Abstract
Background
Although the benefits of laparoscopic hepatectomy (LH) for hepatocellular carcinoma (HCC) in most circumstances are evident, the benefits for large HCC are contentious. This study aimed to compare the perioperative outcomes and survival after LH versus open hepatectomy (OH) in large HCC patients.
Methods
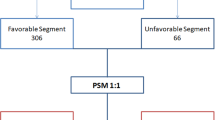
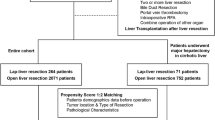
An analysis of prospectively maintained database included 215 hepatectomies for large HCC (diameter ≥ 5 cm). The operative and survival outcomes were compared between the LH group (n = 109) and the OH group (n = 106). Propensity score matching (PSM) 1:1 included 70 patients in each group. The entire cohort multivariable analyses were performed to identify the factors associated with surgical complications and suboptimal recurrence-free survival (RFS).
Results
After PSM, baseline characteristics and the extent of liver resection were similar in both groups. The LH group had a shorter hospital stay than the OH group (7 vs 9.5 days, p = 0.001). The R0 resection rate, complication rate, overall survival, and RFS were similar between the groups. The multivariate analyses revealed two independent factors predicting surgical complication (major resection; p < 0.001 and large volume blood loss; p = 0.042), and 3 independent factors predicting suboptimal RFS including R1 resection (p = 0.011), multifocal HCC (p = 0.005), and microvascular invasion (p = 0.001). LH was not associated with surgical complication and suboptimal RFS.
Conclusion
Our study highlights the benefits of LH by improving the perioperative outcomes, without long-term survival inferiority in selected large HCC patients compared with conventional OH. LH can be an attractive option for large HCC treatment.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Hepatocellular carcinoma (HCC), the commonest primary liver cancer, is one of the leading causes of cancer-related mortality worldwide [1]. Theoretically, the curative treatment of HCC requires one of three therapeutic modalities: tumor ablation, liver transplantation (LT), and hepatectomy [2]. Generally, tumor ablation is only suitable for small HCCs, and there is a shortage of donors for LT. Therefore, hepatectomy is the main curative treatment for HCC in most regions, especially in Asia.
Hepatectomy for large HCC (diameter ≥ 5 cm) is technically difficult and frequently requires major liver resection, which may be associated with higher morbidity and mortality rates than other methods. The long-term survival after liver resection for large HCC has also been reported to be poor [3, 4]. Nonetheless, there have been reports describing encouraging perioperative and long-term outcomes of hepatectomy for large HCC in selected patients [5,6,7,8].
Advances in laparoscopic surgical techniques and instruments have made laparoscopic hepatectomy (LH) an attractive option for the treatment of HCC. However, there are still limitations in the application of the laparoscopic approach. According to the consensus statement on LH [9, 10], the standard recommendation for LH is a single tumor < 5 cm in diameter in a favorable location (anterolateral segments of the liver). Large HCC is not yet accepted as a good indication for LH. Concerns have been raised about the operative difficulties during liver mobilization, tumor manipulation, and parenchymal transection, which may lead to tumor rupture and massive bleeding. The fear of increased operative complications and suboptimal oncological outcomes from LH has made open hepatectomy (OH) the preferred surgical technique for large HCC.
Improved perioperative outcomes and acceptable oncological outcomes with LH have been demonstrated in HCC [11,12,13,14,15]. However, there are limited comparative studies of LH and OH for large HCC. Only a few small series have reported the technical feasibility and potential benefits of the laparoscopic approach for large HCC [16,17,18]. With more experience, the limitations of LH for large HCC can be overcome and LH can offer the benefits of minimal invasiveness and oncological safety. In this study, we compared the perioperative and long-term survival outcomes between LH and OH in patients with large HCC.
Materials and methods
After Institutional Review Board approval was completed (IRB number B-2009-634-107), we reviewed a prospectively maintained database of patients with HCC who underwent hepatectomy between 2003 and 2018 at Seoul National University Bundang Hospital, Seongnam-si, Korea.
Management of patients
Initial assessment and operative candidate
All patients underwent dynamic contrast-enhanced liver imaging [computed tomography (CT) or magnetic resonance imaging (MRI)] for diagnosis and preoperative evaluations. Blood tests included complete blood count, liver function tests (LFT), coagulogram, serum alpha-fetoprotein (AFP) measurement, indocyanine green retention at 15 min (ICGR15), and viral hepatitis status. The treatment options were discussed at a multidisciplinary HCC conference among surgeons, hepatologists, interventional radiologists, and medical oncologists. Three curative therapeutic modalities, hepatectomy, tumor ablation, and LT, were considered as the first choice. For patients with large HCC, hepatectomy is the only curative treatment of choice.
Generally, hepatectomy for large HCC is considered if margin negative resection can be done safely (feasible surgical technique, adequate future liver remnant (FLR), and medically fit for major surgery). The criteria of an adequate liver reserve included (1) limited signs of portal hypertension (2) CTP class A [class B: mostly considered inadequate FLR and class C: absolute contraindication for surgery] (3) FLR > 30–40% (4) ICGR15 < 10–15%. From the oncological standpoint, we prefer resection for patient with single tumor. However, patient with multifocal tumors may be considered resection if preoperative imaging has confirmed of limited numbers of HCC not more than 2–3 lesions. Patient with borderline liver reserve, sequential TACE and PVE might be done to augment FLR volume before hepatectomy. HCC with macrovascular invasion or thrombus in PV or HV are sometime offered surgery if R0 resection is possible.
In most circumstances including large HCC, the operative approaches (LH and OH), are chosen based on the patient’s preference after a detailed explanation of the procedures is given to the patient. The preferred tumor location for LH included segment 2,3,4,5, and 6. In our hospital, size and number of tumor or major vascular invasion are not an absolute contraindication for LH. However, we may consider open approach first for some conditions due to operative and oncological safety concerns including huge tumor in close proximity to major hepatic veins/IVC/PV confluent and HCC with PVTT/BDTT that need thrombectomy or PV resection. We always consider open approach for ruptured HCC. The type of hepatectomy was not modified for the laparoscopic approach.
Surgical technique
The type of hepatectomy was defined according to the Brisbane 2000 terminology for hepatic resection [19]. In general, our team prefers anatomical liver resection as the first choice. However, nonanatomical liver resection is considered for cirrhotic patients with a small remnant volume in order to prevent posthepatectomy liver failure (PHLF). The extent of resection was classified as minor liver resection (the resection of less than 3 liver segments) or major liver resection (the resection at least 3 liver segments). In this study, we classified patients into two groups by the surgical technique used, the LH and OH groups.
In the LH group, all procedures were purely laparoscopic. The patient was placed in the supine position, with legs separated, or in the lithotomy position. We inserted 30-degree rigid or flexible 3D laparoscopes through a 12 mm supra-umbilical or infra-umbilical port. An additional three or four ports were included. Pneumoperitoneum was maintained with a pressure of 12–14 mmHg. Intraoperative evaluations were routinely performed with laparoscopic ultrasonography. We always prepared for the Pringle maneuver but it was only used in selected cases, especially if hemostasis was difficult due to bleeding from diseased parenchyma. Superficial parenchymal transection was performed with a harmonic scalpel (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA). Deep parenchymal transection was performed with a combination of laparoscopic instruments, including the Cavitron ultrasonic surgical aspirator (CUSA) (Valleylab, Inc., Boulder, CO, USA), LigaSure (Valleylab, Inc.), and a harmonic scalpel. Small vessels and bile ducts were secured with Hem-o-lok clips (Teleflex Medical, Research Triangle Park, NC, USA). Large hepatic veins and bile ducts were secured with vascular staplers. The resected tumor were retrieved through an enlarged port site or Pfannenstiel incision.
In the OH technique, the patient was placed in the supine position. A right subcostal incision with a vertical midline extension was made. A special retractor for adequate exposure was used. The tumor and liver were evaluated by manual palpation and with intraoperative ultrasonography to confirm the location of the tumor and its relationships to adjacent structures, and to determine the final operative plan intraoperatively. Parenchymal transection was performed with monopolar and bipolar electric cautery, CUSA, and LigaSure.
Postoperative care
The inpatient postoperative care protocol included daily LFT during the early postoperative days and a CT scan of the liver on postoperative day 5. After hospital discharge, the patient was scheduled for follow-up at 1–2 weeks. Thereafter, outpatient follow-up visits were scheduled every 3 months during the first 2 years and every 6 months thereafter. The outpatient visits included a clinical evaluation, blood tests (AFP and LFT), and imaging (liver CT or MRI).
Assessment of outcomes
The primary outcomes were comparisons of the perioperative outcomes, including operative parameters (blood loss, transfusion requirements, and intraoperative complications), length of hospital stay, and postoperative complications. All complications were classified according to the Clavien–Dindo classification [20]. Liver-specific complications, including PHLF and posthepatectomy bile leakage, were classified according to the International Study Group of Liver Surgery criteria [21, 22]. The secondary outcomes included (1) comparisons of long-term survival, overall survival (OS), and recurrence-free survival (RFS) in patients with large HCC treated with LH or OH (2) to identify factors associated with suboptimal outcomes (operative complications and suboptimal RFS) and to confirm the safety of LH for large HCC treatment by the entire cohort multivariable analyses.
The OS was calculated from the day after surgery to the last day of follow-up or death. The RFS was calculated from the day after surgery to the last day of follow-up, recurrence, or death.
Statistical analysis
Since the study was an observational study to compare the outcomes of two surgical techniques, LH vs. OH, propensity score matching (PSM) was used to reduce the selection bias. Propensity score (PS) was calculated by using binary logistic regression with surgical technique as a binary dependent variable. Seven potential confounders affecting surgeon’s decision in order to select the surgical technique were used in the binary logistic model. The confounders included (1) previous laparotomy (no/yes), (2) cirrhosis by imaging (no/yes), (3) Child–Turcotte–Pugh (CTP) classification (A/B), (4) number of tumor (single/multiple), (5) location of tumor (anterolateral segment/posterosuperior segment), (6) maximum tumor diameter (cm), and (7) extent of resection (minor liver resection/major liver resection). Basically, most surgeons tend to select LH for single large HCC which is located in anterolateral segment planning to do minor liver resection. Additionally, patient with no history of laparotomy, no significant cirrhosis by imaging, and CTP class A were a factor supporting the LH selection as well.
The NCSS software (NCSS, LLC, USA) was used to perform PSM using Mahalanobis distance within PS calipers (0.2*SD of the logit propensity score), 1:1 matching without replacement. Quality of matching was checked by comparison of (1) the distribution of PS, and (2) the absolute standardized mean difference (|d|) of each confounder before and after matching. The |d| of < 10% indicates a negligible difference between groups for that confounder, whereas |d| of 10%-20%, > 20% for acceptable difference and group imbalance, respectively. In this study, the |d| were < 10% for all covariates.
We did not exclude any conversions from the LH group, according to the intention-to-treat analysis. Categorical variables are expressed as numbers and percentages. Continuous variables are expressed as medians and interquartile ranges or as means and standard deviations. The χ2 test or Fisher’s exact test was used, as appropriate, for the comparison of categorical variables, and the Mann–Whitney U test was used for the comparison of continuous variables. Survival (OS and RFS) was calculated with the Kaplan–Meier method and comparisons between groups were assessed with a log-rank test.
The entire cohort multivariable analyses were performed to identify the factors associated with suboptimal outcomes (operative complications and suboptimal RFS) and to confirm the safety of LH for large HCC as well. The binary logistic regression analysis was conducted to identify factor associated with operative complications. The analysis began with a univariable analysis of each potential contributing factors [extent of resection (minor/major), blood loss (< 1000 ml, ≥ 1000 ml), blood transfusion requirement (no/yes), operative technique (LH/OH), cirrhosis by imaging (no/yes), severity of background liver disease (CTP class A/B)] as reported by crude OR (95% CI) and p value. Variables with univariable p value less than 0.10 and variable of interest (operative technique) were then entered into a multiple logistic regression using ENTER approach. The collinearity of these independent variables was checked by variance inflation factor (VIF).
The cox-proportional hazard model was performed to identify factor associated with suboptimal RFS. The potential contributing factors included surgical technique (LH/OH), completeness of resection (R0/R1), microvascular invasion (no/yes), number of HCC (single/multiple) and AFP (≤ 10 ng/ml/ > 10 ng/ml) were analyzed as univariate analysis first. Each variable was checked for the assumption of proportional hazard (PH) by visual looking at plots of ln(−ln(S(t)) against time. Variables with univariable p value less than 0.10 and variable of interest (operative technique) were then entered into multiple Cox’s regression model to identify an independent factor affecting suboptimal RFS. All statistical analyses were performed with SPSS version 23 (IBM SPSS Statistics 23, Armonk, NY, USA). A p value < 0.05 was considered to indicate statistical significance.
Results
During the study period, 902 patients with HCC underwent hepatectomy at our hospital. In total, 249 patients with large HCC (at least one tumor with a diameter ≥ 5 cm), diagnosed with preoperative imaging, were included. To avoid any bias in the survival analysis, 17 patients with a history of spontaneously ruptured HCC or other cancer treatment or combined hepato-cholangiocarcinoma on the final pathology report were excluded. Eight patients who underwent combined hepatectomy and intraoperative tumor ablation and nine patients who underwent robot-assisted liver resection were also excluded. After excluding these patients, a total of 215 patients were included in the study and the statistical analyses. Most variables contain missing data of less than 3% except ICGR15 that was missed about 20%. The study population was classified into two groups according to the surgical technique used, the LH group (n = 109) and the OH group (n = 106). Following 1:1 PSM, we included 70 patients in each group, with similar baseline demographics, similar background liver diseases and function, similar preoperative imaging features of HCC (number and location), and similar extents and difficulty of hepatectomy, for the final comparison of outcomes. The diagram to summarize our hepatectomy experience for large HCC and study flow is showed in supplementary Fig. 1.
Patient demographics
The overall patient demographics before and after PSM are showed in Table 1. Before matching, there were significantly greater proportions of female (p = 0.009) and patient with high body mass index (BMI) (p = 0.016) in the LH group than in the OH group. The proportion of patients with history of transarterial chemoembolization (TACE) was lower in LH group than in the OH group (p = 0.028). In terms of the preoperative HCC imaging characteristics, the LH group had smaller maximum tumor diameters (p = 0.005) and a higher proportion of tumors located in an antero-inferior segment (p = 0.048) than the OH group. The numbers of tumors and the baseline liver function, including the CTP class, were similar between the groups. After PSM, the differences in the overall demographics between the groups were clearly reduced. Only the proportion of patients with history of TACE was lower in the LH group than the OH group (p = 0.049). The potential confounding variables that affected the surgeon’s choice of surgical technique included cirrhotic status, history of previous laparotomy and the radiographic features of HCC were not differ between the two groups.
Surgical and pathological results
The surgical and pathological results before and after matching are presented in Table 2. Before matching, the extent of resection, which determines the difficulty and outcomes of hepatectomy, differed significantly between the groups. The LH group had a higher proportion of patients with minor liver resection, a higher proportion of patients with noncomplex hepatectomy (nonanatomical resection and left lateral sectionectomy) than the OH group (p < 0.001 and 0.001, respectively). After matching, the extent of resection and the complexity of hepatectomy were clearly balanced, with no statistically significant differences between the groups (p = 0.866 and 0.079, respectively). When the perioperative outcomes were compared between the groups after matching, the LH group had a significantly shorter length of hospital stay than the OH group 7 (6–10 days) and 9.5 (7–13 days) days, respectively, p = 0.001). The operative blood loss, operative time, and transfusion requirements did not differ between the groups. The conversion rate in the LH group was 15.6% before matching and 18.6% after matching. The causes of conversions were bleeding, difficulty of tumor manipulation with the laparoscopic approach, severe adhesion, and intolerance of pneumoperitoneum. The pathological results after matching were similar in the two groups, with similar tumor sizes, tumor numbers, completeness of resection rates (R0 vs R1), margin width, and tumor histology (tumor grading, satellite lesions, and microvascular invasion).
Operative complications
A summary of the operative complications is presented in Table 3. There were no differences in the intraoperative complication rates of the two groups. We did not observe any intraoperative patient mortality in this study. In a comparison of the postoperative complication rates, the LH group had a significantly lower overall complication rate than the OH group before matching (19.3% and 32.1%, respectively, p = 0.031), but the complication rate was similar in both groups after matching (24.3% and 25.7%, respectively, p = 0.845). The LH group also had a lower high-grade complication rate (Clavien–Dindo grade ≥ 3) than the OH group before matching (11.9% and 21.7%, respectively, p = 0.055), but the differences was not differ between the groups after matching (17.1 and 18.6%, p = 1.000). There were no differences in rate of other complications including postoperative bleeding, reoperation, bile leakage, liver insufficiency, surgical-site infection, or medical complications between two groups.
Survival outcomes
The 1-, 3-, and 5-year RFS after hepatectomy for the entire cohort of patients with large HCC were 56.3%, 42.4%, and 38.9% respectively. The 1-, 3-, 5-year OS rates of patients after hepatectomy for large HCC in this study were 94.2%, 87.8%, and 77.9%, respectively.
A comparison of Kaplan–Meier survival curves for the groups before and after matching is presented in Fig. 1. Before matching, the 1-, 3-, and 5-year RFS rates in the LH group were 59.0%, 46.8%, and 44.2%, respectively, and those in the OH group were 53.6%, 38.1%, and 32.7% respectively (p = 0.193). The 1-, 3-, and 5-year OS rates in the LH group were 94.1%, 89.1%, and 84.6%, respectively, and those in the OH group were 94.2%, 86.3, and 72.0%, respectively (p = 0.401). After matching, the 1-, 3-, and 5-year RFS rates in the LH group were 53.6%, 38.1%, and 32.7%, respectively, and those in the OH group were 49.8%, 37.3%, and 33.5%, respectively (p = 0.138). The 1-, 3-, and 5-year OS rates in the LH group were 93.8%, 90.3%, and 85.5%, respectively, and those on the OH group were 95.6%, 90.4%, and 77.3%, respectively (p = 0.668).
Multivariable analysis of operative complications and suboptimal RFS
To achieve the best hepatectomy outcomes for patients with large HCC, we performed multivariable analyses to identify factors associated with postoperative complications and suboptimal RFS. On the other hand, we would like to check if LH is safe for large HCC treatment in term of operative and oncological safety standpoints. The results of the multivariable analyses are presented in Tables 4 and 5.
The postoperative complications and potential contributing factors (operative technique [OH], extent of resection [major liver resection], cirrhosis of liver background, CTP class B, large operative blood loss [> 1000 ml], and requirement for blood transfusion) were included in the binary logistic regression analysis. However, based on the rule of thumb of 10 events per variable, only 5 independent variables were seemed appropriate for the analysis of 55 events in this study. Collinearity among three candidate variables for multiple logistic regression, i.e., extent of resection (minor/major), blood loss (< 1000 ml, ≥ 1000 ml), and blood transfusion (no/yes) were examined. Blood loss was found to be highly related with blood transfusion requirement. Therefore, we did not include blood transfusion requirement into the multivariable analysis. Finally, the multiple logistic regression revealed that major liver resection (p < 0.001) and operative blood loss > 1000 ml (p = 0.042) were an independent factor predicting postoperative complications in this study (Table 4).
The potential factors predicting poor oncological outcome associated with suboptimal RFS, including operative technique (OH), completeness of resection (R1), largest tumor size (≥ 10 cm), background liver cirrhosis, presence of microvascular invasion, number of HCCs (multiple lesions), AFP-producing HCC (AFP ≥ 10 ng/ml), presence of postoperative complications, and requirement for blood transfusion during surgery, were included. The multiple Cox’s proportional hazard model of RFS demonstrated that the completeness of resection (R1 resection), the presence of microvascular invasion, and multifocal tumors were independent poor prognostic factors related to suboptimal RFS (p = 0.011, 0.001, and 0.005, respectively) (Table 5).
Subgroup analysis of hepatectomy for huge HCC larger than 10 cm
A comparison of operative and survival outcomes between the LH group and the OH group is presented in Table 6. Due to small sample size, the PSM was inappropriate to conduct. There were no differences in the baseline tumor characteristics of the two groups. The OH group had significant higher proportion of major liver resection than the LH group (p = 0.008). The conversion rate in the LH group was 23.1%, which was higher than the conversion rate of the LH group entire cohort. There were no significant differences in operative time, blood loss, R0 resection rate, post-operative complications rate and long-term survival between the groups. The LH group had trend toward shorter length of hospital stay than the OH group (p = 0.006). The Kaplan–Meier survival curves comparison RFS and OS between the groups are presented in supplementary Figs. 2 and 3.
Discussion
Since the first statement of the consensus conference on laparoscopic hepatectomy (LH) was reported in 2009 [9], the laparoscopic approach to the surgical treatment of HCC has become more widely used. The benefits of LH for improving perioperative outcomes, reducing the complication rate, and shortening the length of the hospital stay under most circumstances are now clearly evident. However, the laparoscopic technique has several limitations, which might be considered a relative contraindication in many centers, that include LH for large HCC. It is difficult to manipulate large tumors during liver mobilization and parenchymal transection, which may increase the risk of tumor rupture and subsequent intraoperative bleeding. Moreover, to achieve an adequate resection margin for large tumors, major liver resection is usually necessary, which may result in PHLF. There are currently few reports of LH for large HCC, and comparative studies of LH and OH are particularly sparse.
With our increasing experience of LH, a large tumor size is not considered an absolute contraindication for LH at our center. The feasibility of LH for large HCC has been previously reported [16]. In that report, the study population was quite small and the proportion of patients treated with major liver resection was significantly lower in the large HCC group. However, that preliminary report supported the proposition that LH can be performed safely in well-selected patients with large HCC.
To date, there have been few comparative studies of large HCC treated with LH vs OH [23, 24]. Although the operative procedure is technically challenging, LH for patients with large HCC could improve their perioperative outcomes, with acceptable oncological outcomes [18, 23, 24]. Theoretically, a randomized controlled trial (RCT) is the best way to test this hypothesis, but an RCT in patients with large HCC is challenging because the number of patients is limited and there is significant heterogeneity among patients. Therefore, we used PSM to minimize the selection bias in this study. The key variables affecting the decision of surgeon to choose the surgical technique (LH or OH) and the operative outcomes, including the preoperative radiographic features of HCC (number, location, and diameter of tumor), cirrhotic status and portal hypertension by imaging, severity of background liver disease (CTP classification), history of previous laparotomy, and the extent or difficulty of hepatectomy, were well balanced in both groups after matching. This approach made the comparisons of the outcomes between the two surgical techniques both feasible and reasonable.
In terms of the operative outcomes, this study demonstrated similar rates of R0 resection, operative times, blood loss, and transfusion requirements between the two groups. Therefore, although LH is considered to be difficult for large HCC, the operative outcomes are similar between LH and OH. After matching, the conversion rate in the LH group were 18.6% for entire cohort and 23.1% for huge HCC subgroup. Most conversions were due to bleeding, poor access, and risk of tumor rupture with the laparoscopic technique. The conversion rate in this study was slightly higher than that in previous reports of LH (6–13%) [13, 25,26,27]. Unplanned conversion may lead to intraoperative complications, such as massive bleeding or tumor rupture, which cause suboptimal short- and long-term outcomes [28, 29]. Therefore, the threshold for open conversion in large HCC should be lower than usual. Although we did not exclude any conversion from LH group due to the intention to treat analysis, patients who underwent LH experienced statistically significantly shorter hospital stay, confirming the benefit of LH in enhancing postoperative recovery. A likely explanation is that LH results in less postoperative pain, early ambulation, and an early return to daily activities.
In terms of the surgical complication rates, our study showed that LH had significantly lower overall and high-grade complication rates than OH before matching, but these differences were not statistically significant after matching. The rates of other complications, including PHLF, bile leakage, reoperation, postoperative bleeding, wound infection, and medical complications, were similar in both groups. The only factor associated with postoperative complications was major liver resection. The surgical technique (LH or OH) was not associated with postoperative complications, which confirms the operative safety of LH for selected large HCC patients.
To compare operative outcomes with the resection of small HCC that LH is usually related with less blood loss and complication rate than OH [12, 13], the resection of large HCC resulted in equal blood loss and complication rate between the groups. These findings might be unique for the resection of large HCC. Similarly, the conversion rate of LH for large HCC is higher than usual as well.
In the long-term survival analyses, our study demonstrated similar OS and RFS in the two groups before and after PSM. A multivariate analysis showed that the prognostic factors for suboptimal RFS were R1 resection, multifocal HCC, and microvascular invasion. However, the surgical technique (LH or OH) was not related to the prognosis. Based on these findings and previous reports on the significance of clear/wide resection margin (0.5–1 cm) [30, 31], we recommended R0 resection regardless of the surgical techniques for large HCC to achieve the best survival outcomes, even though some reports advocated R1 resection for parenchymal saving was an acceptable for HCC treatment in some locations especially, major vascular structures [32, 33]. In addition to the previous report [13] regarding the comparable survival outcomes between LH and OH for HCC patients, these results support the propositions that LH is feasible and safe for the treatment of large HCC in selected cases and yields similar long-term survival rates to OH.
The subgroup analysis focused on hepatectomy for huge HCC (diameter > 10 cm) revealed comparable perioperative and survival outcomes between the LH group and the OH group. These findings have supported the feasibility of LH in well selected super-challenging cases. Unfortunately, although the LH showed the trend toward shorten hospital stay, it was not reach statistical significance due to very small sample size.
To the best of our knowledge, this study is the largest comparative study to demonstrate the benefits of LH over OH for the treatment of large HCC in term of improving the perioperative outcomes, with similar long-term survival. Based on the results of this study, LH should be considered more frequently as an attractive option for hepatectomy in patients with large HCC. Our experience suggests that the keys to success include appropriate patient selection (avoiding too difficult tumors for LH based on preoperative imaging, such as huge HCC in close proximity to major hepatic veins or IVC, HCC with tumor thrombus in main portal vein or bile duct, and HCC with adjacent organs invasion) and a timely intraoperative decision to continue the operation laparoscopically or to convert to OH (the unplanned conversion of a laparoscopic approach may result in massive bleeding or tumor rupture).
This study had a few limitations. First, although the selection bias of the surgeon to choose the surgical technique was reduced and baseline characteristic differences were balanced between the groups by PSM, the study still involved some unavoidable bias due to retrospective design. The randomized clinical trial is needed to avoid all selective bias and to ensure the similar baseline characteristics between two groups. Second, the study population after PSM was relatively small. Therefore, although the surgical results indicated a tendency toward a reduced complication rate before matching, this trend was not statistically significant after matching.
In conclusion, LH for the treatment of large HCC is feasible and safe in a center experienced in minimally invasive liver surgery. Our study demonstrates the benefit of LH in improving posthepatectomy recovery, with rates of long-term survival equivalent to those of OH. Therefore, LH should be considered a reasonable treatment option for selected patients with large HCC. Based on our results, the size of the HCC alone should not be considered a contraindication for LH.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Bruix J, Reig M, Sherman M (2016) Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 150:835–853
Yeh CN, Lee WC, Chen MF (2003) Hepatic resection and prognosis for patients with hepatocellular carcinoma larger than 10 cm: two decades of experience at Chang Gung memorial hospital. Ann Surg Oncol 10:1070–1076
Zhao HC, Wu RL, Liu FB et al (2016) A retrospective analysis of long term outcomes in patients undergoing hepatic resection for large (>5 cm) hepatocellular carcinoma. HPB 18:943–949
Poon RT, Fan ST, Wong J (2002) Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg 194:592–602
Yang LY, Fang F, Ou DP, Wu W, Zeng ZJ, Wu F (2009) Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg 249:118–123
Chang YJ, Chung KP, Chang YJ, Chen LJ (2016) Long-term survival of patients undergoing liver resection for very large hepatocellular carcinomas. Br J Surg 103:1513–1520
Lim C, Compagnon P, Sebagh M et al (2015) Hepatectomy for hepatocellular carcinoma larger than 10 cm: preoperative risk stratification to prevent futile surgery. HPB 17:611–623
Buell JF, Cherqui D, Geller DA et al (2009) The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg 250:825–830
Wakabayashi G, Cherqui D, Geller DA et al (2015) Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261:619–629
Cheung TT, Poon RT, Yuen WK et al (2013) Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg 257:506–511
Kabir T, Tan ZZ, Syn NL et al (2021) Laparoscopic versus open resection of hepatocellular carcinoma in patients with cirrhosis: meta-analysis. Br J Surg 109:21–29
Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y (2015) Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol 63:643–650
Shehta A, Han HS, Yoon YS, Cho JY, Choi Y (2016) Laparoscopic liver resection for hepatocellular carcinoma in cirrhotic patients: 10-year single-center experience. Surg Endosc 30:638–648
Dumronggittigule W, Han HS, Ahn S, Yoon YS, Cho JY, Choi Y (2020) Laparoscopic versus open hepatectomy for hepatocellular carcinoma in elderly patients: a single-institutional propensity score matching comparison. Dig Surg 37:495–504
Kwon Y, Han HS, Yoon YS, Cho JY (2015) Are large hepatocellular carcinomas still a contraindication for laparoscopic liver resection? J Laparoendosc Adv Surg Tech A 25:98–102
Gil E, Kwon CHD, Kim JM et al (2017) Laparoscopic liver resection of hepatocellular carcinoma with a tumor size larger than 5 cm: review of 45 cases in a tertiary institution. J Laparoendosc Adv Surg Tech A 27:799–803
Levi Sandri GB, Spoletini G, Vennarecci G, Francone E, Abu Hilal M, Ettorre GM (2018) Laparoscopic liver resection for large HCC: short- and long-term outcomes in relation to tumor size. Surg Endosc 32:4772–4779
Strasberg SM, Belghiti J, Clavien PA et al (2000) The Brisbane 2000 terminology of liver anatomy and resections. HPB 2:333–339
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Rahbari NN, Garden OJ, Padbury R et al (2011) Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 149:713–724
Koch M, Garden OJ, Padbury R et al (2011) Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 149:680–688
Shelat VG, Cipriani F, Basseres T et al (2015) Pure laparoscopic liver resection for large malignant tumors: does size matter? Ann Surg Oncol 22:1288–1293
Ai JH, Li JW, Chen J, Bie P, Wang SG, Zheng SG (2013) Feasibility and safety of laparoscopic liver resection for hepatocellular carcinoma with a tumor size of 5–10 cm. PLoS ONE 8:e72328
Takahara T, Wakabayashi G, Beppu T et al (2015) Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci 22:721–727
Soubrane O, Goumard C, Laurent A et al (2014) Laparoscopic resection of hepatocellular carcinoma: a French survey in 351 patients. HPB 16:357–365
Lee W, Han HS, Yoon YS et al (2016) Comparison of laparoscopic liver resection for hepatocellular carcinoma located in the posterosuperior segments or anterolateral segments: a case-matched analysis. Surgery 160:1219–1226
Stiles ZE, Glazer ES, Deneve JL, Shibata D, Behrman SW, Dickson PV (2019) Long-term implications of unplanned conversion during laparoscopic liver resection for hepatocellular carcinoma. Ann Surg Oncol 26:282–289
Halls MC, Cipriani F, Berardi G et al (2018) Conversion for unfavorable intraoperative events results in significantly worse outcomes during laparoscopic liver resection: lessons learned from a multicenter review of 2861 cases. Ann Surg 268:1051–1057
Lee W, Han HS, Ahn S, Yoon YS, Cho JY, Choi Y (2018) Correlation between resection margin and disease recurrence with a restricted cubic spline model in patients with resected hepatocellular carcinoma. Dig Surg 35:520–531
Aoki T, Kubota K, Hasegawa K et al (2020) Significance of the surgical hepatic resection margin in patients with a single hepatocellular carcinoma. Br J Surg 107:113–120
Matsui Y, Terakawa N, Satoi S et al (2007) Postoperative outcomes in patients with hepatocellular carcinomas resected with exposure of the tumor surface: clinical role of the no-margin resection. Arch Surg 142:596–602
Donadon M, Terrone A, Procopio F et al (2019) Is R1 vascular hepatectomy for hepatocellular carcinoma oncologically adequate? Analysis of 327 consecutive patients. Surgery 165:897–904
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Wethit Dumronggittigule, Ho-Seong Han, Chulaluk Komoltri, Mizelle D'Silva, Boram Lee, Jai Young Cho have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
464_2022_9812_MOESM1_ESM.tif
Supplementary file1 (TIF 634 kb) The summary diagram demonstrating our hepatectomy experience for large HCC and the study flow.
464_2022_9812_MOESM2_ESM.tif
Supplementary file2 (TIF 983 kb) Kaplan–Meier curve demonstrating recurrence-free survival of patients with huge hepatocellular carcinoma treated with laparoscopic hepatectomy or open hepatectomy.
464_2022_9812_MOESM3_ESM.tif
Supplementary file3 (TIF 923 kb) Kaplan–Meier curve demonstrating overall survival of patients with huge hepatocellular carcinoma treated with laparoscopic hepatectomy or open hepatectomy.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dumronggittigule, W., Han, HS., Komoltri, C. et al. Laparoscopic versus open hepatectomy for large hepatocellular carcinoma: a single center propensity-score-matching comparative analysis of perioperative outcomes and long-term survival. Surg Endosc 37, 2997–3009 (2023). https://doi.org/10.1007/s00464-022-09812-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09812-8