Abstract
Background
Several studies have been conducted comparing laparoscopic liver resection (LLR) versus open liver resection (OLR) for hepatocellular carcinoma (HCC), however, the optimal therapeutic approach has not been established. Therefore, we conducted a systematic review and meta-analysis of studies comparing LLR versus OLR for HCC.
Methods
MEDLINE and Cochrane Central Register of Controlled Trials database were systematically searched for relevant studies.
Results
Fifty-one studies were identified including a total of 6812 patients (2786 patients underwent LLR and 4026 patients were subjected to OLR). Blood transfusion rate, hospital stay in days, 30-days mortality rate and morbidity were significantly lower in LLR comparing with OLR (odds ratio (OR) 0.45; 95% confidence interval (CI) 0.30–0.69; P = 0.001; I2 = 55.83%), (MD − 3.87; 95% CI − 4.86 to − 2.89; P = 0.001; I2 = 87.35%), (OR 0.32; 95% CI 0.16–0.66; P = 0.001; I2 = 0%), and (OR 0.42; 95% CI 0.34–0.52; P = 0.001; I2 = 39.64), respectively. There was no significant difference between LLR and OLR regarding the operative time in minutes, resection margin in centimeter and R0 resection (MD 18.29; 95% CI − 1.58 to 38.15; p = 0.07; I2 = 91.73%), (MD 0.04; 95% CI − 0.06 to 0.14; P = 0.41; I2 = 48.03%) and (OR 1.31; 95% CI 0.98–1.76; P = 0.07; I2 = 0%), respectively. The 1-year overall survival (1-OS) and 5-OS rates were significantly higher in LLR comparing with OLR (OR 1.45; 95% CI 1.06–1.99; P = 0.02; I2 = 25.59%) and (OR 1.36; 95% CI 1.07–1.72; P = 0.01; I2 = 14.88%), respectively.
Conclusion
LLR is superior to OLR regarding intraoperative blood loss, blood transfusion rate, hospital stay in days, 30-days mortality and morbidity, however, randomized controlled trials are needed to identify the superiority of either strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatocellular carcinoma is the third most common causes of cancer-related death in the world [1]. Liver resection is considered the most widely used treatment for patients with hepatocellular carcinoma (HCC) [2]. Laparoscopic liver resection (LLR) has increasingly been adopted since it was first introduced in 1991 [3]. There has been a growing body of evidence that LLR is associated with lower mortality and morbidity rates in comparison with open liver resection (OLR) [4]. However, LLR is still a challenging approach for patients as well as surgeons since most HCC are developed on top of cirrhotic liver or chronic hepatitis [5,6,7]. Furthermore, LLR is associated with technical difficulties, relatively longer operative time and bleeding risk in parenchymal resection [8, 9]. Although many studies have been conducted comparing LLR versus OLR for HCC, data are still relatively controversial, given the recent advancements in laparoscopic devices and techniques over the last few years and the increase in surgeons’ experience [10,11,12,13,14,15,16]. The recently published meta-analysis comparing between LLR and OLR did not differentiate between propensity score-matched studies and unmatched studies, therefore, its results might have an inherent risk of confounding bias limiting the causal relationship between both interventions and the observed clinical outcomes [17, 18]. Moreover, several studies have reported favorable clinical outcomes associated with LLR for right HCC, however, the efficacy of LLR in right hepatectomy has not been well established [19, 20]. Therefore, we sought to conduct a systematic review and meta-analysis of studies comparing LLR versus OLR in patients with right and left HCC and reported clinical outcomes in propensity-score matched and unmatched cohorts, to mitigate the potential risk of confounding bias, to identify the safety and efficacy of LLR in terms of surgical and oncological outcomes.
Methods
This systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) [21]. MEDLINE and Cochrane Central Register of Controlled Trials database were systematically searched through July 2018 using the following search terms: (1) hepatocellular carcinoma, liver neoplasms, liver cirrhosis/complications, malignant liver tumor; (2) hepatectomy, open liver resection, open hepatectomy (3) laparoscopy, laparoscopic hepatectomy. We had no restrictions on studies’ design. We included prospective and retrospective observational studies and randomized controlled trials (RCTs). Studies had to fulfill the following prespecified criteria to be considered qualified for inclusion in our meta-analysis; (1) comparing in a head to head fashion between LLR and OLR in patients with right or left HCC; (2) reported oncological and surgical outcomes, as mentioned below, in both treatment groups; (3) included patients with primary HCC. In case of duplicate publications reflecting the same population, we included the report with the longer follow-up duration. Our outcomes of interest were operative time in minutes (min), amount of blood loss in milliliter (ml), rate of blood transfusion, length of hospital stay in days, 30-days mortality, morbidity, recurrence rate, resection margin in centimeter (cm), R0 resection, 1-year over-all survival (1-OS), 3-years overall survival (3-OS), 5-years overall survival (5-OS), 1-year disease-free survival (1-DFS), 3-years disease-free survival (3-DFS), and 5-years disease-free survival (5-DFS) rates.
Two reviewers (Meng and Xu) independently screened databases for relevant studies based on the abovementioned criteria. After title and abstract screening, the full text of the selected articles was evaluated for eligibility. The two reviewers independently extracted the relevant data in a standardized extraction form, and third reviewer’s opinion was sought in case of disagreements (Duan). We performed a subgroup analysis restricted to studies reported clinical outcomes in propensity-score matched cohorts to mitigate the potential risk of confounding bias in data gleaned from observational studies. We conducted a subgroup analysis focused on studies exclusively recruited patients with right HCC to investigate the role of LLR in comparison with OLR in right HCC.
This meta-analysis is exempt from the need for IRB approval.
Statistical analysis
For categorical variables, summary estimates were expressed as odds ratio (OR) with corresponding 95% confidence interval (CI), and for continuous variables, summary estimates were expressed as mean difference (MD) with corresponding CI. The OR of our outcomes of interest was calculated according to the DerSimonian-Laird random effects model [22]. Heterogeneity across studies was assessed using Q-statistic and I2 –statistic [23]. The I2 statistic describes the percentage of total variation across studies that is due to heterogeneity. Sensitivity analyses were performed using the one-study-removed method to show how the summary estimate changes if the study that has the largest effect size is removed. Egger’s regression test and visual inspection of funnel plots were used to assess for potential publication bias since studies with statistically significant results are more likely to be published than studies with non-significant findings [24]. The statistical level of significance was 2-tailed P < 0.05. All Analyses were performed using random-effects model. All analyses were performed using Comprehensive Meta-analysis version 3.0 software (Biostat, Inc., New Jersey, USA).
Results
All studies meta-analysis
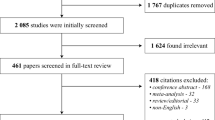
The process of studies selection is displayed in Fig. 1. Out of 1004 studies screened, the full text of 72 articles was reviewed. Fifty-one studies were identified including a total of 6812 patients, 2786 patients underwent LLR and 4026 patients were subjected to OLR. Studies’ characteristics are shown in Table 1. Patients’ characteristics are shown in Table 2. The recruitment periods of the included studies was between 1990 and 2017. The follow-up duration of the included studies ranged from 3 to 78.5 months.
The mean of operative time in minutes was reported in 26 studies including 3664 patients. There was no significant difference between LLR and OLR regarding the operative time in min (MD 18.29; 95% CI − 1.58 to 38.15; P = 0.07; I2 = 91.73%), Fig. 2. The operative blood loss in ml was reported in 19 studies including 2112 patients. Operative blood loss was significantly lower in LLR in comparison with OLR (MD − 124.09; 95% CI − 188.21 to − 59.97; P = 0.001; I2 = 94.09%). The incidence of blood transfusion was significantly lower in LLR comparing with OLR (OR 0.45; 95% CI 0.30–0.69; P = 0.001; I2 = 55.83%).
The mean of hospital stay in days, 30-days mortality rate and morbidity were significantly lower in LLR comparing with OLR (MD − 3.87; 95% CI − 4.86 to − 2.89; P = 0.001; I2 = 87.35%), (OR 0.32; 95% CI 0.16–0.66; P = 0.001; I2 = 0%), and (OR 0.42; 95% CI 0.34–0.52; P = 0.001; I2 = 39.64), respectively, Fig. 3.
Resection margin in centimeter (cm) and R0 resection were not significantly different between LLR and OLR groups (MD 0.04; 95% CI − 0.06 to 0.14; P = 0.41; I2 = 48.03%) and (OR 1.31; 95% CI 0.98 —1.76; P = 0.07; I2 = 0%), respectively. The recurrence rate was significantly higher in the OLR group than LLR group (OR 0.83; 95% CI 0.71–0.98; P = 0.03; I2 = 0%), Fig. 4.
The 1-OS and 5-OS were significantly higher in LLR comparing with OLR group (OR 1.45; 95% CI 1.06–1.99; P = 0.02; I2 = 25.59%) and (OR 1.36; 95% CI 1.07–1.72; P = 0.01; I2 = 14.88%), respectively, Fig. 5. 3-OS was not significantly different between both groups (OR 1.07; 95% CI 0.70–1.63; P = 0.77; I2 = 56.6). 1-DFS was significantly higher in LLR group comparing with OLR group (OR 1.42; 95% CI 1.032–1.972; P = 0.032; I2 = 55.57%), however, 3-DFS and 5-DFS did not differ significantly between both approaches (OR 1.349; 95% CI 1.349; 0.939–1.938; P = 0.105; I2 = 78.66%) and (OR 0.79; 95% CI 0.48–1.31; P = 0.36; I2 = 81.49%), respectively, Fig. 6.
Propensity score-matched studies subgroup analysis
Our subgroup analysis restricted to propensity score-matched studies did not show significant difference between both methods in terms of operative time in min (MD 11.64; 95% CI − 20.02 to 43.33; P = 0.47; I2 = 87.53%), blood loos in ml (MD − 95.62; 95% CI − 206.17 to 14.93; P = 0.09; I2 = 82.32), but significantly reduced blood transfusion rate with LLR than with OLR (OR 0.54; 95% CI 0.38–0.78; P = 0.001; I2 = 0%), Fig. 7.
Forest plot showing: A the mean operative time of laparoscopic liver resection (LLR) in comparison with open liver resection (OLR) in propensity score-matched studies, B The mean blood loss in ml in LLR in comparison with OLR in propensity score matched studies, and C The rate of blood transfusion in LLR in comparison with OLR in propensity score-matched studies
The mean of hospital stay in days, 30-days mortality, and morbidity were significantly lower in LLR in comparison with OLR (MD − 4.306; 95% CI − 5.79 to − 2.81; P = 0.001; I2 = 62.68%), (OR 0.31; 95% CI 0.11–0.84; P = 0.02; I2 = 0%), and (OR 0.51; 95% CI 0.39–0.67; P = 0.001; I2 = 33.98%), respectively, Fig. 8. Resection margin in cm and R0 resection did not differ from all-studies analysis, Fig. 9. However, recurrence rate showed non-significant difference between both approaches when the analysis was limited to propensity-score matched studies (OR 0.93; 95% CI 0.74–1.16; P = 0.50; I2 = 13.86%).
Forest plot showing: A the mean difference of hospital stay in days in laparoscopic liver resection (LLR) in comparison with open liver resection (OLR) in propensity score matched studies, B The 30-days mortality rate of LLR in comparison with OLR in propensity score-matched studies, and C The rate of morbidity in LLR in comparison with OLR in propensity score-matched studies
Forest plot showing: A the mean difference of resection margin in CM of laparoscopic liver resection (LLR) in comparison with open liver resection (OLR) in propensity score-matched studies, B The R0 resection rate of LLR in comparison with OLR in propensity score-matched studies, and C The recurrence in LLR in comparison with OLR in propensity score-matched studies
1-OS showed a trend favoring LLR over OLR (OR 1.53; 95% CI 0.94–2.47; P = 0.09; I2 = 39.4%). 3-OS and 5-OS did not differ from all-studies analysis, Fig. 10. 1-DFS and 3-DFS showed a trend favoring LLR over OLR (OR 1.527; 95% CI 0.99–2.34; P = 0.05; I2 = 68.13%) and (OR 1.24; 95% CI 1.01–1.53; P = 0.04; I2 = 18.18%), Fig. 11. 5-DFS did not differ from all-studies analysis.
Right hepatectomy subgroup analysis
When the analysis was restricted to studies of right hepatectomy, operative time was significantly lower in the OLR group than LLR group (MD 135.05; 95% CI 47.83–222.27; P = 0.001; I2 = 70.57%). Blood loss showed a trend of reduced blood loss amount with LLR than with OLR group (MD 43.88; 95% CI − 162.54 to 9.48; P = 0.08; I2 = 45.05%). Hospital stay in days and morbidity were significantly lower in LLR comparing with OLR (MD -3.96; 95% CI − 6.19 to − 1.743; P = 0.001; II2 = 83.04%) and (OR 0.16; 95% CI 0.06–0.43; P = 0.001; II2 = 0%), respectively, Fig. 12. Resection margin did not significantly differ between both approaches (MD 0.03; 95% CI − 0.20 to 0.25; P = 0.82; I2 = 60.00%).
Forest plot showing: A The mean difference of operative time in minutes in laparoscopic liver resection (LLR) in comparison with open liver resection (OLR) in right hepatectomy, B The mean difference of blood loss in ML in LLR in comparison with OLR in right hepatectomy, C The mean difference of hospital stay in days in LLR in comparison with OLR in right hepatectomy, D The morbidity rate in LLR comparing with OLR in right hepatectomy, and E the mean difference of resection margin in CM in LLR in comparison with OLR in right hepatectomy
Our sensitivity analysis using one-study-removal approach did not show any change in any outcomes of our interest, Supplemental Figs. 1, 2, and 3. Funnel plots of publication bias didn’t show any risk of publication bias with our outcomes of interest, Supplemental Fig. 4. Egger’s test showed a significant risk of publication bias with morbidity (Egger’s regression intercept − 1.37; 95% CI − 2.06 to − 0.68; P = 0.001), R0 resection (Egger’s regression intercept 0.88; 95% CI 0.18 –.57; P = 0.015) and 5-years DFS (Egger’s regression intercept 5.56; 95% CI 3.38–7.74; P = 0.001), Table 3. Egger’s test did not show any significant risk of publication bias with any other outcome.
Discussion
Our meta-analysis showed that blood loss, blood transfusion rate, 30-days mortality, hospital stay in days and recurrence rate were significantly lower in the LLR group in comparison with OLR group. 1-OS, 5-OS and 1-DFS rates showed a significantly favorable outcome associated with LLR in comparison with OLR. There was no significant difference between both groups regarding operative time in min, 3-OS, 3-DFS and 5-DFS. In a subgroup analysis restricted to right hepatectomy, our meta-analysis did not find any significant difference between both groups in terms of operative time in min and resection margin in cm. There was a strong trend, albeit non-significant, favoring LLR over OLR regarding blood loss. Hospital stay in days and morbidity were significantly lower in LLR comparing with OLR. Our subgroup analysis restricted to studies reported clinical outcomes in propensity-score matched populations showed a non-significant difference regarding blood loss and recurrence rate between both approaches, however LLR was associated with a significantly reduced blood transfusion rate.
In consistent with previous studies, we found favorable outcomes associated with LLR regarding operative blood loss and blood transfusion [18, 25]. The reduction in bleeding with LLR may be explained by the less risk of hepatic vein or vena cava injury because of the meticulous parenchymal dissection provided by the laparoscopy modality and the hemostatic effect of pneumoperitoneum which might have controlled bleeding from hepatic vein branches [26]. Furthermore, the magnification provided by laparoscopy allows better identification of small blood vessels which might have reduced the risk of blood loss and subsequently the blood transfusion rate.
In line with previous meta-analyses, LLR was significantly associated with lower postoperative 30-days mortality and shorter hospital stay in days comparing with OLR group [18, 25]. This might be explained by the less manipulation of abdominal organs, smaller incision, decreased rate of complications, less severe pain, lower need for narcotic pain medications, and early ambulation in the LLR group in comparison with OLR group.
In contradiction with previous studies, our all-studies meta-analysis showed that LLR was significantly associated with decreased recurrence rate comparing with OLR [25], which came as no surprise because of the decreased operative blood loss in the LLR group. Katz et al. concluded that intraoperative blood loss is an independent predictable factor to the tumor recurrence and survival rates [27]. Furthermore, although resection margin status was not significantly different between both groups in our analysis, there were other different preoperative and postoperative factors, such as Child–Pugh classification, amount of blood loss, and resected liver volume between LLR and OLR groups which might have influenced the recurrence rate [14, 28,29,30,31]. Therefore, the recurrence rate between both groups was not significantly different after restricting the analysis to propensity score-matched studies.
The second international consensus conference rated the quality of evidence supporting the superiority of LLR as low since most of the evidence derived from observational studies comparing LLR versus OLR [8, 32, 33]. The jury of the conference strongly recommended launching studies comparing between LLR and OLR in a head-to-head randomized fashion. Currently, there are a few ongoing randomized controlled trials randomizing patients with HCC to LLR versus OLR (NCT01768741), (NCT00606385), and (NCT02526043). Hopefully, these studies can provide a valid, non-biased evidence regarding the superiority of either strategy.
Our meta-analysis demonstrated a favorable survival rate at 5 years in LLR in all-studies analysis, and this survival benefit persisted even after restricting the analysis to propensity score-matched studies. Previous studies that investigated the correlation between resection margin and the OS and DFS concluded that resection margin can significantly predict the prognosis of HCC [34, 35]. Our meta-analysis showed a non-significant difference between both groups in terms of resection margin, therefore, the difference in survival benefits was most probably driven by other postoperative clinical outcomes, such as blood loss, postoperative complications, morbidity and 30-days mortality. Furthermore, the more manipulation and compression of the tumor during OLR might have resulted in more dissemination of tumor cells through portal vein to systematic circulation and this could have impacted the survival rate [36]. Our results are inconsistent with Poon et al’s findings that concluded that the resection margin was not associated with postoperative recurrence pattern and subsequently survival rate [37].
Our study did not find a statistically significant difference between LLR and OLR regarding 3-OS which might be explained by the limited number of studies reported 3-OS in comparison to studies reported 1-OS. Although there was a limited number of studies reported 5-OS, this might be compensated by the relatively large number of events over at 5 years follow-up.
Our meta-analysis has several limitations; (1) none of the included studies were a randomized controlled trial which makes our study has a risk of confounding and selection bias, however we run a subgroup analysis focused on propensity score-matched studies to mitigate the inherent risk of confounding bias associated with observational studies. RCTs comparing between both resection approaches are needed to accurately identify the superiority of either strategy. (2) there was an inherent heterogeneity regarding the definition of clinical outcomes across the included studies. (3) Despite our extensive literature search, we found a limited number of studies recruited patients with right hepatectomy, therefore, the results of our study should be interpreted carefully regarding the safety and efficacy of LLR in comparison with OLR in right hepatectomy. Further studies investigating the clinical outcomes of both approaches in right hepatectomy are still needed. (4) We could not run a subgroup analysis based on tumor classification since most of the studies did not stratify the HCC into stages or based on Endmondson classification and report clinical outcomes accordingly. (5) Our results regarding morbidity, R0 resection, and 5-DFS should be interpreted with caution since our analysis found a potential publication bias with these outcomes. The potential publication bias might have arisen from that tertiary care centers, that are well equipped, are more likely to publish data about LLR than community hospitals.
In conclusion, LLR was significantly associated with shorter operative time, less blood loss, less blood transfusion rate, shorter hospital stay in days, lower 30-days mortality rate, and lower morbidity. There was no significant difference between LLR and OLR regarding resection margin. There was no significant difference between both groups in terms of 3-DFS, 5-DFS, 3-OS, nevertheless LLR was associated with a significantly higher 1-OS, 5-OS and 1-DFS rates. RCTs are needed to identify the efficacy and safety of LLR in comparison with OLR in patients with HCC. Further studies investigating LLR in right hepatectomy are needed.
References
Mittal S, El-Serag HB (2013) Epidemiology of HCC: consider the Population. J Clin Gastroenterol 47:S2–6
Ryder S (2003) Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut 52:1-8
Reich H, McGlynn F, DeCaprio J, Budin R (1991) Laparoscopic excision of benign liver lesions. Obstet Gynecol 78:956–958
Parks KR, Kuo YH, Davis JM, OB B, Hagopian EJ (2014) Laparoscopic versus open liver resection: a meta-analysis of long-term outcome. HPB (Oxford) 16:109–118
Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362:1907–1917
Ziogas IA, Tsoulfas G (2017) Advances and challenges in laparoscopic surgery in the management of hepatocellular carcinoma. World J Gastrointest Surg 9:233–245
Goumard C, Farges O, Laurent A, Cherqui D, Soubrane O, Gayet B, Pessaux P, Pruvot FR, Scatton O, French Association for Hepatobiliary and Pancreatic Surgery (2015) An update on laparoscopic liver resection: the French Hepato-Bilio-Pancreatic Surgery Association statement. J Visc Surg 152:107–112
Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, OʼRourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schön MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM (2015) Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261:619–6
McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, Nicholl J, Collaboration Balliol, Aronson JK, Barkun JS, Blazeby JM, Boutron IC, Campbell WB, Clavien PA, Cook JA, Ergina PL, Feldman LS, Flum DR, Maddern GJ, Nicholl J, Reeves BC, Seiler CM, Strasberg SM, Meakins JL, Ashby D, Black N, Bunker J, Burton M, Campbell M, Chalkidou K, Chalmers I, de Leval M, Deeks J, Ergina PL, Grant A, Gray M, Greenhalgh R, Jenicek M, Kehoe S, Lilford R, Littlejohns P, Loke Y, Madhock R, McPherson K, Meakins J, Rothwell P, Summerskill B, Taggart D, Tekkis P, Thompson M, Treasure T, Trohler U, Vandenbroucke J (2009) No surgical innovation without evaluation: the IDEAL recommendations. Lancet 374:1105–1112
Amato B, Aprea G, De Rosa D, Milone M, di Domenico L, Amato M, Compagna R, Santoro M, Johnson LB, Sanguinetti A, Polistena A, Avenia N (2017) Laparoscopic hepatectomy for HCC in elderly patients: risks and feasibility. Aging Clin Exp Res 29:179–183
Landi F, De’ Angelis N, Scatton O, Vidal X, Ayav A, Muscari F, Dokmak S, Torzilli G, Demartines N, Soubrane O, Cherqui D, Hardwigsen J, Laurent A (2017) Short-term outcomes of laparoscopic vs. open liver resection for hepatocellular adenoma: a multicenter propensity score adjustment analysis by the AFC-HCA-2013 study group. Surg Endosc 31:4136–4144
Li W, Zhou X, Huang Z, Zhang K, Luo X, Zhong J, Chen Y (2017) Short-term and long-term outcomes of laparoscopic hepatectomy, microwave ablation, and open hepatectomy for small hepatocellular carcinoma: a 5-year experience in a single center. Hepatol Res 47:650–657
Chen J, Li H, Liu F, Li B, Wei Y (2017) Surgical outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma for various resection extent. Medicine (Baltimore) 96:e6460
Xiang L, Li J, Chen J, Wang X, Guo P, Fan Y, Zheng S (2016) Prospective cohort study of laparoscopic and open hepatectomy for hepatocellular carcinoma. Br J Surg 103:1895–1901
Cheung TT, Poon RT, Dai WC, Chok KS, Chan SC, Lo CM (2016) Pure laparoscopic versus open left lateral sectionectomy for hepatocellular carcinoma: a single-center experience. World J Surg 40:198–205
Tanaka S, Takemura S, Shinkawa H, Nishioka T, Hamano G, Kinoshita M, Ito T, Kubo S (2015) Outcomes of Pure laparoscopic versus open hepatic resection for hepatocellular carcinoma in cirrhotic patients: a case-control study with propensity score matching. Eur Surg Res 55:291–301
Jiang B, Yan XF, Zhang JH (2018) Meta-analysis of laparoscopic versus open liver resection for hepatocellular carcinoma. Hepatol Res 48:635–663
Sotiropoulos GC, Prodromidou A, Kostakis ID, Machairas N (2017) Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. Updates Surg 69:291–311
Yoon YI, Kim KH, Kang SH, Kim WJ, Shin MH, Lee SK, Jung DH, Park GC, Ahn CS, Moon DB, Ha TY, Song GW, Hwang S, Lee SG (2017) Pure laparoscopic versus open right hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score matched analysis. Ann Surg 265:856–863
Tarantino G, Magistri P, Serra V, Berardi G, Assirati G, Ballarin R, Di Benedetto F (2017) Laparoscopic liver resection of right posterior segments for hepatocellular carcinoma on cirrhosis. J Laparoendosc Adv Surg Tech A 27:559–563
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 1986:177–188
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Bmj 327:557–560
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Jiang B, Yan XF, Zhang JH (2018) Meta-analysis of laparoscopic versus open liver resection for hepatocellular carcinoma. Hepatol Res 48:635–663
Jaskille A, Schechner A, Park K, Williams M, Wang D, Sava J (2005) Abdominal insufflation decreases blood loss and mortality after porcine liver injury. J Trauma 2005:1305–1308
Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, Fong Y, D’Angelica MI, Blumgart LH, Dematteo RP (2009) Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg 249(4):617–623
Ker CG, Chen JS, Kuo KK, Chuang SC, Wang SJ, Chang WC, Lee KT, Chen HY, Juan CC (2011) Liver surgery for hepatocellular carcinoma: laparoscopic versus open approach. Int J Hepatol 2011:596792
Ahn S, Cho A, Kim EK, Paik KY (2016) Favorable long-term oncologic outcomes of hepatocellular carcinoma following laparoscopic liver resection. J Laparoendosc Adv Surg Tech A 26:447–452
Lai C, Jin R, Liang X, Cai X (2016) Comparison of laparoscopic hepatectomy, percutaneous radiofrequency ablation and open hepatectomy in the treatment of small hepatocellular carcinoma. J Zhejiang Univ Sci B 17:236–246
Yamashita Y, Ikeda T, Kurihara T, Yoshida Y, Takeishi K, Itoh S, Harimoto N, Kawanaka H, Shirabe K, Maehara Y (2014) Long-term favorable surgical results of laparoscopic hepatic resection for hepatocellular carcinoma in patients with cirrhosis: a single-center experience over a 10-year period. J Am Coll Surg 219:1117–1123
Nguyen KT, Gamblin TC, Geller DA (2009) World review of laparoscopic liver resection-2,804 patients. Ann Surg 250:831–841
Lin NC, Nitta H, Wakabayashi G (2013) Laparoscopic major hepatectomy: a systematic literature review and comparison of 3 techniques. Ann Surg 257:205–213
Hu W, Pang X, Guo W, Wu L, Zhang B (2015) Relationship of different surgical margins with recurrence-free survival in patients with hepatocellular carcinoma. Int J Clin Exp Pathol 8:3404–3409
Zhong FP, Zhang YJ, Liu Y, Zou SB (2017) Prognostic impact of surgical margin in patients with hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 96:e8043
Hayashi N, Egami H, Kai M, Kurusu Y, Takano S, Ogawa M (1999) No-touch isolation technique reduces intraoperative shedding of tumor cells into the portal vein during resection of colorectal cancer. Surgery 125:369–374
Poon RT, Fan ST, Ng IO, Wong J (2000) Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg 231:544–551
Takahara T, Wakabayashi G, Beppu T, Aihara A, Hasegawa K, Gotohda N, Hatano E, Tanahashi Y, Mizuguchi T, Kamiyama T, Ikeda T, Tanaka S, Taniai N, Baba H, Tanabe M, Kokudo N, Konishi M, Uemoto S, Sugioka A, Hirata K, Taketomi A, Maehara Y, Kubo S, Uchida E, Miyata H, Nakamura M, Kaneko H, Yamaue H, Miyazaki M, Takada T (2015) Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci 22:721–727
Cheung TT, Dai WC, Tsang SH, Chan AC, Chok KS, Chan SC, Lo CM (2016) Pure Laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma in 110 patients with liver cirrhosis: a propensity analysis at a single center. Ann Surg 264:612–620
Jiang H, Cao J (2015) Impact of laparoscopic versus open hepatectomy on perioperative clinical outcomes of patients with primary hepatic carcinoma. Chin Med Sci J 30:80–83
Luo L, Zou H, Yao Y, Huang X (2015) Laparoscopic versus open hepatectomy for hepatocellular carcinoma: short- and long-term outcomes comparison. Int J Clin Exp Med 8:18772–18778
Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y (2015) Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol 63:643–650
Komatsu S, Brustia R, Goumard C, Perdigao F, Soubrane O, Scatton O (2016) Laparoscopic versus open major hepatectomy for hepatocellular carcinoma: a matched pair analysis. Surg Endosc 30:1965–1974
Xiao L, Xiang LJ, Li JW, Chen J, Fan YD, Zheng SG (2015) Laparoscopic versus open liver resection for hepatocellular carcinoma in posterosuperior segments. Surg Endosc 29:2994–3001
Cho JY, Han HS, Yoon YS, Choi Y, Lee W (2015) Outcomes of laparoscopic right posterior sectionectomy in patients with hepatocellular carcinoma in the era of laparoscopic surgery. Surgery 158:135–141
Lee JJ, Conneely JB, Smoot RL, Gallinger S, Greig PD, Moulton CA, Wei A, McGilvray I, Cleary SP (2015) Laparoscopic versus open liver resection for hepatocellular carcinoma at a North-American Centre: a 2-to-1 matched pair analysis. HPB (Oxford) 17:304–310
Yoon SY, Kim KH, Jung DH, Yu A, Lee SG (2015) Oncological and surgical results of laparoscopic versus open liver resection for HCC less than 5 cm: case-matched analysis. Surg Endosc 29:2628–2634
Siniscalchi A, Ercolani G, Tarozzi G, Gamberini L, Cipolat L, Pinna AD, Faenza S (2014) Laparoscopic versus open liver resection: differences in intraoperative and early postoperative outcome among cirrhotic patients with hepatocellular carcinoma-a retrospective observational study. HPB Surg 2014:871251
Ahn KS, Kang KJ, Kim YH, Kim TS, Lim TJ (2014) A propensity score-matched case-control comparative study of laparoscopic and open liver resection for hepatocellular carcinoma. J Laparoendosc Adv Surg Tech A 24:872–877
Memeo R, de’Angelis N, Compagnon P, Salloum C, Cherqui D, Laurent A, Azoulay D (2014) Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: a case-control study. World J Surg 38:2919-2926
Kim SJ, Jung HK, Lee DS, Yun SS, Kim HJ (2014) The comparison of oncologic and clinical outcomes of laparoscopic liver resection for hepatocellular carcinoma. Ann Surg Treat Res 86:61–67
Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, Fan ST, Lo CM (2013) Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg 257:506–511
Ai JH, Li JW, Chen J, Bie P, Wang SG, Zheng SG (2013) Feasibility and safety of laparoscopic liver resection for hepatocellular carcinoma with a tumor size of 5-10 cm. PLoS ONE 8:e72328
Kobayashi S, Nagano H, Marubashi S, Kawamoto K, Wada H, Eguchi H, Tanemura M, Umeshita K, Doki Y, Mori M (2013) Hepatectomy based on the tumor hemodynamics for hepatocellular carcinoma: a comparison among the hybrid and pure laparoscopic procedures and open surgery. Surg Endosc 27:610–617
Hu BS, Chen K, Tan HM, Ding XM, Tan JW (2011) Comparison of laparoscopic vs open liver lobectomy (segmentectomy) for hepatocellular carcinoma. World J Gastroenterol 17:4725–4728
Lee KF, Chong CN, Wong J, Cheung YS, Lai P (2011) Long-term results of laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: a case-matched analysis. World J Surg 35:2268–2274
Kim HH, Park EK, Seoung JS, Hur YH, Koh YS, Kim JC, Cho CK, Kim HJ (2011) Liver resection for hepatocellular carcinoma: case-matched analysis of laparoscopic versus open resection. J Korean Surg Soc 80:412–419
Truant S, Bouras AF, Hebbar M, Boleslawski E, Fromont G, Dharancy S, Leteurtre E, Zerbib P, Pruvot FR (2011) Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: a case-matched study. Surg Endosc 25:3668–3677
Aldrighetti L, Guzzetti E, Pulitano C, Cipriani F, Catena M, Paganelli M, Ferla G (2010) Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol 102:82–86
Tranchart H, Di Giuro G, Lainas P, Roudie J, Agostini H, Franco D, Dagher I (2010) Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc 24:1170–1176
Alemi F, Kwon E, Freise C, Kang SM, Hirose R, Stewart L, Corvera CU (2010) Hepatic surgery at a VA tertiary medical center: lessons learned. Am J Surg 200:591–595
Sarpel U, Hefti MM, Wisnievsky JP, Roayaie S, Schwartz ME, Labow DM (2009) Outcome for patients treated with laparoscopic versus open resection of hepatocellular carcinoma: case-matched analysis. Ann Surg Oncol 16:1572–1577
Lai EC, Tang CN, Ha JP, Li MK (2009) Laparoscopic liver resection for hepatocellular carcinoma: ten-year experience in a single center. Arch Surg 144:143–147
Cai XJ, Yang J, Yu H, Liang X, Wang YF, Zhu ZY, Peng SY (2008) Clinical study of laparoscopic versus open hepatectomy for malignant liver tumors. Surg Endosc 22:2350–2356
Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL (2003) Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg 138:763–769
Shimada M, Hashizume M, Maehara S, Tsujita E, Rikimaru T, Yamashita Y, Tanaka S, Adachi E, Sugimachi K (2001) Laparoscopic hepatectomy for hepatocellular carcinoma. Surg Endosc 15:541–544
Cheung TT, Ma KW, She WH, Dai WC, Tsang SHY, Chan ACY, Chok KSH, Lo CM (2018) Pure laparoscopic hepatectomy with augmented reality-assisted indocyanine green fluorescence versus open hepatectomy for hepatocellular carcinoma with liver cirrhosis: a propensity analysis at a single center. Asian J Endosc Surg 11:104–111
Xu X, Chen J, Wang F, Ni Q, Naimat U, Chen Z (2017) Recurrence of hepatocellular carcinoma after laparoscopic hepatectomy: risk factors and treatment strategies. J Laparoendosc Adv Surg Tech A 27:676–684
Xu HW, Liu F, Li HY, Wei YG, Li B (2018) Outcomes following laparoscopic versus open major hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score-matched analysis. Surg Endosc 32:712–719
Ryu T, Honda G, Kurata M, Kobayashi S, Sakamoto K, Honjo M (2018) Perioperative and oncological outcomes of laparoscopic anatomical hepatectomy for hepatocellular carcinoma introduced gradually in a single center. Surg Endosc 32:790–798
Zhang Y, Chen XM, Sun DL (2016) Short-term outcomes of laparoscopic versus open right hemihepatectomy for hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech 26:e157–e160
Zhang Y, Huang J, Chen XM, Sun DL (2016) A comparison of laparoscopic versus open left hemihepatectomy for hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech 26:146–149
Sposito C, Battiston C, Facciorusso A, Mazzola M, Muscarà C, Scotti M, Romito R, Mariani L, Mazzaferro V (2016) Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg 103:871–880
Sotiropoulos GC, Machairas N, Stamopoulos P, Kostakis ID, Dimitroulis D, Mantas D, Kouraklis G (2016) Laparoscopic versus open liver resection for hepatocellular carcinoma: initial experience in Greece. Ann Gastroenterol 29:521–529
Jiang X, Liu L, Zhang Q, Jiang Y, Huang J, Zhou H, Zeng L (2016) Laparoscopic versus open hepatectomy for hepatocellular carcinoma: long-term outcomes. J buon 21:135–141
Acknowledgements
We thank all members of the Hepatobiliary Surgery Department for valuable comments on this study and helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Meng Xiangfei, Xu Yinzhe, Pan Yingwei, Lu Shichun and Duan Weidong have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
464_2019_6781_MOESM1_ESM.tif
Supplementary material 1 (TIFF 5796 kb). Supplemental Appendix Fig. 1. Sensitivity analysis using one- study removal approach of operative in minutes, blood loss in ML, and blood transfusion rate in laparoscopic liver resection (LLR) in comparison with open liver resection (OLR)
464_2019_6781_MOESM2_ESM.tif
Supplementary material 2 (TIFF 8858 kb). Supplemental Appendix Fig. 2. Sensitivity analysis using one- study removal approach of hospital stay in days, mortality rate and morbidity rate in laparoscopic liver resection (LLR) in comparison with open liver resection (OLR)
464_2019_6781_MOESM3_ESM.tif
Supplementary material 3 (TIFF 4785 kb). Supplemental Appendix Fig. 3. Sensitivity analysis using one-study removal approach of mean difference of resection margin in CM, R0 resection rate, and recurrence rate in laparoscopic liver resection (LLR) in comparison with open liver resection (OLR)
464_2019_6781_MOESM4_ESM.tif
Supplementary material 4 (TIFF 4168 kb). Supplemental Appendix Fig. 4. Funnel plot showing the risk of publication bias of A) operative time, B) blood loss in ML, C) blood transfusion rate, D) hospital stay in days, E) 30-days mortality rate, and F) morbidity rate
Rights and permissions
About this article
Cite this article
Xiangfei, M., Yinzhe, X., Yingwei, P. et al. Open versus laparoscopic hepatic resection for hepatocellular carcinoma: a systematic review and meta-analysis. Surg Endosc 33, 2396–2418 (2019). https://doi.org/10.1007/s00464-019-06781-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06781-3