Abstract
A series of spirochromene-tethered 1,2,3-triazoles (1,2,3-triazolylspirochromenes) were designed and synthesized via click-chemistry-based one-pot five-component reaction between N-propargyl isatins, malononitrile, dimedone (or 4-hydroxyl-6-methyl-2H-pyran-2-one), arylalkyl halides, and sodium azide using cellulose-supported CuI nanoparticles (Cell-CuI NPs) as heterogeneous catalyst. All synthesized compounds were screened for inhibitory activity against Mycobacterium tuberculosis H37Ra (ATCC 25177) and Mycobacterium bovis BCG (ATCC 35743), in active as well as dormant state. During screening, compounds 6h, j, l were found to exhibit promising antimycobacterial activity against M. bovis BCG, while compounds 7d, h, l showed promising antimycobacterial activity against M. tuberculosis H37Ra as well as M. bovis BCG. The active compounds were found to be noncytotoxic to three human cancer cell lines (MCF-7, HCT116, and A549). The active compounds exhibited selectivity index >10, indicating potential as antitubercular agents. The active compounds were also evaluated for in vitro antibacterial activity, with five (6h, l, 7b, f, l) showing good antibacterial activity against Gram-positive as well as Gram-negative bacteria. Compounds 6h, j, l exhibiting promising activity against M. bovis BCG can serve as good leads for further modification and optimization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis, remaining one of the greatest health problems even in the 21st century [1, 2]. The main problem associated with spread of tuberculosis is emergence of strains resistant to one or more drugs and the very long time required for treatment of multiple-drug-resistant tuberculosis (MDR-TB) as well as extensively drug-resistant tuberculosis (XDR-TB) [3,4,5,6]. For the last few years, the combination of rifampicin, ethambutol, and isoniazid remains the effective therapy for treatment of TB [7] and as far as discovery of new, effective drugs to encounter TB is concerned, except bedaquiline, no remarkable breakthroughs have been achieved in the recent past. With the possibility that tubercular bacilli will acquire resistance to all these drugs, there is an urgent need to develop new antitubercular agents [8]. Amongst various approaches developed for introduction of new antitubercular agents, molecular hybridization involving design of new chemical entities by covalent fusion of two or more pharmacophores is an attractive strategy [9,10,11,12]. Synthesis of such hybrid molecules may involve separate synthesis of two or more subunits with known bioprofiles followed by their combination in a final step, or synthesis in a single step using a multicomponent synthesis strategy [13,14,15,16,17]. Amongst these approaches, the multicomponent condensation pathway is certainly advantageous in terms of atom economy, yield, selectivity, efficiency, and minimum waste generation [18]. With all these concepts in mind and our continued interest in multicomponent synthesis [19,20,21,22,23,24,25], we undertook synthesis of novel hybrid molecules as possible antitubercular agents.
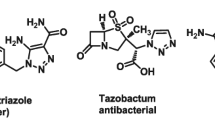
It is well known that, owing to their diverse biological properties, heterocyclic compounds play a very important role in organic chemistry. Among various heterocyclic compounds, spiroheterocycles, especially spirooxindoles, represent a medicinally privileged scaffold present in many naturally occurring bioactive alkaloids (Fig. 1a) [26,27,28,29]. In recent years, spirooxindole derivatives fused with 2-amino-4H-chromene unit, viz. spirochromenes (Fig. 1b), have become immensely important due to their diuretic, spasmolytic, anticancer, anticoagulant, and antianaphylactic activities [30,31,32]. Like spirochromenes, compounds containing 1,2,3-triazole structural motif are also known to possess antitubercular, anticancer, antifungal, antiallergic, antiviral, antimicrobial, antiepileptic, as well as anti-human immunodeficiency virus (HIV) properties (Fig. 1c–f) [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. Considering the biological potential of spirochromenes as well as of 1,2,3-triazoles, we surmised that hybrid molecules containing both of these structural units (Fig. 2) may possess enhanced antitubercular activity.
From the retrosynthetic viewpoint, synthesis of such hybrid molecules involves construction of the spirochromene motif in a first step then construction of the 1,2,3-triazole unit in a subsequent step, or vice versa. The best known pathway for synthesis of spirochromenes involves multicomponent condensation between isatin, active methylene compound (malononitrile/alkyl cyanoacetate), and cyclic 1,3-dicarbonyl compound (dimedone, 4-hydroxyl-6-methyl-2H-pyran-2-one, etc.). Such condensation is reported to proceed under the influence of base as well as CuI as catalyst [49, 50]. On the other hand, copper-catalyzed azide–alkyne cycloaddition (CuAAC, click chemistry) remains the approach of choice for synthesis of 1,2,3-triazoles [51,52,53,54,55] (Scheme 1a, b).
We previously reported cellulose-supported cuprous iodide nanoparticles (Cell-CuI NPs) as heterogeneous and reusable catalyst for synthesis of 1,4-disubstituted 1,2,3-triazoles [56] and recently also reported environmentally benign protocols for synthesis of 2-amino-4H-chromenes [19,20,21]. Based on these results, we speculated that, if cuprous iodide nanoparticles (Cell-CuI NPs) are effective for construction of spirochromene moiety, it would be possible to synthesize the targeted hybrid molecules using a one-pot five-component condensation approach. As an outcome of this philosophy, we report herein synthesis of spirochromene-tethered 1,2,3-triazoles by one-pot five-component condensation between N-propargyl isatins, malononitrile, dimedone (or 4-hydroxy-6-methyl-2H-pyran-2-one), arylalkyl halides, and sodium azide using Cell-CuI NPs as reusable catalyst (Scheme 2).
Results and discussion
Chemistry
The synthetic route started with preparation of N-propargyl isatins. Various substituted N-propargylated isatins were prepared by potassium-carbonate-catalyzed propargylation of isatins with propargyl bromide using dimethylformamide (DMF) as solvent and tetra-n-butylammonium bromide (TBAB) as phase-transfer catalyst [56].
We first focused on exploring the feasibility of using Cell-CuI NPs as catalyst for synthesis of spirochromenes. Accordingly, a model reaction was performed using N-propargyl isatin, malononitrile, and dimedone as substrates (Scheme 3). Thus, to an equimolar mixture of N-propargyl isatin, malononitrile, and dimedone (1 mmol, each) in ethanol (5 mL) were added Cell-CuI NPs (100 mg, 3.7 mol% CuI) followed by continuous stirring at ambient temperature for 12 h. We did not observe any appreciable reaction progress, so the reaction was continued under reflux condition. Timely analysis of the reaction mixture by thin-layer chromatography (TLC) indicated formation of a new product in major quantity. Upon reaction completion, the resultant product was isolated, purified by column chromatography, and identified as the expected spirochromene, A (78%, Scheme 3). Our primary aim with this trial reaction was simply to check the feasibility of using Cell-CuI NPs in synthesis of spirochromenes; however, as the desired spirochromene A was obtained in acceptable yield (78%) during the trial reaction, no further attempts were made towards improving the yield of A (Scheme 3).
Encouraged by this initial success, we next planned to undertake one-pot synthesis of 1,2,3-triazolylspirochromene 6a (Scheme 2, R1 = R2 = H) as model compound. Accordingly, to well-stirred solution of N-propargyl isatin, malononitrile, dimedone, benzyl bromide (1 mmol, each), and sodium azide (1.1 mmol) in ethanol (5 mL) were added Cell-CuI NPs (200 mg, 7.4 mol% CuI). Stirring was continued under reflux, and reaction progress monitored by TLC. Upon reaction completion followed by work-up as well as spectral analysis, we were pleased to note formation of expected 1,2,3-triazolylspirochromene 6a in acceptable yield (72%). Thereafter, attempts were directed towards improving the yield of desired 6a. In this context, the model reaction was repeated in different reaction media. It was observed that, in common organic solvents such as toluene, acetonitrile, as well as tetrahydrofuran, the yield of the desired product 6a, remained nearly the same. However, with water, water–ethanol, as well as water–acetonitrile as reaction medium, significantly increased yield of desired 6a was found (Table 1, entries 3, 7, 8). However, with these water-based reaction media, product isolation proved to be slightly cumbersome. Hence, the model reaction was then carried out in dimethylformamide–water (2:1, v/v) as mixed solvent system (Table 1, entries 9–11). This modification proved to be useful in terms of both yield as well as isolation of the desired product 6a (Table 1, entry 9). The results summarized in Table 1 also reveal that use of less than the optimized amount of catalyst failed to furnish the desired product 6a in the same yield (Table 1, entries 10, 11). It is noteworthy that, in absence of added catalyst, the reaction furnished desired product 6a in low yield (35%).
Having established appropriate reaction conditions, we turned our attention to examine the generality of the reaction conditions. Thus, the benzyl bromide component of the model reaction was replaced by 4-methyl (4b), 4-fluoro (4c), and 4-chlorobenzyl bromide (4d). Under the optimized reaction conditions, corresponding 1,2,3-triazolylspirochromenes 6b–d were produced in excellent yield (Table 2). To examine the scope of the reaction conditions, various N-propargylated isatins derived from 5-chloroisatin (1b), 5-bromoisatin (1c), and 5-fluoroisatin (1d) were then used. Once again, the reaction furnished expected 1,2,3-triazolylspirochromenes 6e–p in acceptable yield (Table 2). Finally, the dimedone component in each successful reaction was replaced with 4-hydroxyl-6-methyl-2H-pyran-2-one (3b) (Scheme 2). Most gratifyingly, in each case, corresponding 1,2,3-triazolylspirochromenes 7a–p were produced in acceptable yield (Table 2). In this multicomponent synthesis of 1,2,3-triazolylspirochromenes under the influence of copper catalyst, formation of the chromene unit precedes construction of the 1,2,3-triazole unit (TLC). A plausible mechanism for the formation of the desired 1,2,3-triazolylspirochromene is depicted in Fig. 3.
In heterogeneously catalyzed reactions, reusability as well as stability of the catalyst is of importance from both economic and environmental points of view. Hence, upon completion of the model reaction, the catalyst was separated by filtration, washed repeatedly with ethyl acetate as well as acetone, dried in air, and used in the next run with the same substrates. The recovered catalyst also furnished desired 1,2,3-triazolylspirochromene 6a in nearly the same yield (Fig. 4). Subsequent studies on reusability of the catalyst revealed that the catalyst could be used for four consecutive cycles without appreciable change in the yield of the desired triazole 6a.
In copper-catalyzed click synthesis of biologically active molecules, study of catalyst leaching is also of prime importance. Therefore, upon reaction completion followed by catalyst separation, resultant filtrate was analyzed by atomic absorption spectroscopy, revealing no detectable copper content.
Biological evaluation
Antitubercular studies
As well as designing and synthesizing novel organic molecules, we also aimed to screen their antitubercular activity. Hence, all synthesized compounds were screened for in vitro antitubercular activity against M. tuberculosis H37Ra (MTB) (ATCC 25177) and M. bovis BCG (ATCC 35743) by twofold dilution technique. The minimum inhibitory concentration (MIC) and 50% inhibition concentration (IC50) values of the tested compounds are presented in Table 3. Rifampicin, a drug in regular clinical use, was applied as reference standard. During primary screening, of 32 synthesized compounds, 20 were found to be active against both species with MIC in the range of 2.87–10.00 µg/mL (Table 3). In particular, compounds 6h, j, l were found to be most active against M. bovis BCG (MIC from 2.87 to 6.23 µg/mL) in active as well as dormant state. On the other hand, compounds 7d, h, l were found to be promisingly active (MIC < 7 µg/mL) against both M. tuberculosis H37Ra as well as M. bovis BCG. From the antimycobacterial activity data summarized in Table 3, it is evident that the activity depends upon the nature of the substituents R1 and R2 present on the phenyl rings. Replacement of hydrogen atoms on phenyl rings at R1 and R2 by halogen resulted in increased antitubercular activity. Amongst halogens, presence of chlorine substituent on phenyl as well as isatin ring proved to be useful for enhanced antitubercular activity. Regarding the choice of the 1,3-diketone, replacement of dimedone with 4-hydroxyl-6-methyl-2H-pyran-2-one did not cause any significant change in the antimycobacterial activity against M. bovis BCG, while for M. tuberculosis H37Ra it was increased to moderate extent.
Cytotoxicity
All synthesized compounds (6a–7p) were then evaluated for their in vitro cytotoxicity against three human cancer cell lines, viz. MCF-7, HCT116, and A549, by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay with paclitaxel as positive control [57,58,59]. The results are summarized in Table 4. The 50% growth inhibition concentration (GI50) values (>100 µg/mL) of the active compounds 6a–7f, m–p indicate that these compounds are potent and specific inhibitors of MTB. Especially 7g–l were found to be active antiproliferative compounds with GI50 in the range of 22.08–71.68 μg/mL against MCF-7 cell line but not against HCT116 or A549.
GI50 values obtained from cytotoxicity studies allow calculation of the selectivity index, which reflects the concentration of a compound at which it is active against mycobacteria but not toxic towards host cells. Higher selectivity index indicates that the compound can be used as a therapeutic agent. The selectivity of triazolylspirochromenes 6a–7p towards human cell lines and against M. bovis BCG was therefore described in terms of the selectivity index, and the results are presented in Table 5. It is evident that compounds 6h, j, l, 7b, d, h, l exhibited selectivity index value >10, indicating potential as antitubercular agents, thus they should be investigated further as possible anti-TB agents.
Antibacterial studies
Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis are common pathogens that have harmful effects on human health. We thought it worthwhile to evaluate the antimicrobial activity of 1,2,3-triazolylspirochromenes against these microorganisms [60]. This assay was performed by well plate method (zone of inhibition) using ampicillin and kanamycin as reference standards. The results are summarized in Table 6 (ESI, Table S6). Five compounds (6h, l, 7b, f, l) showed more than 90% inhibition at concentration of 3 µg/mL. Compounds showing good antibacterial activity were further analyzed to determine MIC values using dose–response curves. Compounds 6h, l, 7b, f, l showed good antibacterial activity against E. coli, P. aeruginosa, S. aureus, and B. subtilis, with MIC values varying from 1.1 to 5.29 µg/mL.
All the results above clearly indicate that target compound 6h is mycobacteria specific and can be further explored as a potential antitubercular drug.
Conclusions
Based on the structure of compounds with proven biological activity and our experience in click chemistry, green chemistry, and multicomponent reactions, studies were undertaken on the design and one-pot synthesis of novel compounds as possible antitubercular agents. We designed and synthesized for the first time a series of 1,2,3-triazolylspirochromenes by one-pot five-component condensation between N-propargyl isatin, malononitrile, dimedone (or 4-hydroxyl-6-methyl-2H-pyran-2-one), arylalkyl halide, and sodium azide using cellulose-supported CuI nanoparticles (Cell-CuI NPs) as heterogeneous catalyst. Simple experimental procedure, wide scope, and catalyst reusability for five consecutive runs are the main merits of this ecobenign protocol. The synthesized compounds were screened for their antimycobacterial activity. Among all the compounds, 6h, j, l showed very good antimycobacterial activity against M. bovis BCG. Some of the synthesized 1,2,3-triazolylspirochromenes (7d, h, l) showed promising antimycobacterial activity against Mycobacterium tuberculosis H37Ra as well as M. bovis BCG. The active compounds were found to be noncytotoxic to three cell cancer lines. Selectivity studies revealed that these active compounds have potential as possible antitubercular agents. The specificity of these compounds was checked by screening their antibacterial activity against four bacterial strains. Compounds 6h, l, 7b, f, l showed good antibacterial activity. All the above screening results clearly indicate that 1,2,3-triazolylspirochromenes can be further explored as potential antitubercular drugs via chemical modification.
References
B.H. Herzog, Respiration 65, 5 (1998)
Global Tuberculosis Control: WHO report 2014
R.P. Tripathi, N. Tewari, N. Dwivedi, V.K. Tiwari, Med. Res. Rev. 25, 93 (2005)
D.-B. Young, M.D. Perkin, K. Duncanan, C.E. Barry, J. Clin. Invest. 118, 1255 (2008)
C. Lienhardt, A. Vernon, M.C. Raviglione, Curr. Opin. Pulm. Med. 16, 186 (2010)
M.M. Sankar, J. Singh, S.C.A. Diana, S. Singh, Tuberculosis 93, 75 (2013)
M.M. Singh, Indian J. Tuberc. 54, 1 (2007)
L.T. Nao, J.J. Okogun, W.R. Folk, Nat. Prod. Rep. 30, 584 (2013)
R. Maia, C. Do, C.A.M. Fraga, Curr. Enzyme Inhib. 6, 171 (2010)
C. Viegas-Junior, A. Danuello, V. da Silva Bolzani, E.J. Barreiro, C.A.M. Fraga, Curr. Med. Chem. 14, 1829 (2007)
C.A.M. Fragae, Drug. Discov. 4, 605 (2009)
E.M. Guantai, K. Ncokazi, T.J. Egan, J. Gut, P.J. Rosenthal, P.J. Smith, K. Chibale, Bioorg. Med. Chem. 18, 8243 (2010)
K. Kurnaravel, G. Vasuki, Curr. Org. Chem. 13, 1820 (2009)
M. Syamala, Org. Prep. Proced. Int. 41, 1 (2009)
A. Dömling, W. Wang, K. Wang, Chem. Rev. 112, 3083 (2012)
V.A. Gulevich, G.A. Zhdanko, V.A.O. Romano, G.V. Nenajdenko, Chem. Rev. 110, 5235 (2010)
C. De Graaff, E. Ruijter, R.V. Orru, Chem. Soc. Rev. 41, 3969 (2012)
J.E. Biggs- Houck, A. Younai, J.T. Shaw, Curr. Opin. Chem. Biol. 14, 371 (2010)
K.S. Pandit, R.V. Kupwade, P.V. Chavan, U.V. Desai, P.P. Wadgaonkar, K.M. Kodam, ACS Sustain. Chem. Eng. 4, 3450 (2016)
K.S. Pandit, P.V. Chavan, U.V. Desai, M.A. Kulkarni, P.P. Wadgaonkar, New J. Chem. 39, 4452 (2015)
M.A. Kulkarni, V.R. Pandurangi, U.V. Desai, P.P. Wadgaonkar, C. R. Chim. 15(9), 745 (2012)
A.M. Kulkarni, K.S. Pandit, P.V. Chavan, U.V. Desai, P.P. Wadgaonkar, RSC Adv. 5, 70586 (2014)
K.S. Pandit, P.V. Chavan, U.V. Desai, M.A. Kulkarni, P.P. Wadgaonkar, New J. Chem. 39, 4452 (2015)
S.D. Mitragotri, D.M. Pore, U.V. Desai, P.P. Wadgaonkar, Catal. Commun. 9, 1822 (2008)
D.M. Pore, U.V. Desai, T.S. Thopate, P.P. Wadgaonkar, Aust. J. Chem. 60, 435 (2007)
J.F.M. da Silva, S.J. Garden, A.C. Pinto, J. Braz. Chem. Soc. 12, 273 (2001)
A.E. Medvedev, A. Clow, M. Sandler, V. Glover, Biochem. Pharmacol. 52, 385 (1996)
M. Yamazaki, E. Okuyama, Tetrahedron Lett. 22, 135 (1981)
A. Dandia, R. Singh, S. Khauria, C. Merienne, G. Morgant, A. Loupy, Bioorg. Med. Chem. 14, 2409 (2006)
J. Skommer, D. Wlodkowic, M. Matto, M. Eray, J. Pelkonen, Leuk. Res. 30, 322 (2006)
N. Yu, J.M. Aramini, M.W. Germann, Z. Huang, Tetrahedron Lett. 41, 6993 (2000)
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Eur. J. Med. Chem. 28, 517 (1993)
V.V. Rostovtsev, L.G. Green, V.V. Fokin, K.B. Sharpless, Angew. Chem. 114, 2708 (2002)
V.V. Rostovtsev, L.G. Green, V.V. Fokin, K.B. Sharpless, Angew. Chem. Int. Ed. 41, 2596 (2002)
C.W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 67, 3057 (2002)
N. Boechat, V.F. Ferreira, S.B. Ferreira, M.D.L.G. Ferreira, FdC da Silva, M.M. Bastos, M.D.S. Costa, M.C.S. Lourenço, A.C. Pinto, A.U. Krettli, A.C. Aguiar, B.M. Teixeira, N.V. da Silva, P.R.C. Martins, F.A.F.M. Bezerra, A.L.S. Camilo, G.P. da Silva, C.C.P. Costa, J. Med. Chem. 54, 5988 (2011)
S.R. Patpi, L. Pulipati, P. Yogeeswari, D. Sriram, N. Jain, B. Sridhar, R. Murthy, T. Anjana Devi, S.V. Kalivendi, S. Kantevari, J. Med. Chem. 55, 3911 (2012)
R.J. Naik, M.V. Kulkarni, K.S.R. Pai, P.G. Nayak, Chem. Biol. Drug Des. 80, 516–523 (2012)
F. Mir, S. Shaf, M.S. Zaman, N.P. Kalia, V.S. Rajput, C. Mulakayal, N. Mulakayala, I.A. Khan, M.S. Alam, Eur. J. Med. Chem. 76, 274 (2014)
D. Kumar, G. Beena, S. Khare, A.K. Kidwai, R. Tyagi, D.S.Rawat Singh, Eur. J. Med. Chem. 81, 301 (2014)
D. Addla, A. Jallapally, D. Gurram, P. Yogeeswari, D. Sriram, S. Kantevari, Bioorg. Med. Chem. Lett. 24, 1974 (2014)
M.H. Shaikh, D.D. Subhedar, L. Nawale, D. Sarkar, F.A. Kalam Khan, J.N. Sangshetti, B.B. Shingate, Med. Chem. Commun. 6, 1104 (2015)
J.M. Altimari, S.C. Hockey, H.I. Boshoff, A. Sajid, L.C. Henderso, ChemMedChem 10, 787 (2015)
T. Lee, M. Cho, S.Y. Ko, H.J. Youn, D.J. Baek, W.J. Cho, C.Y. Kang, S. Kim, J. Med. Chem. 50, 585 (2007)
Y. Xia, Z. Fan, J. Yao, Q. Liao, W. Li, F. Qu, L. Peng, Bioorg. Med. Chem. Lett. 16, 2693 (2006)
Y.-C. Duan, Y.-C. Ma, E. Zhang, X.-J. Shi, M.-M. Wang, X.-W. Ye, H.-M. Liu, Eur. J. Med. Chem. 62, 11 (2013)
Y. Zhang, Z. Lv, H.A. Zhong, D. Geng, M. Zhang, T. Zhang, Y. Li, K. Li, Eur. J. Med. Chem. 53, 356 (2012)
G.I. Wei, W. Luan, S. Wang, S. Cui, F. Li, Y. Liu, Y. Liu, M. Cheng, Org. Biomol. Chem. 13, 1507 (2015)
K. Rad-Moghadam, L. Youseftabar-Miri, Tetrahedron 67, 5693 (2011)
Z. Jamshidi, A.A. Esmaeili, J.T. Mague, J. Chem. Res. 40, 471 (2016)
S.A. Bakunov, S.M. Bakunova, T. Wenzler, M. Ghebru, K.A. Werbovetz, R. Brun, R.R. Tidwell, J. Med. Chem. 53, 254 (2010)
H. Singh, J. Sindhu, J.M. Khurana, C. Sharma, K.R. Aneja, Eur. J. Med. Chem. 77, 145 (2014)
P. Thirumurugan, D. Matosiuk, K. Jozwiak, Chem. Rev. 113, 4905 (2013)
S.G. Agalave, S.R. Maujan, V.S. Pore, Chem. Asian J. 6, 269 (2011)
R. Berg, B.F. Straub, Beilstein J. Org. Chem. 9, 2715 (2013)
P.V. Chavan, K.S. Pandit, U.V. Desai, M.A. Kulkarni, P.P. Wadgaonkar, RSC Adv. 4, 42137 (2014)
T. Mosmann, J. Immunol. Methods 65(1–2), 55 (1983)
G. Ciapetti, E. Cenni, L. Pratelli, A. Pizzoferrato, Biomaterials 14, 359 (1993)
D. Sreekanth, A. Syed, S. Sarkar, D. Sarkar, B. Santhakumari, A. Ahmad, I. Khan, J. Microbiol. Biotechnol. 19, 1342 (2009)
R. Singh, L.U. Nawale, M. Arkile, U.U. Shedbalkar, S.A. Wadhwani, D. Sarkar, B.A. Chopade, Int. J. Antimicrob. Agents 46(2), 183 (2015)
U. Singh, S. Akhtar, A. Mishra, D. Sarkar, J. Microbiol. Methods 84(2), 202 (2011)
A. Khan, D. Sarkar, J. Microbiol. Methods 73, 62 (2008)
S. Sarkar, D. Sarkar, J. Biomol. Screen. 17(7), 966 (2012)
M. Protopopova, C. Hanrahan, B. Nikonenko, R. Samala, P. Chen, J. Gearhart, L. Einck, C.A. Nacy, J. Antimicrob. Chemother. 56, 968 (2005)
M. Poggi, R. Barroso, A.J. Costa-Filho, H.B. de Barros, F. Pavan, C.Q. Leite, D. Gambino, M.H. Torre, J. Mex. Chem. Soc. 57, 198 (2013)
L.L. Gundersen, J. Nissen-Meyer, B. Spilsberg, J. Med. Chem. 45, 1383 (2002)
Acknowledgements
U. V. D. and P. V. C. thank the University Grants Commission (UGC), New Delhi, India for financial assistance [F. 43—221/2014 (SR)] and for the award of a teacher fellowship, respectively. We gratefully acknowledge Dr. K. G. Kanade, Principal, Yashavantrao Chavan Institute of Science, Satara, for encouragement.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Experimental
General
Isatins (Aldrich), benzyl halides (Aldrich), sodium azide (S. D. Fine-Chem. Limited, Mumbai), dimedone, malononitrile, copper(I) iodide (Spectrochem, Mumbai), and microcrystalline cellulose (SRL, Mumbai) were used as received. Cell-CuI NPs as catalyst were prepared according to the procedure developed and reported by us earlier [56]. All melting points were recorded using Kumar melting point apparatus. Infrared (IR) spectra were recorded neat using a Thermo Scientific Nicolet iS10 FT-IR spectrometer. 1H nuclear magnetic resonance (NMR) (300 MHz) and 13C NMR (75.4 MHz) spectra were recorded using Bruker Avance II spectrometer. High-resolution mass spectra (HRMS) were recorded using Thermo Scientific Q-Exactive, Accela 1250 pump, instrument.
Representative procedure for one-pot synthesis of 1,2,3-triazolylspirochromenes
N-Propargyl isatin (1 mmol), malononitrile (1 mmol), dimedone (1 mmol), benzyl bromide (1 mmol), and sodium azide (1.1 mmol) were placed in a round-bottomed flask. DMF–water (1:2, 6 mL, v/v) and cellulose-CuI NPs as catalyst (0.2 g) were added, and the reaction mixture was heated at 70 °C. After reaction completion (TLC), the reaction mixture was filtered and the filter was washed with ethyl acetate (4 × 10 mL). The organic extract was washed with water and brine and dried over Na2SO4. Removal of solvent under vacuum furnished corresponding 1,4-disubstituted 1,2,3-triazolylspirochromene as solid product. The resultant solid was successively washed using a mixture of hexane–chloroform (80: 20 v/v), and dried. None of the resultant products required any further purification. The recovered catalyst was washed with acetone, dried in air, and reused for five consecutive runs.
Biological methods
Antimycobacterial activity
All synthesized compounds were screened for their in vitro activity against M. tuberculosis H37Ra (MTB) (ATCC 25177) and M. bovis BCG (ATCC 35743) using twofold dilution technique to determine the minimum inhibitory concentration (MIC). Screening against M. tuberculosis H37Ra was carried out by XTT reduction menadione assay (XRMA) by reading the absorbance at 470 nm, while screening of M. bovis BCG was carried out by nitrate reductase (NR) assay [61,62,63]. The optical density was read on a microplate reader using a 470-nm filter for XTT and a 540-nm filter for NR against blank prepared from cell-free wells. Absorbance given by cells treated with vehicle alone was taken as 100% cell growth. Initially, primary screening was done at 30, 10, and 3 µg/ml. Compounds showing 90% inhibition of bacilli at or lower than 30 µg/ml were selected for further dose–response curve analysis. All experiments were performed in triplicate, with values expressed as average ± standard deviation. MIC and IC50 values of selected compounds were calculated from dose–response curves using Origin 6 software. Percentage inhibition was calculated using the formula: % inhibition = [(control − CMP)/(control − blank)] × 100, where “control” is the activity of mycobacteria without compounds, “CMP” is the activity of mycobacteria in presence of compound, and “blank” is the activity of culture medium without mycobacteria.
Cytotoxicity and selectivity index
To check the selectivity, all compounds were assayed for their cytotoxic effects against three different cell lines (MCF-7, A549, and HCT116) by MTT assay (Table 4) [64,65,66]. The cell lines were maintained at 37 °C under 5% CO2/95% air humidified environment. The concentration range for each compound was selected as 30, 10, and 3 µg/mL. Each concentration was tested in duplicate in a single experiment. GI50 and MIC values were calculated using OriginPro software. Viability and growth in presence of test material were calculated using the following formula: % cytotoxicity = [(average absorbance of control − absorbance of compound)/(absorbance of control − absorbance of blank)] × 100, where control is the culture medium with cells and dimethyl sulfoxide (DMSO), and blank is the culture medium without cells. The selectivity index (SI) was calculated by dividing the 50% growth inhibition concentration (GI50) value for each cell line (MCF-7, A549, and HCT116) by the MIC for in vitro activity against active/dormant MTB and BCG [60].
Antibacterial activity
All bacterial cultures were first grown in Luria-Bertani (LB) medium at 37 °C at 180 RPM. Once the culture reached 1 OD, it was used for antibacterial assay. Bacterial strains E. coli (NCIM 2688), P. aeruginosa (NCIM 2036) as Gram-negative and B. subtilis (NCIM 2079), S. aureus (NCIM 2010) as Gram-positive strains were obtained from NCIM (NCL, Pune) and grown in Luria-Bertani medium. Screening was carried out by adding 0.1% 1-OD inoculated culture to each well of a 96-well plate containing the compounds to be tested and measuring the optical density at 620 nm after 8 h for Gram-negative or 12 h for Gram-positive bacteria.
Rights and permissions
About this article
Cite this article
Chavan, P.V., Pandit, K.S., Desai, U.V. et al. Click-chemistry-based multicomponent condensation approach for design and synthesis of spirochromene-tethered 1,2,3-triazoles as potential antitubercular agents. Res Chem Intermed 43, 5675–5690 (2017). https://doi.org/10.1007/s11164-017-2955-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2955-y