Connor et al. 2007; Figueiredo et al. 2014). Changes to species dispersal level among patches will affect the degree of connectivity between those patches. Connectivity among marine ecosystems is considered in conservation planning (e.g. 50 reefs initiative—Beyer et al. 2018) but spatial conservation planning has not considered how best to protect patches of bistable ecosystems whose connectivity is changing.
While previous studies have explored the influence of dispersal on alternative stable state ecosystem dynamics, the influence of different dispersal levels on the stability of complex multi-species ecosystems is still not well understood. Dispersal has been shown to increase species diversity (Tilman 1994) with intermediate dispersal levels maximizing biodiversity in metacommunity models (Loreau et al. 2003) but it is not well understood how different dispersal levels may change the existence and composition of stable states in ecosystems with alternative stable states. Starting with the simplest bistable systems and networks, Amarasekare (1998), Kang and Lanchier (2011), and Knipl and Röst (2016) modelled two one-population patches, each with a population that exhibited bistability due to Allee effects showing that the number of stable states increases when the populations are connected. van Nes and Scheffer (2005) took this a step forward and modelled a one-dimensional network of bistable patches (with simple consumer-resource ecosystems) at different dispersal levels and showed that the response of the system to perturbations depends on the direction of change and the heterogeneity of the system. Such analyses may also be pertinent to more speciose and complex alternative stable state ecosystems, such as coral reefs, which are of importance for conservation.
Coral reef ecosystems have long been hypothesized to have alternative stable states (Hughes 1994). Mumby et al. (2007a) showed that a model of coral-macroalgal spatial competition that exhibits coral-macroalgal bistability through grazer-mediated transitions (maintained by hysteresis feedbacks) can qualitatively explain the loss of coral cover on Jamaican reefs that follows from a loss of grazers and hurricane disturbance. In Mumby et al. (2007a)’s model, reefs with high grazing rates have only a coral-dominated stable state, reefs with low grazing rates have only a macroalgal-dominated stable state and at intermediate grazing rates, both the coral-dominated state and the macroalgal-dominated state are stable and separated by a mixed coral-macroalgal state that has mixed stability (a saddle). The model developed by Mumby et al. (2007a) has served as the basis for subsequent models of coral reef cover dynamics that consider the contribution of sponges (González-Rivero et al. 2011), more explicit parrotfish dynamics (Blackwood et al. 2012), and multiple coral species with different responses to thermal stress (Baskett et al. 2014)—among others. Others (e.g. Fung et al. 2011) have also suggested that changing nutrient enrichment and sedimentation levels on coral reefs may also change whether one or both of the coral-dominated and macroalgal-dominated stable states are stable. While some have argued that reefs exhibit a continuum of coral and macroalgal composition rather than bistability (Bruno et al. 2009; Żychaluk et al. 2012) or that there exists greater support for alternative stable states in Caribbean reefs (more likely to be in the necessary parameter range—Bellwood et al. 2004; Hughes et al. 2010; Fung et al. 2011; Roff and Mumby 2012), direct tests of the theory have shown diverging trajectories to either a coral or macroalgal-dominated state on Indo-Pacific reefs (Graham et al. 2015). Also, there have been experimental demonstrations of hysteresis between coral and macroalgal-dominated stable states on Indo-Pacific reefs (Schmitt et al. 2019) and of coral and macroalgal-dominated stable states on Indo-Pacific and Caribbean reefs (Done 1992a,b; Hughes 1994; Nyström et al. 2000; Rogers and Miller 2006; Cheal et al. 2010).
Bistability is often considered in coral reef conservation planning (e.g. Hughes et al. 2017; Steneck et al. 2018)—because the consequences of incorrectly assuming that the system does not have multiple stable states (Dudgeon et al. 2010) leads to conservation actions that could be more detrimental to coral reef health than actions that assume reefs are bistable (Knowlton 1992) (e.g. more stringent management thresholds). If a stable coral-dominated state exists under the conditions present in a particular reef system, it means that a coral-dominated state is an achievable management goal for that system. Knowing the conditions that promote the existence of a stable coral-dominated state in a reef system and knowing how to push the system into the coral-dominated state is thus crucial for coral reef management. Ensuring coral dominance on reefs is crucial for maintaining diverse fish populations (Wilson et al. 2006; Graham and Nash 2013; Darling et al. 2017) which in turn provide food and livelihood for the human communities that live near coral reefs in many parts of the world.
We do not know how present-day changes to macroalgal and coral dispersal (e.g. due to habitat degradation or climate change) will affect the resilience of the coral-dominated stable state in reef systems, particularly how it may interact with the algal grazing rates of herbivores. Coral dominance is thought to be maintained through positive-feedback loops with grazers (more coral, more habitat for grazers; more grazers, more room for coral—Williams and Polunin 2001; Hoey and Bellwood 2011; Mumby et al. 2007b) and macroalgal dominance is maintained through the inhibition of coral recruitment by macroalgae (Hughes 1996; McCook et al. 2001; Kuffner et al. 2006; Evensen et al. 2019). These feedback loops may be affected by the amount of coral and macroalgal recruits present on a reef (Nyström et al. 2000; Carpenter and Edmunds 2006; Mumby 2009) which itself is dependent on the coral and macroalgal dispersal levels that exist among reefs. However, the effects of different dispersal levels on the stability of the coral-dominated stable state have not been fully explored. Previous theoretical models explore the effect of a fixed rate of coral recruitment from external sources (Fung et al. 2011; Fabina et al. 2015), or seasonal vs. constant recruitment of coral from external sources (McManus et al. 2019), or a fixed rate of coral recruitment between two patches (Baskett et al. 2010) or the effect of various rates of external coral and macroalgal recruitment into a single reef (Elmhirst et al. 2009)—but none has explored how changing the levels of coral and macroalgal dispersal between explicit reefs might affect coral reef dynamics.
Given the uncertainty in how coral reef cover dynamics are affected by changing dispersal levels among two bistable reefs, the possibility of new stable states emerging, spatial maintenance of states, or spatial propagation of a regime shift remain unexplored. A first step, and our objective here, is understanding the stability properties and resilience of two bistable reefs with heterogeneous abundances of grazers that are interconnected by dispersal of coral and macroalgae. We develop and analyze a mathematical model that extends Mumby et al. (2007a) to the situation of two reefs connected by dispersal of coral and macroalgae recruits. Specifically, we explore a wide range of coral and macroalgal dispersal levels and herbivore grazing rates to determine at which dispersal levels and grazing rates the system maintains a stable coral-dominated state. We show that at intermediate grazing rates on both reefs, a spatial mosaic of multiple stable states emerges at a low (> 0) dispersal level. When the grazing rates between the two reefs are allowed to differ, dispersal from a high grazing reef can “tip” a low grazing reef from exhibiting a coral-dominated stable state to being bistable. Conversely, dispersal from a high-grazing reef can “tip” a low grazing reef from exhibiting a macroalgal-dominated stable state to being bistable, thereby spatially propagating ecosystem recovery or collapse.
Model
Overview
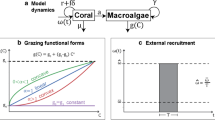
We extended the single-reef cover models of Mumby et al. (2007a) and Elmhirst et al. (2009) to a model of two reefs connected by the dispersal of coral larvae and macroalgal gametes (i.e. recruits) (Fig. 1; Eqs. (1.1–1.4)). We built from these continuous, deterministic models (Mumby et al. 2007a; Elmhirst et al. 2009) because we wanted to see how adding dispersal would alter the stability patterns that they observed. Our model is ‘closed’—all recruits either disperse to the same reef or to the other reef, none are lost and none come from the ‘outside’. The dispersal ‘level’ is defined as the percent of recruits of coral or macroalgae that disperse to the other reef instead of staying in the same reef—i.e. a ‘high coral dispersal level’ means that a high percent of coral recruits disperse to the other reef. In this way, the ‘dispersal level’ may change due to changes in species composition (as different species of coral or macroalgae may be more/less dispersive), changes in ocean current patterns, changes in propagule development time due to temperature changes, or changes in coral reef cover extent (as this may change the distance between reefs). We also incorporate herbivore grazing on macroalgae and turf algae into our model. We focus on grazing and dispersal as these are considered key drivers of coral reef dynamics and are prominent in spatial conservation planning of marine protected areas (Graham et al. 2015; Hughes et al. 2017; Steneck et al. 2018; Beyer et al. 2018). Specifically, we explore three different scenarios (explained below): (1) equal grazing and equal dispersal, (2) equal grazing taxa-specific dispersal, and (3) heterogeneous grazing, equal dispersal. We did not explore the heterogeneous grazing, taxa-specific dispersal scenario as we did not anticipate that the results would qualitatively differ from scenario 3 given that we did not see qualitative differences (i.e. no new stable states) between scenarios 1 and 2.
Description of model
The two-reef model captures how reef cover changes over time for two reefs interconnected by dispersal-modelling changes in the proportional cover of coral (C), macroalgae (M), and turf algae (T) in each of reefs 1 and 2. The dynamics are given by:
with the subscripts 1 and 2 indicating the reef identity and the state variables and parameters as defined in Table 1. The dynamics of turf algae are given implicitly by \(1= {M}_{1}+{C}_{1}+{T}_{1}\) and \(1= {M}_{2}+{C}_{2}+{T}_{2}\)—this model does not allow for any free space. The grazing rate of macroalgae and turf algae by parrotfish, urchins, and other grazers, is represented by gi, where a higher gi indicates a higher abundance of grazers. The \(\frac{{g}_{i}{M}_{i}}{{M}_{i}+{T}_{i}}\) (i:{1,2}) term in Eqs. (1.1 and 1.3) describes the indiscriminate grazing on turf algae and macroalgae by these grazers, specifically showing the loss of macroalgae in each reef due to grazing (see Blackwood et al. 2018). The \(a{M}_{i}{C}_{i}\) (i:{1,2}) term in Eqs. (1.1–1.4) captures the overgrowth of coral by macroalgae and the \(d{C}_{i}\) (i:{1,2}) term in Eqs. (1.1 and 1.3) represents the loss of coral due to natural coral mortality, where a represents the rate macroalgae vegetatively overgrows coral and d represents the rate of natural coral mortality. Dispersal is modelled by parameters qj and pj (j:{M,C}) (Table 1), which control the percent of coral or macroalgal recruits that move from reef 1 to reef 2 or reef 2 to reef 1, respectively. 1-qj or 1-pj (j:{M,C}) indicate the percent of coral or macroalgal recruits that stay in reef 1 or reef 2, respectively. As in Elmhirst et al. (2009), the coral and macroalgal recruits (after arriving at their destination reef) settle onto and displace turf algae (Wilson et al. 2003; Birrell et al. 2005, 2008) at rate ɣ (macroalgae) or rate r (coral). For example, in Eq. (1.1), the expression \(\gamma \left(1-{q}_{m}\right){M}_{1}{T}_{1}\) describes the self-recruitment of macroalgae from reef 1 (1-qm) onto turf present in reef 1, and in Eq. (1.2), the expression \(r{p}_{c}{C}_{2}{T}_{1}\) describes the recruitment of coral from reef 2 (pc) onto turf in reef 1.
Analysis
We analyzed the two-reef model Eqs. (1.1–1.4) to assess how equilibria, stability of equilibria, resilience of stable nodes, and associated reef compositions change in relation to dispersal levels and grazing rates. Equilibria are specific values of coral and macroalgae cover at which no further change in the system occurs, and their stability properties determine whether they attract trajectories (stable node), repel trajectories (unstable node), or repel at least one component of a trajectory (saddles, which tend to separate the basins of attraction surrounding stable nodes) (e.g. see Fig. 2, Supplementary Information—Fig. S1). Because our state variables are constrained to only take values between 0 and 1 (since each state variable is a proportion and since \(1= {M}_{i}+{C}_{i}+{T}_{i}\) for each reef), the resilience of a stable node is easily quantified as the proportion of all possible state values (i.e. state space) that will ultimately converge on that equilibrium (i.e. the stable node’s basin of attraction). This relates directly to the classic definition of the resilience of a state as being its capacity to absorb (resist or return from) disturbance (Holling 1973; Carpenter et al. 2001; Ives and Carpenter 2007; Côté and Darling 2010) as these basins show how far the system can be pushed away from a stable node and still return to it. We conducted our analyses using MATLAB (MATLAB 2019 v. 9.6.0) to determine the equilibria and R (v3.5.3, R Core Team 2019) to simulate the model and visualize the results (see Supplementary Information—S1).
Number and stability of equilibria under scenario 1. The graphs on the left are grazing bifurcation diagrams taken at a variety of different dispersal levels (qm = pm = qc = pc) (indicated by the headers in the grey bars). Grazing bifurcation diagrams show the stability of the equilibria in the system and proportion of coral cover in reef 1 at different grazing rates (g1 = g2). Note that ‘Dispersal Level = 0.06’ means that 6% of the coral and 6% of the macroalgal recruits are dispersing out of their initial reef, to the other reef, while ‘Coral Cover Reef 1’ refers to the proportion of coral cover present in reef 1. The graphs on the right are phase-plane plots that show how the system of differential equations behaves starting from a representative set of initial conditions at the dispersal level indicated and a grazing rate of 0.29 (corresponding to the red box in the bifurcation diagrams). Due to the multidimensionality of the system, some equilibria are hidden under one another in the bifurcation diagram (but see Supplementary Information—Fig. S4 for separation of equilibria by stability). The dots in the bifurcation diagram represent equilibria of the system of equations and their colour represents their stability (white dot indicates unresolved equilibria, i.e. a Jacobian eigenvalue of 0). The phase-plane plots show the equilibria using symbols that are consistent across all 4 plots with the same stability colour-coding as in the bifurcation diagrams. The lines on the phase-plane plots represent different trajectories—each starting at a particular point in the allowable state space (1 = M + C + T, also see ‘Analysis’ section) and progressing to one of the stable nodes (represented by black symbols). The colours of the lines correspond with the type of the stable equilibria that each trajectory lands on
Study design
We examined three different scenarios of grazing rates and dispersal levels, described below. In all of the scenarios (unless otherwise specified), we varied both the grazing rates and dispersal levels. We conducted the stability and resilience analyses for grazing rates that varied from 0.01 to 0.99 (at increments of 0.01) and coral dispersal levels from 0 to 0.99 (at increments of 0.01). This enabled us to look at a large range of grazing values around those that lead to bistability in Mumby et al. (2007a) and Elmhirst et al. (2009) [~ 0.2–0.4] as well as dispersal scenarios that range from two independent reefs to two fully connected reefs. We did not exclude any possible coral dispersal levels since the percentage of coral dispersing is highly variable. It is highly variable because it is dependant on the amount of coral in each reef, size of each reef, and potential dispersal distance of each coral species (i.e. coral pelagic larval duration, for which there is much variability and uncertainty across coral taxa (Baums et al. 2006 (20–30 days); Treml et al. 2008 (up to 80 days); Treml et al. 2012 (up to 60 days); Wood et al. 2014 (up to 120 days); Hock et al. 2017 (7–14 days)). Dispersal level and grazing rate values will be communicated as either low (0–0.2), intermediate (0.2–0.4), or high (0.4–0.99). For simplicity, we focus on symmetric dispersal (the same dispersal rate to and from each reef) because symmetric dispersal kernels have been observed in some coral reef systems and so are not unrealistic (D’Aloia et al. 2015; also used in Baskett et al. 2014) but also because we did not anticipate that asymmetric dispersal would qualitatively change the stability patterns compared to those observed in the symmetric dispersal case. In scenarios 1 and 2, the two reefs are indistinguishable as they are always given identical parameter values. The parameter values for the fixed parameters (Table 1) were all taken from Elmhirst et al. (2009).
Scenario 1: Equal grazing, equal dispersal
In this scenario, the coral and macroalgal dispersal rates are equal and symmetric (qm = pm = qc = pc) and grazing rates in both reefs are the same (g1 = g2). Macroalgal dispersal levels are thought to be low (Deysher and Norton 1981; Stiger and Payri 1999) but it is hard to eliminate the possibility that the levels are similar to that of coral dispersal as not much is known about the spatial scales of macroalgal dispersal (Roff and Mumby 2012). Scenario 1 will serve as the base case.
Scenario 2: Equal grazing, taxa-specific dispersal
Next, we allow dispersal of coral to be higher than macroalgae but maintain equal grazing rates in both reefs (g1 = g2). Here, the percent of coral dispersing (i.e. the coral dispersal level) is higher relative to the percent of macroalgae dispersing (i.e. the macroalgal dispersal level) but dispersal within each taxon are the same to and from each reef (symmetric) (qm = pm < qc = pc). In this scenario, we explore four different levels of macroalgal dispersal (macroalgal dispersal 50%, 25%, 10%, 5% of coral dispersal). These levels were chosen because available data indicates that it is unrealistic for macroalgal dispersal to exceed 50% of that of coral or be effectively non-existent (i.e. lower than 5% of coral dispersal) (Deysher and Norton 1981; Stiger and Payri 1999; Mumby 2006). The ‘10% of coral dispersal’ results are included in the supplementary material as they did not differ much from the 5% level (Supplementary Information—Fig. S2).
Scenario 3: Heterogeneous grazing, equal dispersal
The third scenario investigates the effect of heterogeneous grazing rates among reefs over the range of symmetric dispersal levels. To enable disambiguation of the influence of different dispersal levels from that of heterogeneous grazing rates, the dispersal levels of coral and macroalgae in this scenario are symmetric and equal (qm = pm = qc = pc). Also, to simplify analysis of this scenario, we only considered a few different grazing rates (denoted, ‘grazer levels’)—reef 1 has either a high (g1 = 0.5), intermediate (g1 = 0.3), or low (g1 = 0.1) grazer level while the grazing rate of reef 2 (g2) varies systematically between 0.01 and 0.99. These grazer levels were chosen in line with the grazing levels that were found to lead to coral dominance, bistability, and macroalgal dominance (respectively) by Mumby et al. (2007a) and Elmhirst et al. (2009). This scenario allows exploration of different management scenarios—allowing one to see how the number and stability of equilibria in the system may change when the reefs are under different fishing regimes (for example).
Results
Scenario 1: Assuming symmetric coral and macroalgal recruitment
The inclusion of dispersal between two reefs altered the qualitative coral and macroalgal cover dynamics by changing the possible types of stable states of a single reef and for the two connected reefs. At very low dispersal levels (~ 0%), a coral-dominated reef and a macroalgal-dominated reef are able to coexist as the two reefs behave independently, with each reef exhibiting bistability of coral-dominated and macroalgal-dominated stable states (Fig. 2a; see Supplementary Information—Fig. S4 for more details) at intermediate grazing rates and the system of two reefs having three possible types of stable states (Both Coral-Dominated, Both Macroalgal-Dominated, One Coral-Dominated One Macroalgal-Dominated stable state (OCDOMD state)—Table 3) (Fig. 3d, h, and p). At higher dispersal levels (> 0%–6%), a new mixed coral and macroalgal type of stable state (Both Mixed Coral-Macroalgal Stable State (BMCM state)—Table 3) emerges where both reefs have some coral and some macroalgal cover (Figs. 2b, 3l) at intermediate grazing rates. At these dispersal levels, each reef is tristable at intermediate grazing rates and the system of two reefs again has three possible types of stable states (Both Coral-Dominated, Both Macroalgal-Dominated, BMCM)—though these types of states differ from those at very low (~ 0) dispersal levels (Fig. 3d, h, and l—‘Equal’ column). Finally, at higher dispersal levels (> 6%), the two-reef system behaves as if it is only one reef, with both reefs always having the same stability properties—each reef is bistable at intermediate grazing rates and the system of two reefs only has two possible types of stable states (Both Coral-Dominated, Both Macroalgal-Dominated—Table 3) (Figs. 2c, 3d and h). In summary, across all dispersal-grazing combinations possible under this scenario, the two-reef system has four possible types of stable states but five different possible configurations of those stable states at any particular dispersal-grazing combination (Fig. 3d, h, l, and p): (1) Both Coral-Dominated only, (2) Both Macroalgal-Dominated only, (3) Both Coral-Dominated, Both Macroalgal-Dominated (two-reef system is bistable), (4) Both Coral-Dominated, Both Macroalgal-Dominated, BMCM (two-reef system is tristable), and (5) Both Coral-Dominated, Both Macroalgal-Dominated, OCDOMD (two-reef system is tristable) (Fig. 2).
Basin of attraction size of the different equilibria for scenarios 1 and 2. Each panel is a heat map—the colour of every pixel in the heat maps represents the proportion of the total allowable state space (see bar on right; see ‘Analysis’ section) that is encompassed by the basin of attraction of the stable node(s) representing the type of stable state displayed in that row (‘Both Coral-Dominated’, ‘Both Macroalgal-Dominated, ‘Mixed Coral-Macroalgal’, ‘Coral-Dominated, Macroalgal-Dominated’; see Table 3), at the respective grazing rate and coral dispersal level. Thus, each row depicts where in the parameter space each type of stable state is stable and if you add all of the rows together, it shows where in the parameter space different combinations of stable state types are stable. Grey or N/A pixels indicate that no trajectories in the total allowable state space at that grazing rate and coral dispersal level were attracted to the stable node of that row. The macroalgal dispersal level (pm, qm) of each pixel is either equal to the coral dispersal level (pc, qc) (last column), or 5%/25%/50% respectively (5%, 25%, 50% columns; 10% column in Supplementary Information—Fig. S2). This figure shows the results from scenarios 1 and 2 only. No (C) or (D) type equilibria were observed at a coral dispersal level greater than 0.45, so higher coral dispersal levels are not shown. See Supplementary Information—S1 for more details
Based on our numerical simulation and graphical assessment, the BMCM stable state (Table 3) at low dispersal levels is bounded by a supercritical pitchfork bifurcation at a dispersal level of 0% and a subcritical pitchfork bifurcation at a dispersal level between 6 and 7% (Supplementary Information—Fig. S3). As the dispersal levels change (once dispersal level > 0), the range of grazing rates at which either the Both Coral-Dominated state or the Both Macroalgal-Dominated state is fairly stable, only changing by ~ 0.01 grazing rate in either as the dispersal level changes (Fig. 3d, h, l, and p). The emergence of BMCM stable states at dispersal levels > 6% results in a decrease in size of the basin of attractions for the Both Coral-Dominated stable state and the Both Macroalgal-Dominated stable state (Fig. 3d, h, l, and p). The Both Coral-Dominated stable state has the highest resilience at higher dispersal levels (above 6%) (Fig. 3d, h, l, and p), when the two highly connected reefs behave like a single reef.
Scenario 2: Asymmetric recruitment
The possible types of stable states of the system of two reefs does not change when the assumption of equal recruitment of coral and macroalgae is relaxed (qm = pm < qc = pc; Supplementary Information—Fig. S1; Supplementary Information—Fig. S5 for more details), but the resilience of the BMCM stable states increases as the percent of macroalgae dispersing decreases relative to that of coral (Fig. 3i–l). The resilience of the BMCM stable states increases to encompass ~ 40% of the state space (for particular parameter combinations) as macroalgae connectivity is decreased relative to the coral connectivity, with an accompanying decrease in the resilience of the Both Coral-Dominated stable state and the Both Macroalgal-Dominated stable states (Fig. 3a–d, m–p). Also, the range of dispersal levels in which the BMCM stable states exist (Fig. 3i–l) and the level of coral and macroalgal cover within each reef while in this state (Supplementary Information—Fig. S1) (in the BMCM states) becomes larger as the macroalgae dispersal level decreases relative to the coral dispersal level. In this scenario, the OCDOMD stable states (Table 3) are still only evident at a coral dispersal level of 0% (Fig. 3m–p).
Scenario 3: Heterogeneous grazing
Under this scenario, traditional coral-macroalgal bistability on a reef (à la Mumby et al. 2007a) emerges at all three grazer levels (g1:{0.1, 0.3, 0.5}) but tri-stability only emerges if at least one reef has an intermediate grazing rate (Fig. 4). A key result from this scenario is that a reef with low grazing that would, in isolation, be in the macroalgal-dominated state can instead be in a stable coral-dominated state when it experiences dispersal from a reef that has a high grazing rate [0.31–0.99] (Fig. 4a). However, the opposite scenario is also possible, where a reef with a high grazing rate can be ‘tipped’ to a macroalgal-dominated stable state if connected by high coral and macroalgal dispersal to a reef with a low grazing rate (Fig. 4c), since at high dispersal, the macroalgal-dominated state is also stable in the high grazing rate reef. Essentially, when there is one reef with high grazing connected by coral and macroalgal dispersal to another reef with low grazing, both reefs are bistable and the Both Coral-Dominated state and the Both Macroalgal-Dominated state are both stable states of the two-reef system. The higher the dispersal level, the less coral or macroalgae needed in both reefs to ensure that the Both Coral-Dominated or Both Macroalgal-Dominated states are stable (respectively) (Supplementary Information—Fig. S6) (when there is one reef with high grazing and one with low). The Both Coral-Dominated stable state (Table 3) is the only achievable stable state under intermediate to high dispersal levels when one reef has an intermediate grazing rate and the other has a high grazing rate, or when both have high grazing rates (Fig. 4a–c). The OCDOMD states (Table 3) are still only achievable at dispersal levels of 0% but emerge at all three grazer levels (Fig. 4j–l). The BMCM stable states are the only achievable stable states when one of the reefs has low grazing rates and the other has a high grazing rate and there are low or low-intermediate dispersal levels between the two (Fig. 4g–i). In summary, across all dispersal-grazing combinations possible under this scenario, the two-reef system has four possible types of stable states (Table 3) and eleven different possible configurations of those stable states at any particular dispersal-grazing combination—each type of stable state can be the only achievable stable state and all types of bistability and tristability are achievable except the BMCM states and the OCDOMD states are never both stable (Fig. 4g–i, j–l; Supplementary Information—Fig. S7).
Basin of attraction size of the different equilibria for scenario 3. Each panel is a heat map—the colour of every pixel in the heat maps represents the proportion of the total allowable state space (see bar on right; also see ‘Analysis’ section) that is encompassed by the basin of attraction of the stable node(s) representing the type of stable state displayed in that row (‘Both Coral-Dominated’, ‘Both Macroalgal-Dominated, ‘Mixed Coral-Macroalgal’, ‘Coral-Dominated, Macroalgal-Dominated’; see Table 3), at the respective grazing rate of reef 2 (g2) and coral dispersal level (pc, qc). Thus, each row depicts where in the parameter space each type of stable state is stable and if you add all of the rows together, it shows where in the parameter space different combinations of stable state types are stable. Grey or N/A pixels indicate that no trajectories in the total allowable state space at that grazing rate and coral dispersal level were attracted to the stable node of that row. Grazing rate of reef 1 (g1) of a particular pixel on the heat maps is indicated by the column header (low grazer level (g1 = 0.1), medium grazer level (g1 = 0.3), high grazer level (g1 = 0.5)) and grazing rate of reef 2 of a pixel is indicated by the x-axis. The macroalgal dispersal level (pm, qm) is the same as the coral dispersal level for every pixel. No (D) type equilibria were observed at a coral dispersal level greater than 0.45, so higher coral dispersal levels are not shown in the final row of the figure—the y-axis of the rest of the rows runs from 0 to 0.99. See Supplementary Information—S1 for more details
Discussion
Our results show that the combination of dispersal and local bistability in a system of two interconnected coral reefs is predicted to lead to new dynamics and a new type of stable state—the both mixed coral-macroalgal dominance stable state (BMCM state—Table 3) where both reefs have some coral cover and some macroalgae cover. The model also predicts that the BMCM states are stable at even higher coral dispersal levels when the macroalgal dispersal level is set to be lower than the coral dispersal level (Fig. 3i–l and Supplementary Information—Fig S1) (as is generally thought to be the case—Deysher and Norton 1981; Stiger and Payri 1999; Mumby 2006). These BMCM states are reminiscent of the intermediate stable state (i.e. both populations not extinct or at carrying capacity) that emerges in a model of two bistable populations (bistability arising from Allee effects) when there is dispersal of individuals between the populations (Amarasekare 1998; Kang and Lanchier 2011; Knipl and Röst 2016). The existence of these BMCM states in real coral reef systems has, in the past, been used as evidence against the alternative stable states hypothesis on reefs by various studies (Bruno et al. 2009; Żychaluk et al. 2012 but see Mumby et al. 2013; Jouffray et al. 2015)—Jouffray et al. (2015) even specifically mentioned a third state, though it is unclear if this third state is the same type as the one we describe here. Specifically, these studies find a high proportion of BMCM states in reefs around the world, and our results show that these BMCM states can be interpreted as consistent with the alternative stable states theory with the inclusion of dispersal among explicit reefs.
Our findings demonstrate that considering dispersal between coral reefs is consequential for spatial conservation planning. In particular, at fairly high dispersal levels, reefs with a high abundance of grazers (e.g. protected areas) may tip reefs with few grazers to coral dominance. Yet, such dispersal levels may also allow macroalgal-dominated reefs with few grazers to tip reefs with an abundance of grazers into a macroalgal-dominated state. Thus, the combination of dispersal and spatially heterogeneous grazer abundance, for example arising from fishing and protection, can produce a spatial propagation of coral or macroalgal dominance. This result shows how empirical findings (Williams and Polunin 2001; Aronson and Precht 2006; Mumby 2009; Dudgeon et al. 2010; Donovan et al. 2018) of macroalgal and coral levels associated with grazing rates that seem incongruent with traditional coral-macroalgal alternative stable states theory or that simply found instances of dispersal maintaining coral dominance on low grazing reefs (Done 1992a) may actually not be incongruent with theory (Knowlton 1992; Scheffer et al. 2001; Mumby et al. 2007a) when dispersal is included in the model. Both of our results imply that coral reef management policies meant to ensure coral dominance on a reef should consider not only the abundance of grazers on the reef but also the amount of coral and macroalgal larvae that disperse among reefs.
The results from this study expand on the relationship between grazing rate and reef cover state hypothesized by past theory on single reefs (Mumby et al. 2007a; Elmhirst et al. 2009). Previous models (e.g. Mumby et al. 2007a) have suggested high grazing rates are likely to support a coral-dominated stable state on a single reef and that coral reefs have either a coral-dominated stable state or a macroalgal-dominated stable state. Our results provide a spatial extension to those findings—that a reef abundant with coral and grazers can also support a coral-dominated stable state in a second reef depauperate of grazers via dispersal of coral larvae (Fig. 4a and Supplementary Information – Fig. S6) and that BMCM stable states are possible at low dispersal levels. The existence of these stable BMCM states imply that a stable mosaic of intermediate coral-macroalgal composition is also theoretically possible for coral reef networks, in addition to the stable states of coral or macroalgal dominance. In addition, when the grazing rate and dispersal level are such that these BMCM states are predicted to be stable, the resilience of the ‘Both Coral-Dominated’ and the ‘Both Macroalgal-Dominated’ states (Table 3) are both predicted to be lowered, thereby increasing the threshold grazing rate and dispersal level needed to ensure that only the coral-dominated state is predicted to be stable. Thus, while these BMCM stable states provide other stable states with some coral present, the coral-dominated state is harder to achieve. In this way, these BMCM stable states are not ideal if managers want to ensure that their systems trend towards a coral-dominated stable state but they provide a preferable management objective and some level of protection from the macroalgal-dominated stable state. Also, the finding that the intended benefits of protection may ‘spillover’ to maintain a coral-dominated stable state in surrounding (connected) unprotected reefs that may have fishery removal of grazers is relevant for the design of fisheries and conservation management tools, such as marine reserves. Here, the mechanism is not the spread of grazers from protected areas but rather the movement of incoming coral larvae, which can maintain a coral-dominated state that in turn maintains live coral habitat to support reef fish and fisheries (Harborne et al. 2011; Chong-Seng et al. 2012; Sheppard et al. 2017). However, these results also show that a reef with a lower grazing rate that is connected to one with a high grazing rate may tip that high grazing reef into a macroalgal-dominated state, if the low grazing reef has high macroalgal levels (Supplementary Information—Fig. S6). Which stable state is achieved in these situations depends on the initial level of coral and macroalgae in both reefs. Thus, anthropogenic interventions such as fisheries restrictions on one reef may also affect the stable states obtained in other nearby reefs.
While this study extends previous theory on the resilience of coral reefs, there are nonetheless aspects of coral reef biology that we did not include. For example, we did not incorporate other species that compete for space with coral, turf algae, and macroalgae such as sponges. Past models have shown that alternative stable states with sponges are possible (e.g. sponges—González-Rivero et al. 2011), and so spatial extensions of such work may have analogous results to ours. We also did not consider the effects of other press perturbations that affect coral reefs such as nutrient enrichment, sedimentation, and acidification, which likely alter the parameter spaces in which coral is dominant through their harmful effects on coral persistence (Done 1992a; Chauvin et al. 2011; Fung et al. 2011; Koop et al. 2001; Celis-Plá et al. 2015; Tebbett et al. 2018; Tebbett and Bellwood 2019; Wenger et al. 2020). We also did not explicitly consider pulse perturbations such as bleaching (explored in Baskett et al. 2014; Fabina et al. 2015) that could move the system past an equilibrium of mixed stability (i.e. a saddle) and into the basin of attraction of a different stable state. However, by fully characterizing the state space (Figs. 3 and 4) for different dispersal levels, grazing rates, and cover types, the potential of pulse perturbations such as bleaching events to cause state shifts may be anticipated. For a deeper exploration of press and pulse perturbations in an ecosystem with multiple stability, refer to Ratajczak et al. (2017).
Lastly, we only considered a system of two reefs connected by symmetric dispersal. The amount of pelagic dispersal of coral larvae, macroalgal gametes, and other larvae in the ocean varies over time because oceanic currents, larval production, and ocean temperature (O’Connor et al. 2007; Figueiredo et al. 2014) vary across years and within years. Pelagic larval dispersal between two reefs or among larger networks of reefs may also be asymmetric among reefs due to asymmetric ocean currents or differences in reef size and reef larval production. In this model, to gain a basic idea of the effects of dispersal on the stable states present on coral reefs, we did not consider asymmetric or time-varying dispersal (note that Barnett and Baskett (2015) find that variable recruitment only minorly impacts the resilience of a two patch alternative stable state fish community) but they should be considered in future studies. However, we do not anticipate asymmetric dispersal will qualitatively change the outcomes we see here (i.e. the existence of new stable states or the ability of one reef to tip another), although the parameter space in which we show this to occur is likely to be altered. Time-varying dispersal may cause the stability landscapes we quantified to also vary in time, causing the basins of attraction and even existence of stable equilibria to also vary through time, which obviously would affect the dynamics. Interestingly, McManus et al. (2019) found the average rate of external recruitment more important than whether recruitment was seasonal or constant, implying that approximating the dynamics with constant average dispersal rates may be useful for predicting the resilience of the coral-dominated state under time-varying dispersal. Future work should focus on how a variety of asymmetric, temporally changing dispersal levels affects dynamics in systems of multiple reefs in relation to network structure (McManus et al. 2020) and grazer heterogeneity, particularly considering whether there can be localized pockets of a certain state or not in different spatially explicit networks of connected coral reefs (van Nes and Scheffer 2005; Karatayev and Baskett 2020).
By including many levels of coral and macroalgal dispersal in a standard coral reef cover model, we demonstrate theoretically the possibility of mixed coral-macroalgal (BMCM) stable states and expand the range of conditions that allow for the existence of a coral-dominated stable state on a reef. These results illustrate that a broader set of empirical observations of coral reef states are consistent with coral reef alternative stable states theory (Knowlton 1992; Mumby et al. 2007a) than previously realized—further enforcing the importance of considering its conclusions when making management decisions. Our results suggest that the resilience of coral-dominated states on a particular reef depends on the coral and macroalgal dispersal and grazing rate it experiences and the dispersal, grazing, and reef cover of surrounding reefs. More generally, our results further our understanding of how altering dispersal among bistable ecosystems impacts the stability and existence of the states of a spatially extended bistable ecosystem. Other ecosystems posited to be bistable such as temperate reefs (i.e. urchin barren-kelp ecosystems, Filbee-Dexter and Scheibling 2014; Karatayev and Baskett 2020), savannah grasslands (Belsky 1986), freshwater lakes (Scheffer 1989), and arctic tundra ecosystems (McLaren and Jefferies 2004) might have similar responses to the incorporation of dispersal. Humans are altering marine connectivity via climate change (O’Connor et al. 2007; Figueiredo et al. 2014; Munday et al. 2009) and through habitat destruction (McCauley et al. 2015) at the same time as they are altering fishing levels on coral reefs (Pauly et al. 2002; Srinivasan et al. 2010; Sumaila et al. 2011)—this study illustrates the importance of accounting for connectivity and herbivore abundance as interacting forces, as the effect that each has on the stability of coral-dominated states of a reef is dependent on the level of the other. Thus, careful consideration of coral reef herbivore and dispersal levels in the design of coral reef fisheries and conservation management plans may be essential for conserving coral reef ecosystems and thus sustaining the livelihoods and food security of hundreds of millions of people around the world.
References
Amarasekare P (1998) Interactions between local dynamics and dispersal: insights from single species models. Theor Popul Biol 53:44–59. https://doi.org/10.1006/tpbi.1997.1340
Aronson RB, Precht WF (2006) Conservation, precaution, and Caribbean reefs. Coral Reefs 25:441–450. https://doi.org/10.1007/s00338-006-0122-9
Balkenhol N, Cushman SA, Storfer AT, Waits LP (2015) Introduction to landscape genetics–concepts, methods, applications. In: Balkenhol N, Cushman SA, Storfer AT, Waits LP (eds) Landscape Genetics. Wiley, Hoboken, NJ, pp 1–8
Bailey S (2007) Increasing connectivity in fragmented landscapes: an investigation of evidence for biodiversity gain in woodlands. Forest Ecol Manag 238:7–23. https://doi.org/10.1016/j.foreco.2006.09.049
Barnett LA, Baskett ML (2015) Marine reserves can enhance ecological resilience. Ecol Lett 18:1301–1310. https://doi.org/10.1111/ele.12524
Baskett ML, Nisbet RM, Kappel CV, Mumby PJ, Gaines SD (2010) Conservation management approaches to protecting the capacity for corals to respond to climate change: a theoretical comparison. Glob Change Biol 16:1229–1246. https://doi.org/10.1111/j.1365-2486.2009.02062.x
Baskett ML, Fabina NS, Gross K (2014) Response diversity can increase ecological resilience to disturbance in coral reefs. Am Nat 184:E16–E31. https://doi.org/10.1086/676643
Baums IB, Paris CB, Chérubin LM (2006) A bio-oceanographic filter to larval dispersal in a reef-building coral. Limnol Oceanogr 51:1969–1981. https://doi.org/10.4319/lo.2006.51.5.1969
Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429:827–833. https://doi.org/10.1038/nature02691
Belsky AJ (1986) Population and community processes in a mosaic grassland in the Serengeti, Tanzania. J Ecol 74:841–856. https://doi.org/10.2307/2260402
Beyer HL, Kennedy EV, Beger M, Chen CA, Cinner JE, Darling ES et al (2018) Risk-sensitive planning for conserving coral reefs under rapid climate change. Conserv Lett 11:e12587. https://doi.org/10.1111/conl.12587
Birrell CL, McCook LJ, Willis BL (2005) Effects of algal turfs and sediment on coral settlement. Mar Pollut Bull 51:408–414. https://doi.org/10.1016/j.marpolbul.2004.10.022
Birrell CL, McCook LJ, Willis BL, Diaz-Pulido GA (2008) Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr Mar Biol Annu Rev 46:25–63
Blackwood JC, Hastings A, Mumby PJ (2012) The effect of fishing on hysteresis in Caribbean coral reefs. Theor Ecol 5:105–114. https://doi.org/10.1007/s12080-010-0102-0
Blackwood JC, Okasaki C, Archer A, Matt EW, Sherman E, Montovan K (2018) Modeling alternative stable states in Caribbean coral reefs. Nat Resour Model 31. https://doi.org/10.1111/nrm.12157
Bruno JF, Sweatman H, Precht WF, Selig ER, Schutte VG (2009) Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology 90:1478–1484. https://doi.org/10.1890/08-1781.1
Carpenter S, Walker B, Anderies JM, Abel N (2001) From metaphor to measurement: resilience of what to what? Ecosystems 4:765–781. https://doi.org/10.1007/s10021-001-0045-9
Carpenter RC, Edmunds PJ (2006) Local and regional scale recovery of Diadema promotes recruitment of scleractinian corals. Ecol Lett 9:271–280. https://doi.org/10.1111/j.1461-0248.2005.00866.x
Celis-Plá PS, Hall-Spencer JM, Horta PA, Milazzo M, Korbee N, Cornwall CE, Figueroa FL (2015) Macroalgal responses to ocean acidification depend on nutrient and light levels. Front Mar Sci 2:26. https://doi.org/10.3389/fmars.2015.00026
Chauvin A, Denis V, Cuet P (2011) Is the response of coral calcification to seawater acidification related to nutrient loading? Coral Reefs 30:911. https://doi.org/10.1007/s00338-011-0786-7
Cheal AJ, MacNeil MA, Cripps E, Emslie MJ, Jonker M, Schaffelke B, Sweatman H (2010) Coral–macroalgal phase shifts or reef resilience: links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs 29:1005–1015. https://doi.org/10.1007/s00338-010-0661-y
Chong-Seng KM, Mannering TD, Pratchett MS, Bellwood DR, Graham NA (2012) The influence of coral reef benthic condition on associated fish assemblages. PLoS ONE 7:e42167. https://doi.org/10.1371/journal.pone.0042167
Côté IM, Darling ES (2010) Rethinking ecosystem resilience in the face of climate change. PLoS Biol 8:e1000438. https://doi.org/10.1371/journal.pbio.1000438
Cowen RK, Sponaugle S (2009) Larval dispersal and marine population connectivity. Annu Rev Mar Sci 1:443–466. https://doi.org/10.1146/annurev.marine.010908.163757
D’Aloia CC, Bogdanowicz SM, Francis RK, Majoris JE, Harrison RG, Buston PM (2015) Patterns, causes, and consequences of marine larval dispersal. PNAS 112:13940–13945. https://doi.org/10.1073/pnas.1513754112
Darling ES, Graham NA, Januchowski-Hartley FA, Nash KL, Pratchett MS, Wilson SK (2017) Relationships between structural complexity, coral traits, and reef fish assemblages. Coral Reefs 36:561–575. https://doi.org/10.1007/s00338-017-1539-z
Deysher L, Norton TA (1981) Dispersal and colonization in Sargassum muticum (Yendo) Fensholt. J Exp Mar Biol Ecol 56:179–195. https://doi.org/10.1016/0022-0981(81)90188-X
Done T (1992a) Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247:121–132. https://doi.org/10.1007/BF00008211
Done T (1992b) Constancy and change in some great barrier Reef coral communities: 1980–1991. Am Zool 32:655–662. https://doi.org/10.1093/icb/32.6.655
Donovan MK, Friedlander AM, Lecky J, Jouffray JB, Williams GJ, Wedding LM et al (2018) Combining fish and benthic communities into multiple regimes reveals complex reef dynamics. Sci Rep 8:1–11. https://doi.org/10.1038/s41598-018-35057-4
Dudgeon SR, Aronson RB, Bruno JF, Precht WF (2010) Phase shifts and stable states on coral reefs. Mar Ecol Prog Ser 413:201–216. https://doi.org/10.3354/meps08751
Elmhirst T, Connolly SR, Hughes TP (2009) Connectivity, regime shifts and the resilience of coral reefs. Coral Reefs 28:949–957. https://doi.org/10.1007/s00338-009-0530-8
Evensen NR, Doropoulos C, Wong KJ, Mumby PJ (2019) Stage-specific effects of Lobophora on the recruitment success of a reef-building coral. Coral Reefs 38:489–498. https://doi.org/10.1007/s00338-019-01804-w
Fabina NS, Baskett ML, Gross K (2015) The differential effects of increasing frequency and magnitude of extreme events on coral populations. Ecol Appl 25:1534–1545. https://doi.org/10.1890/14-0273.1
Figueiredo J, Baird AH, Harii S, Connolly SR (2014) Increased local retention of reef coral larvae as a result of ocean warming. Nat Clim Change 4:498–502. https://doi.org/10.1038/nclimate2210
Filbee-Dexter K, Scheibling RE (2014) Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar Ecol Prog Ser 495:1–25. https://doi.org/10.3354/meps10573
Fung T, Seymour RM, Johnson CR (2011) Alternative stable states and phase shifts in coral reefs under anthropogenic stress. Ecology 92:967–982. https://doi.org/10.1890/10-0378.1
González-Rivero M, Yakob L, Mumby PJ (2011) The role of sponge competition on coral reef alternative steady states. Ecol Model 222:1847–1853. https://doi.org/10.1016/j.ecolmodel.2011.03.020
Graham NAJ, Nash KL (2013) The importance of structural complexity in coral reef ecosystems. Coral Reefs 32:315–326. https://doi.org/10.1007/s00338-012-0984-y
Graham NAJ, Jennings S, MacNeil MA, Mouillot D, Wilson SK (2015) Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518:94–97. https://doi.org/10.1038/nature14140
Harborne AR, Mumby PJ, Kennedy EV, Ferrari R (2011) Biotic and multi-scale abiotic controls of habitat quality: their effect on coral-reef fishes. Mar Ecol Prog Ser 437:201–214. https://doi.org/10.3354/meps09280
Harrison A, Bjorndal KA (2006) Connectivity and wide-ranging species in the ocean. Conservation Biology Series-Cambridge 14:213
Hock K, Wolff NH, Ortiz JC, Condie SA, Anthony KR, Blackwell PG, Mumby PJ (2017) Connectivity and systemic resilience of the Great Barrier Reef. PLoS Biol 15:e2003355. https://doi.org/10.1371/journal.pbio.2003355
Hoey AS, Bellwood DR (2011) Suppression of herbivory by macroalgal density: a critical feedback on coral reefs? Ecol Lett 14:267–273. https://doi.org/10.1111/j.1461-0248.2010.01581.x
Holling CS (1973) Resilience and stability of ecological systems. Annu Rev Ecol Syst 4:1–24
Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551. https://doi.org/10.1126/science.265.5178.1547
Hughes TP (1996) Demographic approaches to community dynamics: a coral reef example. Ecology 77:2256–2260. https://doi.org/10.2307/2265718
Hughes TP, Graham NAJ, Jackson JB, Mumby PJ, Steneck RS (2010) Rising to the challenge of sustaining coral reef resilience. TREE 25:633–642. https://doi.org/10.1016/j.tree.2010.07.011
Hughes TP, Barnes ML, Bellwood DR, Cinner JE, Cumming GS, Jackson JB et al (2017) Coral reefs in the Anthropocene. Nature 546:82–90. https://doi.org/10.1038/nature22901
Ives AR, Carpenter SR (2007) Stability and diversity of ecosystems. Science 317:58–62. https://doi.org/10.1126/science.1133258
Jones GP, Almany GR, Russ GR, Sale PF, Steneck RS, Van Oppen MJH, Willis BL (2009) Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges. Coral Reefs 28:307–325. https://doi.org/10.1007/s00338-009-0469-9
Jouffray JB, Nyström M, Norström AV, Williams ID, Wedding LM, Kittinger JN, Williams GJ (2015) Identifying multiple coral reef regimes and their drivers across the Hawaiian archipelago. Philos T R Soc B 370:20130268. https://doi.org/10.1098/rstb.2013.0268
Kang Y, Lanchier N (2011) Expansion or extinction: deterministic and stochastic two-patch models with Allee effects. J Math Biol 62:925–973. https://doi.org/10.1007/s00285-010-0359-3
Karatayev VA, Baskett ML (2020) At what spatial scales are alternative stable states relevant in highly interconnected ecosystems? Ecology 101:e02930. https://doi.org/10.1002/ecy.2930
Knipl D, Röst G (2016) Spatially heterogeneous populations with mixed negative and positive local density dependence. Theor Popul Biol 109:6–15. https://doi.org/10.1016/j.tpb.2016.01.001
Knowlton N (1992) Thresholds and multiple stable states in coral reef community dynamics. Am Zool 32:674–682. https://doi.org/10.1093/icb/32.6.674
Koop K, Booth D, Broadbent A, Brodie J, Bucher D, Capone D et al (2001) ENCORE: the effect of nutrient enrichment on coral reefs. Synthesis of results and conclusions. Mar Pollut Bull 42:91–120. https://doi.org/10.1016/S0025-326X(00)00181-8
Krosby M, Tewksbury J, Haddad NM, Hoekstra J (2010) Ecological connectivity for a changing climate. Conserv Biol 24:1686–1689
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS (2006) Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:07–117. https://doi.org/10.3354/meps323107
Loreau M, Mouquet N, Gonzalez A (2003) Biodiversity as spatial insurance in heterogeneous landscapes. PNAS 100:12765–12770. https://doi.org/10.1073/pnas.2235465100
Manel S, Schwartz MK, Luikart G, Taberlet P (2003) Landscape genetics: combining landscape ecology and population genetics. TREE 18:189–197. https://doi.org/10.1016/S0169-5347(03)00008-9
MATLAB (2019) version 9.6.0 (R2019a). Natick, Massachusetts: The MathWorks Inc
McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH, Warner RR (2015) Marine defaunation: animal loss in the global ocean. Science 347:6219. https://doi.org/10.1126/science.1255641
McCook L, Jompa J, Diaz-Pulido G (2001) Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 19:400–417. https://doi.org/10.1007/s003380000129
McLaren JR, Jefferies RL (2004) Initiation and maintenance of vegetation mosaics in an Arctic salt marsh. J Ecol 92:648–660. https://doi.org/10.1111/j.0022-0477.2004.00897.x
McManus LC, Watson JR, Vasconcelos VV, Levin SA (2019) Stability and recovery of coral-algae systems: the importance of recruitment seasonality and grazing influence. Theor Ecol 12:61–72. https://doi.org/10.1007/s12080-018-0388-x
McManus LC, Vasconcelos VV, Levin SA, Thompson DM, Kleypas JA, Castruccio FS et al (2020) Extreme temperature events will drive coral decline in the Coral Triangle. Glob Change Biol 26:2120–2133. https://doi.org/10.1111/gcb.14972
Munday PL, Leis JM, Lough JM, Paris CB, Kingsford MJ, Berumen ML, Lambrechts J (2009) Climate change and coral reef connectivity. Coral Reefs 28:379–395. https://doi.org/10.1007/s00338-008-0461-9
Mumby PJ (2006) The impact of exploiting grazers (Scaridae) on the dynamics of Caribbean coral reefs. Ecol Appl 16:747–769. https://doi.org/10.1890/1051-0761(2006)016[0747:TIOEGS]2.0.CO;2
Mumby PJ, Hastings A, Edwards HJ (2007a) Thresholds and the resilience of Caribbean coral reefs. Nature 450:98. https://doi.org/10.1038/nature06252
Mumby PJ, Harborne AR, Williams J, Kappel CV, Brumbaugh DR, Micheli F et al (2007b) Trophic cascade facilitates coral recruitment in a marine reserve. PNAS 104:8362–8367. https://doi.org/10.1073/pnas.0702602104
Mumby PJ (2009) Phase shifts and the stability of macroalgal communities on Caribbean coral reefs. Coral Reefs 28:761–773. https://doi.org/10.1007/s00338-009-0506-8
Mumby PJ, Steneck RS, Hastings A (2013) Evidence for and against the existence of alternate attractors on coral reefs. Oikos 122:481–491. https://doi.org/10.1111/j.1600-0706.2012.00262.x
Nyström M, Folke C, Moberg F (2000) Coral reef disturbance and resilience in a human-dominated environment. TREE 15:413–417. https://doi.org/10.1016/S0169-5347(00)01948-0
O’Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, Kinlan BP, Weiss JM (2007) Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. PNAS 104:1266–1271. https://doi.org/10.1073/pnas.0603422104
Paris-Limouzy CB (2011) Reef interconnectivity/larval dispersal. In: Cabioch G, Davies P, Done T, Gischler E, Macintyre IG, Wood R, Woodroffe C (eds) Encyclopedia of modern coral reefs: structure, form and process. Berlin, Germany, pp 881–889
Pauly D, Christensen V, Guénette S, Pitcher TJ, Sumaila UR, Walters CJ et al (2002) Towards sustainability in world fisheries. Nature 418:689–695. https://doi.org/10.1038/nature01017
Petraitis PS, Dudgeon SR (2004) Detection of alternative stable states in marine communities. J Exp Mar Biol Ecol 300:343–371. https://doi.org/10.1016/j.jembe.2003.12.026
Petraitis P (2013) Multiple stable states in natural ecosystems. Oxford, UK
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ratajczak Z, D’Odorico P, Collins SL, Bestelmeyer BT, Isbell FI, Nippert JB (2017) The interactive effects of press/pulse intensity and duration on regime shifts at multiple scales. Ecol Monogr 87:198–218. https://doi.org/10.1002/ecm.1249
Roff G, Mumby PJ (2012) Global disparity in the resilience of coral reefs. TREE 27:404–413. https://doi.org/10.1016/j.tree.2012.04.007
Rogers CS, Miller J (2006) Permanent “phase shifts” or reversible declines in coral cover? Lack of recovery of two coral reefs in St. John, US Virgin Islands. Mar Ecol Prog Ser 306:103–114. https://doi.org/10.3354/meps306103
Scheffer M (1989) Alternative stable states in eutrophic, shallow freshwater systems: a minimal model. Hydrobiol Bull 23:73–83. https://doi.org/10.1007/BF02286429
Scheffer M, Carpenter S, Foley JA, Folke C, Walker B (2001) Catastrophic shifts in ecosystems. Nature 413:591. https://doi.org/10.1038/35098000
Schmitt RJ, Holbrook SJ, Davis SL, Brooks AJ, Adam TC (2019) Experimental support for alternative attractors on coral reefs. PNAS 116:4372–4381. https://doi.org/10.1073/pnas.1812412116
Schröder A, Persson L, De Roos AM (2005) Direct experimental evidence for alternative stable states: a review. Oikos 110:3–19. https://doi.org/10.1111/j.0030-1299.2005.13962.x
Sheppard C, Davy S, Pilling G, Graham N (2017) The biology of coral reefs. Oxford, UK
Srinivasan UT, Cheung WW, Watson R, Sumaila UR (2010) Food security implications of global marine catch losses due to overfishing. J Bioecon 12:183–200. https://doi.org/10.1007/s10818-010-9090-9
Staver AC, Archibald S, Levin SA (2011) The global extent and determinants of savanna and forest as alternative biome states. Science 334:230–232. https://doi.org/10.1126/science.1210465
Steneck RS, Mumby PJ, MacDonald C, Rasher DB, Stoyle G (2018) Attenuating effects of ecosystem management on coral reefs. Sci Adv 4:eaao5493. https://doi.org/10.1126/sciadv.aao5493
Stiger V, Payri CE (1999) Spatial and temporal patterns of settlement of the brown macroalgae Turbinaria ornata and Sargassum mangarevense in a coral reef on Tahiti. Mar Ecol Prog Ser 191:91–100. https://doi.org/10.3354/meps191091
Sumaila UR, Cheung WW, Lam VW, Pauly D, Herrick S (2011) Climate change impacts on the biophysics and economics of world fisheries. Nature Clim Change 1:449–456. https://doi.org/10.1038/nclimate1301
Tebbett SB, Bellwood DR, Purcell SW (2018) Sediment addition drives declines in algal turf yield to herbivorous coral reef fishes: implications for reefs and reef fisheries. Coral Reefs 37:929–937. https://doi.org/10.1007/s00338-018-1718-6
Tebbett SB, Bellwood DR (2019) Algal turf sediments on coral reefs: what’s known and what’s next. Mar Pollut Bull 149:110542. https://doi.org/10.1016/j.marpolbul.2019.110542
Tilman D (1994) Competition and biodiversity in spatially structured habitats. Ecology 75:2–16. https://doi.org/10.2307/1939377
Treml EA, Halpin PN, Urban DL, Pratson LF (2008) Modeling population connectivity by ocean currents, a graph-theoretic approach for marine conservation. Landscape Ecol 23:19–36. https://doi.org/10.1007/s10980-007-9138-y
Treml EA, Roberts JJ, Chao Y, Halpin PN, Possingham HP, Riginos C (2012) Reproductive output and duration of the pelagic larval stage determine seascape-wide connectivity of marine populations. Integr Comp Biol 52:525–537. https://doi.org/10.1093/icb/ics101
van Nes EH, Scheffer M (2005) Implications of spatial heterogeneity for catastrophic regime shifts in ecosystems. Ecology 86:1797–1807. https://doi.org/10.1890/04-0550
Veron JEN (1995) Corals in space and time: the biogeography and evolution of the Scleractinia. Ithaca, USA
Wenger AS, Harris D, Weber S, Vaghi F, Nand Y, Naisilisili W et al (2020) Best-practice forestry management delivers diminishing returns for coral reefs with increased land-clearing. J Appl Ecol 57:2381–2392. https://doi.org/10.1111/1365-2664.13743
Williams I, Polunin N (2001) Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs 19:358–366. https://doi.org/10.1007/s003380000121
Wilson SK, Bellwood DR, Choat JH, Furnas MJ (2003) Detritus in the epilithic algal matrix and its use by coral reef fishes. Oceanogr Mar Biol 41:279–310
Wilson SK, Graham NA, Pratchett MS, Jones GP, Polunin NV (2006) Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Glob Change Biol 12:2220–2234. https://doi.org/10.1111/j.1365-2486.2006.01252.x
Wood S, Paris CB, Ridgwell A, Hendy EJ (2014) Modelling dispersal and connectivity of broadcast spawning corals at the global scale. Global Ecol Biogeogr 23:1–11. https://doi.org/10.1111/geb.12101
Żychaluk K, Bruno JF, Clancy D, McClanahan TR, Spencer M (2012) Data-driven models for regional coral-reef dynamics. Ecol Lett 15:151–158. https://doi.org/10.1111/j.1461-0248.2011.01720.x
Acknowledgements
We wish to thank Compute Canada for providing computational resources and Z. Ahmed for his guidance in this process. We wish to thank A. McLeod and E. Tekwa for helpful comments on the project.
Funding
This research was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Canada Graduate Scholarship to A.G. and NSERC Discovery Grants (no. 4922 to M.K. and no. 5134 to M.-J. F.) and Canada Research Chairs to M.K and M.-J.F.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. A.G. led the design of the study, ran the model and wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Greiner, A., S. Darling, E., Fortin, MJ. et al. The combined effects of dispersal and herbivores on stable states in coral reefs. Theor Ecol 15, 321–335 (2022). https://doi.org/10.1007/s12080-022-00546-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-022-00546-w