Abstract
Background
The safety and oncologic outcomes of patients with advanced cirrhosis undergoing laparoscopic liver resection (LLR) compared to open resection (OLR) for hepatocellular carcinoma (HCC) remain unclear.
Methods
Patients with HCC resection during 2010–2014 were identified from the National Cancer Database. Patients with severe fibrosis; single lesions; M0; and known grade, margin status, tumor size, length of hospital stay, 30- and 90-day mortality, 30-day readmission, surgical approach, and complete follow-up were included. A 1:1 propensity score matching analysis of LLR:OLR was performed. Prognostic effect of LLR was assessed by multivariable Cox proportional hazards model.
Results
A total of 1799 hepatectomy patients (minor (n = 491, 27.3%); major (n = 1308, 72.7%)) were included. Of 193 (10.7%) LLR patients, 190 were eligible for matching. The LLR vs OLR did not differ for patient characteristics, resection margin status, and 30-day (p = 0.141), 90-day mortality (p = 0.121), or 30-day readmission (p = 0.784). Median hospital stay was shorter for LLR (6 vs 8 days, p = 0.001). Median overall survival (OS) was similar for LLR vs OLR (44.2 and 39.5 months, respectively, p = 0.064). Predictors of worse OS were older age (hazard ratio (HR) 1.04, p = 0.034), > 2 comorbidities (HR 1.29, p = 0.012), grade 3–4 disease (HR 1.81, p = 0.025), N1 disease (HR 1.04, p = 0.048), and R1 margins (HR 1.34, p = 0.002). After adjustment for confounders, LLR vs OLR was not a significant risk factor for OS (HR 1.14, 95% CI 0.76–1.71, p = 0.522).
Conclusion
While LLR in advanced cirrhosis for patients with HCC proved safe, optimal patient selection based on the preoperatively available factors comorbidities, age, degree of underlying liver disease, and high-quality oncologic surgery will determine long-term survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common indication for laparoscopic liver resection (LLR) worldwide.1,2 While reports from consensus conferences3,4,5 and expert centers6,7,8,9,10,11,12,13,14,15 suggest a benefit from LLR vs open liver resection (OLR) in patients with HCC specifically, these studies are from high-volume centers with significant expertise in managing liver cirrhosis and treating HCC.13,16,17 Whether the data from these expert centers is generalizable to non-specialized practices remains unclear to date. In addition, most series on LLR for HCC are from Asian centers, where HCC is the most common indication for liver resection and where the etiology for the liver cirrhosis leading to HCC is different from the West. These general differences in the surgical treatment of HCC between Eastern and Western countries have been reported.18 However, it remains unclear whether the surgical approach specifically (LLR vs OLR) impacts outcomes in Western patients.
While HCC is intimately associated with liver cirrhosis (4 out of 5 cases), it is poor liver function rather than HCC that has the greatest negative impact on short- and long-term survival in patients with potentially curable HCC and cirrhosis. In these cirrhotic patients at risk for decompensation, LLR vs OLR has been shown to be associated with less surgical trauma and a lower incidence of peritoneal dissection without compromising oncologic safety.19 Moreover, in this patient population of curable HCC with cirrhosis, LLR specifically is associated with lower rates of postoperative liver failure and ascites than OLR. Reports on this association of surgical approach and outcome are from specialized centers with significant experience in managing cirrhosis and treating HCC and from carefully selected patient populations.7,9,12,15,20,21,22
To help resolve the controversy regarding any specific benefit of LLR in Western patients with HCC and advanced cirrhosis and whether these benefits can be predicted preoperatively, we compared short- and long-term outcomes of LLR and OLR for HCC in a US population-based cohort. To ensure results are reflective of national trends and generalizable to non-expert centers, we used data from the National Cancer Database (NCDB), a nationwide, facility-based (specialized and non-specialized centers) comprehensive cancer surveillance database that captures information of at least 70% of all newly diagnosed cancers in the USA. Additionally, to minimize the impact of selection bias on the study findings (less advanced cases may be operated via LLR), propensity score matching was employed.
Materials and Methods
Patient Selection Criteria and Data Source
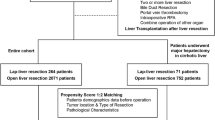
The NCDB was queried to identify patients aged 18 years or older with a histologically confirmed diagnosis of HCC during 2010–2014. Cases of HCC were identified using the International Classification of Diseases for Oncology, third edition, primary tumor site code for liver (C22) and morphology codes for HCC (8170/3, 8171/3, 8172/3, 8173/3, 8174/3, and 8175/3). Patients were excluded if they did not have severe cirrhosis (International Association for Study of the Liver score > 423), if they had M1 disease or multiple liver lesions, if they did not undergo surgery for HCC, if the procedure was converted to open, or if data were unavailable regarding comorbidities (Charlson-Deyo score), type of operation (LLR vs OLR), tumor size, tumor grade, margin status, number of tumors, nodal status, intrahepatic vascular invasion status, length of hospital stay, 30-day and 90-day mortality, or 30-day unplanned readmission (Fig. 1).
Outcomes
The outcomes measured were death within 30 days after liver resection, death within 90 days after liver resection,24 readmission within 30 days after liver resection,25 length of hospital stay after liver resection, and overall survival (OS), defined in the NCDB and in our study as the time from liver resection until death of any cause.
Statistical Analysis
Propensity score matching to minimize differences in baseline characteristics between the patients who underwent LLR and the patients who underwent OLR was used (Fig. 2). Factors considered the most important confounders also contributing to survival risk were chosen for the propensity score algorithm (age, sex, hospital volume, comorbidities, tumor size, tumor grade, type of resection, and vascular invasion). These factors were chosen on the basis of previously reported preoperatively available factors predictive of outcome26,27,28 and consensus of the investigators achieved via structured communication technique. A 1:1 match on the propensity score, without replacement, was performed with a conservative caliper width of 10% of the standard deviation of the log of propensity score. Given the paired nature of the data, the Mann-Whiney U test was used to compare binary outcomes, and the Wilcoxon signed-rank test was used to compare continuous outcomes. Matched pairs with missing data for either patient were excluded during analysis of the missing outcome.
OS was evaluated using the Kaplan–Meier method and compared using the log-rank test. To adjust for possible confounders (age, sex, comorbidities, tumor size, tumor grade, nodal status, margin status, type of hepatectomy (minor or major), and surgical approach), Cox multivariable regression was performed to identify factors affecting OS and the effect of surgical approach on OS. Major hepatectomy was defined as resection of more than two segments.
All statistical computations were performed using SPSS, version 24 (IBM Inc., Armonk, NY).
Results
A total of 76,799 patients aged 18 years or older who were diagnosed with HCC were identified in NCDB during 2010–2014 (Fig. 1). Patients with incomplete data, who did not have severe fibrosis or recorded fibrosis data, did not undergo surgery, or with missing data about tumor grade or margin status, were excluded. This left 1799 patients to be included in the analysis.
Characteristics of the Entire Cohort
Of the 1799 patients, 1606 (89.3%) underwent OLR, and 193 (10.7%) underwent LLR; 491 (27.3%) underwent minor and 1308 (72.7%) major hepatectomies.
The demographic and clinicopathologic characteristics of the entire cohort are summarized in Table 1. No significant differences were observed between patients who underwent OLR and those who underwent LLR with respect to sex, histologic grade, tumor size, N category, or proportion of patients with R1 resection margins. The OLR group was older and had higher rates of 2 or more comorbidities, lymphovascular invasion, and major hepatectomy; the LLR group had a higher rate of minor hepatectomy.
Characteristics of the Matched Cohort
Of the 193 patients who underwent LLR, 190 patients were eligible for 1:1 matching (Table 1). In the matched cohort, no significant differences were observed between the patients who underwent OLR and those who underwent LLR with respect to any measured demographic or clinicopathologic characteristic and facility type where the surgery was performed (Supplementary Table 1), including the proportion of patients with R1 resection margins.
Mortality, Readmission Rate, and Hospital Stay in Patients with OLR vs LLR
In the matched cohort, the OLR and LLR groups did not differ with respect to 30-day mortality, 90-day mortality, or 30-day readmission rate. Median hospital stay was significantly shorter for the LLR group (6 vs 8 days, p = 0.001) (Table 2).
Predictors of Readmission and Mortality
On multivariable analysis, predictors of 30-day and 90-day mortality were older age, 2 or more comorbidities, and major hepatectomy (Table 3). LLR vs OLR was not a significant risk factor for mortality. Predictors of 30-day readmission were older age, 2 or more comorbidities, and major hepatectomy (Table 3). LLR vs OLR was not a significant risk factor for 30-day readmission.
Overall Survival in Patients with OLR vs LLR
In the matched cohort, median OS was 42.3 months. Median OS was similar (not inferior) for the OLR group (39.5 months) and LLR group (44.2 months) (log-rank p = 0.064) (Fig. 3).
Predictors of OS
On multivariable analysis (Table 3), predictors of poor OS were older age, 2 or more comorbidities, histologic grade 3 or 4, larger tumor size, vascular invasion, positive lymph nodes, and R1 resection margins. Major hepatectomy was a predictor of better OS. LLR vs OLR was not a significant predictor of survival.
Discussion
In this study, we found that, in a Western population–based cohort, LLR in patients with advanced cirrhosis was similar to OLR in terms of OS, 30-day and 90-day mortality, and 30-day readmission rate. Moreover, in the multivariable analysis, the only factors related to mortality and 30-day readmission were advanced age, 2 or more comorbidities, and major hepatectomy. Surgical approach was not related to short-term or long-term outcome.
Prior studies of LLR for HCC in patients with cirrhosis6,7,10,14,15,20 have suggested that LLR leads to the same oncologic outcome and mortality as OLR in selected patients. However, most of these earlier studies were conducted at specialized centers in a carefully selected Asian population, whereas the current study is based on a representative sample of Western patients treated in both academic and non-academic centers. Therefore, the findings presented are more representative of typical Western practice patterns compared to prior reports.
Further, our results indicate that LLR is comparable to OLR regarding 90-day mortality and OS, even after adjusting for major liver resection which was a significant component of the study population (31.6%). It has been suggested that LLR vs OLR results in less disruption of collateral circulation through the abdominal wall due to the smaller incisions required for ports, less liver mobilization preserving perihepatic collaterals, and lower demand for intravenous fluid replacement due to less sensible losses decreasing the risk of third spacing. These factors result in a decreased risk of prolonged postoperative ascites and liver decompensation after surgery.7,29 For these reasons, the beneficial effect of laparoscopy may be more prominent in major hepatectomies and in patients with advanced cirrhosis, which was demonstrated here.15,30 In the LLR group, the incidence of ascites was significantly less than that in the OLR group (9.4 vs 31.3%, p = 0.030), while the blood loss, the transfusion requirement, the rate of overall postoperative complications, and the OS and disease-free survival were similar.30,31 Therefore, in appropriately selected HCC patients with advanced cirrhosis, LLR should be encouraged, when it can be performed safely by surgeons that have surpassed the minimal learning/teaching curve for complex laparoscopic liver surgery of 45 to 75 cases at the minimum.32
Until recently, it has been thought that for the subgroup of patients with HCC and cirrhosis10 the literature is insufficient to support LLR. This concept that LLR in general should not be recommended for patients with cirrhosis is being confronted by the findings of this study that show that outcomes of appropriately selected LLR patients are at least similar regarding 30- and 90-day mortality and 30-day readmission to OLR in a population-based setting. These results are in agreement with some reports that show no significant difference between LLR and OLR in terms of blood loss, blood transfusions, operative time, Pringle maneuver duration, overall morbidity, and postoperative mortality.33 Even liver-specific complications, such as ascites decompensation and postoperative liver failure, have been shown to be similar between LLR and OLR in patients with HCC and early cirrhosis.7,14,29 However, these reports are from European and Asian expert centers with significant experience in managing cirrhosis and HCC. In a recent study from the USA6 of LLR in patients with HCC and moderate to advanced cirrhosis, morbidity and mortality did not differ significantly between the 26 patients with Child–Pugh class B (n = 20) or class C (n = 6) disease and the 80 patients with Child–Pugh class A disease. The decision to perform LLR on 6 patients with Child–Pugh class C can be viewed as controversial evidenced by the resulting severe morbidity and a 90-day mortality rate of 16.6%. These results underscore the results of this study showing that the main determinants of outcome are patient selection and quality of surgery rather than the surgical approach.
While this study showing comparative outcomes between LLR and OLR is representative of a wide range of practice patterns, a recent study13 reported a volume–outcome relationship between hospital volume and outcome for patients undergoing LLR for primary liver cancer. The mortality in low-volume centers was similar for OLR and LLR and therefore the adoption of LLR does not explain the inferior performance of low-volume centers reported in this study and suggests that outcomes for patients at low-volume centers are more likely related to variations in perioperative care. In the present study, after matching, no difference related to the facility type was found (Supplementary Table 1).
To our knowledge, this is the largest study to date comparing LRR and OLR for HCC in patients with advanced cirrhosis, which accounts for selection bias and minimizes confounding (propensity score matching), there are several limitations. While it is well known that non-quantifiable or unknown measures may impact the treatment, selection cannot be controlled for with this approach or any other method apart from a randomized controlled trial. The statistical approach chosen here may control the most for selection bias while helping to answer the question whether LLR for HCC in advanced cirrhosis is appropriate. Another limitation of this study is that the NCDB, the source of our data, is subject to coding error. To minimize this risk, incongruent data were excluded resulting in a reduction of the source data from 76,799 to reliable 1799 items. Further, in the NCDB, cirrhosis is reported as severe liver fibrosis and traditionally used factors to determine degree of cirrhosis such as Child–Pugh score, portal hypertension, esophageal varices, platelet count, or splenomegaly were not available. Nevertheless, the use of this dataset permitted comparison of LLR vs OLR for HCC in a large sample of patients with cirrhosis treated at both expert and non-expert centers across the USA, a topic that has previously not been investigated aside from small studies from single-institution expert centers. Our finding that only 11% of the patients with advanced cirrhosis who underwent liver resection for HCC in 2010–2014 underwent LLR shows that LLR remains in an early phase practiced by a few innovators and early adopters.34 This suggests that the patients in this study were treated by surgeons who may have reached a plateau on their learning curve. While few cirrhotic patients with HCC may benefit from LLR today, the presented data suggest that dissemination of safe implementation of LRR across various practice patterns will allow well-selected patients to have easy access of care while reducing morbidity and postoperative decompensation from LLR.
Conclusion
The presented data show that LLR in patients with cirrhosis and HCC leads to comparable oncologic outcome and mortality as OLR. Further, not the surgical approach but age, tumor characteristics, and comorbidities are the main determinants of outcome. Since it was demonstrated in this population-based cohort of Western patients that the main determinants of outcome are factors that are available preoperatively, careful selection of cirrhotic patients with HCC for LLR will reduce morbidity and hepatic decompensation, while allowing improved access to care encouraging mortality and oncologic outcome.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- HR:
-
Hazard ratio
- LLR:
-
Laparoscopic liver resection
- NCDB:
-
National Cancer Database
- OLR:
-
Open liver resection
- OS:
-
Overall survival
References
Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg. 2016;263(4):761–77. https://doi.org/10.1097/sla.0000000000001413.
Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250(5):831–41. https://doi.org/10.1097/SLA.0b013e3181b0c4df.
Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, O’Rourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schon MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261(4):619–29. https://doi.org/10.1097/sla.0000000000001184
Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D'Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250(5):825–30.
Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, Aroori S, Belli G, Besselink M, Briceno J, Gayet B, D'Hondt M, Lesurtel M, Menon K, Lodge P, Rotellar F, Santoyo J, Scatton O, Soubrane O, Sutcliffe R, Van Dam R, White S, Halls MC, Cipriani F, Van der Poel M, Ciria R, Barkhatov L, Gomez-Luque Y, Ocana-Garcia S, Cook A, Buell J, Clavien PA, Dervenis C, Fusai G, Geller D, Lang H, Primrose J, Taylor M, Van Gulik T, Wakabayashi G, Asbun H, Cherqui D. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg. 2018;268(1):11–8. https://doi.org/10.1097/sla.0000000000002524.
Beard RE, Wang Y, Khan S, Marsh JW, Tsung A, Geller DA. Laparoscopic liver resection for hepatocellular carcinoma in early and advanced cirrhosis. HPB (Oxford). 2018. 20(6):521–9 https://doi.org/10.1016/j.hpb.2017.11.011.
Belli G, Limongelli P, Fantini C, D'Agostino A, Cioffi L, Belli A, Russo G. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2009;96(9):1041–8. https://doi.org/10.1002/bjs.6680.
Beppu T, Wakabayashi G, Hasegawa K, Gotohda N, Mizuguchi T, Takahashi Y, Hirokawa F, Taniai N, Watanabe M, Katou M, Nagano H, Honda G, Baba H, Kokudo N, Konishi M, Hirata K, Yamamoto M, Uchiyama K, Uchida E, Kusachi S, Kubota K, Mori M, Takahashi K, Kikuchi K, Miyata H, Takahara T, Nakamura M, Kaneko H, Yamaue H, Miyazaki M, Takada T. Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22(10):711–20. https://doi.org/10.1002/jhbp.261.
Cherqui D, Laurent A, Tayar C, Chang S, Van Nhieu JT, Loriau J, Karoui M, Duvoux C, Dhumeaux D, Fagniez PL. Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspectives. Ann Surg. 2006;243(4):499–506. https://doi.org/10.1097/01.sla.0000206017.29651.99.
Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, Fan ST, Lo CM. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg. 2013;257(3):506–11. https://doi.org/10.1097/SLA.0b013e31827b947a.
Sarpel U, Hefti MM, Wisnievsky JP, Roayaie S, Schwartz ME, Labow DM. Outcome for patients treated with laparoscopic versus open resection of hepatocellular carcinoma: case-matched analysis. Ann Surg Oncol. 2009;16(6):1572–7. https://doi.org/10.1245/s10434-009-0414-8.
Tranchart H, Di Giuro G, Lainas P, Roudie J, Agostini H, Franco D, Dagher I. Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc. 2010;24(5):1170–6. https://doi.org/10.1007/s00464-009-0745-3.
Varley PR, Tohme ST, Chidi AP, Goswami J, van der Windt D, Geller DA, Tsung A. Dissemination of Minimally Invasive Liver Resection for Primary Malignancy: Reevaluating Effectiveness. Ann Surg Oncol. 2018;25(3):808–17. https://doi.org/10.1245/s10434-017-6308-2.
Yamashita Y, Ikeda T, Kurihara T, Yoshida Y, Takeishi K, Itoh S, Harimoto N, Kawanaka H, Shirabe K, Maehara Y. Long-term favorable surgical results of laparoscopic hepatic resection for hepatocellular carcinoma in patients with cirrhosis: a single-center experience over a 10-year period. J Am Coll Surg. 2014;219(6):1117–23. https://doi.org/10.1016/j.jamcollsurg.2014.09.003.
Yoon YI, Kim KH, Kang SH, Kim WJ, Shin MH, Lee SK, Jung DH, Park GC, Ahn CS, Moon DB, Ha TY, Song GW, Hwang S, Lee SG. Pure Laparoscopic Versus Open Right Hepatectomy for Hepatocellular Carcinoma in Patients With Cirrhosis: A Propensity Score Matched Analysis. Ann Surg. 2017;265(5):856–63. https://doi.org/10.1097/sla.0000000000002072.
He J, Amini N, Spolverato G, Hirose K, Makary M, Wolfgang CL, Weiss MJ, Pawlik TM. National trends with a laparoscopic liver resection: results from a population-based analysis. HPB (Oxford). 2015;17(10):919–26. https://doi.org/10.1111/hpb.12469.
Spolverato G, Ejaz A, Kim Y, Hall BL, Bilimoria K, Cohen M, Ko C, Pitt H, Pawlik TM. Patterns of care among patients undergoing hepatic resection: a query of the National Surgical Quality Improvement Program-targeted hepatectomy database. J Surg Res. 2015;196(2):221–8. https://doi.org/10.1016/j.jss.2015.02.016.
Kokudo T, Hasegawa K, Kokudo N, Kokudo T, Uldry E, Demartines N, Halkic N. Hepatocellular Carcinoma: The Gap Between Eastern and Western Clinical Practice. Ann Surg. 2018;267(2):e27-e8. https://doi.org/10.1097/sla.0000000000001960.
Laurent A, Tayar C, Andreoletti M, Lauzet JY, Merle JC, Cherqui D. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16(3):310–4. https://doi.org/10.1007/s00534-009-0063-0.
Sposito C, Battiston C, Facciorusso A, Mazzola M, Muscara C, Scotti M, Romito R, Mariani L, Mazzaferro V. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg. 2016;103(7):871–80. https://doi.org/10.1002/bjs.10137.
Belli G, Fantini C, Belli A, Limongelli P. Laparoscopic liver resection for hepatocellular carcinoma in cirrhosis: long-term outcomes. Dig Surg. 2011;28(2):134–40. https://doi.org/10.1159/000323824.
Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamazoe S, Yamamoto S, Kubo S. Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surg Endosc. 2013;27(7):2592–7. https://doi.org/10.1007/s00464-013-2795-9.
Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–9.
Mise Y, Vauthey JN, Zimmitti G, Parker NH, Conrad C, Aloia TA, Lee JE, Fleming JB, Katz MH. Ninety-day Postoperative Mortality Is a Legitimate Measure of Hepatopancreatobiliary Surgical Quality. Ann Surg. 2015;262(6):1071–8. https://doi.org/10.1097/sla.0000000000001048.
Mise Y, Day RW, Vauthey JN, Brudvik KW, Schwarz L, Prakash L, Parker NH, Katz MH, Conrad C, Lee JE, Fleming JB, Aloia TA. After Pancreatectomy, the “90 Days from Surgery” Definition Is Superior to the “30 Days from Discharge” Definition for Capture of Clinically Relevant Readmissions. J Gastrointest Surg. 2016;20(1):77–84; discussion https://doi.org/10.1007/s11605-015-2984-z.
Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, Curley SA, Ellis LM, Regimbeau JM, Rashid A, Cleary KR, Nagorney DM. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20(6):1527–36. https://doi.org/10.1200/jco.2002.20.6.1527.
Shindoh J, Andreou A, Aloia TA, Zimmitti G, Lauwers GY, Laurent A, Nagorney DM, Belghiti J, Cherqui D, Poon RT, Kokudo N, Vauthey JN. Microvascular invasion does not predict long-term survival in hepatocellular carcinoma up to 2 cm: reappraisal of the staging system for solitary tumors. Ann Surg Oncol. 2013;20(4):1223–9. https://doi.org/10.1245/s10434-012-2739-y.
Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211(3):277–87.
Kaneko H, Tsuchiya M, Otsuka Y, Yajima S, Minagawa T, Watanabe M, Tamura A. Laparoscopic hepatectomy for hepatocellular carcinoma in cirrhotic patients. J Hepatobiliary Pancreat Surg. 2009;16(4):433–8. https://doi.org/10.1007/s00534-009-0123-5.
Xu HW, Liu F, Li HY, Wei YG, Li B. Outcomes following laparoscopic versus open major hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score-matched analysis. Surg Endosc. 2018;32(2):712–9. https://doi.org/10.1007/s00464-017-5727-2.
Komatsu S, Brustia R, Goumard C, Perdigao F, Soubrane O, Scatton O. Laparoscopic versus open major hepatectomy for hepatocellular carcinoma: a matched pair analysis. Surg Endosc. 2016;30(5):1965–74. https://doi.org/10.1007/s00464-015-4422-4.
Nomi T, Fuks D, Kawaguchi Y, Mal F, Nakajima Y, Gayet B. Learning curve for laparoscopic major hepatectomy. Br J Surg. 2015;102(7):796–804. https://doi.org/10.1002/bjs.9798.
Cipriani F, Fantini C, Ratti F, Lauro R, Tranchart H, Halls M, Scuderi V, Barkhatov L, Edwin B, Troisi RI, Dagher I, Reggiani P, Belli G, Aldrighetti L, Abu Hilal M. Laparoscopic liver resections for hepatocellular carcinoma. Can we extend the surgical indication in cirrhotic patients? Surg Endosc. 2018;32(2):617–26. https://doi.org/10.1007/s00464-017-5711-x.
Wilson CB. Adoption of new surgical technology. Bmj. 2006;332(7533):112–4. https://doi.org/10.1136/bmj.332.7533.112.
Acknowledgements
The authors thank Stephanie Deming for her invaluable editorial support.
Author information
Authors and Affiliations
Contributions
Substantial contributions to:
- The conception or design of the work:
Eduardo A. Vega, MD; Onur C. Kutlu, MD; Katharina Joechle, MD; Nestor De La Cruz, MD; Dicken Ko, MD; Claudius Conrad, MD, PhD
- The acquisition, analysis, or interpretation of data for the work:
Eduardo A. Vega, MD; Onur C. Kutlu, MD; Katharina Joechle, MD; Nestor De La Cruz, MD; Dicken Ko, MD; Claudius Conrad, MD, PhD
Drafting the work or revising it critically for important intellectual content: all authors
Final approval of the version to be published: all authors
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all authors
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Oral presentation
59th Society for Surgery of the Alimentary Tract Annual Meeting, 2018, Washington, DC.
Electronic Supplementary Material
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Vega, E.A., Kutlu, O.C., Joechle, K. et al. Preoperative Prognosticators of Safe Laparoscopic Hepatocellular Carcinoma Resection in Advanced Cirrhosis: a Propensity Score Matching Population-Based Analysis of 1799 Western Patients. J Gastrointest Surg 23, 1157–1165 (2019). https://doi.org/10.1007/s11605-019-04139-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04139-7