Abstract
Extreme disturbances often lead to community reorganisations, yet sometimes ecosystems unexpectedly fail to recover. Such surprising outcomes may pinpoint important yet overlooked mechanisms that drive ecosystems into undesirable states. Using long-term field observations, experimental manipulations and mechanistic modelling, we document the drivers of an unexpected phase shift from coral to macroalgal dominance following typhoon disturbance on reefs in Palau (Micronesia). After extensive coral mortality, an ephemeral bloom of a canopy-forming macroalga (Liagora sp.) provided physical refuge from herbivore grazing, resulting in the establishment of a secondary, understory macroalga (Lobophora spp.). After disappearance of Liagora canopies and resulting loss of grazing refuge, the Lobophora patches continued to expand and led to a macroalgal (Lobophora-) dominated state that has persisted for more than 2 years. We developed a mechanistic model of Lobophora patch dynamics parameterised with rates of growth measured in situ to simulate the observed proliferation of Lobophora under variable grazing refuges in space and time. Model simulations showed that short-term escapes from grazing were pivotal in allowing establishment of patches of Lobophora. Ephemeral grazing refuges created an opportunity to reach a cover above which Lobophora growth exceeds grazing, so that Lobophora could expand after disappearance of Liagora canopies. Critically, in the absence of grazing refuge, herbivore biomass was sufficient to prevent the establishment of Lobophora patches. Our model demonstrates that with rapid algal growth and low grazing, a relatively minor grazing refuge (6 month) is sufficient to escape herbivore control after extensive coral mortality, leading to unexpected recovery failure. Transient fluctuations in the intensity of control mechanisms, such as herbivore grazing, can have disproportionate and long-lasting effects on community structure. Overall, this study stresses that our perception of reef dynamics must integrate the time scales at which reefs can be sensitive to transient changes in mechanisms promoting coral dominance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In an era of rapid environmental change, predicting the dynamics of ecological systems is critical in anticipating the potential loss of ecosystem functions and services. As an emerging science, predictive systems ecology advocates the development of mechanistic, process-based ecological models to forecast future changes in ecosystem state and functioning (Cuddington and others 2013; Evans and others 2013). Yet predicting the behaviour of ecological systems is challenging because of the complexity of ecological interactions and variability of fluctuating environments, and sometimes ecosystems respond unexpectedly to disturbances. Examples of such ecological surprises (Paine and others 1998) include the unanticipated disruption of species interactions or demographic rates (Doak and others 2008; Lindenmayer and others 2010), which can scale up to ecosystem-level shifts in structure and function (Holling 1996; Scheffer and Carpenter 2003). Because such community shifts often lead to undesirable ecosystem states that might be difficult to reverse, their anticipation is the focus of increasing research efforts (Scheffer and others 2009).
Ecological surprises refer to disparity between the observed behaviour of a system and a priori expectations, and as such do not reflect pure ignorance but rather contradict well-established knowledge about the dynamics of a system (Doak and others 2008). Although ecological surprises are often treated as outliers because they perform outside a range of expectations, they may actually identify important yet overlooked mechanisms and even challenge the current understanding of ecosystem functioning (Lindenmayer and others 2010). Surprising ecological outcomes after disturbances are particularly problematic because they challenge the ability to predict recovery trajectories of nonequilibrial systems. Unanticipated failure of (or unusual pathways to) recovery has been linked to the synergistic effects of compounded perturbations (Paine and others 1998; Denny and others 2009), whereby the temporal alignment of multiple stressors affects the expected sequence of ecological succession. Extreme values, levels of variance, and complex temporal patterns of a single environmental variable can affect demographic rates significantly (Benedetti-Cecchi 2003; Denny and others 2009). Yet model-based explorations of system dynamics are often conducted by simulating the average effects of environmental or ecological drivers, with little attempt at integrating more complex scenarios whereby the driving process exhibits some specified temporal trend (Gaines and Denny 1993; Denny and others 2009). Moreover, the effect of changing the temporal scale of the modelled processes is rarely considered, yet different time steps may generate different outcomes depending on the transient dynamics of control mechanisms (Hastings 2004). Accounting for the transient dynamics of ecological controls in model simulations may help explaining the emergence of surprising outcomes in natural systems. This is important as ecological surprises may become more prevalent in the future with more intense and frequent disturbances (Williams and Jackson 2007; Lindenmayer and others 2010).
Coral reefs are one of the several model ecosystems to study nonequilibrium dynamics and community shifts (Scheffer and Carpenter 2003; Mumby and others 2007). Healthy reefs are typically dominated by scleractinian corals which maintain a carbonate framework that provides habitat for a multitude of organisms and support ecosystem functions such as productive fisheries and coastal protection. Resilient reef ecosystems generally recover from acute disturbances (for example, storms) and a central component to successful coral recovery is the intensity of grazing that promotes competitive dominance of corals over fleshy macroalgae (Bellwood and others 2004; McManus and Polsenberg 2004; Mumby and Steneck 2008). Coral mortality events open up the reef substratum to new colonisation, and insufficient grazing after disturbance can result in the establishment of macroalgae. Such a transition from coral to macroalgal dominance in response to acute disturbance represents a phase shift (sensu Done 1992), and persistence of a macroalgal-dominated state can reveal the existence of an alternative attractor created by reinforcing (that is, positive) ecological feedbacks (Mumby and Steneck 2008). In particular, abundant macroalgae deter coral settlement and decrease post-settlement coral survival (Kuffner and others 2006; Doropoulos and others 2016). Furthermore, fish herbivores are more efficient at consuming the initial (that is, diminutive algal turf) rather than later stages of algal successions (for example, Steneck 1988), so that large fleshy macroalgae are difficult to remove from reefs once established. Understanding the dynamics of coral–algal phase shifts is therefore of critical importance to anticipate and perhaps avoid critical transitions that could be potentially difficult to reverse.
Owing to the multiplicity of factor affecting coral and macroalgal demographics and their interaction, identifying the driving mechanisms of coral–algal phase shifts using experimental approaches is challenging. Yet, model simulation of incremental changes in one or multiple ecological drivers, such as grazing intensity (Mumby and others 2007; Fung and others 2011; Sandin and McNamara 2012; Bozec and others 2016), algal productivity (Fung and others 2011; Mumby and others 2014), or coral growth and mortality (Anthony and others 2011; Bozec and Mumby 2015), allows the systematic exploration of environmental conditions favouring macroalgal dominance. Although simulation-based investigations of coral–algae equilibria have improved the understanding of critical transitions on coral reefs, such approaches tend to treat reef ecosystems as a linear set of joint parameter values and ignore the potential impact of the transient dynamics of control mechanisms (Scheffer and others 2008). Investigating the importance of transient time scales on coral-reef dynamics may help explain unexpected pathways towards macroalgal dominance.
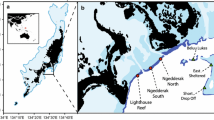
To date, most accounts of coral–algal phase shifts have occurred in the Caribbean where reefs are less resilient and with a predilection towards rapid macroalgal blooms (Roff and Mumby 2012; but see Cheal and others 2010; Graham and others 2015). However, a recent case study challenges our current understanding about critical transitions on coral reefs. In Palau (Micronesia, West Pacific), a relatively intact and supposedly resilient reef environment (Golbuu and others 2007) has undergone a macroalgal phase shift following catastrophic typhoon impacts to coral populations (Doropoulos and others 2014; Roff and others 2015a, b). In early December 2012, super-typhoon Bopha caused an almost complete loss of corals along the entire eastern barrier reef (~ 150 km). Several weeks after the typhoon, a bloom of the upright, red foliose macroalga Liagora sp. (Figure 1A) persisted for 6 months, as is typical for this alga following severe storms (for example, Hughes 1994). The ephemeral Liagora canopies triggered secondary macroalgal succession to the encrusting fleshy brown macroalga Lobophora spp. (Figure 1B). Experimental manipulations during the Liagora bloom (Roff and others 2015b) revealed that its canopy created a localised refuge from fish grazing which appeared to facilitate the colonisation of Lobophora as an understory species. Yet, the Lobophora population continued to expand even after Liagora disappeared (Roff and others 2015b) and has continued to maintain a substantive cover for at least 2 years post-typhoon (Figure 1C). Interestingly, coral recovery has been limited on these reefs likely in part because of the high sensitivity of some Pacific corals to allelopathic interactions with Lobophora (Mumby and others 2016; Vieira and others 2016; Doropoulos and others 2017).
A Bloom of the red foliose macroalga Liagora sp. following super-typhoon Bopha and subsequent coral loss. B Patch of the encrusting fleshy brown macroalga Lobophora spp. growing under the Liagora canopies. C Mean (± SE) percentage cover of Liagora, Lobophora and hard corals in the three reef sites (n = 9 transects per survey) between March 2012 and October 2014. D Timeline of fish and benthic surveys (open circles: Doropoulos and others 2014; Roff and others 2015a, b; filled circle: this study) and the herbivore-exclusion experiment (this study) (Color figure online).
The existence of a persistent Lobophora bloom on a forereef environment in Palau appears to be unprecedented in recent history (Roff and others 2015b). Specifically, this macroalgal phase shift was unexpected because a previous episode of extensive coral loss occurred with the 1998 bleaching event and was not followed by a bloom of Lobophora (Bruno and others 2001; Golbuu and others 2007). Moreover, one might anticipate reefs that became even more resilient during the decade preceding the typhoon due to prohibition of fishing. Here, we ask whether this unanticipated phase shift from coral to Lobophora dominance can be explained by the transient time scale and magnitude of the refuge from grazing; is a severe six-month reduction in grazing within Liagora canopies sufficient to allow Lobophora to further escape control by herbivores at the reef scale? We develop and test a mechanistic understanding of the facilitative effect of Liagora on Lobophora described by Roff and others (2015b) by quantifying the importance, timing and limits of the demographic processes involved in this macroalgal phase shift. We conducted additional experiments and used more detailed field observations to assess the bottom-up and top-down controls of macroalgal dynamics. We then created a simulation model of algal patch dynamics to evaluate the relative contribution of different demographic mechanisms to the reef-scale expansion of Lobophora, where mechanisms include rates of macroalgal recruitment and growth, empirical estimates of herbivory, and an explicit escape from grazing in time and space. Overall, we demonstrate the importance of an ephemeral protection from grazing for macroalgal expansion and emphasise the relevance of assessing the transient dynamics in nonequilibrium systems to reduce surprise.
Methods
We develop a process-based (mechanistic) approach to understand the dynamics of the macroalgal (Lobophora) expansion documented by Roff and others (2015b) on Eastern Palauan reefs. A necessary step was to capture the bottom-up and top-down controls of algal dynamics in situ. We first quantified macroalgal productivity during the phase shift using a long-term (27 months) herbivore-exclusion experiment deployed at the study site. Second, we analysed the biomass composition of herbivorous fish assemblages to explore their variability in time and space and capture the functional characteristics of herbivore grazing at the reef scale. Third, we built a mechanistic model of algal dynamics with the empirical rates of algal demographics and reconstructed by simulation the post-typhoon expansion of Lobophora with an explicit grazing escape provided by ephemeral Liagora canopies. We then used the model to circumscribe the functional levels of grazing intensity on the reefs conditional to an acceptable fit to observations and tested an alternative scenario whereby no ephemeral escape from grazing would have occurred after the typhoon. Finally, we investigated the relative importance of macroalgal growth, recruitment, grazing and physical refuge from grazing to Lobophora dynamics through sensitivity analysis, and explored by simulation the impact of a grazing escape at different scales of space and time.
Study Site
The study took place at Lighthouse Reef and Ngederrak Reef located on the eastern side of Palau (Figure S1). These reefs have demonstrated a remarkable ability to recover high cover of diverse branching acroporid corals following extensive coral mortality (Golbuu and others 2007). The two reefs have a different management status, with Ngederrak Reef being a no-entry, no-fishing zone since 2001. Previous studies (Doropoulos and others 2014; Roff and others 2015a, b) have surveyed benthic cover and roving herbivorous fish (that is, all scarids, acanthurids and siganids) on the forereef slope (depth 4–6 m) of three sites located about 1 km apart (Lighthouse North, Ngederrak South, Ngederrak North). As a result, data on benthic cover and herbivorous fish biomass data are available before and after category 5 typhoon Bopha caused widespread coral losses in early December 2012 (Figure 1D).
Herbivore-Exclusion Experiment
Macroalgal growth rates were assessed from April 2013 to July 2015 (Figure 1D) using herbivore-exclusion cages (50 × 50 × 20 cm) deployed on six permanent plots at about 7 m depth at Lighthouse North. Cages were made of PVC-coated wire mesh (mesh size 2.4 cm) and positioned 6–12 m apart. The reef substratum was not cleared of turf or macroalgae prior to affixing the cages so that the enclosed surface was representative of a carbonate framework following the natural die-back of Liagora canopies. The enclosed reef surface was photographed eight times during the 27 months of the experiment (Figure 2). Photographs were taken perpendicular to the reef substratum with close-up planar views. The resulting 50 × 50 cm quadrats were divided into four adjacent frames and the percentage cover of biotic and nonliving substrata was visually estimated within each frame by units of 5%. The estimated covers were averaged at the cage level.
Photograph series of a herbivore-exclusion cage (50 × 50 × 20 cm) on the reef slope (~ 7 m depth) of the northern section of Lighthouse Reef (7°16′41.76″N, 134°27′50.58″E) from April 2013 to July 2015 (number of days after cage deployment in brackets) showing fluctuations in foliose macroalgae throughout the study period.
The reef substratum was classified following eight major groups: sand, rubble, carbonate reef framework, sessile invertebrates (including corals), crustose coralline algae (CCA), uncropped algal turf, encrusting fleshy macroalgae and upright macroalgae. Carbonate reef framework designates hard surfaces covered by a mix of sparse and diminutive (< 5 mm) cropped algal turf, CCA and detritus, which is commonly referred as the epilithic algal matrix (EAM) (Wilson and others 2003). In contrast, uncropped algal turf (hereafter simply referred to as “algal turf”) was defined as a cover of dense and thick filamentous algae that are long enough to be visible in the photographs, and typically forms an early successional stage of macroalgal communities (Lewis 1986; Hixon and Brostoff 1996). Encrusting fleshy macroalgae essentially refer to the crustose and decumbent forms of Lobophora spp. (Coen and Tanner 1989), whereas upright macroalgae were mostly represented by the corticated foliose Dictyota and Padina, the articulated calcareous Halimeda, and remnant holdfasts of Liagora at the time of cage deployment.
Macroalgal Growth Rates
Assessing algal dynamics in terms of substratum colonisation, with areal cover as a metric of population abundance, implies that algal populations are limited by the available space. We assumed that the growth of Lobophora (that is, encrusting fleshy algae) and upright macroalgae followed a standard logistic model whereby colonisable space was the only resource (carrying capacity) that limited population size. As a result, macroalgal growth rate is proportional to the space yet to be occupied: it decreases as the macroalga grows and the invadable space reduces, so that macroalgal cover expands following a sigmoid curve. In an idealised planar space with only one population, the carrying capacity is constant and attains 100% if the space is fully colonisable. However, modelling a system with multiple colonisers implies that the carrying capacity of each population will change as the colonisable space is taken by the other colonisers. In the absence of negative allelopathy and overtopping canopy (that is, no overgrowth), each population asymptotes to a specific cover that is fully determined by the intrinsic growth rate and initial cover of all competitors. This simplifies the system to a race for space whereby the fastest growing competitor has an obvious advantage because it takes up available space faster than others.
Nonlinear parametric regression using the “nls” function of R package “stats” (Ritz and Streibig 2008) was conducted to fit the logistic curve to the temporal cover data of Lobophora and upright macroalgae separately, with time expressed in days. Percentage cover was converted to the proportion of total colonisable space by excluding sand and sessile invertebrates, so that the covers of macroalgae and available space sum to 100% at every time point. We restricted the fit to an initial growth phase whereby available space was considered large enough to not affect macroalgal expansion. Fitting the model to this phase of relatively unconstrained growth allowed assuming a carrying capacity of 100% for each macroalgal group, thus restraining the estimation procedure to two parameters: the intrinsic growth rate and the initial cover of each macroalga.
To evaluate the validity of our fitting assumptions (that is, no allelopathy, no overtopping and unconstrained growth phase), we asked whether a logistic growth of the two macroalgae expanding concurrently (that is, with their respective carrying capacity being reduced over time) would reproduce the patterns of macroalgal colonisation observed over the full experiment. We thus simulated the expansion of the two macroalgae growing with daily increments and their carrying capacity re-calculated every day by excluding the space already taken by the competitor. Fitting assumptions were considered acceptable provided that simulations would predict the average cover of Lobophora and upright macroalgae observed at equilibrium (that is, after the phase of presumed unconstrained growth).
Spatio-Temporal Patterns of Fish Herbivory
Despite important declines in reef structural complexity caused by the typhoon, no significant change was detected on the total biomass of fish herbivores between March 2012 and February 2013 (Roff and others 2015a). Here we add another fish survey (October 2014) and ask whether the reef-scale expansion of Lobophora can be linked to changes in fish herbivory. We hypothesised that changes in habitat quality following the disappearance of Liagora canopies (that is, after April 2013) may have led to different assemblages of fish herbivores with a reduced ability to control macroalgal expansion. Moreover, spatial differences in fish herbivory could be anticipated between Ngederrak (protected from fishing) and Lighthouse (unprotected) with a potential impact on macroalgal dynamics. Each herbivore species was assigned a functional group defined by a dominant feeding behaviour and presumed impact on algae (Table S1). We then conducted a principal component analysis (PCA) on log-transformed species biomasses using the “ade4” R package (Dray and Dufour 2007) to detect spatio-temporal variations in assemblage composition. The ordination of species (34 species × 72 transects) was interpreted in terms of their functional impact on reef algae, that is, their ability to remove different types of algal substratum irrespective of the resource that is actually ingested and/or assimilated (for example, cyanobacteria, microalgae, detritus, see Clements and others 2016). Differences among years (that is, 2012, 2013 and 2014) and sites (n = 6–10 replicate transects per site and year) on the scores of the most significant factorial axes were tested using linear models performed with the lm function of the R software (R Core Team 2015).
Mechanistic Model of Algal Patch Dynamics
A spatially explicit, process-based model of algal patch dynamics was developed to investigate the possible mechanisms underlying the expansion of Lobophora on the Palauan reef sites. Model structure and parameterisation are detailed in Online Appendix 2 so the model is only briefly described here. Model simulations can be run from scripts encoded in MATLAB® and available in Online Appendix 3.
The model is a two-dimensional square lattice of 1 m2 cells representing a horizontal 20 × 20 m reef surface. Each cell can be occupied by a mixture of EAM, uncropped algal turf, Lobophora and upright macroalgae. Simulations follow a monthly time step and integrate explicit rates of recruitment, growth and grazing on each algal type with stochastic spatial variations. As an early stage of algal succession (Lewis 1986; Hixon and Brostoff 1996; Doropoulos and others 2017), uncropped algal turf forms the breeding ground of macroalgae and defines the space available for macroalgal colonisation: if a cell is left ungrazed for one month, the areal cover (cm2) of EAM converts to algal turf that allows macroalgal propagules to settle and grow. Monthly increments of the two macroalgae are determined by their intrinsic growth rate and their current carrying capacity in the cell. In addition, a cell can be overgrown by the surrounding Lobophora patches that expand horizontally at a rate determined by the neighbouring vegetation.
Grazing is spatially constrained (Williams and others 2001) and is expressed as the proportion of the total reef surface efficiently maintained in a cropped state every month, which essentially represents the overall net impact of grazing resulting from the balance between continuous growth and consumption of algae at the reef scale (Mumby and others 2007). At each time step, this net impact of grazing converts to a surface of algae to be removed from the grid. Algal removal is conducted randomly across the grid and can be reduced on selected cells to represent local refuges from grazing. Localised grazing reductions are compensated at the reef scale to achieve the specified amount of consumed algae. At every time step, grazing is distributed among each algal group following specified feeding preferences, which are merely rules of consumption reflecting community-wide algal selectivity of fish herbivores. All consumed algal surfaces are converted into EAM (that is, cropped algae) for the next model iteration. A shortfall in any algal group is compensated by an equivalent consumption on the next preferred group (see details in Online Appendix 2). We point out that our modelling of grazing behaviour is functional and does not imply that these algae are the primary sources of nutrition for herbivorous fish.
Simulating Post-typhoon Reef Dynamics in Palau
The model was first used to reproduce the observed dynamics of Lobophora with the explicit simulation of a transient Liagora canopy leading to localised grazing reductions. The grid was initialised by randomly designating a number of cells representative of a Liagora canopy, whereby individual thalli sway with water motion and create an area that deter fish grazing (Roff and others 2015b). The area under the influence of swaying Liagora thalli is typically wider than just the areal cover of the canopy, so that a realistic representation of these grazing refuges requires estimates of the swept substrates underneath the Liagora canopy (Online Appendix 2 and Figure S2). A cell is either “swept” or “exposed”, and the proportion of swept cells at a given time step matches the proportional area of swept substrates estimated over time on the studied reefs. In addition, the grazable area is adjusted at every time step to the amount of ungrazable substrates (that is, the proportional cover of sand and sessile invertebrates) estimated from the benthic surveys (Online Appendix 2 and Figure S3).
In February 2013, the estimated cover of Lobophora was less than 0.5% (Figure 1C). Although this might be a reasonable estimate for Liagora-free substrates, it is likely the extensive canopy of Liagora deterred the detection of small underlying Lobophora thalli. Assuming that Liagora canopies effectively provided a refuge against herbivores, Lobophora would have recruited under the canopy and most likely close to the holdfasts of Liagora. Lobophora tolerates reduced light intensity, and this allows it to proliferate in shaded environments such as the canopy of branching corals (Diaz-Pulido and others 2009; Bennett and others 2010). That Lobophora successfully recruited and started to grow around the holdfasts of Liagora is supported by significant correlations between the two macroalgae in April 2013 (Figure S4). We thus assumed that small yet cryptic amounts of Lobophora had been overlooked in February 2013, and deduced them by hindcasting the logistic growth model from April back to February 2013; this produced values of the initial Lobophora cover in the range 2.2–3.8% across the three sites (Online Appendix 2). As no significant cover of upright macroalgae (that is, other than Liagora) was observed in February 2013, their initial cover was set to a minimum 0.5% as virtually absent. Contrary to Lobophora, upright macroalgae are more likely to be negatively affected by light reductions and the sway movement of Liagora thalli. Hence, no upright macroalgae were observed following disappearance of Liagora canopies after April 2013.
Empirical evidence from the Caribbean suggests that the net grazing impact (that is, the proportion of the reef surface maintained in a cropped state) may be limited to 30–40% on reefs with relatively healthy herbivorous fish populations (Williams and others 2001; Mumby 2006; Mumby and others 2007). Such information is lacking on Indo-Pacific reefs; whereas levels of grazing intensity can be inferred from species-specific feeding rates (for example, Fox and Bellwood 2007), this alone does not inform about the functional impact of grazing, that is, the efficient control of algal expansion at monthly time scales. To circumscribe the levels of functional herbivory on the three Palauan sites, we ran simulations for increasing grazing impacts from 10 to 60%, with relative feeding preferences set to 0.95 on turf/EAM, 0.04 on upright macroalgae and only 0.01 on Lobophora (Online Appendix 2). These proportions reflect the large dominance of fish grazing either on EAM or turf (Figure S5A–B) with macroalgal browsers (Naso lituratus and Naso unicornis) accounting for 2–8% of the herbivorous fish biomass across the three study sites. Furthermore, experimental studies have demonstrated extremely low preferential feeding on Lobophora (Paul and Hay 1986; Pillans and others 2004). For each model simulation, grazing impact in the designated swept cells was reduced to 20% of the input value following observation of lower bite rates inside the Liagora canopies (Roff and others 2015b).
Assessing the Drivers of the Lobophora Bloom
Model simulations were used as virtual experiments to assess the relative importance of the modelled mechanisms in the bloom of Lobophora. First, we asked whether Lobophora would have been able to bloom if fully exposed to herbivores after the typhoon. This alternative scenario was explored for the three sites by simulating post-typhoon reef dynamics using the same grazing values but this time without Liagora canopies. This also allowed us to test whether an ephemeral refuge from grazing was the key to the alga’s escape. Second, we explored the individual effect of model parameters (Table S2) on the predictions of Lobophora dynamics. This sensitivity analysis was performed by simulating algal dynamics in Lighthouse North with a 40% grazing impact (best-fit value) while imposing a ± 20% change for each parameter value. Parameter sensitivity was evaluated from the deviation to a 39.6% cover of Lobophora predicted after 20 months with this best-fit grazing impact.
We then investigated the effects of a transient grazing escape driven by hypothetical blooms of Liagora simulated with varying spatial extent (swept area from 10 to 80% of the modelled reef) and persistence (from 1 to 20 months starting from February 2013). Model simulations were performed for Lighthouse North using the best-fit value of grazing impact. We compared the cover of Lobophora predicted after 20 months under each scenario. Because the initial cover of Lobophora is assumed to be intrinsically dependent on the extent of the Liagora canopy, each simulation was run with an initial Lobophora cover that was proportional to swept substrates as predicted in February 2013 (from 0.6 to 4.8%). A second set of simulations assessed the impact of the initial Lobophora cover per se, independently from the spatial extent of a Liagora bloom. Here, the initial cover of Lobophora was increased from 0 to 6% but swept areas were kept constant at 50% of the reef substratum.
Results
Macroalgal Growth Dynamics
At the time of cage deployment (end of April 2013), the enclosed reef substratum was colonised by an average cover (± SE) of 10.8 ± 2.2% of Lobophora and 3.5 ± 1.3% of upright macroalgae (essentially remnant Liagora holdfasts), consistent with broader scale estimates from contemporaneous benthic surveys (respectively, 9.3 ± 2.0% and 6.9 ± 1.2%). Reduced herbivory within cages resulted in a dramatic increase in macroalgae over the first 6 months (Figure 3A), leading to a total cover of macroalgae of 70–80% which persisted throughout the 2-year duration of the experiment. Once colonisable space was reduced to around 10–20% at approximately 6 months, Lobophora (Figure 3B) and upright macroalgae (Figure 3C) exhibited patterns of dominance that fluctuated over time leading to a strong negative correlation (Figure 3D). Blooming species of upright macroalgae varied among cages but most frequently involved Dictyota with persistent, extensive covers. Other blooming upright macroalgae included Padina and Laurencia that formed ephemeral canopies within the same cages (see Figure 2), and Halimeda that emerged as a late (> 1 year) successional stage.
Temporal dynamics (27 months) of macroalgal cover in exclusion cages at Lighthouse North (n = 6 cages, except one lost at the end of the experiment). A Average percentage cover of all macroalgae, without excluding sand and invertebrates (error bars are standard errors). BLobophora and C upright macroalgae covers as percentage of the colonisable space (that is, sand and invertebrates excluded) during the presupposed phases of unconstrained (filled circles) and constrained (open circles) growths. The dashed lines represent the logistic growth curve fitted to the observed covers during the unconstrained growth phase (that is, when the effect of limiting space is minimal). The solid lines correspond to a simulation of successive growth increments of the two macroalgae with their carrying capacity progressively reduced as the space is colonised. D Spatial covariation between upright macroalgae and Lobophora.
The interval 0–77 days (11 weeks) was considered as a plausible period of relatively unconstrained growth; macroalgal-free space (that is, turf) was then 39% on average (sand-corrected cover) and assumed to be large enough so that space as a limiting factor was considered minimal to macroalgal growth. Fitting the logistic growth model during this period (Figure S6) produced estimates of intrinsic growth rate 0.020 and 0.028 day−1 and the initial cover of 11.6 and 2.8% for Lobophora and upright macroalgae, respectively. With these parameters, the equilibrial covers that were achieved after the period of unconstrained growth (that is, between 6 and 24 months, Figure 3B–C) were consistent with predictions made by simulating the concurrent growth of the two macroalgal groups with their carrying capacities being progressively reduced as the available space was colonised.
Spatio-Temporal Variability of Fish Herbivores
The total biomass of herbivorous fish did not differ significantly among sites from March 2012 to October 2014 (Figure S7). However, the PCA on species biomasses showed a marked change in assemblage composition over time (Figure 4). The first PCA axis revealed a temporal shift in species composition (Figure 4A) but negligible changes in the functional composition (Figure 4B) of the herbivore assemblages. This reflects the replacement of species with similar feeding impact (Figure S5B), that is, grazers/croppers of diminutive algae (Siganus vulpinus and Zebrasoma scopas compensated by Acanthurus nigrofuscus) and scrapers (Scarus niger and Scarus dimidiatus compensated by Scarus schlegeli and Scarus psittacus). Species shifts occurred progressively in time (Figure 4C) as indicated by PC1 scores of fish transects decreasing from 2012 to 2013 (p < 0.001) and 2013 to 2014 (p < 0.001). Macroalgal browsers were the only functional group exhibiting significant changes: their biomass slightly decreased from 2012 to 2013 (p = 0.038) then increased substantially from 2013 to 2014 (p < 0.001). No differences were detected among the three study sites (Figure 4D), indicating that temporal variations in species composition were spatially consistent.
Principal component analysis of the herbivorous fish assemblage biomass. A Fish species ordination on the first factorial plane (24% of total inertia) with labels designating parrotfish (Sc: Scarus; Ch: Chlorurus; Ce: Cetoscarus), surgeonfish (Ac: Acanthurus; Na: Naso; Ct: Ctenochaetus; Ze: Zebrasoma) and rabbitfish (Si: Siganus). Labels of species with low contribution are not displayed for clarity. The barplot (top right) represents the eigenvalues. B Projection of the corresponding functional (that is, feeding) groups as supplementary variables (see group definitions in Table S1). C, D Scatterplots of the ordination of fish transects grouped, respectively, per survey and site, with each inertia ellipse encompassing about 2/3 of the associated transects. The black dots represent the centroids of the ellipses.
Simulation of Reef-Scale Macroalgal Dynamics
Assuming herbivory remained constant over time, post-typhoon simulations of macroalgal dynamics reproduced the observed patterns of Lobophora for grazing impact values in the range 30–40% (Figure 5A). Lobophora first exhibited a steep increase before stabilising as space was taken by the colonisation of corals and other sessile invertebrates (10–15% by October 2014). The observed differences in Lobophora trajectory among sites can be explained by differences in the cover of such nongrazable substrates, as a good fit was achieved for all three sites under similar levels of herbivory.
A Reef-scale simulations of Lobophora dynamics in the three Palauan reef sites after super-typhoon Bopha (December 2012). Brown lines represent the average trajectory (n = 40 replicate simulations) of Lobophora cover for input values of grazing impact (proportion of the substratum maintained in a cropped state) increasing from 10 to 60%. Dots with error bars indicate the average cover and associated standard error of Lobophora. Pie charts show the observed covers of (1) swept area within the Liagora canopy, (2) nongrazable area due to patches of sand or sessile invertebrates and (3) grazable area of substrates that can be colonised by algae. B Model simulations in the absence of Liagora canopies with the same grazing impacts. Dots and error bars (light grey) only for comparison with the observed scenario (Color figure online).
Drivers of Lobophora Expansion
Simulations were run with Liagora being prevented from blooming after the typhoon in order to test whether an ephemeral escape from grazing was key to the reef-scale expansion of Lobophora. The model found that Lobophora would only bloom if grazing impact fell below 10% (Figure 5B) but without being able to reproduce the observed trajectory.
The sensitivity analysis showed that grazing impact, the spatial extent of Liagora swept areas, and the intrinsic growth rate of Lobophora were the most influential parameters on Lobophora dynamics (Figure 6A).
A Sensitivity analysis of model simulations to individual changes (± 20%) in parameter values. Effects of parameter changes are reflected by deviations to a predicted 39.6% Lobophora cover after 20 months using a 40% grazing impact. B, C Effect of increasing the temporal and spatial escape from a 40% grazing impact on the predicted cover of Lobophora after 20 months. Spatial grazing escape is represented in B by the surface of swept areas (with a proportional increase in the initial cover of Lobophora) and in C by the initial cover of Lobophora (with constant swept areas fixed to 50% of the reef).
The effects of the transient escape from grazing were further explored by increasing concurrently the spatial extent and the duration of the Liagora canopies (Figure 6B). Simulating an initial vegetation of Lobophora proportional to swept areas, the magnitude of Lobophora blooms was more sensitive to an increase in the cover of swept areas than an increase in their persistence with the benefits of a grazing refuge being achieved within just 6 months. Changing the initial cover of Lobophora for a fixed amount of swept substrates (here, 50% cover) revealed the sensitivity of Lobophora blooms to its initial vegetation (Figure 6C). Indeed, a threshold effect was found whereby a very small increase in the initial cover of Lobophora (here, in the range 0.5–1.5%) leads to drastically different outcomes for the reef (0–30% of Lobophora after 20 months).
Discussion
Mechanisms underlying macroalgal phase shifts after episodic coral mortalities generally imply a functional loss of herbivory, increased algal productivity and/or the recruitment failure of corals able to quickly colonise the open space (Hughes 1994; Aronson and Precht 2001; Mumby and Steneck 2008). The persistent bloom of Lobophora on eastern Palauan reefs challenges our expectations because fish populations have been partially protected from fishing for more than a decade, algal productivity is mostly driven by wave exposure within this oligotrophic environment (Roff and others 2015a; Doropoulos and others 2017) and coral metapopulations are dominated by fast-growing corals (acroporids and pocilloporids, Doropoulos and others 2014) that until recently have triggered rapid reef recoveries in the region (Golbuu and others 2007). By combining in situ macroalgal growth rates with spatially explicit grazing, we provide a mechanistic explanation of the observed macroalgal phase shift, which, rather than challenging our current understanding of coral–algae equilibria, emphasises the importance of transient ecological dynamics in the maintenance of (or lack thereof) reef resilience. Specifically, we show that an ephemeral protection from grazing allows Lobophora to reach population levels where algal growth exceeds consumption by herbivores. Central to the reef-scale expansion of Lobophora is the transient, physical escape from grazing offered by the bloom of Liagora during a vulnerable stage whereby grazing control is normally prevalent, followed by a persistent size escape due to low levels of consumption by herbivores. This grazing escape occurred over a relatively short time (that is, less than 6 months) and underscores the need to quantify and model reef ecosystem processes at fine (< 1 year) temporal scales. Characterising the success of a bloom of Lobophora on a Pacific reef also allowed us to identify threshold values of fish grazing and macroalgal productivity within which Lobophora can escape top-down control. Lessons learned from this unanticipated Lobophora bloom have implications for our ability to understand and anticipate critical transitions on Pacific coral reefs, which, while having been relatively exempt of macroalgal phase shifts in recent history (Bruno and others 2009; Roff and Mumby 2012) may become increasingly sensitive with increasing disturbance regimes under climate change (Anthony and others 2011).
Any model relies on simplifying assumptions but most parameters that support our mechanistic simulations were derived from in situ measurements. Critically, rates of macroalgal growth were quantified using an unprecedented long-term (27-months) herbivore-exclusion experiment that ran concurrently to the proliferation of Lobophora, so that our estimates reflect productivity levels that drove the bloom at a reef scale. This is a rare opportunity that allows for a realistic parameterisation of macroalgal population dynamics on coral reefs. Because macroalgal growth was quantified in terms of horizontal expansion (that is cover) and not biomass (for example, Hixon and Brostoff 1996), factors limiting macroalgal expansion are primarily those affecting the availability of colonisable space. In the exclusion cages, macroalgae quickly overgrew the enclosed bare substrates to reach 70–80% cover after only 6 months. Although comparable covers have been observed in the Caribbean within 6 months of herbivore exclusion (Lewis 1986; Sotka and Hay 2009; Ferrari and others 2012), such rapid growth rates for reef macroalgae may be unprecedented in the Indo-Pacific (Roff and Mumby 2012; but see Diaz-Pulido and others 2009). We note, however, that Lobophora was already covering approximately 11% of the reef substratum at the time of cage deployment. A possible reason for relatively high algal productivity at Lighthouse Reef is wave exposure, which is thought to have triggered the primary bloom of Liagora (Roff and others 2015a). Moreover, high cover of Lobophora in the adjacent backreef and channels of Lighthouse reef (up to ~ 30% cover, unpublished data) may act as a local source population that can disperse to forereef environments. In the cages, Lobophora and upright macroalgae occupied most of the colonisable space and a simple mechanistic simulation of the two macroalgal groups growing concurrently led to realistic predictions of their respective average cover at saturation (that is, after 4 months). Residual fluctuations in the relative proportion of the two macroalgal groups at saturation can be explained by ephemeral blooms of upright macroalgae overtopping Lobophora. Although this indicates occasional biases in the detection of Lobophora patches, such biases were likely minimal during the phase of unconstrained growth (0–77 days) used to model macroagal growth rates. Moreover, ignoring these fluctuations had no consequences on the reproduction of reef trajectories because no upright macroalgae were actually detected on the reef following disappearance of Liagora.
Central to the simulation of a blooming Lobophora was the physical refuge created by Liagora canopies whereby Lobophora could escape the ambient levels of herbivore grazing. Short-term refuges from grazing promoted establishment of patches of Lobophora, and this initial vegetation was key to the macroalga’s reef expansion as model simulations revealed a threshold effect of the initial cover on Lobophora proliferation (Figure 6C). Although we do not describe the early colonisation of Lobophora around Liagora holdfasts (that is, simulations started two months after the typhoon), both the extent of Liagora canopies and the reduced grazing underneath were likely instrumental to the growth initiation of Lobophora. At the end of the Liagora bloom, the cover of Lobophora was moderate (5–10%) and further expansion implies a permanent escape from herbivory control. Critically, in the absence of the ephemeral refuge, grazing was sufficient to prevent the establishment of Lobophora patches as observed following the extensive coral loss caused by the 1998 bleaching event (P. J. Mumby, pers. obs.). Thus, an ephemeral refuge from grazing created an opportunity to reach a cover above which Lobophora growth exceeds the amount removed by herbivores.
The uncontrolled expansion of Lobophora following disappearance of the grazing refuge can be explained by limited fish feeding on Lobophora combined with the spatial dilution of grazing over a large space unoccupied by corals. Removal rates of Lobophora by fish herbivores are generally low on the reef (Hay 1981; Morrison 1988; Bennett and others 2010) and the best-fit values of grazing impact were found within 30–40% under the assumption of a relative (that is, preferential) feeding of 0.01 for Lobophora. The value of these two parameters (that is, grazing impact and relative feeding on Lobophora) likely compensate each other in the amount of Lobophora consumed at every time step, and a systematic exploration of their combined effects (Figure S8) shows that a grazing impact within 10–20% with a relative feeding on Lobophora of 0.05 produces a similarly good prediction of reef trajectories. While the range of possible grazing impact values is large, site-averaged biomasses of fish herbivores fluctuated between 5.7 and 14.0 g m−2 which positions the studied reefs at the lower end of the biomass range reported for Micronesian fish herbivores using the same survey method (Mumby and others 2013). Moreover, the average biomass of scarids (4.4 g m−2) was well below a Caribbean atoll (20 g m−2) for which a 30% grazing impact was considered a reasonable estimate of functional herbivory (Mumby 2006). Collectively, model simulations and empirical data suggest that the grazing impact at Lighthouse–Ngederrak is more likely to be below 30%. Although a relatively low herbivore biomass is not surprising where fishing is permitted (that is, at Lighthouse Reef), our results suggest that about 13 years of fishing protection at Ngederrak Reef had no clear impact on herbivorous fish populations.
With a low grazing pressure on Lobophora, reversing the macroalgal phase shift at Lighthouse–Ngederrak could be difficult unless the relative consumption of Lobophora increases as a response to this new community state. We note that the biomass of the generalised macroalgal browsers, while marginal compared to the biomass of the other functional groups, was significantly higher at the end of the study in all three sites. A similar trend was detected at Ngederrak for the diminutive algal cropper Siganus doliatus which has been observed feeding on Lobophora in assays (Bennett and others 2010). The recruitment and expansion of fast-growing corals could also help reversing the phase shift by progressively reducing the grazable space and intensifying grazing on algal substrates. Although experimental evidence shows that even moderate covers of Lobophora can inhibit coral settlement (Doropoulos and others 2017), the alga may have limited effects on juvenile corals (Mumby and others 2016). Interestingly, corals were observed growing inside the cages despite 40–60% Lobophora covers, which indicates that, in the absence of corallivory, some coral species have the ability to outcompete Lobophora once early settlement bottlenecks are surpassed (Doropoulos and others 2016; Mumby and others 2016). Continued or disrupted coral recovery at Lighthouse/Ngederrak will inform about the reversibility of the macroalgal phase shift, thereby indicating the existence of alternative attractors on eastern Palauan reefs.
Our findings have important implications for the resilience of the studied reefs in Palau, and more generally to phase shifts from coral to Lobophora dominance which are increasingly seen on Pacific reefs (Done and others 2007; Diaz-Pulido and others 2009; Cheal and others 2010). First, reefs in general (and not only in the Caribbean) may be sensitive to blooms of Lobophora because strong deterrence to grazing creates a steep threshold effect in the dynamics of the alga. The threshold cover of uncontrolled reef expansion, that is, the cover past which Lobophora growth exceeds its consumption by herbivores, likely depends on the ambient levels of herbivory, and our model simulations suggest that a Lobophora cover of 5–10% following extensive coral mortality may be enough to escalate a macroalgal phase shift where herbivorous fish biomass is relatively low (that is, < 15 g m−2). Critically, an ephemeral relaxation of grazing can provide the opportunity for Lobophora populations to reach this threshold due to relatively fast growth rates. Although it might not be possible to predict the occurrence of storm-induced blooms of Liagora, partly because their dynamics are poorly understood (Roff and others 2015a), a similar effect may be caused by an episodic pulse of algal productivity, and critical is the ability of herbivores to respond rapidly to counteract macroalgal expansion (Scheffer and others 2008). Efficient management against blooms of low-palatability macroalgae may not only require maintaining high herbivory to shift the algal threshold up, but may also require keeping algal productivity low so that exceeding the threshold cover is unlikely under a transient relaxation of grazing. Finally, the grazing escape promoted by the Liagora bloom occurred in less than 6 months, a time scale shorter than those usually considered by coral-reef ecosystem models (but see Sandin and McNamara 2012). This underscores the need to model at finer temporal scales the processes that control reef macroalgae to improve our ability to explain and anticipate phase shifts.
Escape from herbivory is a key process structuring the distribution of macroalgae on temperate and tropical reefs (Hay 1981; Lubchenco and Gaines 1981). Although spatial escapes at geomorphic scales (that is, zonation) involve the effect of environmental drivers such as wave exposure, tidal regime or depth, local escapes are driven by small-scale interactions with physical and biotic components of the reef habitat, such as substratum topography or habitat-forming species (for example, corals, sponges, large canopy algae). Examples of local escapes from herbivores on coral reefs include the protection offered by live coral branches to Lobophora (Diaz-Pulido and others 2009; Bennett and others 2010) or Halimeda (Castro-Sanguino and others 2016) but this generally does not affect coral persistence unless an external event (for example, mass coral bleaching, Diaz-Pulido and others 2009) provides the opportunity for macroalgal overgrowth. Ephemeral escapes from grazing after extensive coral mortality, such as observed in the present study, may be uncommon in the natural history of coral reefs, or perhaps they go unnoticed because they occur over very short time periods and would be normally buffered in relatively pristine environments (that is, with full herbivore capacity). With the erosion of mechanisms underlying reef resilience and the acceleration of external disturbances with climate change, coral reefs may be increasingly sensitive to short-term escapes from herbivore control. Our findings emphasise the need of increasing monitoring effort over shorter time scales for detecting temporary grazing relaxation or failure.
Ecosystem models generally explore nonequilibrial dynamics across a predetermined set of control parameter values, but these values are kept constant or imposed with stochastic fluctuations during temporal simulations. The reason is that control parameters are often assumed to change slowly relative to the nonequilibrial dynamics of the system. Our study underlines the impact of transient dynamics in the processes that stabilise disturbance-driven ecosystems. An ephemeral dysfunction of some important control mechanism can have disproportionate consequences on ecosystem structure and functioning, and failure to detect it can challenge our understanding of resilience. Simulating the transient dynamics of control mechanisms at multiple time scales may reveal important processes able to trigger a critical transition and improve our ability to anticipate future community shifts.
References
Anthony K, Maynard JA, Diaz-Pulido G, Mumby PJ, Marshall PA, Cao L, Hoegh-Guldberg O. 2011. Ocean acidification and warming will lower coral reef resilience. Glob Change Biol 17:1798–808.
Aronson RB, Precht WF. 2001. White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460:25–38.
Bellwood DR, Hughes TP, Folke C, Nyström M. 2004. Confronting the coral reef crisis. Nature 429:827–33.
Benedetti-Cecchi L. 2003. The importance of the variance around the mean effect size of ecological processes. Ecology 84:2335–46.
Bennett S, Vergés A, Bellwood DR. 2010. Branching coral as a macroalgal refuge in a marginal coral reef system. Coral Reefs 29:471–80.
Bozec Y-M, Mumby PJ. 2015. Synergistic impacts of global warming on the resilience of coral reefs. Philos Trans R Soc B 370:20130267.
Bozec Y-M, O’Farrell S, Bruggemann JH, Luckhurst BE, Mumby PJ. 2016. Tradeoffs between fisheries harvest and the resilience of coral reefs. Proc Natl Acad Sci 113:4536–41.
Bruno J, Siddon C, Witman J, Colin P, Toscano M. 2001. El Nino related coral bleaching in Palau, western Caroline Islands. Coral Reefs 20:127–36.
Bruno JF, Sweatman H, Precht WF, Selig ER, Schutte VG. 2009. Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology 90:1478–84.
Castro-Sanguino C, Lovelock C, Mumby PJ. 2016. The effect of structurally complex corals and herbivory on the dynamics of Halimeda. Coral Reefs 35:597–609.
Cheal AJ, MacNeil MA, Cripps E, Emslie MJ, Jonker M, Schaffelke B, Sweatman H. 2010. Coral–macroalgal phase shifts or reef resilience: links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs 29:1005–15.
Clements KD, German DP, Piché J, Tribollet A, Choat JH. 2016. Integrating ecological roles and trophic diversification on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biol J Linn Soc 120:729–51.
Coen LD, Tanner CE. 1989. Morphological variation and differential susceptibility to herbivory in the tropical brown alga Lobophora variegata. Mar Ecol Prog Ser 54:287–98.
Cuddington K, Fortin M-J, Gerber LR, Hastings A, Liebhold A, O’connor M, Ray C. 2013. Process-based models are required to manage ecological systems in a changing world. Ecosphere 4:20.
Denny MW, Hunt LJ, Miller LP, Harley CD. 2009. On the prediction of extreme ecological events. Ecol Monogr 79:397–421.
Diaz-Pulido G, McCook LJ, Dove S, Berkelmans R, Roff G, Kline DI, Weeks S, Evans RD, Williamson DH, Hoegh-Guldberg O. 2009. Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS ONE 4:e5239.
Doak DF, Estes JA, Halpern BS, Jacob U, Lindberg DR, Lovvorn J, Monson DH, Tinker MT, Williams TM, Wootton JT. 2008. Understanding and predicting ecological dynamics: are major surprises inevitable. Ecology 89:952–61.
Done TJ. 1992. Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247:121–32.
Done T, Turak E, Wakeford M, DeVantier L, McDonald A, Fisk D. 2007. Decadal changes in turbid-water coral communities at Pandora Reef: loss of resilience or too soon to tell? Coral Reefs 26:789–805.
Doropoulos C, Roff G, Bozec Y-M, Zupan M, Werminghausen J, Mumby PJ. 2016. Characterizing the ecological trade-offs throughout the early ontogeny of coral recruitment. Ecol Monogr 86:20–44.
Doropoulos C, Roff G, Visser M-S, Mumby PJ. 2017. Sensitivity of coral recruitment to subtle shifts in early community succession. Ecology 98:304–14.
Doropoulos C, Roff G, Zupan M, Nestor V, Isechal AL, Mumby PJ. 2014. Reef-scale failure of coral settlement following typhoon disturbance and macroalgal bloom in Palau, Western Pacific. Coral Reefs 33:613–23.
Dray S, Dufour A-B. 2007. The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22:1–20.
Evans MR, Bithell M, Cornell SJ, Dall SR, Díaz S, Emmott S, Ernande B, Grimm V, Hodgson DJ, Lewis SL. 2013. Predictive systems ecology. Proc R Soc B Biol Sci 280:20131452.
Ferrari R, Gonzalez-Rivero M, Ortiz JC, Mumby PJ. 2012. Interaction of herbivory and seasonality on the dynamics of Caribbean macroalgae. Coral Reefs 31:683–92.
Fox RJ, Bellwood DR. 2007. Quantifying herbivory across a coral reef depth gradient. Mar Ecol Prog Ser 339:49–59.
Fung T, Seymour RM, Johnson CR. 2011. Alternative stable states and phase shifts in coral reefs under anthropogenic stress. Ecology 92:967–82.
Gaines SD, Denny MW. 1993. The largest, smallest, highest, lowest, longest, and shortest: extremes in ecology. Ecology 74:1677–92.
Golbuu Y, Victor S, Penland L, Idip D Jr, Emaurois C, Okaji K, Yukihira H, Iwase A, Van Woesik R. 2007. Palau’s coral reefs show differential habitat recovery following the 1998-bleaching event. Coral Reefs 26:319–32.
Graham NA, Jennings S, MacNeil MA, Mouillot D, Wilson SK. 2015. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518:94–7.
Hastings A. 2004. Transients: the key to long-term ecological understanding? Trends Ecol Evol 19:39–45.
Hay ME. 1981. Spatial patterns of agrazing intensity on a caribbean barrier reef: herbivory and algal distribution. Aquat Bot 11:97–109.
Hixon MA, Brostoff WN. 1996. Succession and herbivory: effects of differential fish grazing on Hawaiian coral-reef algae. Ecol Monogr 66:67–90.
Holling CS. 1996. Surprise for science, resilience for ecosystems, and incentives for people. Ecol Appl 6:733–5.
Hughes TP. 1994. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265:1547–51.
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS. 2006. Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:107–17.
Lewis SM. 1986. The role of herbivorous fishes in the organization of a Caribbean reef community. Ecol Monogr 56:183–200.
Lindenmayer DB, Likens GE, Krebs CJ, Hobbs RJ. 2010. Improved probability of detection of ecological “surprises”. Proc Natl Acad Sci 107:21957–62.
Lubchenco J, Gaines SD. 1981. A unified approach to marine plant-herbivore interactions. I. Populations and communities. Annu Rev Ecol Syst 12:405–37.
McManus JW, Polsenberg JF. 2004. Coral–algal phase shifts on coral reefs: ecological and environmental aspects. Prog Oceanogr 60:263–79.
Morrison D. 1988. Comparing fish and urchin grazing in shallow and deeper coral reef algal communities. Ecology 69:1367–82.
Mumby PJ. 2006. The impact of exploiting grazers (Scaridae) on the dynamics of Caribbean coral reefs. Ecol Appl 16:747–69.
Mumby PJ, Bejarano S, Golbuu Y, Steneck RS, Arnold SN, Van Woesik R, Friedlander AM. 2013. Empirical relationships among resilience indicators on Micronesian reefs. Coral Reefs 32:213–26.
Mumby PJ, Hastings A, Edwards HJ. 2007. Thresholds and the resilience of Caribbean coral reefs. Nature 450:98–101.
Mumby PJ, Steneck RS. 2008. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol Evol 23:555–63.
Mumby PJ, Steneck RS, Adjeroud M, Arnold SN. 2016. High resilience masks underlying sensitivity to algal phase shifts of Pacific coral reefs. Oikos 125:644–55.
Mumby PJ, Wolff NH, Bozec Y-M, Chollett I, Halloran P. 2014. Operationalizing the resilience of coral reefs in an era of climate change. Conserv Lett 7:176–87.
Paine RT, Tegner MJ, Johnson EA. 1998. Compounded perturbations yield ecological surprises. Ecosystems 1:535–45.
Paul VJ, Hay ME. 1986. Seaweed susceptibility to herbivory: chemical and morphological correlates. Mar Ecol Prog Ser 33:255–64.
Pillans RD, Franklin CE, Tibbetts IR. 2004. Food choice in Siganus fuscescens: influence of macrophyte nutrient content and availability. J Fish Biol 64:297–309.
R Core Team (2015). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Ritz C, Streibig JC. 2008. Nonlinear regression with R. Berlin: Springer.
Roff G, Chollett I, Doropoulos C, Golbuu Y, Steneck RS, Isechal AL, van Woesik R, Mumby PJ. 2015a. Exposure-driven macroalgal phase shift following catastrophic disturbance on coral reefs. Coral Reefs 34:715–25.
Roff G, Doropoulos C, Zupan M, Rogers A, Steneck RS, Golbuu Y, Mumby PJ. 2015b. Phase shift facilitation following cyclone disturbance on coral reefs. Oecologia 178:1193–203.
Roff G, Mumby PJ. 2012. Global disparity in the resilience of coral reefs. Trends Ecol Evol 27:404–13.
Sandin SA, McNamara DE. 2012. Spatial dynamics of benthic competition on coral reefs. Oecologia 168:1079–90.
Scheffer M, Bascompte J, Brock WA, Brovkin V, Carpenter SR, Dakos V, Held H, Van Nes EH, Rietkerk M, Sugihara G. 2009. Early-warning signals for critical transitions. Nature 461:53–9.
Scheffer M, Carpenter SR. 2003. Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol Evol 18:648–56.
Scheffer M, Van Nes EH, Holmgren M, Hughes T. 2008. Pulse-driven loss of top-down control: the critical-rate hypothesis. Ecosystems 11:226–37.
Sotka EE, Hay ME. 2009. Effects of herbivores, nutrient enrichment, and their interactions on macroalgal proliferation and coral growth. Coral Reefs 28:555–68.
Steneck RS. 1988. Herbivory on coral reefs: a synthesis. Proc 6th Int Coral Reef Symp 1:37–49.
Vieira C, Thomas OP, Culioli G, Genta-Jouve G, Houlbreque F, Gaubert J, De Clerck O, Payri CE. 2016. Allelopathic interactions between the brown algal genus Lobophora (Dictyotales, Phaeophyceae) and scleractinian corals. Sci Rep 6:18637.
Williams ID, Polunin NV, Hendrick VJ. 2001. Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar Ecol Prog Ser 222:187–96.
Williams JW, Jackson ST. 2007. Novel climates, no-analog communities, and ecological surprises. Front Ecol Environ 5:475–82.
Wilson SK, Bellwood DR, Choat JH, Furnas MJ. 2003. Detritus in the epilithic algal matrix and its use by coral reef fishes. Oceanogr Mar Biol Annu Rev 41:279–309.
Acknowledgements
The authors would like to thank C. Castro-Sanguino and G. Diaz-Pulido for helpful comments and discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors’ Contribution
Y-MB and PJM conceived the methodology; CD and GR collected the benthic cover data and ran the caging experiment with PJM; PJM conducted fish surveys; Y-MB estimated macroalgal covers from photographs, performed data analysis and modelling, and completed manuscript preparation. All authors assisted in data and model interpretation, and manuscript revision.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bozec, YM., Doropoulos, C., Roff, G. et al. Transient Grazing and the Dynamics of an Unanticipated Coral–Algal Phase Shift. Ecosystems 22, 296–311 (2019). https://doi.org/10.1007/s10021-018-0271-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-018-0271-z