Abstract
Purpose
To analyze the long-term oncological outcomes of Barcelona Clinic Liver Cancer (BCLC) stages 0–A hepatocellular carcinoma (HCC) patients associated with or without microvascular invasion (MVI) treated with laparoscopic versus laparotomic liver resection.
Methods
Clinicopathological data of HCC patients with BCLC stages 0–A from four medical centers were retrospectively reviewed. The survival outcomes of patients who underwent laparoscopic hepatectomy were compared with those who underwent laparotomic hepatectomy. Subgroup analyses in terms of MVI were further performed to explore the effect of surgical approaches on the long-term survival outcomes. Propensity score matching (PSM) analysis was used to match patients between the laparoscopic and laparotomic resection groups in a 1:1 ratio.
Results
495 HCC patients at BCLC stages 0–A were enrolled, including 243 in the laparoscopic resection group and 252 in the laparotomic resection group. Laparoscopic resection group had a shorter operation time, less blood loss, a lower frequency of blood transfusion and postoperative complication rates. The laparoscopic resection group had a significantly better overall survival (OS) and recurrence-free survival (RFS) than the laparotomic resection group before and after PSM. Subgroup analysis demonstrated that OS and RFS of patients without MVI were remarkably better in the laparoscopic resection group compared with the laparotomic resection group. However, no significant differences in OS and RFS between the two groups were found in patients with MVI after PSM.
Conclusions
Pure laparoscopic hepatectomy for patients with BCLC stages 0–A HCC can be performed safely with favorable perioperative and long-term oncological outcomes at high-volume liver cancer centers, regardless of the presence of MVI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common primary malignancies with a poor prognosis. In 2020, HCC accounted for approximately 900,000 new cases and was responsible for more than 830,000 deaths, ranking seventh and second, respectively, for all cancers [1]. Microvascular invasion (MVI), defined as the presence of cancer cell clusters in blood vessels with endothelial cell linings under microscope, is common in HCC. It indicates aggressive biological behavior of HCC and is associated with poor prognosis after hepatectomy or transplantation [2].
Barcelona Clinic Liver Cancer (BCLC) staging system has been proposed to provide a clinical classification of HCC. This staging system incorporates important clinical information regarding tumor burden, liver function and general health status of patients, and it is recommended for treatment allocation and prognostic prediction [3]. For HCC patients at BCLC stages 0–A, liver resection is the most effective and promising treatment option, with acceptable overall survival (OS) and recurrence-free survival (RFS) rates [4,5,6].
Currently, both laparoscopic and laparotomic hepatectomy are both safe and feasible surgical methods for HCC patients. Laparotomic liver resection has traditionally been, and now is still the gold standard operation type to treat HCC. Laparoscopic liver resection has gained wide acceptance among liver surgeons and established as a safe alternative to open resection since this procedure was first reported in 1991 [7]. Compared with open liver resection, the main advantage of laparoscopic resection is minimal invasiveness and faster postoperative recovery [8]. Multiple retrospective studies have confirmed the safety and efficacy of laparoscopic resection applied in HCC patients. Twaij et al. [9] and Zhou et al. [10] reported that laparoscopic resection was superior to open resection in terms of perioperative results and did not compromise the oncological outcomes, suggesting the emergence of the era of laparoscopic hepatectomy.
It is a general consensus that laparoscopic hepatectomy confers better perioperative outcomes than laparotomic resection, but the long-term survival outcomes following these two surgical approaches are still controversial [11,12,13,14]. Previous published studies have several limitations, such as single-center experience and small sample size, making it not conducive to assess the therapeutic effect. Moreover, whether different surgical approaches have a significant effect on the postoperative oncological outcomes of patients with HCC associated with or without MVI still lacks evidence-based research.
In our study, the patients’ cohort from four medical centers in China was established based on different surgical approaches (laparoscopic and laparotomic liver resection). The aim of this study is to analyze and compare the perioperative outcomes and long-term prognoses of HCC patients associated with or without MVI at BCLC stages 0–A who were treated with laparoscopic or open liver resection, providing a higher level of clinical evidence using propensity score matching (PSM) analysis.
Methods
Patient selection
The study was conducted on consecutive HCC patients who underwent either laparoscopic liver resection or open liver resection at four high-volume hospitals, the Eastern Hepatobiliary Surgery Hospital (EHBH), Fujian Provincial Hospital (FPH), Changzhou People’s Hospital (CZPH), and Affiliated Tumor Hospital of Guangxi Medical University (ATHGMU) from March 2015 to February 2018. The clinical and pathological data of patients were retrospectively retrieved from medical electronic systems and prospectively maintained in a central database. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Clinical Research Ethics Committees of the EHBH, FPH, CZPH, and ATHGMU. Written informed consent for clinical research was obtained from all the patients prior to enrollment.
Patients with BCLC stages 0–A HCC who underwent complete liver resection were included in this study. The inclusion criteria were as follows: (I) age 18–75 years; (II) no history of previous major abdominal surgery or other malignant diseases; (III) no previous treatment for HCC before liver resection. These patients were divided into the laparoscopic and laparotomic hepatectomy groups on the basis of surgical operations. The choice of the surgical methods was based on the patient’s wish after full discussion with the operating surgeon. All laparoscopic operations were performed by experienced surgeons who had surpassed the learning curves for laparoscopic liver resection.
Diagnostic standard of HCC and MVI
The diagnosis of HCC was based on the guidelines for Diagnosis and Treatment of Primary Hepatocellular Carcinoma [15]. The diagnosis of MVI was determined by histopathological examination of the resected surgical specimens. Generally, two experienced pathologists independently evaluated MVI status by observing hematoxylin eosin (HE)-stained slices under microscope. If the MVI status was not conclusive, special immunohistochemical staining was used to identify the vessel walls, such as CD31, CD34 (vascular endothelium), SMA (vascular smooth muscle layer), and Podoplanin (lymphatic endothelium) [16,17,18].
Preoperative assessment
Routine preoperative investigations were consisted of medical history taking, hepatitis B serology, full blood counts, liver and renal function tests, blood glucose, serum alpha-fetoprotein (AFP), and coagulation tests. Imaging examinations included plain radiography or computed tomography (CT) scan of the chest, abdominal ultrasonography (US), contrast-enhanced CT, and/or magnetic resonance imaging (MRI), to evaluate tumor resectability. All cases were discussed at weekly multidisciplinary treatment (MDT) meetings which included liver surgeons, hepatologists, gastroenterologists, interventional radiologists, and medical oncologists. The patients were prepared for surgery after a consensus was reached on tumor resectability at the multidisciplinary conferences.
Surgical procedure
The laparoscopic hepatectomy was performed as previously reported [19, 20]. The patient was placed in a supine position with full exposure to the tumor. The camera port was placed above or on the right side of the umbilicus under direct version. Carbon dioxide pneumoperitoneum pressure was maintained at 12–14 mmHg, and three or four additional ports were used. Intraoperative US was routinely used to guide the resection planes. The operative site was mainly determined by the location of the tumor. The liver was prelabeled with an ultrasonic knife. Parenchymal transection was performed by combination of ultrasonic shears (harmonic scalpel; Ethicon Endo-Surgery Inc., Cincinnati, OH, USA), Cavitron ultrasonic surgical aspirator (CUSA; ValleyLab Inc., Boulder, CO, USA), and LigaSure (ValleyLab Inc.). Large vessels were secured by Hem-O-lock clips (Teleflex Medical, Research Triangle Park, NC, USA). The resected tumor specimens were placed into a retrieval bag and extracted through an enlarged incision site. Fibrin glue sealant (Greenplast, Green Cross Corp., Seoul, Korea) was applied to the cut surface of the liver, and a drainage tube was placed after hemostasis.
The open liver resection was performed using the techniques described previously [5]. The patient was placed in a supine position and surgery was performed through a right subcostal incision with upward midline extension. The scope of resection depended on tumor size and residual liver volume. General abdominal evaluation was carried out to exclude metastasis, and liver evaluation was done to determine tumor’s location and size, proximity to adjacent vessels, and to exclude multiplicity of tumors by manual palpation and intraoperative US. Parenchymal transection was performed using an ultrasonic scalpel. Pringle’s maneuver and infrahepatic vena cava clamping were performed as necessary.
Follow-up
All patients were followed up once every 2 months during the first year after hospital discharge, and once every 3 months thereafter, until death or dropout from the follow-up program. Follow-up examinations included laboratory results [white blood cell (WBC) count, hemoglobin, platelets count, serum total bilirubin, albumin (ALB), aspartate aminotransferase (AST), glucose, creatinine, AFP, prothrombin time (PT)], abdominal US, contrast-enhanced CT or MRI. HCC recurrence was diagnosed based upon CT or MRI findings, and elevated serum AFP levels. The patients were regularly followed up once every 3–6 months if there was no evidence of recurrence until 5 years after surgery. The study was censored on June 30, 2021.
Study outcomes
The primary outcomes of this study were long-term oncological outcomes of OS and DFS. Intraoperative outcomes, including blood loss, blood transfusion, operation time, and postoperative short-term outcomes, including length of hospital stay and postoperative complications, were considered as secondary outcomes.
Definitions
Surgical resection margin was defined as the shortest measured distance from the edge of the tumor to the plane of liver transection [21]. In this study, it was classified as wide and narrow resection margin based on a cutoff of 1 cm. Anatomical and non-anatomical resections were defined on the basis of the Brisbane 2000 Nomenclature of Liver Anatomy and Resections [22]. Major liver resection was defined as a resection of three or more Couinaud liver segments; otherwise, it was classified as a minor liver resection. Postoperative liver failure was defined as a serum total bilirubin level exceeding 50 μmol/L and prothrombin time lower than 50% on postoperative day 5 [23]. The definition of bile leakage was based on the criteria of the International Study Group of Liver Surgery [24]. OS was calculated from the date of surgery to the date of death or the last documented visit. RFS was defined as the time interval between surgery and the first diagnosis of HCC recurrence or the last follow-up.
Statistical analysis
Continuous variables with a normal distribution were presented as mean ± standard deviation (SD), and the Student’s t test was used to compare differences between groups. Skewed distributed continuous variables were expressed as median with interquartile range (IQR), and the Mann–Whitney U test was applied. Categorical data were shown as frequencies and percentages, and compared using Chi-square test or Fisher’s exact probability test as appropriate.
Propensity score matching (PSM) analysis was used to minimize the potential confounders and selection bias and balance the patient baseline characteristics between groups. The propensity score was estimated for each patient using a multivariate logistic regression model, and a 1:1 group matching was performed using the nearest-neighbor matching method without replacement. Variables including age, sex, body mass index (BMI), hepatitis B virus (HBV) infection, antiviral therapy, hypertension, diabetes mellitus (DM), cirrhosis, tumor diameter, MVI, resection margin, WBC, hemoglobin, platelets, total bilirubin, ALB, AST, blood glucose, creatinine, serum AFP and PT were matched. A caliper width of 0.2 standard deviations was set to prevent poor matching.
The Kaplan–Meier method was used for survival analyses, and log-rank test was conducted to compare differences. Univariate regression analysis was used to explore potential risk factors associated with OS and RFS. The statistically significant variables in univariate analysis were further incorporated into multivariate analysis. In the Cox proportional hazards regression model, independent prognostic factors were screened out by a backward stepwise selection process with likelihood ratio (LR) method. A p value less than 0.05 was considered to be statistically significant in our study.
All statistical analyses were performed using R program (version 3.6.3, R foundation for Statistical Computing, Vienna, Austria) with the Survival and Survminer packages.
Results
Baseline characteristics of the patients
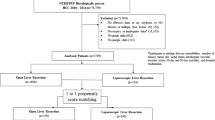
Of the 1104 consecutive HCC patients who underwent hepatectomy during the study period, 435 underwent laparoscopic hepatectomy, 669 underwent open liver resection. As shown in Fig. 1, after excluding 609 patients with BCLC stages B–C, 495 HCC patients with BCLC stage 0 or A, including 243 in the laparoscopic liver resection group and 252 in the open liver resection group, were enrolled into this study. After PSM, 378 patients (189 patients in each group) were matched (Fig. 1).
The baseline clinicopathological characteristics of HCC patients before PSM are shown in Table 1. Compared with the open liver resection group, the laparoscopic liver resection group had fewer diabetes (16.0% vs. 24.2%, p = 0.032), a lower percentage of tumor diameter > 5 cm (42.0% vs. 61.9%, p < 0.001), more positive MVI status (45.7% vs. 35.3%, p = 0.024), more wide resection margin (39.1% vs. 28.6%), a higher percentage of WBC count > 4 × 109/L (90.5% vs. 83.7%, p = 0.034), a lower percentage of AST level > 45 U/L (24.7% vs. 35.7%, p = 0.010), a lower percentage of PT > 13.5 s (18.9% vs. 29.8%, p = 0.007). After PSM, all these clinicopathological features became well balanced and comparable between the two groups (all p > 0.05) (Table 1). The baseline clinicopathological characteristics of patients associated with (n = 200) or without MVI (n = 295) are shown in Tables S1 and S2.
Long-term oncological outcomes of patients in the laparoscopic and laparotomic groups before and after PSM
Before PSM, the OS at 1, 2, 3 and 5 years were 93.7%, 85.9%, 82.6% and 78.8%, respectively, and the RFS at 1, 2, 3 and 5 years were 81.0%, 77.7%, 75.0% and 71.4%, respectively, for the laparoscopic liver resection group. For the laparotomic hepatectomy group, the OS at 1, 2, 3 and 5 years were 88.1%, 77.3%, 67.9% and 55.7%, respectively, and the RFS at 1, 2, 3 and 5 years were 72.8%, 66.1%, 58.7% and 53.6%, respectively. The results indicated that the long-term oncological outcomes were significantly better in the laparoscopic resection group compared with the laparotomic resection group (both p < 0.001) (Fig. 2a, b).
Overall survival (OS) and recurrence-free survival (RFS) of BCLC stages 0–A HCC patients treated with laparoscopic or laparotomic liver resection before and after PSM. OS (a) and RFS (b) of patients with BCLC stages 0–A HCC before PSM. OS (c) and RFS (d) of patients with BCLC stages 0–A HCC after PSM
After PSM, the OS at 1, 2, 3 and 5 years were 93.0%, 84.4%, 80.3% and 75.8%, respectively, and the RFS at 1, 2, 3 and 5 years were 80.5%, 76.9%, 73.4% and 70.9%, respectively, for the laparoscopic liver resection group. For the laparotomic hepatectomy group, the OS at 1, 2, 3 and 5 years were 89.9%, 78.9%, 70.9% and 61.0%, respectively, and the RFS at 1, 2, 3 and 5 years were 73.8%, 68.2%, 62.0% and 56.8%, respectively. The results after PSM still showed a better long-term survival in the laparoscopic resection group compared with the laparotomic resection group (both p = 0.013) (Fig. 2c, d).
Subgroup survival analysis in patients associated with or without MVI in the laparoscopic and laparotomic groups before and after PSM
As shown in Fig. 3, before PSM, both OS and RFS of MVI negative patients were significantly better in the laparoscopic resection group compared with the laparotomic resection group (both p < 0.001) (Fig. 3a, b). The OS and RFS of MVI positive patients were also significantly better in the laparoscopic resection group compared with the laparotomic resection group (both p < 0.05) (Fig. 3c, d).
Overall survival (OS) and recurrence-free survival (RFS) of BCLC stages 0–A HCC patients associated with or without microvascular invasion (MVI) treated with laparoscopic or laparotomic liver resection before PSM. OS (a) and RFS (b) of patients with BCLC stages 0–A HCC without MVI before PSM. OS (c) and RFS (d) of patients with BCLC stages 0–A HCC with MVI before PSM
As shown in Fig. 4, after PSM, both OS and RFS of MVI negative patients were also significantly better in the laparoscopic resection group compared with the laparotomic resection group (both p < 0.05) (Fig. 4a, b). However, no significant differences for OS (p = 0.56) and RFS (p = 0.17) in MVI positive patients were observed between the laparoscopic and laparotomic resection groups (Fig. 4c, d).
Overall survival (OS) and recurrence-free survival (RFS) of BCLC stages 0–A HCC patients associated with or without microvascular invasion (MVI) treated with laparoscopic or laparotomic liver resection after PSM. OS (a) and RFS (b) of patients with BCLC stages 0–A HCC without MVI after PSM. OS (c) and RFS (d) of patients with BCLC stages 0–A HCC with MVI after PSM
Independent risk factors analysis before and after PSM
Before PSM, univariate and multivariate analyses demonstrated that HBV infection, diabetes mellitus, AST level > 45 U/L, AFP level > 400 ng/mL, tumor diameter > 5 cm, presence of MVI, and open liver resection approach were independent prognostic factors of OS. Diabetes mellitus, AST level > 45 U/L, total bilirubin level > 17.5 μmol/L, AFP level > 400 ng/mL, tumor diameter > 5 cm, presence of MVI, and open liver resection approach were independent prognostic factors for RFS (Table 2).
After PSM, as presented in Table 3, univariate and multivariate analyses identified that HBV infection, absence of antiviral therapy, AST level > 45 U/L, tumor diameter > 5 cm, presence of MVI, and open liver resection approach were independent prognostic factors of OS. Besides, AST level > 45 U/L, tumor diameter > 5 cm, presence of MVI and open liver resection approach were identified as independent prognostic factors for RFS.
Intraoperative and postoperative outcomes
Before PSM, the percentage of central-located tumor, blood loss, the frequency of blood transfusion, operation time, and length of hospital stay were significantly lower in the laparoscopic resection group than the laparotomic resection group; whereas the frequency of Pringle’s maneuver (portal inflow occlusion) and its duration were remarkably higher in the laparoscopic resection group compared with the laparotomic resection group (Table 4). In addition, the complication occurrence rates of hydrothorax and ascites were significantly lower in the laparoscopic resection group than the laparotomic resection group (Table 4). Type of hepatectomy, extent of liver resection, complications of hepatic failure, bile leakage, and pulmonary or abdominal infection were comparable between the two groups (Table 4).
After PSM, blood loss, the frequency of blood transfusion, and length of hospital stay were significantly lower in the laparoscopic resection group than the laparotomic resection group; while the percentages of minor hepatectomy and peripheral-located tumor, and Pringle’s maneuver duration were remarkably higher in the laparoscopic resection group compared with the laparotomic resection group (Table S3). Additionally, the complication occurrence rates of ascites were significantly lower in the laparoscopic resection group than the laparotomic resection group (Table S3). Type of hepatectomy, frequency of Pringle’s maneuver, complications of hepatic failure, bile leakage, and pulmonary or abdominal infection were comparable between the two groups (Table S3).
Discussion
During the past decade, the number of minimally invasive hepatectomy performed globally has increased exponentially. The introduction of new surgical equipment and increased experience in laparoscopic liver resection have allowed this procedure to be performed more frequently and safely than before. Previously, a case-matched PSM study revealed comparative perioperative and long-term outcomes between HCC patients who underwent laparoscopic and open liver resection [5]. However, to the best of our knowledge, high-quality studies comparing the perioperative and oncological outcomes in BCLC stages 0–A HCC patients associated with or without MVI after laparoscopic or open hepatectomy are still lacking. Although prospective randomized controlled trials are the gold standard for treatment efficacy comparison and evaluation, it is difficult, and sometimes even unethical to conduct in real-world clinical practice. Instead, PSM analysis has been proposed as a useful alternative to reduce selection bias and increase the evidence level of observational comparative studies.
A meta-analysis in 2013 indicated that both surgical procedures have similar long-term outcomes [11]. Laparoscopic approach did not achieve significant survival improvement compared with conventional open resection approach in early years. However, in this large cohort study across four medical centers from China, we found that the long-term survival outcomes were significantly better in the laparoscopic resection group than its counterpart before and after PSM. There were three possible reasons. Firstly, all surgeons who performed laparoscopic hepatectomy were experienced and had passed the learning curves of laparoscopic technology, thereby ensuring safety, maturity and stability of every operation. Secondly, the magnified visualization of laparoscopy, real-time guidance of intraoperative US and assisting technology of immunofluorescence allow for precise surgical manipulation in laparoscopic surgery. Thirdly, some studies reported that compared with traditional open resection approach, laparoscopic treatment had less inhibitory effect on the immune response of the body, which may play a role in anti-tumor recurrence [25, 26].
The presence of MVI worsens survival outcomes of HCC patients after liver resection. Huang et al. [27] documented that MVI was also an independent risk factor for OS and RFS in HCC patients at BCLC stage A. Wang et al. [28] showed that MVI could predict an adverse recurrence pattern and had the potential to be used as a reference index to decide whether to operate for HCC. In our study, before PSM, the laparoscopic group had a markedly higher percentage of MVI compared with the laparotomic group. Thus, it is unscientific to make subgroup survival analysis only using data before PSM. Uneven distribution of MVI status between the two groups will potentially influence the effect of surgical approaches on patients’ survival. After PSM, the baseline clinicopathological characteristics, including MVI, were well balanced between the groups. Under this circumstance, we can ensure that the survival differences between the two groups derived only from the treatment grouping factor, rather than the PSM performance above.
Subgroup survival analysis showed that the OS and RFS of patients without MVI were significantly improved in the laparoscopic resection group compared to the laparotomic resection group before and after PSM. We speculated that blood loss, length of hospitalization and postoperative complications were potential factors which influenced patients’ survival [14, 29,30,31]. Nevertheless, no significant differences in OS and RFS were obtained in patients with MVI between the laparoscopic and laparotomic resection groups after PSM. We considered that the survival benefits of patients who underwent laparoscopic resection were counteracted by the presence of MVI. Therefore, from a conservative viewpoint, our results can be interpreted to indicate that laparoscopic liver resection is at least not inferior to the standard open resection approach and can be a feasible alternative for HCC patients at BCLC stages 0–A regardless of the presence of MVI.
Intraoperative and postoperative short-term outcomes following the two surgical approaches were also compared and analyzed in this study. Our results showed that laparoscopic hepatectomy had more minor liver resection, more peripheral-located tumors, more required Pringle’s maneuver and higher occlusion duration, less blood loss, a lower frequency of blood transfusion, a shorter operation time, and a shorter hospital stay compared with laparotomic hepatectomy. We considered that the higher operative efficiency and less need for blood transfusion of laparoscopic hepatectomy may be associated with the smaller incision and less surgical trauma. Nonetheless, a previous study demonstrated that laparoscopic right hepatectomy (LRH) was associated with a tendency of prolonged operation time after PSM [32]. We speculated that the prolonged operation time may be related to the relative complexity of this procedure, the limited vision field of right hepatectomy, and less experience and skills of operating surgeons. Additionally, we found that the postoperative complication rates of hydrothorax and ascites were significantly reduced for patients who underwent laparoscopic liver resection. However, a previous study reported that postoperative complication rates were comparable for HCC patients between the laparoscopic and laparotomic groups in the PSM cohort [33]. Hence, the operation time, safety degree and postoperative complication rates of these two surgical approaches for HCC patients are still controversial.
The present study has several limitations. First, this is a nonrandomized retrospective study with its inherent selection bias. Although a 1:1 propensity score matching was performed to minimize baseline differences between the laparoscopic and laparotomic hepatectomy groups, potential confounders may still exist to influence outcomes. Further prospective research needs to be designed and conducted. Second, certain heterogeneity in center experience, surgical procedures and perioperative management may exist among different hospitals. Third, although the number of patients enrolled in our study is relatively large, the sample size is still insufficient to draw a firm conclusion about the effect of MVI on the surgical choice in HCC patients. Last, this study was conducted in China with most patients having a background of HBV infection. It is unknown whether our findings can be extrapolated to other different races and etiologies.
Conclusion
Taken together, our study elucidated that for HCC patients at BCLC stages 0 or A, the long-term outcomes of patients who were treated with laparoscopic liver resection were at least not inferior to those of patients who underwent laparotomic hepatectomy approach. Therefore, laparoscopic liver resection may be a safe and feasible alternative for HCC patients at early stages, regardless of the presence of MVI.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249
Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, et al. Nomogram for preoperative estimation of microvascular invasion risk in Hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016;151:356–363
European Association for the Study of the Liver. Electronic address EEE, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236
Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv238–iv255
Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y. Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol. 2015;63:643–650
Takahara T, Wakabayashi G, Beppu T, Aihara A, Hasegawa K, Gotohda N, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22:721–727
Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gynecol. 1991;78:956–958
Yoshida H, Taniai N, Yoshioka M, Hirakata A, Kawano Y, Shimizu T, et al. Current status of laparoscopic hepatectomy. J Nippon Med Sch. 2019;86:201–206
Twaij A, Pucher PH, Sodergren MH, Gall T, Darzi A, Jiao LR. Laparoscopic vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: systematic review and meta-analysis. World J Gastroenterol. 2014;20:8274–8281
Zhou YM, Shao WY, Zhao YF, Xu DH, Li B. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Dig Dis Sci. 2011;56:1937–1943
Yin Z, Fan X, Ye H, Yin D, Wang J. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol. 2013;20:1203–1215
Goh EL, Chidambaram S, Ma S. Laparoscopic vs open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a meta-analysis of the long-term survival outcomes. Int J Surg. 2018;50:35–42
Jiang S, Wang Z, Ou M, Pang Q, Fan D, Cui P. Laparoscopic versus open hepatectomy in short- and long-term outcomes of the hepatocellular carcinoma patients with cirrhosis: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A. 2019;29:643–654
Xiangfei M, Yinzhe X, Yingwei P, Shichun L, Weidong D. Open versus laparoscopic hepatic resection for hepatocellular carcinoma: a systematic review and meta-analysis. Surg Endosc. 2019;33:2396–2418
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750
Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108–113
Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855
Zhang XP, Chai ZT, Feng JK, Zhu HM, Zhang F, Hu YR, et al. Association of type 2 diabetes mellitus with incidences of microvascular invasion and survival outcomes in hepatitis B virus-related hepatocellular carcinoma after liver resection: a multicenter study. Eur J Surg Oncol. 2022;48:142–149
Li B, Liu T, Zhang Y, Zhang J. Retroperitoneal laparoscopic hepatectomy of recurrent hepatocellular carcinoma: case report and literature review. BMC Gastroenterol. 2020;20:278
Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, et al. The southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg. 2018;268:11–18
Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245:36–43
Popescu I, Câmpeanu I. Surgical anatomy of the liver and liver resection. Brisbane 2000 Terminology. Chirurgia (Bucur). 2009;104:7–10
Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The “50–50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828 (discussion 828-829)
Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688
Tang F, Tie Y, Tu C, Wei X. Surgical trauma-induced immunosuppression in cancer: recent advances and the potential therapies. Clin Transl Med. 2020;10:199–223
Gitzelmann CA, Mendoza-Sagaon M, Talamini MA, Ahmad SA, Pegoli W Jr, Paidas CN. Cell-mediated immune response is better preserved by laparoscopy than laparotomy. Surgery. 2000;127:65–71
Huang C, Zhu XD, Ji Y, Ding GY, Shi GM, Shen YH, et al. Microvascular invasion has limited clinical values in hepatocellular carcinoma patients at Barcelona Clinic Liver Cancer (BCLC) stages 0 or B. BMC Cancer. 2017;17:58
Wang H, Qian YW, Wu MC, Cong WM. Liver resection is justified in patients with BCLC intermediate stage hepatocellular carcinoma without microvascular invasion. J Gastrointest Surg. 2020;24:2737–2747
Ivanics T, Claasen MP, Patel MS, Rajendran L, Shwaartz C, Raschzok N, et al. Long-term outcomes of laparoscopic liver resection for hepatocellular carcinoma: a propensity score matched analysis of a high-volume North American center. Surgery. 2022;171:982–991
Cheung TT, Dai WC, Tsang SH, Chan AC, Chok KS, Chan SC, et al. Pure laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma in 110 patients with liver cirrhosis: a propensity analysis at a single center. Ann Surg. 2016;264:612–620
Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617–623
Chen K, Pan Y, Wang YF, Zheng XY, Liang X, Yu H, et al. Laparoscopic right hepatectomy for hepatocellular carcinoma: a propensity score matching analysis of outcomes compared with conventional open surgery. J Laparoendosc Adv Surg Tech A. 2019;29:503–512
Beppu T, Wakabayashi G, Hasegawa K, Gotohda N, Mizuguchi T, Takahashi Y, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22:711–720
Acknowledgements
This study was supported by Meng Chao Talent Training Program (EHBH2019YC112) and National Natural Science Foundation of China (82172846).
Funding
Meng Chao Talent Training Program (EHBH2019YC112); National Natural Science Foundation of China (82172846).
Author information
Authors and Affiliations
Contributions
Conception and design: W-XG, S-QC, S-YY, M-LY, Y-FD, J-KF, J-ZY; Administrative support: W-XG, S-QC; Provision of study materials or patients: W-XG, S-QC, M-LY, YD, J-ZY; Collection and assembly of data: S-YY, LG, JX; Data analysis and interpretation: S-YY, J-KF, Y-JX, Z-HL; Statistical analysis: S-YY, J-KF, Y-JX, Z-HL; Manuscript writing: all authors; Final approval of manuscript: all authors.
Corresponding authors
Ethics declarations
Conflict of interest
Shi-Ye Yang, Mao-Lin Yan, Yun-Fei Duan, Jin-Kai Feng, Jia-Zhou Ye, Yan-Jun Xiang, Zong-Han Liu, Lei Guo, Jie Xue, Shu-Qun Cheng and Wei-Xing Guo have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate (ethics)
All procedures performed in this study involving human participants were approved by the Institutional Ethics Review Board of the Eastern Hepatobiliary Surgery Hospital, Fujian Provincial Hospital, The Third Affiliated Hospital of Soochow University, and Affiliated Tumor Hospital of Guangxi Medical University. This study was in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent for study participation was obtained from all the patients.
Consent to publish (ethics)
All authors confirm that the work described has not been published before and is not under consideration for publication elsewhere. All authors have seen and gave consent to the publication of this study. The publication of this work has been approved by the responsible authorities at the institution where the work is carried out.
Animal research (ethics)
Not applicable.
Clinical trials registration
Not applicable because this is a retrospective observational study.
Synopsis
For hepatocellular carcinoma (HCC) patients at BCLC stages 0–A with or without microvascular invasion (MVI), laparoscopic liver resection may be a safe and feasible alternative to laparotomic liver resection.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, SY., Yan, ML., Duan, YF. et al. Perioperative and long-term survival outcomes of laparoscopic versus laparotomic hepatectomy for BCLC stages 0–A hepatocellular carcinoma patients associated with or without microvascular invasion: a multicenter, propensity score matching analysis. Hepatol Int 16, 892–905 (2022). https://doi.org/10.1007/s12072-022-10353-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10353-4