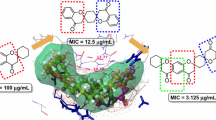
Abstract
A simple and effective three-component one-pot green methodology was employed for the synthesis of a new thiazolidine-2,4-dione based bisspirooxindolo-pyrrolidine derivatives using [Bmim]BF4 ionic liquid via [3 + 2] cycloaddition reaction. It is an environmentally benign, column chromatography-free, shorter reaction time, good yield and easy product isolation method. The synthesized compounds 10a–x, were thoroughly characterized by using various spectroscopic methods like FT-IR, 1H NMR, 13C NMR, Mass spectrometry and finally by single crystal X-ray diffraction method. In vitro anti-tubercular (anti-TB) activity studies were carried out on these synthesized compounds, and they showed good to moderate anti-TB activity against Mycobacterium tuberculosis H37Rv strain. The compound 10a exhibited good anti-TB activity, with an MIC (Minimum Inhibitory Concentration) value of 12.5 µg/mL, and the compounds 10m, 10o and 10r showed moderate activity with an MIC value of 25.0 µg/mL. Remaining compounds exhibited poor activity against Mycobacterium tuberculosis. Ethambutol, rifampicin and isoniazid were used as standard drugs. Furthermore, in silico molecular docking experiments on the TB protein (PDB ID: 1DF7) were carried out to understand the binding interactions, and they showed least binding energy values ranging from −8.9 to −7.2 kcal/mol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) is an infectious disease caused by the aerobic bacterium Mycobacterium tuberculosis. It ranks as the 13th leading cause of death globally and the second most dangerous infectious disease after COVID-19. A report from the World Health Organization (WHO) in 2023 revealed that, globally, TB incidence continued to rise with 10.6 million new cases reported in 2022, an increase from 10.3 million in 2021 and 10.0 million in 2020. TB has more impact than HIV with 1.13 million deaths in HIV-negative individuals in 2022, almost double the number of deaths from HIV/AIDS, which continued to decline to 0.63 million in 2022. Additionally, there were 0.17 million TB-related deaths in people with HIV [1, 2]. The current treatment for TB is known as directly observed treatment short course (DOTS), which involves a combination of first-line anti-TB drugs such as isoniazid, rifampicin, pyrazinamide and ethambutol. This treatment regimen lasts for a minimum of 6 months [3, 4]. However, extended treatment can lead to negative consequences, including complications from the drug treatment plan, lack of compliance and hepatotoxicity [5]. Moreover, the emergence of drug-resistant TB strains, including multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB, presents additional challenges for effectively treating the disease [6, 7]. Hence, it is imperative to develop novel anti-TB drugs that possess high effectiveness, low toxicity levels, and the capacity to enhance the current treatment approach. These agents should also feature innovative mechanisms of action and prove effective against MDR and XDR TB strains [8,9,10].
The biological activity and physical characteristics of natural and synthesized compounds having core structure of oxindole or spirooxindole pose an interesting synthetic challenge. For the past few decades, there has been a steady growth in the number of papers pertaining to the synthesis of spirocyclic oxindoles [11, 12]. Bisspirooxindoles become popular synthetic targets because of their diverse pharmacological properties [13, 14]. There are many bioactive natural compounds that include spirooxindoles such as coerulescine, horsfiline [15], spirotryprostatin A, welwitindolinone A, elacomine and alstonisine [16, 17]. It has also been discovered that the synthetic spirooxindoles exhibit a variety of pharmacological features, including progesterone receptor modulators, anti-HIV, anti-cancer, anti-TB, anti-malarial, and mouse double minute 2 (MDM2) inhibitors Fig. 1 [18,19,20].
Thiazolidine-2,4-diones, including rosiglitazone, pioglitazone, and ciglitazone, belong to the class of insulin-sensitizing drugs. Apart from their widely recognized anti-diabetic properties, various studies, both in vitro and in vivo, have revealed their potential antibacterial, anti-cancer and antifungal activities [21,22,23,24,25,26,27,28,29,30,31]. Heterocyclic compounds are particularly valuable in medicinal chemistry due to their established efficacy. Some examples of effective anti-diabetic agents used in type 2 diabetes treatment include pyrazole, 1,3,4-oxadiazoles, 1,2,3-triazole [32], furan [33], thiazolidine-2,4-dione [34], thiazole, benzothiazole [35], pyrrole, indole [36], benzoxazolone, and oxazolone [37]. Thiazolidine-2,4-diones (TZD’s) are oral anti-diabetic drugs that help to enhance insulin sensitivity, contributing to the management of type-2 diabetes [38].
Multi-component reactions (MCR’s) are widely used in chemical and pharmaceutical combinatorial chemistry [39]. They combine multiple reactions in a single step to create organic compounds, eliminating the need for isolating intermediates and reducing waste, labor, time and cost [40]. MCR’s are eco-friendly and well-suited for constructing complex molecules from easily available starting materials. They are highly effective in generating compound libraries for screening, known for their high productivity, simple procedures, atom economy and ease of execution. By forming multiple covalent bonds in one-pot transformations, MCR’s enable the creation of diverse and complex molecules, approaching the concept of an ideal synthesis. MCR with green chemistry condition has emerged as an essential tool in synthetic chemistry, achieved through the use of eco-friendly solvents, reusable catalyst and non-toxic substances [41]. Ionic liquids have garnered significant attention in green synthesis due to their numerous advantages. These green solvents possess catalytic properties, are easily recyclable, and exhibit chemical and thermal stability, allowing for controlled reactions with shorter reaction time and high yields. Consequently, they became a highly desirable option for the development of green synthetic methods [42]. Therefore, in this paper, we report a three-component, one-pot, environmentally benign synthesis of thiazolidine-2,4-dione-based bisspirooxindolo-pyrrolidine derivatives (Fig. 2). This innovative approach involves the use of the ionic liquid [Bmim]BF4 in [3 + 2] cycloaddition reaction, leading to the formation of these unique compounds. While there were no literature reports on the anti-TB activity of thiazolidine-2,4-dione-based bisspirooxindolo-pyrrolidines, additionally, we investigated their in vitro and in silico anti-TB activity.
Results and discussion
The title compounds were synthesized using thiazolidine-2,4-dione based phenyl benzamide substituted chalcones (dipolarophile) 7a–m, isatin 8a–c and sarcosine 9. The starting material thiazolidine-2,4-dione 1, was synthesized using a previously reported method [50]. The Knoevenagel condensation products 3a–k and N-substituted thiazolidine-2,4-dione chalcones 5a–k were prepared in accordance with the literature [51]. Previous X-ray diffraction studies were demonstrated that the (Z)-5-benzylidenethiazolidine-2,4-dione moiety in 3a–k exclusively provided ‘Z’ geometry shown in Fig. 3 [52, 53].
The intermediate 7a–m were synthesized through an amide coupling reaction between thiazolidine-2,4-dione chalcones 5a–k and various substituted anilines 6a–c. The amide coupling reaction was conducted at room temperature for 6–8 h using HBTU, DIPEA and DMF as the solvent. This synthetic procedure resulted in the formation of intermediate 7a–m with good yields (85–90%) in Fig. 3.
In order to synthesize the target compounds 10a–x, we initially examined the [3 + 2] cyclization reaction with intermediate 7e, and an azomethine ylide was generated in situ by the reaction of isatin 8a and sarcosine 9, to give target compound 10e. Table 1 display the results of the optimization process for the compound 10e. Initially, the reaction was carried out in ethanol solvent at room temperature, but even after 12 h, the reaction was not completed. Subsequently, the reaction was conducted in ethanol under reflux condition for 6–8 h. As a result, compound 10e was successfully formed with a significant yield of 80%. Furthermore, we explored the use of various solvents to enhance the yield and investigate the influence of the solvent on reaction time. The experimental process involved conducting the reactions in different solvents, such as EtOH, MeOH, CH3CN, DMF, and THF (Table 1, entry 1–10). We performed the reactions in all of these solvents at room temperature as well as under reflux condition, but no improvement in the yield was observed and time of the reaction was also not satisfactory.
However, as part of our effort towards adopting green methodologies, we attempted to utilize an ionic liquid [Bmim]BF4 as a catalyst and ethanol as the solvent. The reaction was conducted at both room temperature as well as reflux to optimize the reaction conditions. Notably, we were able to obtain the compound 10e with a remarkable yield 96% at reflux condition in just 30 min (Table 1, Entry 12). This condition was considered as the most optimized for the synthesis of bisspirooxindolo-pyrrolidines 10a–x, compared to the other conditions.
The results obtained from our study suggest that [Bmim]BF4 could be an effective and suitable medium for promoting reactions. The reaction conditions were tested for their substitution tolerance, and it was found that the substituted isatins 8a–c and various substituted dipolarophiles 7a–m reacted efficiently, resulting in the synthesis of new thiazolidine-2,4-dione based bisspirooxindolo-pyrrolidine derivatives 10a–x. The title compounds were synthesized with impressive yield of 87–96% within a reaction time of just 30–60 min (Table 2, Entry 1–24). Our results demonstrate the clear advantages of this green protocol, achieving increased productivity within shorter reaction time and facilitating straightforward separation processes. These findings highlight the potential of ionic liquids as promising solvents for sustainable and eco-friendly chemical processes, which can revolutionize the way chemical reactions are carried out and promote a greener future for the chemical industry.
The reaction mechanism involved in the synthesis of target compounds 10a–x in the presence of [Bmim]BF4 is depicted in Scheme 1. The ionic liquid serves as a catalyst, promoting the polarization of the π-bond in the carbonyl group of molecules through the interaction with its electron-deficient hydrogen atom [54,55,56,57]. This polarization facilitates the reaction with isatin 8a and sarcosine 9, leading to the elimination of H2O and the formation of intermediate 11. Furthermore, intermediate 11 undergoes CO2 elimination to form the highly reactive azomethine ylide 12. The [Bmim]BF4 molecules also establish hydrogen bonds with the carbonyl group of the dipolarophiles. Due to this hydrogen bonding, [Bmim]BF4 promotes the activation of the adjacent double bond, resulting in cyclization with the azomethine ylide and the production of the target compounds 10a–x.
A series of compounds 10a–x were synthesized and characterized thoroughly using various analytical techniques; FT-IR, 1H NMR, 13C NMR, Mass spectrometry and single-crystal X-ray diffraction. For instance, we observed characteristic bands in the IR spectrum of the compound 10e, at 3408 and 3353 cm−1 for the N–H stretching frequencies of oxindole and phenyl benzamide moieties, respectively. The carbonyl stretching frequencies at 1754, 1696, 1678 and 1656 cm−1 belonging to thiazolidine-2,4-dione, isatin moiety and phenyl benzamide respectively. In the 1H NMR spectrum of compound 10e, we detected peaks at δ 4.68–4.55 (m, 2H) for the –CH2 protons attached to the thiazolidine-2,4-dione ring, δ 4.48–4.44 (m, 1H) for the –CH proton in the pyrrolidine ring, δ 3.92 (t, J = 9.6 Hz, 1H), and δ 3.48 (t, J = 8.4 Hz, 1H) for the two diastereotopic protons (–CH2) in the pyrrolidine ring. In the 13C NMR spectrum, we identified peaks at δ 177.08 ppm, δ 175.31 ppm, δ 169.69 ppm, and δ 165.62 ppm for the carbonyl carbon of oxindole, thiazolidine-2,4-dione and phenylbenzamide moieties respectively. The peaks at δ 79.49 ppm and δ 73.37 ppm corresponded to the two spiro carbons of oxindole and thiazolidine-2,4-dione moieties. We confirmed the presence of spiro carbons by their absence in the DEPT-135 NMR spectrum, while these characteristic peaks appeared with negative signs in the 13C-APT (Attached Proton Test) NMR experiment for compound 10e. The molecular ion peak at m/z 603.2064 [M + H]+ in the mass spectrum verified the molecular weight of compound 10e. Further, 13C NMR of the fluoro compound 10k shows coupling constant (13C−19F) at various chemical shift values are: at δ 175.84 (1JCF = 241 Hz), δ 117.93 (2JCF = 24 Hz), δ 114.86 (2JCF = 25 Hz), δ 111.83 (3JCF = 8 Hz) and δ 124.82 (4JCF = 4 Hz). Moreover, SCXRD data of the compound 10u (CCDC: 2,330,413) authenticates the structure and regiochemistry of the synthesized compounds (Fig. 4).
Anti-tubercular activity of bisspirooxindolo-pyrrolidines 10a–x.
In this study, the desired compounds 10a–x were tested in vitro anti-TB screening against M. tuberculosis H37Rv strain (ATCC27294) using the microplate alamar blue assay (MABA) method [58]. The aim was to determine the minimum inhibitory concentration (MIC) values of the compounds 10a–x and compare them with the MIC values of standard drugs ethambutol, rifampicin and isoniazid. The results were summarized in Table 3, which provides the MIC values for each compound and the reference drugs. The MABA method is a reliable and widely used approach for evaluating the anti-TB activity of compounds, allowing for efficient screening and comparison of their effectiveness against the target pathogen. When compared to first-line anti-TB drugs, compounds 10a–x demonstrated good to moderate activity against M. tuberculosis with MIC values ranging from 12.5 to > 25 µg/mL. Notably, the compound 10a exhibited good activity with an MIC value of 12.5 µg/mL, when compare to the standard drugs such as isoniazid, rifampicin and ethambutol. On the other hand, compounds 10m, 10o, and 10r displayed moderate activity with an MIC value of 25 µg/mL. However, the remaining compounds showed poor activity against M. tuberculosis. These results highlight the effectiveness of the compounds 10a, 10m, 10o and 10r in inhibiting the growth of the TB bacteria.
Structure activity relationship (SAR) studies
Structure–activity relationship (SAR) studies suggest that the anti-TB activity of the title compounds was influenced by electron-donating or electron-accepting capabilities of phenyl ring substituents, as well as structural changes. A simple phenyl ring presence demonstrates good anti-TB activity, while substitutions such as methyl (−Me), chloro (−Cl), and bromo (−Br) within the phenyl ring result in milder anti-TB activity compared to other substitutes. However, compounds with substitutions on the isatin ring exhibit poorer activity when compared to unsubstituted isatin. These findings underscore the significance of substituent groups on the phenyl ring as donors and acceptors, as well as the impact of structural alterations on the anti-TB activity of the compounds.
Molecular docking
To investigate the binding locations and interactions of the target compounds 10a–x with M. tuberculosis dihydrofolate reductase (PDB ID: 1DF7), computational molecular docking studies were conducted using the AutoDock Tools software version 1.5.6 [59]. The in silico investigations revealed favourable binding energies (lowest binding energies) of the active molecules with the desired protein, with values ranging from −8.9 to −7.2 kcal/mol. Among them, compound 10j exhibited the lowest binding energy of −8.9 kcal/mol by interacting with the three amino acid residues ARG32 (2.93 Å), GLN28 (2.77 Å) and LYS53 (1.87 Å) of the protein 1DF7 through hydrogen bonding with carbonyl oxygen atoms of thiazolidine-2,4-dione, benzamide and oxindole moieties respectively.
Similarly, compound 10u displayed a least binding energy of −8.6 kcal/mol by interacting with the amino acid residues GLY137 (2.32 Å) and TYR156 (2.13 Å) through hydrogen bonding with the carbonyl oxygen atoms benzamide and thiazolidine-2,4-dione moieties respectively. Table 4 shows the complete hydrogen bonding patterns of the title compounds, reference drugs and standard M.tb.DHFR inhibitors with protein 1DF7, while ligand interactions of the compounds 10j and 10u were depicted in Figs. 5 and 6 respectively. Further, the docking studies of the synthesized compounds were compared with the standard drugs ethambutol, rifampicin and isoniazid. When compared to these standard drugs, most of the synthesized compounds show least binding energies. Notably, the binding energies and hydrogen bonding interactions observed in the docking study aligned well with the results obtained from the in vitro anti-TB investigations.
In addition, we have also compared the docking studies of the synthesized compounds with standard M.tb.DHFR inhibitors such as methotrexate and trimethoprim. Methotrexate exhibited the lowest binding energy of −9.1 kcal/mol and formed seven hydrogen bonds with amino acid residues ASP27 (2.48 Å), GLY18 (2.18 Å), GLY96 (2.96 Å), ILE5 (2.49 Å), ILE94 (2.49 Å) and THR46 (1.92, 2.77 Å). Trimethoprim, on the other hand, demonstrated a binding energy of −6.9 kcal/mol and formed two hydrogen bonds with amino acid residues ASP27 (2.15 Å) and SER49 (2.23 Å) (Table 4, entry 28 and 29). These results indicate that the target compounds show least binding energies and they are comparable with the M.tb.DHFR inhibitors.
ADME prediction
The ADME (absorption, distribution, metabolism, excretion) provides an easy path to know and identify molecules of drugs of the required therapeutic dose maintaining a high safety profile. In addition to it, the risk of drug failure in the final stages of clinical trials can be reduced by in silico prediction of pharmacokinetic parameters [60]. Table 5. represents the ADME prediction results of synthesized compounds.
Lipophilicity was examined by LogP which was actually the estimation of octanol/water partition coefficient. Predicted lipophilicity values were ranging from 1.96 to 4.45 which revealed good lipophilicity of the compounds. The expected aqueous solubility (LogS) values for synthesized compounds varies from −5.70 to −8.05, indicating moderate solubility in aqueous media due to presence of lipophilic groups. Apart from LogP and LogS values, the TPSA (topological polar surface area) values indicate compounds having moderate bioavailability. The result from LogPapp (apparent permeability co-efficient) suggests all the compounds have moderate Caco–2 permeability ranging from −0.205 × 10–6 to 0.903 × 10–6. Good high human intestinal absorption (HIA: 88.756—100%), and remarkably high values of the blood–brain partition coefficient (logBB) indicate favourable pharmacological activity of the compounds. All the above results suggest acceptable pharmacokinetic parameters and give us key to lead molecules for development of potential drugs.
Conclusion
We have designed and synthesized a new series of bisspirooxindolo-pyrrolidines from thiazolidine-2,4-dione based chalcones and isatin within a single framework. A green methodology was employed by using [Bmim]BF4 ionic liquid for the synthesis of bisspirooxindolo-pyrrolidine derivatives 10a–x, under ethanol reflux via [3 + 2] cycloaddition reaction. This synthetic process has several advantages, including environmental friendly process, column chromatography free, high yield in a short reaction time and easy product isolation. The synthesized compounds were well characterized and determined by FT-IR, 1H-NMR, 13C-NMR, Mass spectrometry, DEPT-135, APT and SCXRD method. Further the compounds were investigated for their in vitro and in silico anti-TB activity. The compound 10a showed good anti-TB activity 12.5 μg/mL against TB bacteria and the compounds 10n, 10p and 10s showed moderate activity 25.0 μg/mL with respect to standard drugs like ethambutol, rifampicin and isoniazid. The results of the in silico docking studies of the target compounds were comparable with the standard drugs and M.tb.DHFR inhibitors. Based on the in vitro, in silico and ADME predictions, the synthesized compounds 10a–x were suggested to act as promising pharmacophores for future generation of anti-TB agents.
Typical procedure for the synthesis of (Z)-4-((5-benzylidene-2,4-dioxothiazolidin-3-yl)methyl)-N-phenylbenzamide 7a–m [61, 62].
A mixture of (Z)-4-((5-benzylidene-2,4-dioxothiazolidin-3-yl)methyl)benzoic acid 5a–k (1.0 mmol), DIPEA (1.5 mmol), and HBTU (1.0 mmol) in DMF was stirred at room temperature for 20 min. Subsequently, aniline 6a–c (1.2 mmol) was added to the reaction mixture, which was stirred at room temperature until the reaction was complete (typically 6–8 h), as confirmed by TLC analysis. After the reaction was completed, cold water was added, resulting in the formation of a precipitate. This precipitate was filtered, followed by recrystallization in methanol to obtain pure compounds for direct use in the next step.
Typical procedure for the synthesis of title compounds bisspirooxindolo-pyrrolidines 10a–x.
A mixture of chalcones 7a–m (1.0 mmol), isatin 8a–c (1.2 mmol), sarcosine 9 (1.2 mmol) and [Bmim]BF4 (50 mol%) was vigorously stirred in ethanol under reflux condition for 30 min. The progress of the reaction was continuously monitored using TLC. Once the reaction reached completion, the reaction mixture was allowed to cool to room temperature and cold water was added. The resulting precipitate was then filtered and subsequently recrystallized in methanol to obtain pure compounds 10a–x.
References
World Health Organization (2023) Global tuberculosis report 2023. World Health Organization, Geneva (https://www.who.int/publications/i/item/9789240083851)
Komakech K, Nakiyingi L, Fred A, Achan B, Joloba M, Kirenga BJ, Ssengooba W (2024) Effect of mixed mycobacterium tuberculosis infection on rapid molecular diagnostics among patients starting MDR-TB treatment in uganda. BMC Infect Dis 24:1–8. https://doi.org/10.1186/s12879-023-08968-5
Bhowruth V, Dover LG, Besra GS (2007) 4 tuberculosis chemotherapy: recent developments and future perspectives. Prog Med Chem 45:169–203. https://doi.org/10.1016/S0079-6468(06)45504-1
Preez ID, Loots DT (2018) Novel insights into the pharmacometabonomics of first-line tuberculosis drugs relating to metabolism, mechanism of action and drug-resistance. Drug Metab Rev 50:466–481. https://doi.org/10.1080/03602532.2018.1559184
Molla Y, Wubetu M, Dessie B (2021) Anti-tuberculosis drug induced hepatotoxicity and associated factors among tuberculosis patients at selected hospitals, ethiopia. Hepatic Med Evid Res 13:1–8. https://doi.org/10.2147/hmer.s290542
Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, Soolingen DV, Jensen P, Bayona J (2010) Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 375:1830–1843. https://doi.org/10.1016/S0140-6736(10)60410-2
Muthukrishnan L (2021) Multidrug resistant tuberculosis – diagnostic challenges and it’s conquering by nanotechnology approach – an overview. Chem Biol Interact 337:109397. https://doi.org/10.1016/j.cbi.2021.109397
Allaka BS, Basavoju S, Madhu Rekha E, Sriram D, Rama Krishna G (2022) Design and synthesis of novel quinazolinyl-bisspirooxindoles as potent anti-tubercular agents: an ultrasound-promoted methodology. Mol Divers. https://doi.org/10.1007/s11030-022-10500-x
Borah P, Deb PK, Venugopala KN, Al-Shar’i NA, Singh V, Deka S, Srivastava A, Tiwari V (2020) Tuberculosis: an update on pathophysiology, molecular mechanisms of drug resistance, newer anti-TB drugs, treatment regimens and host- directed therapies. Curr Top Med Chem 21:547–570. https://doi.org/10.2174/1568026621999201211200447
Tetali SR, Kunapaeddi E, Mailavaram RP, Singh V, Borah P, Deb PK, Venugopala KN, Hourani W, Tekade RK (2020) Current advances in the clinical development of anti-tubercular agents. Tuberculosis 125:101989. https://doi.org/10.1016/j.tube.2020.101989
Ball-Jones NR, Badillo JJ, Franz AK (2012) Strategies for the enantioselective synthesis of spirooxindoles. Org Biomol Chem 10:5165–5181. https://doi.org/10.1039/c2ob25184a
Dai W, Jiang XL, Wu Q, Shi F, Tu SJ (2015) Diastereo and enantioselective construction of 3,3-pyrrolidinyldispirooxindole framework via catalytic asymmetric 1,3-dipolar cycloadditions. J Org Chem 80:5737–5744. https://doi.org/10.1021/acs.joc.5b00708
Baddepuri S, Allaka BS, Gamidi RK, Faizan M, Pawar R, Basavoju S (2023) An ultrasound assisted green protocol for the synthesis of quinoxaline based bisspirooxindoles: crystal structure analysis, enone umpolung, DFT calculations, anti-cancer activity, and molecular docking studies. Synth Commun 53:835–854. https://doi.org/10.1080/00397911.2023.2199360
Kulkarni MG, Dhondge AP, Chavhan SW, Borhade AS, Shaikh YB, Birhade DR, Desai MP, Dhatrak NR (2010) Total synthesis of (±)-coerulescine and (±)-horsfiline. Beilstein J Org Chem 6:876–879. https://doi.org/10.3762/bjoc.6.103
Hilton ST, Jones K (2007) The tandem radical route to indole alkaloids: an unusual rearrangement reaction. ARKIVOC 2007:120–128. https://doi.org/10.3998/ark.5550190.0008.b11
Hu Y, Zou Y, Wu H, Shi D (2012) A facile and efficient ultrasound-assisted synthesis of novel dispiroheterocycles through 1,3-dipolar cycloaddition reactions. Ultrason Sonochem 19:264–269. https://doi.org/10.1016/j.ultsonch.2011.07.006
Lotfy G, El Ashry ESH, Said MM, El Tamany ES, Abdel Aziz YM, Al-Dhfyan A, Al-Majid AM, Barakat A (2018) Regio and stereoselective synthesis of new spirooxindoles via 1,3-dipolar cycloaddition reaction: anticancer and molecular docking studies. J Photochem Photobiol B, Biol 180:98–108. https://doi.org/10.1016/j.jphotobiol.2018.01.026
Tiwari S, Pathak P, Sagar R (2016) Efficient synthesis of new 2,3-dihydrooxazole-spirooxindoles hybrids as antimicrobial agents. Bioorganic Med Chem Lett 26:2513–2516. https://doi.org/10.1016/j.bmcl.2016.03.093
Yu B, Yu DQ, Liu HM (2015) Spirooxindoles: promising scaffolds for anticancer agents. Eur J Med Chem 97:673–698. https://doi.org/10.1016/j.ejmech.2014.06.056
Takashima T, Fujiwara Y, Higuchi K, Arakawa T, Yano Y, Hasuma T, Otani S (2001) PPAR-gamma ligands inhibit growth of human esophageal adenocarcinoma cells through induction of apoptosis, cell cycle arrest and reduction of ornithine decarboxylase activity. Int J Oncol 19:465–471. https://doi.org/10.3892/ijo.19.3.465
Galli A, Ceni E, Crabb DW, Mello T, Salzano R, Grappone C, Milani S, Surrenti E, Surrenti C, Casini A (2004) Antidiabetic thiazolidinediones inhibit invasiveness of pancreatic cancer cells via PPARγ independent mechanisms. Gut 53:1688–1697. https://doi.org/10.1136/gut.2003.031997
Yoshizumi T, Ohta T, Ninomiya I, Terada I, Fushida S, Fujimura T, Nishimura GI, Shimizu K, Yi S, Miwa K (2004) Thiazolidinedione, a peroxisome proliferator-activated receptor-gamma ligand, inhibits growth and metastasis of HT-29 human colon cancer cells through differentiation-promoting effects. Int J Oncol 25:631–639. https://doi.org/10.3892/ijo.25.3.631
Betz MJ, Shapiro I, Fassnacht M, Hahner S, Reincke M, Beuschlein F (2005) Peroxisome proliferator-activated receptor-γ agonists suppress adrenocortical tumor cell proliferation and induce differentiation. J Clin Endocrinol Metab 90:3886–3896. https://doi.org/10.1210/jc.2004-1267
Turturro F, Friday E, Fowler R, Surie D, Welbourne T (2004) Troglitazone acts on cellular PH and DNA synthesis through a peroxisome proliferator-activated receptor γ-independent mechanism in breast cancer-derived cell lines. Clin Cancer Res 10:7022–7030. https://doi.org/10.1158/1078-0432.CCR-04-0879
Shiau CW, Yang CC, Kulp SK, Chen KF, Chen CS, Huang JW, Chen CS (2005) Thiazolidenediones mediate apoptosis in prostate cancer cells in part through inhibition of Bcl-XL/Bcl-2 functions independently of PPARγ. Cancer Res 65:1561–1569. https://doi.org/10.1158/0008-5472.CAN-04-1677
Han SW, Roman J (2006) Rosiglitazone suppresses human lung carcinoma cell growth through PPARγ-dependent and PPARγ-independent signal pathways. Mol Cancer Ther 5:430–437. https://doi.org/10.1158/1535-7163.MCT-05-0347
Kaminskyy D, Zimenkovsky B, Lesyk R (2009) Synthesis and in vitro anticancer activity of 2,4-azolidinedione-acetic acids derivatives. Eur J Med Chem 44:3627–3636. https://doi.org/10.1016/j.ejmech.2009.02.023
Li Q, Wu J, Zheng H, Liu K, Guo TL, Liu Y, Eblen ST, Grant S, Zhang S (2010) Discovery of 3-(2-aminoethyl)-5-(3-phenyl-propylidene)-thiazolidine-2,4-dione as a dual inhibitor of the Raf/MEK/ERK and the PI3K/Akt signaling pathways. Bioorganic Med Chem Lett 20:4526–4530. https://doi.org/10.1016/j.bmcl.2010.06.030
Kilcigil GA, Altanlar N (2000) Synthesis of 3-substituted phenacyl-5- [2-phenyl-4H-4-oxo-1-benzopyran-6- yl)methylenyl]-thiazolidine-2,4-diones and evaluation of their antimicrobial activity. Arzneimittelforschung 50:154–157. https://doi.org/10.1055/s-0031-1300181
Ponnuchamy S, Sumesh RV, Ranjith Kumar R (2015) Regioselective synthesis of novel dispiro oxindole-pyrrolizine-thiazolidine-2,4-dione hybrids. Tetrahedron Lett 56:4374–4376. https://doi.org/10.1016/j.tetlet.2015.05.090
Datar PA, Jadhav SR (2015) Design and synthesis of pyrazole-3-one derivatives as hypoglycaemic agents. Int J Med Chem 2015:1–10. https://doi.org/10.1155/2015/670181
Mariappan G, Saha BP, Datta S, Kumar D, Haldar PK (2011) Design, synthesis and antidiabetic evaluation of oxazolone derivatives. J Chem Sci 123:335–341. https://doi.org/10.1007/s12039-011-0079-2
Naim MJ, Alam O, Alam MJ, Shaquiquzzaman M, Alam MM, Naidu VGM (2018) Synthesis, docking, in vitro and in vivo antidiabetic activity of pyrazole-based 2,4-thiazolidinedione derivatives as PPAR-γ modulators. Arch Pharm (Weinheim) 351:3–4. https://doi.org/10.1002/ardp.201700223
Meltzer-Mats E, Babai-Shani G, Pasternak L, Uritsky N, Getter T, Viskind O, Eckel J, Cerasi E, Senderowitz H, Sasson S, Gruzman A (2013) Synthesis and mechanism of hypoglycemic activity of benzothiazole derivatives. J Med Chem 56:5335–5350. https://doi.org/10.1021/jm4001488
Xu Q, Huang L, Liu J, Ma L, Chen T, Chen J, Peng F, Cao D, Yang Z, Qiu N, Qiu J, Wang G, Liang X, Peng A, Xiang M, Wei Y, Chen L (2012) Design, synthesis and biological evaluation of thiazole and indole-based derivatives for the treatment of type II diabetes. Eur J Med Chem 52:70–81. https://doi.org/10.1016/j.ejmech.2012.03.006
Singha T, Singh J, Naskar A, Ghosh T, Mondal A, Kundu M, Harwansh RK, Maity TK (2012) A series of potential bioactive compounds, 4-amino-5-mercapto-3-(4-chlorophenyl. Indian J Pharm Educ Res 46:346–351
Altaff SKM, Raja Rajeswari T, Subramanyam C (2020) Synthesis, α-amylase inhibitory activity evaluation and in silico molecular docking study of some new phosphoramidates containing heterocyclic ring. Phosphorus Sulfur Silicon Relat Elem 196:389–397. https://doi.org/10.1080/10426507.2020.1845679
(a) Dömling A, Ugi I (2000) Multicomponent reactions with isocyanides. Angew Chemie-Int Ed 39:3168–3210. https://doi.org/10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u (b) Dömling A (2006) Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 106:17–89. https://doi.org/10.1021/cr0505728
Zhu J, Bienaymé H (2005) Multicomponent Reactions. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. https://doi.org/10.1002/3527605118
Esmaeili AA, Amini-Ghalandarabad S, Mesbah F, Tasmimi M, Izadyar M, Fakhari AR, Salimi AR (2015) Efficient synthesis of novel spiro[indole-3,6′-pyrano[2,3-d][1,3]thiazolo[3,2-a]pyrimidine derivatives through an organobase-catalyzed, three-component reaction. Tetrahedron 71:2458–2462. https://doi.org/10.1016/j.tet.2015.01.055
Sagar Vijay KP, Suresh L, Vinodkumar T, Reddy BM, Chandramouli GVP (2016) Zirconium doped ceria nanoparticles: an efficient and reusable catalyst for a green multicomponent synthesis of novel phenyldiazenyl-chromene derivatives using aqueous medium. ACS Sustain Chem Eng 4:2376–2386. https://doi.org/10.1021/acssuschemeng.6b00056
(a) Duchet L, Legeay JC, Carrié D, Paquin L, Vanden Eynde JJ, Bazureau JP (2010) Synthesis of 3,5-disubstituted 1,2,4-oxadiazoles using ionic liquid-phase organic synthesis (IoLiPOS) methodology. Tetrahedron 66:986–994. https://doi.org/10.1016/j.tet.2009.11.079 (b) Martins M A P, Frizzo C P, Moreira D N, Zanatta N, Bonacorso H G (2008) Ionic liquids in heterocyclic synthesis. Chem Rev 108:2015–2050. https://doi.org/10.1021/cr078399y (c) Pogaku V, Krishna V S, Sriram D, Rangan K, Basavoju S (2019) Ultrasonication-ionic liquid synergy for the synthesis of new potent anti-tuberculosis 1,2,4-triazol-1-yl-pyrazole based spirooxindolopyrrolizidines. Bioorganic Med Chem Lett 29:1682–1687. https://doi.org/10.1016/j.bmcl.2019.04.026
Shaikh FM, Patel NB, Rajani D (2013) Synthesis of new thiazolidine-2,4-dione derivatives and their antimicrobial and anti-tubercular activity. Indian J Res Pharm Biotechnol 1:496–503. https://doi.org/10.1016/j.bioorg.2020.103676
Ponnuchamy S, Kanchithalaivan S, Ranjith Kumar R, Ashraf Ali M, Soo Choon T (2014) Antimycobacterial evaluation of novel hybrid arylidene thiazolidine-2,4-diones. Bioorganic Med Chem Lett 24:1089–1093. https://doi.org/10.1016/j.bmcl.2014.01.007
Trotsko N, Golus J, Kazimierczak P, Paneth A, Przekora A, Ginalska G, Wujec M (2020) Design, synthesis and antimycobacterial activity of thiazolidine-2,4-dione-based thiosemicarbazone derivatives. Bioorg Chem 97:103676. https://doi.org/10.1016/j.bioorg.2020.103676
Mhiri C, Boudriga S, Askri M, Knorr M, Sriram D, Yogeeswari P, Nana F, Golz C, Strohmann C (2015) Design of novel dispirooxindolopyrrolidine and dispirooxindolopyrrolothiazole derivatives as potential antitubercular agents. Bioorganic Med Chem Lett 25:4308–4313. https://doi.org/10.1016/j.bmcl.2015.07.069
Arumugam N, Almansour AI, Kumar RS, Siva KV, Sriram D, Dege N (2021) Stereoselective synthesis and discovery of novel spirooxindolopyrrolidine engrafted indandione heterocyclic hybrids as antimycobacterial agents. Bioorg Chem 110:5–11. https://doi.org/10.1016/j.bioorg.2021.104798
Araujo DM, Maste MM, Alegaon S, Saxena A (2018) Synthesis, antitubercular evaluation and docking studies of novel benzimidazole analogues. IJPSR 9:3696–3704. https://doi.org/10.13040/IJPSR.0975-8232.9(9).3696-04
Subhedar DD, Shaikh MH, Nawale L, Yeware A, Sarkar D, Shingate BB (2016) [Et3NH][HSO4] catalyzed efficient synthesis of 5-arylidene-rhodanine conjugates and their antitubercular activity. Res Chem Intermed 42:6607–6626. https://doi.org/10.1007/s11164-016-2484-0
Kumar H, Deep A, Marwaha RK (2020) Design, synthesis, in silico studies and biological evaluation of 5-((E)-4-((E)-(substituted aryl/alkyl)methyl)benzylidene)thiazolidine-2,4-dione derivatives. BMC Chem 14:1–15. https://doi.org/10.1186/s13065-020-00678-2
Alegaon SG, Alagawadi KR (2012) New thiazolidine-2,4-diones as antimicrobial and cytotoxic agent. Med Chem Res 21:3214–3223. https://doi.org/10.1007/S00044-011-9876-X
Bruno G, Costantino L, Curinga C, Maccari R, Monforte F, Nicolò F, Ottanà R, Vigorita MG (2002) Synthesis and aldose reductase inhibitory activity of 5-arylidene-2,4-thiazolidinediones. Bioorganic Med Chem 10:1077–1084. https://doi.org/10.1016/S0968-0896(01)00366-2
Ottanà R, MacCari R, Barreca ML, Bruno G, Rotondo A, Rossi A, Chiricosta G, Di Paola R, Sautebin L, Cuzzocrea S, Vigorita MG (2005) 5-arylidene-2-imino-4-thiazolidinones: design and synthesis of novel anti-inflammatory agents. Bioorganic Med Chem 13:4243–4252. https://doi.org/10.1016/j.bmc.2005.04.058
Ghorbani M, Noura S, Oftadeh M, Zolfigol MA, Soleimani MH (2015) Preparation of neutral ionic liquid [2-eim] OAc with dual catalytic-solvent system roles for the synthesis of 2-amino-3-cyano-7-hydroxy-4-(aryl)-4H-chromene derivatives. J Mol Liq 212:291–300. https://doi.org/10.1016/j.molliq.2015.09.024
Venkatesan K, Pujari SS, Lahoti RJ, Srinivasan KV (2008) An efficient synthesis of 1,8-dioxo-octahydro-xanthene derivatives promoted by a room temperature ionic liquid at ambient conditions under ultrasound irradiation. Ultrason Sonochem 15:548–553. https://doi.org/10.1016/j.ultsonch.2007.06.001
Zare L, Mahmoodi NO, Yahyazadeh A, Nikpassand M (2012) Ultrasound-promoted regio and chemoselective synthesis of pyridazinones and phthalazinones catalyzed by ionic liquid [Bmim]Br/AlCl3. Ultrason Sonochem 19:740–744. https://doi.org/10.1016/j.ultsonch.2011.11.008
Kia Y, Osman H, Kumar RS, Basiri A, Murugaiyah V (2014) Ionic liquid mediated synthesis of mono and bis-spirooxindole-hexahydropyrrolidines as cholinesterase inhibitors and their molecular docking studies. Bioorganic Med Chem 22:1318–1328. https://doi.org/10.1016/j.bmc.2014.01.002
Krishna VS, Zheng S, Rekha EM, Guddat LW, Sriram D (2019) Discovery and evaluation of novel mycobacterium tuberculosis ketol-acid reductoisomerase inhibitors as therapeutic drug leads. J Comput Aided Mol Des 33:357–366. https://doi.org/10.1007/S10822-019-00184-1/FIGURES/10
Trott O, Olson AJ (2009) AutoDock vina: improving the speed and accuracy of docking with a new scoring function, efcient optimization, and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:1–13. https://doi.org/10.1038/srep42717
Barasa L, Yoganathan S (2018) An efficient one-pot conversion of carboxylic acids into benzimidazoles via an HBTU-promoted methodology. RSC Adv 8:35824–35830. https://doi.org/10.1039/C8RA07773H
Vrettos EI, Sayyad N, Mavrogiannaki EM, Stylos E, Kostagianni AD, Papas S, Mavromoustakos T, Theodorou V, Tzakos AG (2017) Unveiling and tackling guanidinium peptide coupling reagent side reactions towards the development of peptide-drug conjugates. RSC Adv 7:50519–50526. https://doi.org/10.1039/c7ra06655d
Acknowledgements
The authors R. N. V. and S. B thank the Director of NIT Warangal for providing the facilities. R. N. V. expresses gratitude to Council of Scientific and Industrial Research (CSIR) and the Ministry of Education (MoE) of India for the fellowship. S. B. thank the CSIR-EMR(II) [02(0300)/17] for funding.
Author information
Authors and Affiliations
Contributions
Rukya Naik V-Design and synthesized the compounds, G. Rama Krishna-SXRD data collection and solved the structure, Jyothi Kumari- Anti-TB activity, Dharmarajan Sriram-Anti-TB analysis, Srinivas Basavoju- Corresponding author
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rukyanaik, V., Gamidi, R.K., Kumari, J. et al. A Green one-pot three component synthesis of thiazolidine-2,4-dione based bisspirooxindolo-pyrrolidines with [Bmim]BF4: their in vitro and in silico anti-TB studies. Mol Divers (2024). https://doi.org/10.1007/s11030-024-10853-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-024-10853-5