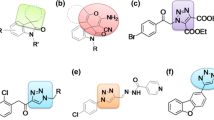
Abstract
A simple, green and catalyst-free novel protocol is developed for the synthesis of medicinally important spiro[indole-3,2′[1,3]-thiazine]-2,4′-dione and spiro[acenaphthylene-1,2′-[1,3]thiazine]dione libraries by the tandem reaction of readily available reagents in 1-butyl-3-methylimidazolium hexafluorophosphate [bmim][PF6]. The ionic liquid has been used as a solvent as well as catalyst for this reaction. This reaction proceeded smoothly in good to excellent yields and offered several other advantages including short reaction time, simple experimental workup procedure and no by-products. The synthesized compounds were subjected to antimycobacterial efficacy against Mycobacterium tuberculosis H37Rv strain and DNA cleavage activity.

A highly practical and efficient preparation of spiro[indole-3,2′[1,3]-thiazine]-2,4′-dione and spiro[acenaphthylene-1,2′-[1,3]thiazine]dione derivatives was developed via an ionic liquid mediated and promoted multi-component reaction of indole-2,3-dione/acenaphthalene-1,2-dione, aniline and 3-mercaptopropionic acid. The synthesized compounds were subjected to antimycobacterial efficacy against M. tuberculosis H37Rv strain and DNA cleavage activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Deoxyribonucleic acid (DNA) is the intracellular target for wide range of anticancer and antibiotic drugs.[1] Studies on interaction of drug molecules with DNA have become an active research in recent years as they facilitate molecular interaction studies which may result in devising new drugs with different mechanisms and models of action. Despite recent progress in cancer chemotherapy, high toxicity and low specificity of current medications are motivating scientists to search for safer and more effective anticancer drugs.[2] Metal-based molecules are the foremost and widely used anticancer drugs in cancer therapy,[3] but these possess inherent side effects, solubility and acquired drug resistance. Therefore, considerable attempts are being made to replace these drugs with suitable alternatives.
Thia-azaheterocycles have attracted considerable attention because of their wide biological and pharmacological activities.[4] Moreover, thia-azaheterocycles exhibited potent antitumour activities against non-small cell lung cancer cell line H460, paclitaxel-resistant H460taxR, human colon cancer cell line HT-29 and human breast cancer cell line MDA-MB-231.[5] Thiazine and its derivatives are an important class of hete rocyclic compounds possessing broad biological activities, such as COX-1 inhibition,[6] antiinflammatory,[7] antiproliferative,[8] antihistaminic,[9] and anti-HIV activities.[10] These are also known as anti-radiation agents and used as radiation-sickness drugs.[11] Furthermore, antibiotic activity of cephalosporins is due to the presence of 1,3-thiazine nucleus.[12] As regards chemical viewpoint, 1,3-thiazines are important synthetic intermediates in organic syntheses.[13]
Organo fluorine compounds have been receiving significant attention in materials and pharmaceutical sciences due to their unique physical and biological properties such as the increased membrane permeability, enhanced hydrophobic binding and stability against metabolic oxidation.[14] Among these compounds, trifluoromethyl group-containing molecules are especially important, and continue to attract increasing attention from various fields.[15] Since fluorine is virtually absent in the living tissue,[16] fluorinated pharmaceuticals might possess comparatively less environmental and mammalian toxicity.
Indole derivatives constitute an important class of therapeutic agents in medicinal chemistry including anticancer,[17] antioxidant,[18] antirheumatoidal and anti-HIV[19] properties and also play a vital role in the immune system.[20] Spirooxindole possesses a myriad of biological activities such as inhibition of the mammalian cell cycle at G2/M phase,[21] inhibition of microtubule assembly,[22] modulation of the function of muscarinic serotonin receptors,[23] antitumour activity against human brain cancer cell lines, neuroplastoma SKN-BE, and malignant glioma GAMG.[24] In addition, spiroindoles containing thia-azaheterocyclic ring system as a structural motif are present in many pharmacologically important synthetic and naturally occurring compounds (as typified by spirobrassinin).[25]
The above-mentioned biological importance inspired us to attach spirooxindoles to the thiazine scaffold, and the combination of two privileged structures in one molecule leads to drug-like molecules.
A number of methods have been reported for the preparation of spirooxindole derivatives involving the synthon thioacids.[26] All these processes use plenty of organic solvents, reactions have suffered from long reaction time and a narrow scope of substrates. The spiro[indole-3,2′[1,3]-thiazine]-2,4′-diones incorporating two biodynamic heterocyclic thiazine and indole moieties[27] through a spiro carbon atom appear to be of great interest and were earlier synthesized by reacting 3-indolylimine (intermediate such as Shiff-base formed by condensation of isatin and aniline) and 3-mercaptopropionic acid using high boiling carcinogenic hydrocarbons with continuous azeotropic removal of water.[28] Therefore, it is necessary to develop an efficient and new versatile method for the synthesis of these compounds.
Multi-component reactions (MCRs) are of increasing importance in organic and medicinal chemistry, because the strategies of MCR offer significant advantages over conventional linear-type syntheses.[29] MCRs leading to interesting heterocyclic scaffolds are particularly useful for the creation of diverse chemical libraries of ‘drug-like’ molecules for biological screening, since the combination of three or more small molecular weight building blocks in a single operation leads to a high combinatorial efficacy.
In recent years, ionic liquids (ILs) have received recognition as green media in organic synthesis due to the ease of tuning their physical properties, such as good solvating capability, wide liquid range, negligible vapour pressure, tunable polarity, high thermal stability and ease of recyclability.[30] The rising number of publications is indicative of the potential of ILs as ‘designer solvents’ for various chemical reactions.
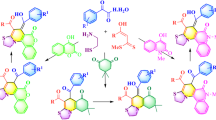
Considering the fact that ILs have been used as useful and efficient media for esterification reactions in spite of the ‘in situ’ formation of water[31] because they can create an opportunity to drive the equilibrium and in line with our interest on spiro indoles,[32] we studied the possibility of employing the easily made 1,3-dialkylimidazolium cation-based IL as green and efficient solvent for the preparation of spiro[indole-3,2′[1,3]-thiazine]-2,4′-dione by three-component reaction of isatin 1, aniline 2 and mercaptopropionic acid 3 in IL without any catalyst (scheme 1). We were also interested in knowing the probable impact of assembling of 1,3-thiazine with oxindoles moiety in search of novel anticancer agents through DNA cleavage procedure.
2 Experimental
Melting points of all compounds were determined on a Toshniwal apparatus. Purity of compounds was checked on thin layers of silica Gel-G coated glass plates and n-hexane: ethyl acetate (8:2) as eluent. IR spectra were recorded on a Shimadzu FT IR–8400S spectrophotometer using KBr pellets. 1H and 13C NMR spectra were recorded in DMSO-d 6 and CDCl3 using tetramethylsilane (TMS) as an internal standard on a Bruker Avance spectrophotometer at 300 and 75 MHz, respectively. Mass (ESI) spectra of compounds were recorded on JEOL SX-102 spectrometer at 70 eV. Elemental analyses were carried out on a Carlo-Erba 1108 CHN analyzer.
2.1 Syntheses of spiro[indole-3,2′ [1,3]-thiazine]-2, 4′-diones (4a-i) and spiro[acenaphthylene-1,2′[1,3]- thiazine]diones (6a–f)
A mixture of appropriate indole-2,3-dione (1) / acenaphthalene-1,2-dione (5) (1 mmol), aniline (2) (1 mmol) and 3-mercaptopropionic acid (3) (1.5 mmol) and ionic liquid, [bmim]PF6 (2 mL) were taken in a conical flask. Contents of the flask were stirred magnetically at 80°C. After completion of the reaction (as monitored by thin layer chromatography (TLC)), the product was extracted with diethyl ether and ethereal extracts were evaporated to give a crystalline material. The pure products were characterized by spectral data (1H nuclear magnetic resonance (NMR), 13C NMR and Mass). 1H NMR spectra of 4a–i and 6a–f showed three sets of multiplets due to thiazine ring protons which can be explained according to an first order spectra (AMM′X) splitting pattern (schemes 1 and 2), indicating different environment for HA and HX protons. Similar environment for HM and H\(_{\rm M^\prime}\) protons, with little difference in chemical shift, meant that their signals could not be separated and a complex multiplet integrating for two protons appeared. Spectral data for some products are given below.
2.2 Characterization of compounds (4a–i)
2.2a 1-Benzyl-3′(4-chlorophenyl)-spiro[indole-3,2′[1,3]- thiazine]-2,4′(1H)-dione ( 4a ): IR (KBr, υ, cm − 1) 1695, 1710 (C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 2.64–2.76 (m, 1H, HA), 3.18–3.31 (m, 2H, H\(_{\rm MM^\prime })\), 4.16–4.26 (m, 1H, HX), 4.89 (d, 1H, CH, J = 15.6 Hz) and 5.10 (d, 1H, CH, J = 15.6 Hz), 6.74–7.32 (m, 13H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 29.4, 32.3, 54.90, 78.58, 119.01, 121.95, 123.57, 124.56, 125.05, 125.78, 127.68, 128.58, 129.09, 129.99, 131.58, 134.01, 138.23, 142.68, 168.16, 176.09; MS (ESI, m/z, M + ): 434.0; Anal. calcd. for C24H19ClN2O2S: C, 66.28; H, 4.40; N, 6.44%. Found; C, 66.12; H, 4.35; N, 6.39%.
2.2b 1-Benzyl-3′(3-fluorophenyl)-spiro[indole-3,2′ [1,3]-thiazine]-2,4′(1H)-dione ( 4b ): Mp 274–276°C; IR (KBr, υ, cm − 1) 1690, 1715 (C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 2.63–2.77 (m, 1H, HA), 3.17–3.33 (m, 2H, H\(_{\rm MM^\prime })\), 4.19–4.28 (m, 1H, HX), 4.92 (d, 1H, CH, J = 15.6 Hz) and 5.14 (d, 1H, CH, J = 15.6 Hz), 6.72–7.35 (m, 13H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 28.48, 32.30, 56.99, 78.58, 117.24, 119.34, 120.91, 122.09, 124.01, 125.98, 127.58, 128.73, 129.09,130.89, 131.59, 132.19, 133.08, 141.68, 142.32, 154.69, 168.56, 176.68; MS (ESI, m/z, M + ): 418.1; Anal. calcd. for C24H19FN2O2S: C, 68.88; H, 4.58; N, 6.69%. Found: C, 68.73; H, 4.51; N, 6.62%.
2.2c 1-Benzyl-3′(3-trifluoromethylphenyl)-spiro[indole- 3,2′[1,3]-thiazine]-2,4′(1H)-dione ( 4c ): Mp 250–252°C; IR (KBr, υ, cm − 1) 1690, 1713 (C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 2.68–2.77 (m, 1H, HA), 3.19–3.35 (m, 2H, H\(_{\rm MM^\prime })\), 4.18–4.30 (m, 1H, HX), 4.91 (d, 1H, CH, J = 15.5 Hz), 5.13 (d, 1H, CH, J = 15.5 Hz), 6.76–7.38 (m, 13H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 29.67, 32.60, 58.65, 81.58, 117.01, 119.37, 120.90, 123.70, 124.67, 126.70, 127.60, 127.80, 128.30, 129.0, 130.0, 131.20, 133.58, 138.59, 141.89, 142.83, 142.40, 169.02, 177.69; MS (ESI, m/z, M + ): 468.1; Anal. calcd. for C25H19F3 N2O2S: C, 64.09; H, 4.09; N, 5.98% Found: C, 64.22; H, 4.02; N, 5.92%.
2.2d 1-Allyl-3′(4-chlorophenyl)-spiro[indole-3,2′-tetrahydro-1,3-thiazine]-2,4′(1H)-dione ( 4d ): Mp 254–256°C; IR (KBr, υ, cm − 1) 1690, 1712 (C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 2.62–2.75 (m, 1H, HA), 3.19–3.28 (m, 2H, H\(_{\rm MM^\prime })\), 4.10–4.22 (m, 1H, HX), 4.29 (dd, 1H, CH2, J 1 = 16.1 Hz, J 2 = 7.9 Hz), 4.58 (dd, 1H, CH2, J 1 = 16.1 Hz, J 2 = 7.9 Hz), 5.25 (d, 1H, CH2, J = 4.4 Hz), 5.43 (d, 1H, CH2, J = 4.4 Hz), 5.84 (m, 1H, CH), 6.72–7.23 (m, 8H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 29.32, 32.15, 56.97, 77.80, 118.01, 120.90, 121.80, 124.67, 127.60, 128.09, 129.99, 130.09, 131.58, 134.58, 138.90, 140.68, 167.16, 178.09; MS (ESI, m/z, M + ): 384.1; Anal. calcd. for C20H17ClN2O2S: C, 62.41; H, 4.45; N, 7.28% Found: C, 62.23; H, 4.39; N, 7.22%.
2.2e 1-Allyl-3′(3-fluorophenyl)-spiro[indole-3,2′-tetrahydro-1,3-thiazine]-2,4′(1H)-dione ( 4e ): Mp 255–257°C; IR (KBr, υ, cm − 1) 1695, 1715 (C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 2.64–2.76 (m, 1H, HA), 3.18–3.31 (m, 2H, H\(_{\rm MM^\prime })\), 4.16–4.26 (m, 1H, HX), 4.35 (dd, 1H, CH2, J 1 = 14.4 Hz, J 2 = 8.1 Hz), 4.54 (dd, 1H, CH2, J 1 = 14.4 Hz, J 2 = 8.1 Hz), 5.35 (d, 1H, CH2, J = 4.3 Hz), 5.48 (d, 1H, CH2, J = 4.3 Hz), 5.99 (m, 1H, CH), 6.74–7.32 (m, 8H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 29.33, 32.17, 58.91, 78.80, 111.10, 116.01, 120.90, 122.36, 124.67, 126.58, 127.60, 128.09, 129.39, 130.09, 131.54, 138.90, 140.68, 142.41, 166.16, 175.09; MS (ESI, m/z, M + ): 368.1; Anal. calcd. for C20H17FN2O2S: C, 65.20; H, 4.65; N, 7.60% Found: C, 65.1 0; H, 4.59; N, 7.56%.
2.2f 1-Allyl-3′(3-trifluoromethylphenyl)-spiro[indole-3,2′[1,3]-thiazine]-2,4′(1H)-dione ( 4f ): Mp 260–262°C; IR (KBr, υ, cm − 1) 1695, 1713 (C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 2.56–2.70 (m, 1H, HA), 3.17–3.22 (m, 2H, H\(_{\rm MM^\prime })\), 4.21–4.26 (m, 1H, HX), 4.31 (dd, 1H, CH2, J 1 =14.2 Hz, J 2 = 8.0 Hz), 4.52 (dd, 1H, CH2, J 1 =14.2 Hz, J 2 = 8.0 Hz), 5.28 (d, 1H, CH2, J 1 = 3.2 Hz), 5.48 (d, 1H, CH2), 5.89 (m, 1H, CH), 6.98–7.43 (m, 8H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 28.90, 31.91, 57.58, 80.58, 117.23, 119.34, 120.90, 121.22, 122.84, 123.36, 126.58, 128.32, 129.39, 130.09, 131.54, 134.64, 139.21, 138.90, 142.41, 166.16, 175.09; MS (ESI, m/z, M + ): 418.1; Anal. calcd. for C21H17F3N2O2S: C, 60.28; H, 4.10; N, 6.69% Found: C, 60.12; H, 4.06; N, 6.62%.
2.2g 1-Ethyl-3′(4-Chlorophenyl)-spiro[indole-3,2′[1,3]-thiazine]-2,4′(1H)-dione ( 4g ): Mp 275–277°C; IR (KBr, υ, cm − 1) 1690, 1715 (C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 1.33 (t, 3H, J = 6.8 Hz), 2.62–2.70 (m, 1H, HA), 3.28–3.39 (m, 2H, H\(_{\rm MM^\prime })\), 3.85 (q, 2H, J = 6.8 Hz), 4.26–4.29 (m, 1H, HX), 6.94–7.32 (m, 8H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 11.33, 28.41, 30.32, 55.73, 87.58, 119.01, 120.50, 123.16, 124.29, 126.98, 129.99, 130.59, 132.24, 133.89, 138.90, 167.28, 176.09; MS (ESI, m/z, M + ): 372.0; Anal. calcd. for C19H17ClN2O2S: C, 61.28; H, 4.40; N, 6.44%. Found: C, 61.08; H, 4.44; N, 6.41%.
2.2h 1-Ethyl-3′(3-fluorophenyl)-spiro[indole-3,2′[1,3]- thiazine]-2,4′(1H)-dione ( 4h ): Mp 270–272°C; IR (KBr, υ, cm − 1) 1690, 1710(C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 1.34 (t, 3H, J = 6.8 Hz), 2.69–2.73 (m, 1H, HA), 3.24–3.42 (m, 2H, H\(_{\rm MM^\prime })\), 3.88 (q, 2H, J = 6.8 Hz), 4.29–4.32 (m, 1H, HX), 6.84–7.32 (m, 8H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 11.83, 29.42, 32.36, 57.73, 89.58, 118.29, 120.22, 122.16, 123.39, 124.58, 125.42, 126.98, 128.99, 130.59, 131.54, 134.67, 139.90, 169.16, 177.09; MS (ESI, m/z, M + ): 356.1; Anal. calcd. for C19H17FN2O2S: C, 60.28; H, 4.41; N, 6.24%. Found: C, 60.42; H, 4.46; N, 6.19%.
2.2i 1-Ethyl-3′(3-trifluoromethylphenyl)-spiro[indole-3, 2′[1,3]-thiazine]-2,4′(1H)-dione ( 4i ): Mp 271–273°C; IR (KBr, υ, cm − 1) 1695, 1715 (C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 1.23 (t, 3H, J = 6.5 Hz), 2.66–2.72 (m, 1H, HA), 3.26–3.36 (m, 2H, H\(_{\rm MM^\prime })\), 3.88 (q, 2H, J = 6.5 Hz), 4.20–4.25 (m, 1H, HX), 6.98–7.32 (m, 8H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 12.33, 28.12, 31.36, 58.73, 81.58, 119.01, 121.36, 122.50, 123.16, 124.66, 125.98, 126.39, 127.99, 130.59, 131.54, 138.90, 142.41, 144.29, 166.90, 175.09; MS (ESI, m/z, M + ): 406.1. Anal. calcd. for C20H17F3N2O2S : C, 61.28; H, 4.40; N, 6.44% Found: C, 61.24; H, 4.45; N, 6.40%.
2.3 Characterization of compounds (6a–f)
2.3a 3′(2-Fluorophenyl)-spiro[acenaphthylene-1,2′[1,3]- thiazine]-2,4′(1H)-dione ( 6a ): Mp 257–259°C; IR (KBr, υ, cm − 1) 1700, 1715 (C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 2.59–2.68 (m, 1H, HA), 3.22–3.35 (m, 2H, H\(_{\rm MM^\prime })\), 4.25–4.33 (m, 1H, HX), 7.02–7.73 (m, 10H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 25.7, 35.9, 94.9, 115.70, 121.50, 123.16, 124.89, 126.98, 127.99, 128.59, 129.01, 129.89, 130.59, 131.54, 132.79, 133.08, 133.68, 138.90, 154.41, 175.09, 192.89; MS (ESI, m/z, M + ): 363.0; Anal. calcd. for C21H14FNO2S: C, 69.41; H, 3.88; N, 3.85%. Found: C, 69.30; H, 3.82; N, 3.79%.
2.3b 3′(3-Trifluoromethylphenyl)-spiro[acenaphthylene- 1,2′[1,3]-thiazine]-2,4′(1H)-dione ( 6b ): Mp 227–229°C; IR (KBr, υ, cm − 1) 1700, 1720 (C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 2.60–2.70 (m, 1H, HA), 3.24–3.34(m, 2H, H\(_{\rm MM^\prime })\), 4.34–4.39 (m, 1H, HX), 7.10–7.74 (m, 10H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 24.12, 34.33, 95.90, 117.24, 119.35, 120.90, 121.50, 123.16, 124.32, 126.98, 127.99, 129.01, 129.81, 130.52, 131.50, 132.23, 133.02, 133.56, 138.88, 141.68, 174.09, 192.65; MS (ESI, m/z, M + ): 413.0; Anal. calcd. for C22H14F3NO2S: C, 63.92; H, 3.41; N, 3.39%. Found: C, 63.85; H, 3.32; N, 3.34%.
2.3c 3′(4-Chloro,3-trifluoromethylphenyl)-spiro[acenaphthylene-1,2′[1,3]-thiazine]-2,4′(1H)-dione ( 6c ): Mp 145–147°C; IR (KBr, υ, cm − 1) 1705, 1685 (C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 2.50–2.60 (m, 1H, HA), 3.04–3.14(m, 2H, H\(_{\rm MM^\prime })\), 4.14–4.22 (m, 1H, HX), 7.02–7.64 (m, 9H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 24.02, 34.03, 94.20, 110.23, 117.24, 121.50, 123.16, 125.13, 126.25, 127.99, 128.01, 129.89, 130.59, 131.54, 132.79, 133.08, 133.68, 139.68, 142.39, 150.21, 175.09, 192.65; MS (ESI, m/z, M + ): 447.0; Anal. calcd. for C22H13ClF3NO2S: C, 59.00; H, 2.93; N, 3.13%. Found: C, 58.84; H, 2.84; N, 3.08%.
2.3d 3′(4-Fluorophenyl)-spiro[acenaphthylene-1,2′ [1,3]-thiazine]-2,4′(1H)-dione ( 6d ): Mp 193–195°C; IR (KBr, υ, cm − 1) 1705, 1715 (C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 2.60–2.65 (m, 1H, HA), 3.40–3.44 (m, 2H, H\(_{\rm MM^\prime })\), 4.24–4.32 (m, 1H, HX), 7.02–7.68 (m, 10H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 25.01, 34.03, 94.90, 115.23, 123.10, 125.13, 126.25, 126.98, 127.99, 128.01, 128.98, 129.89, 130.59, 131.54, 132.79, 133.08, 140.28, 172.09, 190.65; MS(m/z): 363.0; Anal. calcd. for C21H14FNO2S: C, 69.25; H, 3.88; N, 3.85%. Found: C, 69.31; H, 3.82; N, 3.74%.
2.3e 3′(4-Methoxyphenyl)-spiro[acenaphthylene-1,2′ [1,3]-thiazine]-2,4′(1H)-dione ( 6e ): Mp 131–133°C; IR (KBr, υ, cm − 1) 1695, 1705 (C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 2.66–2.70 (m, 1H, HA), 3.29–3.38 (m, 2H, H\(_{\rm MM^\prime })\), 4.20–4.26 (m, 1H, HX), 3.65 (s, 3H, OCH3), 6.82–7.78 (m, 10H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 25.70, 34.89, 56.09, 93.90, 114.23, 121.24, 123.10, 125.03, 126.50, 127.99, 128.01, 128.98, 129.89, 130.59, 131.54, 132.79, 134.08, 142.08, 173.09, 190.65; MS (ESI, m/z, M + ): 375.0; Anal. calcd. for C22H17NO3S: C, 70.38; H, 4.56; N, 3.73% Found: C, 70.22; H, 4.50; N, 3.68%.
2.3f 3′(4-Chlorophenyl)-spiro[acenaphthylene-1,2′ [1,3]-thiazine]-2,4′(1H)-dione ( 6f ): Mp 205–207°C; IR (KBr, υ, cm − 1) 1700, 1715 (C=O); 1H NMR (300 MHz, DMSO-d 6) δ(ppm): 2.63–2.70 (m, 1H, HA), 3.34–3.40 (m, 2H, H\(_{\rm MM^\prime })\), 4.35–4.43 (m, 1H, HX), 7.02–7.68 (m, 10H, Ar-H); 13C NMR (75 MHz, DMSO-d 6) δ(ppm): 25.79, 35.96, 92.92, 118.19, 121.50, 123.16, 124.89, 126.98, 127.99, 128.59, 129.01, 129.49, 130.89, 131.54, 133.68, 138.90, 141.42, 171.09, 190.22; MS (ESI, m/z, M + ): 379.0; Anal. calcd. for C21H14ClNO2S: C, 66.40; H, 3.71; N, 3.69%. Found: C, 66.28; H, 3.42; N, 3.62%.
3 Results and discussion
3.1 Chemistry
To achieve suitable conditions for the synthesis of spirooxindole 4, various reaction conditions and catalysts have been investigated in the reaction of N-benzylisatin 1a, 4-chloro aniline 2a and 3-mercaptopropionic acid 3 as a model reaction (table 1).
It turned out that the multi-component reactions of 1a, 2a and 3 proceeded smoothly in an IL, [bmim][PF6], 1-butyl-3-methylimidazolium hexafluorophosphate, and gave the corresponding spiro[indole-3,2′[1,3]-thiazine]-dione (4a). The yield of 4a increased remarkably with the temperature increasing until 80°C (table 1, entries 1–5). Interestingly, of the two ILs studied, namely [bmim][PF6] and [bmim][BF4], [bmim][PF6] gave better result (table 1, entries 4 and 6), presumably due to its hydrophobic activation activity. It is postulated that water formed ‘in situ’ from the condensation process is miscible with hydrophilic [bmim][BF4] and thus detained, which prevents completion of the reaction. In contrast, the hydrophobic nature of [bmim][PF6] would create a micro-environment to drive the equilibrium by extruding water out of the IL phase and thus result in a higher conversion.
The same reaction was also run in several conventional organic solvents and the results are also included in table 1. Comparing with CH3CN, DMF, EtOH and water, ILs exhibited enhanced reactivity by reducing reaction time and improving the yields significantly. Recovery and reuse of [bmim][PF6] were also studied, and 1a, 2a and 3 were used as model substrates. Upon completion of the condensation process, 4a was obtained by thorough extraction with diethyl ether and the remaining IL phase was recycled in subsequent reactions. Further studies showed that the recovered [bmim][PF6] could be successively recycled for at least five times without obvious loss in its efficiency (table 2). Under the above optimized conditions, we have synthesized several spiro[indole-3,2′[1,3]-thiazine]-2,4′-diones by reaction of various amines and substituted isatins (table 3). Compounds 4a–i are stable solids whose structures were established by IR, 1H, 13C NMR and mass spectroscopy and elemental analysis.
Encouraged by the results obtained above, we extended and to further explore the potential of this protocol for synthesis of spiro-heterocyclic compounds, isatin was replaced by acenaphthalene-1,2-dione 5 and spiro[acenaphthylene-1,2′-[1,3] thiazine] dione derivatives 6a–f were obtained in good yield under the same reaction conditions (table 4).
A plausible mechanism for the formation of the cycloadducts 4 is proposed in scheme 3. Firstly, there is condensation between the isatin and aniline leading to formation of the imine derivative [A] by the loss of water molecule followed by reaction between 3-mercaptopropionic acid and imine derivative yields spiro[indole-3,2′[1,3]-thiazine]-2,4′-dione 4. Role of the IL may be postulated in terms of some Brønsted acidity due to hydrogen atom of imidazolium cation leading to its interaction with the heteroatoms, thereby increasing polarization and promoting the condensation reaction.
4 Biological evaluation
Synthesized compounds were subjected to antimycobacterial efficacy against Mycobacterium tuberculosis H37Rv strain and DNA cleavage activity.
4.1 In vitro evaluation of antimycobacterial activity
Minimum inhibitory concentration (MIC) of compounds was determined against M. tuberculosis H37Rv strain by using Lowenstein–Jensen (LJ) medium (conventional method) as described by Rattan.[33] Determination of MIC of the test compounds against M. tuberculosis H37Rv was performed by LJ agar (MIC) method where primary (1000, 500 and 250 mg/ml) and secondary (200, 100, 62.5, 50, 25, 12.5, 6.25 and 3.25 mg/ml) dilutions of each test compound were added to liquid LJ medium and then media were sterilized by inspissation method. A culture of M. tuberculosis H37Rv growing on LJ medium was harvested in 0.85% saline in Bijou bottles. First stock solution of 2000 mg/ml concentration of all test compounds was prepared in DMSO. These tubes were then incubated at 37°C for 24 h followed by streaking of M. tuberculosis H37Rv (5 × 104 bacilli per ml). These tubes were then incubated at 37°C. Growth of bacilli was seen after 12 days, 22 days and finally 28 days of incubation. Tubes having the compounds were compared with control tubes where medium alone was incubated with M. tuberculosis H37Rv. Concentration at which no development of colonies occurred or <20 colonies was taken as MIC concentration of test compound. The standard strain M. tuberculosis H37Rv was tested with known drug isoniazid. All the compounds exhibited very poor antitubercular activities against M. tuberculosis H37Rv (table 5).
4.2 DNA cleavage activity
A number of studies have shown that clinical efficacies of many drugs correlate with their ability to induce enzyme-mediated DNA cleavage. Inhibitory potency of the test compounds was assessed by comparing the cleavage of DNA by control and the title compound. DNA cleavage experiments were done according to literature.[34]
Nutrient broth (peptone, 10; yeast extract, 5; NaCl, 10; in (g/l)) was used for culturing the pathogen Staphylococcus aureus. Media (50 mL) was prepared, and autoclaved for 15 min at 121°C under 15 lb pressures. The autoclaved media was inoculated for 24 h at 37°C.
4.2a Isolation of DNA: Fresh bacterial culture (1.5 mL) is centrifuged to obtain the pellet which was then dissolved in 0.5 mL of lysis buffer (100 mM tris pH 8.0, 50 mM ethylenediaminetetraacetic acid (EDTA), 50 mM lysozyme). To this, 0.5 mL of saturated phenol was added and incubated at 55°C for 10 min, then centrifuged at 10,000 rpm for 10 min and to the supernatant, equal volume of chloroform: isoamyl alcohol (24:1) and 1/20th volume of 3 M sodium acetate (pH 4.8) was added. After centrifuging at 10,000 rpm for 10 min, to the supernatant, 3 volumes of chilled absolute alcohol were added. The precipitated DNA was separated by centrifugation and the pellet was dried and dissolved in tris-acetate-EDTA (TAE) buffer (100 mM tris, pH 8.0 adjusted with glacial acetic acid, 10 mM EDTA) and stored in cold condition.
4.2b Treatment of DNA with the samples: The final compounds 4a–i (100 mg) were added separately to the DNA sample. Sample mixtures were incubated at 37°C for 2 h.
During agarose gel electrophoresis,[34] agarose (200 mg) was dissolved in TAE buffer (25 mL) (4.84 g Tris base, pH 8.0, 0.5 M EDTA/1 L) by boiling. When the gel attained ≈55°C, it was poured into the gel cassette fitted with a comb. The gel was then allowed to solidify. The comb was carefully removed and the gel was placed in the electrophoresis chamber flooded with TAE buffer. DNA sample (20 mL, mixed with bromophenol blue dye at 1:1 ratio), was loaded carefully into the wells, along with standard DNA marker and constant 50 V of electricity was passed for around 45 min. The gel was removed and carefully stained with ethidium bromide (ETBR) solution (10 μg/ml) for 10–15 min and the bands were observed under UV transilluminator.
Results are compared with standard DNA marker. All the samples have shown complete cleavage of DNA (figure 1). It was observed from the photograph that compounds 4a–i after treatment with DNA (S. aureus) has cleaved it completely.
5 Conclusion
In conclusion, an efficient and green method has been found for the synthesis of nitrogen and sulphur containing spiro heterocycles via three-component in [bmim][PF6]. Features of this procedure include mild reaction conditions, high yields, one-pot, operational simplicity and environmentally benign reactions. DNA cleavage studies revealed that the test compounds in the series have exhibited promising cleavage activity. Based on these results, selected novel compounds are being screened for anticancer activity which will be reported in due course.
References
Sun Y, Bi S, Song D, Qiao C, Mu D and Zhang H 2008 Sensors and Actuators B 129 799
Indumathi T, Fronczek F R and Prasad K J R 2012 J. Mol. Struct. 1016 134
(a) Gruber B, Kataev E, Aschenbrenner J, Stadlbauer S and Koenig B 2011 J. Am. Chem. Soc. 133 20704; (b) Shao Y, Sheng X, Li Y, Jia Z L, Zhang J J, Liu F and Lu G Y 2008 Bioconjugate Chem. 19 1840
(a) Reifchneider W, Bisabari-Ershadi B, Dripps J E and Bonron J B 1991 U.S. Patent 5,075,293, Chem. Abstr. 116, 1991, 129249f; (b) Rovnyak G C, Narayanan V L and Haugwitz R D U.S. Patent 4,053, 613, 1975; Chem. Abstr. 88, 1978, 22892; (c) Ali S and Alam M 1994 Arch. Pharmacol Res. 17 131
(a) Zhou H Y, Wu S H, Zhai S M, Liu A F, Sun Y, Li R S, Zhang Y, Ekins S, Swaan P W, Fang B L, Zhang B and Yan B 2008 J. Med. Chem. 51 1242; (b) Ottana R, Carotti S, Maccari R, Landini I, Chiricosta G, Caciagli B, Vigorita M G and Mini E 2005 Bioorg. Med. Chem. Lett. 15 3930; (c) Eriksson B, Kurz G, Hedberg C and Westman J, WO2007010273, 2007
Look G C, Schullek J R, Holmes C P, Chinn J P, Gordon E M and Gallop M A 1996 Bioorg. Med. Chem. Lett. 6 707
Allen S, Newhouse B, Anderson A S, Fauber B, Allen A, Chantry D, Eberhardt C, Odingo J and Burgess L E 2004 Bioorg. Med. Chem. Lett. 14 1619
(a) Ottana R, Carotti S, Maccari R, Landini I, Chiricosta G, Caciagli B, Vigorita M G and Mini E 2005 Bioorg. Med. Chem. Lett. 15 3930; (b) Gududuru V, Hurh E, Dalton J T and Miller D D 2004 Bioorg. Med. Chem. Lett. 14 5289
Diurno M V, Mazzoni O, Piscopo E, Calignano A, Giordano F and Bolognese A 1992 J. Med. Chem. 35 2910
Rawal R K, Tripathi R, Katti S B, Pannecouque C and DeClercq E 2007 Bioorg. Med. Chem. 15 3134
Campaigne E and Nargund P K 1964 J. Org. Chem. 29 224
Barret G C, Kane V V and Lowe G 1964 J. Chem. Soc. 783 23
Schmidt R R 1972 Synthesis 333 7
(a) Kuznetsova L, Ungureanu M I, Pepe A, Zanardi I, Wu X and Ojima I 2004 J. Fluorine Chem. 125 415; (b) Kirsch P 2004 Modern fluoroorganic chemistry, synthesis, reactivity and applications, (New York/Heidelberg: Wiley-VCH)
(a) Quan M L, Lam P Y S, Han Q, Pinto D J P, He M Y, Li R H, Ellis C D, Clark C A, Sun J H, Alexander R S, Bai S, Luettgen J M, Knabb R M, Wong P C and Wexler R R 2005 J. Med. Chem. 48 1729; (b) Abid M and Torok B 2005 Adv. Synth. Catal. 347 1797
O’Hagan S C, Cobb S L, Hamilton J T G, Cormac D and Murphy C D 2002 Nature 416 279
Chen I, Safe S and Bjeldanes L 1996 Biochem. Pharmacol. 51 1069
Suzen S and Buyukbingol E 2000 Il Farmaco 55 246
(a) Buyukbingol E, Suzen S and Klopman G 1994 Il Farmaco 49 443; (b) Suzen S and Buyukbingol E 1998 Il Farmaco 53 525
(a) Lieberman P M, Wolfler A, Felsner P, Hofer D and Schauenstien K 1997 Int. Arch. Allergy. Immunol. 112 203; (b) Page D, Yang H, Brown W, Walpole C, Fleurent M, Fyfe M, Gaudreault F and Onge S S 2007 Bioorg. Med. Chem. Lett. 22 6183
(a) Cui C -B, Kakeya H, Okada G, Onose R and Osada H 1996 J. Antibiot. 49 527; (b) Cui C -B, Kakeya H and Osada H 1996 Tetrahedron 52 12651
(a) Khafagy M M, El-Wahas A H F A, Eid F A and El-Agrody A M 2002 Farmaco 57 715; (b) Sebahar P R and Williams R M 2000 J. Am. Chem. Soc. 122 5666
Kang T H, Matsumoto K, Murakami Y, Takayama H, Kitajima M, Aimi N and Watanabe H 2002 Eur. J. Pharmacol. 444 39
Garcia Prado E, Garcia Gimenez M D, De la Puerta Vazquez R, Espartero Sanchez J L and Saenz Rodriguez M T 2007 Phytomedicine 14 280
Kutschy P, Suchy M, Monde K, Harada N, Maruskova R, Curillova Z, Dzurilla M, Miklosova M, Mezencev R and Majzis J 2002 Tetrahedron Lett. 43 9489
(a) Chen H and Shi D 2011 Tetrahedron 67 5686; (b) Khanna. P, Saxena A, Khanna L, Bhagat S and Jain S C 2009 Arkivoc VII 119; (c) Mashelkar U C, Rane D M and Kenny R S 2008 J. Heterocycl. Chem 45 865; (d) Jain S C, Khanna P, Bhagat S, Jain M and Sakhuja R 2005 Phosphorus, Sulfur Silicon Relat. Elem. 180 1829; (e) Azizian J, Morady A V, Jadidi K, Mehrdad M and Sarrafi Y 2000 Synth. Commun. 30 537; (f) Rajopadhye M and Popp F D 1984 J. Heterocycl. Chem. 21 289
(a) Houlihan W J, Remers W A and Brown R K 1992 Indoles: Part I. (New York: Wiley); (b) Sundberg R J 1996 The Chemistry of Indoles (New York: Academic Press)
(a) Popp F D and Rajopadhye M 1985 J. Heterocycl. Chem. 22 93; (b) Joshi K C, Dandia A and Ahm N 1986 Heterocycle 24 2479
(a) Domling A 2002 Curr. Opin. Chem. Biol. 6 306; (b) Ugi I and Heck S 2001 Comb. Chem. High Throughput Screening 4 1; (c) Zhu J and Bienayme H 2005 Multicomponent reactions (Weinheim: Wiley-VCH); (d) Nicolaou K C, Edmonds D J and Bulger P G 2006 Angew. Chem. Int. Ed. 45 7134; (e) Simon C, Vinod A U, Constantieux T J and Roadriguez K 2004 Eur. J. Org. Chem. 4957 2004
(a) Dzyuba S V and Bartsch R A 2003 Angew. Chem., Int. Ed. 42 148; (b) Wilker J S 2002 Green Chem. 4 73; (c) Welton T 1999 Chem. Rev. 99 2071; (d) Plaquevent J-C, Levillain J, Guillen F, Malhiac C, Gaumont A-C 2008 Chem. Rev. 108 5035; (e) Martins M A P, Frizzo C P, Moreira D N, Zanatta N and Bonacorso H G 2008 Chem. Rev. 108 201
Joseph T, Sahoo S and Halligudi S B 2005 J. Mol. Catal. A: Chem. 234 107
(a) Dandia A, Laxkar A K, Singh R 2012 Tetrahedron Lett. 53 3012; (b) Dandia A, Singh R and Bhaskaran S 2011 Green Chem. 13 1852; (c) Dandia A, Jain A K and Bhati D S 2011 Tetrahedron Lett. 52 5333; (d) Dandia A, Parewa V, Jain A K and Rathore K S 2011 Green Chem. 13 2135; (e) Dandia A, Singh R and Bhaskaran S 2010 Ultrason. Sonochem. 17 399; (f) Dandia A, Singh R and Bhaskaran S 2011 Ultrason. Sonochem. 18 1113; (g) Dandia A, Jain A K, Bhati D S and Sharma G N 2011 Ultrason. Sonochem. 18 1143
Rattan A 2000 Antimicrobials in laboratory medicine. Churchill B I, Livingstone New Delhi, 85
Sambrook J, Fritsch E F and Maniatis T 1989 Molecular cloning, A Laboratory Manual, 2nd edn. (Cold Spring Harbor, New York: Cold Spring Harbor Laboratory)
Acknowledgements
Financial assistance from the Council of Scientific and Industrial Research (CSIR), New Delhi is gratefully acknowledged. RS is thankful to CSIR for the award of SRA (No. 13 (8424-A) 2010-Pool). We are also thankful to the Central Drug Research Institute (CDRI), Lucknow for the spectral and elemental analyses.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
DANDIA, A., SINGH, R. & SAINI, D. Ionic liquid-mediated three-component synthesis of fluorinated spiro-thiazine derivatives and their antimycobacterial and DNA cleavage activities. J Chem Sci 125, 1045–1053 (2013). https://doi.org/10.1007/s12039-013-0493-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0493-8