Abstract
An eco-friendly approach for the synthesis of new thiazoloquinolines, thiazolopyridines, and thiazolonaphthyridines scaffolds has been achieved using α-enolicdithioesters, cysteamine, arylglyoxal monohydrate, and cyclic 1,3-diketones (dimedone, 4-hydroxycoumarin, and 4-hydroxy-1-methyl-2(1H)-quinolone) under thermal solvent-free conditions. The highlight of this protocol is the generation of two heterocyclic rings, using a domino, one-pot, four-component reaction. In producing heterocyclic rings first, a thiazole ring generated from the reaction of α-enolicdithioesters and cysteamine is achieved. Second heterocyclic ring formed by the condensation of conjugated thiazole with the Knoevenagel product achieved from arylglyoxal monohydrate and 1,3-diketones followed by an N-cyclization. Catalyst- and solvent-free, short reaction times, high yields and a simple work-up make it an attractive protocol for the preparation of diversified thiazoloquinoline, thiazolopyridine, and thiazolonaphthyridine derivatives.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The organic synthesis field has achieved many inventive technical developments in designing environmental protocols that reduce toxic reagents [1]. Increasing detrimental effects of chemicals on the environment has made more researchers devote significant efforts to protect it. The ideal synthesis in organic chemistry should be a composition of environmentally acceptable, atom efficient, safe, one-step, simple reactions. Green chemical syntheses include designing processes that can facilitate operations of products and work-up, avoiding the employment of catalysts and/or solvents or using green catalysts and/or solvents with the goal of preserving the environment [2, 3]. In the last decade, catalyst-free and solvent-free reactions were developed eschewing the use of catalyst and hazardous solvents in organic reactions. By synthesis protocols improvement, the use of hazardous solvents is prevented and these milieus are better to be removed or replaced by green solvents [4]. In other words, catalyst- and solvent-free reactions significantly simplify the procedures by reducing waste and its impact on the environment [5].
Through multi-component reactions (MCRs), diverse complex molecules are generated in one-pot reaction from simple substances, without isolation and purification of intermediates [6]. MCRs technology has largely impacted drug synthesis processes and is endorsed by academia and industry [7]. Besides, MCRs are an important source of molecular diversity consuming less time and reducing costs [8].
Quinolone derivatives have drawn researchers’ attention, because these structures are found in natural alkaloids and are precursors of some compounds with pharmacological features [9, 10]. Furthermore, quinolones are strong antagonists of many receptors, and when they fuse to sulfur-containing heterocycles can exhibit cytostatic activity versus a wide domain of malignant cell lines [11]. In the literature, these heterocyclic compounds are introduced as strong antibacterial [12] and mycobacterial [10] agents such as 5H-thiazolo[3,2-a]quinoline-4-carboxylic acid derivatives (Fig. 1a), benzothiazolo[3,2-a]quinolones (Fig. 1b), and 2-arylthiazolo[4,5-c]quinoline-5H-ones (Fig. 1c). Pyridine frameworks are also important structures that have various applications in many fields. Pyridine-attached thiazoles exhibit antimicrobial [13] and antifungal activities [14]. Besides, thiazolo[3,2-a]pyridines, have shown many significant bioactivities [15] such as 6-substituted-5-aryl-7-imino-3-phenyl-7H-thiazolo[3,2-a]pyridine-8-carbonitriles (Fig. 1d), and 5-amino-2-(1,3-diphenylpyrazol-4-yl)methylidene-3-oxo-6,8-dicyano-2,3,7-trihydro-7-aryl-thiazolo[3,2-a]pyridines (Fig. 1e). Another important class of heterocycles is the naphthyridines which can have biological and pharmacological activities [16]. Its derivatives have antibacterial activities and they also are employed as diverse kinds of inhibitors [17] such as 8-alkyl(or aryl)thiazolo[4,5-b] [1, 6] naphthyridin-2(3H)-ones (Fig. 1f). Literature procedures, which have been used for preparation of the thiazoloquinolines, thiazolopyridines, and thiazolonaphthyridines, suffer from multi-linear steps, harsh conditions, long reaction times, low yields, and expensive reagents [10, 12, 13, 16, 18,19,20,21,22]. We attempted to explore an efficient procedure that resolves these problems to synthesize compounds of the same scope. Among various methods of synthesizing these compounds, the multi-component reaction is the best to be used in our study. Instead of synthesizing by means of multi-steps, we used a one-pot, multi-component reaction in which instead of using different benzaldehyde derivatives, we applied more active derivatives of arylglyoxals. Thus, without any catalyst or additives, it succeeded in preparing new derivatives from the family of these compounds for the first time with short reaction times and good efficiency under very simple green conditions.
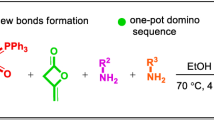
Herein, we represent a facile green synthesis of novel series of thiazoloquinoline, thiazolopyridine, and thiazolonaphthyridine under solvent-free conditions. In this work, we described a one-pot four-component reaction involving α-enolicdithioesters, cysteamine, arylglyoxal monohydrate and cyclic 1,3-diketones (dimedone, 4-hydroxycoumarin, and 4-hydroxy-1-methyl-2(1H)-quinolone) as reactants for synthesis of thiazoloquinoline, thiazolopyridine, and thiazolonaphthyridine derivatives (Scheme 1). The merit of this approach includes simplicity, short reaction time, easy work-up, catalyst-free, solvent-free, one-step process.
Results and discussion
First, we initiated the synthesis of α-enolicdithioester (1) with the reaction of aromatic methylketones (10) and trithiocarbonate (11) (Table 1) [23, 24]. The α-enolicdithioester (1) is an ideal starting material that can bear different functional groups, such as the aromatic and heteroaromatic rings for synthesis of diverse products.
In continuation of our studies on MCRs reactions [25], we started the synthesis of diverse thiazoloquinoline derivatives as novel products, with the domino one-pot four-component reaction of the α-enolicdithioesters (1) (1 mmol) and cysteamine (2) (1 mmol) that followed by addition of arylglyoxal monohydrate (3) (1 mmol) and cyclic 1,3-diketones (4, 6, 8) (1 mmol) under solvent-free conditions at 90 °C (Scheme 1, Tables 2, 3, 4). The corresponding thiazoloquinolines (5, Table 2), thiazolopyridines (7, Table 3), and thiazolonaphthyridines (9, Table 4) were produced within 20–70 min in good-to-excellent yields. The desired products were collected and purified by crystallization with H2O–EtOH.
In exploring electronic effects of arylglyoxal monohydrate, we employed compounds with electron-donating and electron-withdrawing groups on the phenyl ring. The electron-poor arylglyoxal monohydrate gave higher yields than electron-rich compounds, because the arylglyoxal monohydrate plays an electrophilic role in the reaction. It must be taken into consideration that electron-withdrawing groups, increase electrophilic properties and electron-donating groups decrease its activity.
The proposed mechanism for generation of thiazoloquinoline, thiazolopyridine, and thiazolonaphthyridine derivatives is represented in Scheme 2. According to literature [21, 25] and our procedure, first thiazole ring (I) formed through the reaction of α-enolicdithioester and cysteamine. Second, the conjugated thiazole (I) formed in previous step, attacked to Knoevenagel product (II) formed from arylglyoxal monohydrate and 1,3-diketone (dimedone, 4-hydroxycoumarin, and 4-hydroxy-1-methyl-2(1H)-quinolone) and produced intermediate (III), which is in balance with intermediate (IV). Finally, intramolecular N-cyclization of intermediate (IV), which followed by dehydration, the desired thiazoloquinoline, thiazolopyridine, and thiazolonaphthyridine derivatives were formed.
Conclusions
In summary, we represented a facile approach for synthesis of novel thiazoloquinolines, thiazolopyridines, and thiazolonaphthyridines via one-pot, four-component, and domino reaction between α-enolicdithioesters, cysteamine, arylglyoxal monohydrate and cyclic 1,3-diketones (dimedone, 4-hydroxycoumarin, and 4-hydroxy-1-methyl-2(1H)-quinolone) under solvent-free conditions at 90 °C. This approach integrated the advantages such as broad functional scope, cost-effectiveness, short reaction times, easy work-up and purification, and high yields. This straightforward approach provided diversified novel thiazoloquinoline, thiazolopyridine, and thiazolonaphthyridine scaffolds, which can be applied as precursors of medicinal compounds. In our opinion, this is an important protocol based on economical and environmental points of view, which complete avoiding catalysts and solvents during reactions. We also avoided column purifications and products were purified by crystallizations with H2O–EtOH. That is another great advantage of applying this protocol.
Experimental
General methods and materials
The commercially available reagents were purchased from Merck and Sigma-Aldrich companies without further purification. The α-enolicdithioesters were prepared by the reported method [23, 24]. The NMR spectra were recorded on a Bruker Avance 300 MHz instrument in CDCl3. Infrared (IR) spectra were provided with a JASCO FT-IR460 Plus spectrophotometer. Mass spectra were recorded applying an Agilent technologies 5973 network mass selective detector (MSD) operating at an ionization potential of 70 eV. Melting points were determined in open capillaries using a BUCHI510 melting point apparatus. Thin layer chromatography (TLC) was performed on silica-gel Poly Gram SIL G/UV 254 plates.
General procedure for the synthesis of thiazoloquinolines analogues (5)
A round-bottom flask was charged with the appropriate α-enolicdithioesters (1 mmol), cysteamine (1 mmol) and they were stirred at 90 °C over a pre-heated oil bath under solvent-free conditions. When conjugated thiazole was formed (monitored by TLC), arylglyoxal monohydrate (1 mmol) and dimedone (1 mmol) were added to reaction mixture and stirring continued for an appropriate period of time. The completion of reaction was monitored by TLC. Then, the yellow solid product was solved in ethanol and quenched with distilled water. The obtained crude was dried and crystallized with H2O–EtOH to produce the pure product.
4-Benzoyl-5-(hydroxyl(phenyl)methylene)-8,8-dimethyl-1,2,5,7,8,9-hexahydro-6H-thiazolo[3,2-a]quinolin-6-one (5{1, 1})
Yellow solid; m.p. 200–202 °C (yield = 75%). 1H NMR (300 MHz, CDCl3): δ 7.36(d, 2H, J = 7.5 Hz), 7.24–7.05(m, 9H), 3.97(t, 2H, J = 14.7 Hz), 3.40 (t, 2H, J = 14.4 Hz), 1.95–1.64 (m, 4H), 0.72 (S, 3H), 0.59 (S, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 197.17, 191.48, 171.23, 141.98, 139.53, 132.65, 131.13, 130.80, 128.73, 128.55, 128.36, 128.06, 116.96, 113.78, 111.01, 50.60, 48.23, 41.85, 34.82, 31.21, 30.07, 29.70, 26.69 ppm; MS (EI, 70 eV) m/z (%) = 443 (M+, 67), 384 (17), 338 (53), 105 (100); FT-IR (KBr, cm−1): 3433, 2924, 1598, 1480, 1366, 1155, 700.
4-Benzoyl-5-(hydroxyl(4-methoxyphenyl)methylene)-8,8-dimethyl-1,2,5,7,8,9-hexahydro-6H-thiazolo[3,2-a]quinolin-6-one (5{1, 2})
Yellow solid; m.p. 188–190 °C (yield = 73%). 1H NMR (300 MHz, CDCl3): δ 7.36 (d, 2H, J = 7.2 Hz), 7.26–7.12 (m, 3H), 6.97 (d, 2H, J = 8.4 Hz), 6.63 (d, 2H, J = 8.7 Hz), 6.59(br, 1H), 3.94 (t, 2H, J = 14.7 Hz), 3.56 (s, 3H), 3.39 (t, 2H, J = 14.7 Hz), 1.90–1.70 (m, 4H), 0.731 (s, 3H), 0.607(s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 197.22, 191.42, 171.09, 159.54, 141.56, 139.59, 131.09, 130.07, 128.36, 128.05, 123.15, 116.85, 114.00, 113.23, 111.10, 55.28, 50.64, 48.10, 41.82, 34.80, 31.23, 30.03, 29.70, 26.79 ppm; MS (EI, 70 eV) m/z (%) = 473 (M+, 84), 368 (46), 105 (100), 77 (50); FT-IR (KBr, cm−1): 3435, 2957, 1605, 1466, 1367, 1248, 1031, 840, 743.
4-(Furan-2-carbonyl)-5-(hydroxyl(phenyl)methylene)-8,8-dimethyl-1,2,5,7,8,9-hexahydro-6H-thiazolo[3,2-a]quinolin-6-one (5{5, 1})
Yellow solid; m.p. 184–186 °C (yield = 86%). 1H NMR (300 MHz, CDCl3): δ 7.53 (s, 1H), 7.44–7.19 (m, 7H), 6.46 (d, 1H, J = 1.8 Hz), 4.32–4.04 (m, 2H), 3.57(t, 2H, J = 14.1 Hz), 2.46–2.01 (m, 4H), 0.98 (s, 3H), 0.80(s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 197.22, 177.37, 172.04, 152.15, 146.04, 139.26, 133.14, 130.97, 128.87, 128.51, 128.23, 118.57, 114.27, 112.19, 111.29, 50.82, 48.22, 42.42, 35.02, 31.28, 29.81, 29.70, 27.07 ppm; MS (EI, 70 eV) m/z (%) = 433 (M+, 100), 374 (42), 338 (50), 95 (100), 55 (12); FT-IR (KBr, cm−1): 3197, 2926, 1735, 1600, 1368, 1011, 866, 767, 752, 701.
4-(Furan-2-carbonyl)-5-(hydroxyl(4-methoxyphenyl)methylene)-8,8-dimethyl-1,2,5,7,8,9-hexahydro-6H-thiazolo[3,2-a]quinolin-6-one (5{5, 2})
Yellow solid; m.p. 202–204 °C (yield = 84%). 1H NMR (300 MHz, CDCl3): δ 7.62 (br, 1H), 7.29–7.23(m, 4H), 6.88 (dd, 2H, J = 6 Hz), 6.55 (m, 1H), 4.21(q, 2H, J = 15 Hz), 3.83 (s, 3H), 3.66 (t, 2H, J = 12 Hz), 2.50–2.07 (m, 4H), 1.08 (s, 3H), 0.92 (S,3H) ppm; 13C NMR (75 MHz, CDCl3): δ 197.36, 177.33, 159.49, 152.15, 145.99, 138.89, 132.93, 130.18, 123.33, 118.48, 113.95, 113.74, 112.16, 111.35, 55.29, 50.83, 48.09, 42.40, 35.00, 31.31, 29.77, 27.17 ppm; MS (EI, 70 eV) m/z (%) = 463 (M+, 100), 404 (31), 368 (40), 135 (25), 95 (56); FT-IR (KBr, cm−1): 3436, 2923, 1613, 1409, 1367, 1249, 1084, 469.
5-(Hydroxyl(phenyl)methylene)-8,8-dimethyl-4-(thiophene-2-carbonyl)-1,2,5,7,8,9-hexahydro-6H-thiazolo[3,2-a]quinolin-6-one (5{6, 1})
Yellow solid; m.p. 174–176 °C (yield = 83%). 1H NMR (300 MHz, CDCl3): δ 7.53 (d, 2H, J = 13.2 Hz), 7.39 (d, 1H, J = 4.8 Hz), 7.06-7.00 (m, 5H), 6.88 (s, 1H), 4.05–3.89 (m, 2H), 3.39 (t, 2H, J = 14.4 Hz), 2.23–1.93 (m, 4H), 0.77 (s, 3H), 0.62 (s,3H) ppm; 13C NMR (75 MHz, CDCl3): δ 204.65, 197.04, 183.27, 172.71, 143.46, 139.17, 133.64, 133.24, 131.08, 128.86, 128.51, 127.51, 117.73, 114.17, 111.26, 50.77, 48.28, 42.62, 35.10, 31.16, 29.86, 29.70, 26.98 ppm; MS (EI, 70 eV) m/z (%) = 449 (M+, 90), 390 (33), 338 (77), 111 (100); FT-IR (KBr, cm−1): 3420,2958,2923,2619, 1736, 1592, 1475, 1369, 845, 707.
5-(Hydroxyl(4-methoxyphenyl)methylene)-8,8-dimethyl-4-(thiophene-2-carbonyl)-1,2,5,7,8,9-hexahydro-6H-thiazolo[3,2-a]quinolin-6-one (5{6, 2})
Yellow solid; m.p. 142–144 °C (yield = 95%). 1H NMR (300 MHz, DMSO): δ 7.27–7.18 (m, 2H), 6.91–6.86 (m, 1H), 6.71–6.66 (m, 2H), 3.82 (q, 2H, J = 13.2 Hz), 3.428 (s, 3H), 3.31–3.25 (m, 2H), 2.03–1.79 (m, 4H), 0.64 (s, 3H), 0.53 (S, 3H) ppm; 13C NMR (75 MHz, DMSO): δ 206.87, 197.50, 182.56, 171.86, 159.12, 143.99, 139.06, 132.25, 131.74, 130.02, 127.03, 123.73, 117.13, 113.69, 110.65, 55.16, 50.73, 48.03, 42.70, 34.75, 31.29, 30.86, 29.61, 27.12 ppm; MS (EI, 70 eV) m/z (%) = 479 (M+, 94), 420 (20), 368 (64), 111 (100); FT-IR (KBr, cm−1): 3438, 2927, 1612, 1466, 1248, 1029, 837.
4-(4-Bromobenzoyl)-5-(hydroxyl(phenyl)methylene)-8,8-dimethyl-1,2,5,7,8,9-hexahydro-6H-thiazolo[3,2-a]quinolin-6-one (5{2, 1})
Yellow solid; m.p. 182–184 °C (yield = 85%). 1H NMR (300 MHz, CDCl3): δ 7.55 (d, 2H, J = 8.1 Hz), 7.48 (d, 2H, J = 9 Hz), 7.37–7.29 (m, 6H), 4.23 (t, 2H, J = 7.5 Hz), 3.69 (t, 2H, J = 7.2 Hz), 2.15–1.95 (m, 4H), 1.0 (s, 3H), 0.86 (S, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 197.21, 190.17, 171.20, 142.17, 138.34, 132.71, 131.25, 130.59, 129.91, 128.69,128.61, 128.38, 116.65, 113.72, 110.84, 50.59, 48.25, 41.78, 34.84, 31.20, 30.01, 29.70 ppm; MS (EI, 70 eV) m/z (%) = 523 (M+, 48), 338 (72), 183 (90), 105 (59), 43 (100); FT-IR (KBr, cm−1): 3435, 2925, 1689, 1066.
4-(4-Chlorobenzoyl)-5-(hydroxyl(phenyl)methylene)-8,8-dimethyl-1,2,5,7,8,9-hexahydro-6H-thiazolo[3,2-a]quinolin-6-one (5{3, 1})
Yellow solid; m.p. 166–168 °C (yield = 82%). 1H NMR (300 MHz, CDCl3): δ 7.55 (d, 2H, J = 8.1 Hz), 7.55 (d, 2H, J = 8.1 Hz), 7.38–7.27 (m, 8H), 4.22 (t, 2H, J = 9 Hz), 3.67 (t, 2H, J = 9 Hz), 2.08–1.06 (m, 4H), 0.99 (s, 3H), 0.85 (S, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 197.15, 190.11, 171.12, 142.09, 137.89, 137.31, 132.66, 130.65, 129.81, 128.69,128.59, 128.27, 116.67, 113.86, 110.83, 50.58, 48.25, 41.78, 34.85, 31.22, 29.99, 29.68 ppm; MS (EI, 70 eV) m/z (%) = 477 (M+, 0.3), 139 (8), 43 (100); FT-IR (KBr, cm−1): 3435, 2925, 1723, 1625,1097.
5-(Hydroxyl(phenyl)methylene)-4-(4-methoxybenzoyl)-8,8-dimethyl-1,2,5,7,8,9-hexahydro-6H-thiazolo[3,2-a]quinolin-6-one (5{4, 1)
Yellow solid; m.p. 166–168 °C (yield = 92%). 1H NMR (300 MHz, CDCl3): δ 7.65 (d, 2H, J = 9 Hz), 7.29–7.19 (m, 6H), 6.83 (d, 2H, J = 8.7 Hz), 4.17–4.06 (m, 2H),3.79 (s, 3H), 3.55 (t, 2H, J = 9 Hz), 2.30–1.98 (m, 4H), 1.01 (s, 3H), 0.78 (S, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 196.99, 189.96, 170.92, 140.23, 138.23, 133.0, 132.92, 131.42, 131.21, 128.84,128.51, 128.14, 117.54, 113.45, 111.44, 55.45, 50.75, 48.24, 42.50, 34.99, 31.10, 30.0, 29.70 ppm; MS (EI, 70 eV) m/z (%) = 473 (M+, 25), 135 (72), 71 (44), 43 (100); FT-IR (KBr, cm−1): 3430, 2926, 1744, 1601, 1170.
General procedure for the synthesis of thiazolopyridines analogues (7)
A round-bottom flask was charged with the appropriate α-enolicdithioesters (1 mmol), cysteamine (1 mmol) and they were stirred at 90 °C over a pre-heated oil bath under solvent-free conditions. When conjugated thiazole was formed (monitored by TLC), arylglyoxal monohydrate (1 mmol) and 4-hydroxycoumarin (1 mmol) were added to reaction mixture and stirring continued for an appropriate period of time. The completion of reaction was monitored by TLC. Then, the yellow to orange solid product was solved in ethanol and quenched with distilled water. The obtained crude was dried and crystallized with H2O–EtOH to produce the pure product.
4-Benzoyl-5-(hydroxy(4-nitrophenyl)methylene)-1,2-dihydro-5H,6H-chromeno[3,4-e]thiazolo[3,2-a]pyridin-6-one (7{1, 3})
Orange solid; m.p. 118–120 °C (yield = 89%). 1H NMR (300 MHz, CDCl3): δ 10.70 (br, 1H), 8.18 (d, 2H, J = 8.4 Hz), 8.03 (d, 2H, J = 8.4 Hz), 7.83 (d, 2H, J = 8.4 Hz), 7.67–7.41 (m, 3H), 7.33–7.20 (m, 4H), 4.50 (q, 1H, J = 18.6 Hz), 4.20–4.06 (m, 1H), 3.66 (t, 2H, J = 15 Hz) ppm; 13C NMR (75 MHz, CDCl3): δ 193.55, 165.07, 162.39, 152.97, 147.19, 143.36, 137.52, 137.37, 133.12, 132.50, 131.98, 129.63, 128.88, 128.60, 124.37, 124.18, 124.03, 118.34, 116.63, 116.33, 101.70, 48.77, 35.30, 29,70 ppm; MS (EI, 70 eV) m/z (%) = 510 (M+, 0.04), 327 (17), 267 (62), 134 (69), 98 (100), 57 (81); FT-IR (KBr, cm−1): 3430, 2923, 2862, 1676, 1609, 1516, 1342, 1104, 757.
4-(Furan-2-carbonyl)-5-(hydroxy(phenyl)methylene)-1,2-dihydro-5H,6H-chromeno[3,4-e]thiazolo[3,2-a]pyridin-6-one (7{5, 1})
Yellow solid; m.p. 134–136 °C (yield = 83%). 1H NMR (300 MHz, CDCl3): δ 10.10 (br, 1H), 7.57 (d, 1H, J = 0.6 Hz), 7.44–7.39 (m, 2H), 7.33 (d, 2H, J = 3.3 Hz), 7.22 (br, 3H), 7.19 (br, 3H), 4.37–4.28 (m, 1H), 4.13–4.03 (m, 1H), 3.68–3.54 (m, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ 178.56, 164.09, 162.24, 153.02, 151.53, 147.23, 139.60, 134.27, 131.90, 130.86, 128.76, 128.46, 124.30, 123.65, 120.65, 116.85, 116.19, 113.72, 112.64, 102.62, 48.41, 35.29, 29.70 ppm; MS (EI, 70 eV) m/z (%) = 455 (M+, 24),121 (82), 95 (100), 77 (64); FT-IR (KBr, cm−1): 3413, 2926, 1687, 1620, 1465, 760.
4-(Furan-2-carbonyl)-5-(hydroxy(4-methoxyphenyl)methylene)-1,2-dihydro-5H,6H-chromeno[3,4-e]thiazolo[3,2-a]pyridin-6-one (7{5, 2})
Yellow solid; m.p. 148–150 °C (yield = 77%). 1H NMR (300 MHz, CDCl3): δ 7.91 (d, 1H, J = 9 Hz), 7.53 (s, 1H), 7.44–7.39 (m, 1H), 7.29 (d, 1H, J = 3 Hz), 7.21–7.12 (m, 4H), 6.75 (d, 2H, J = 6 Hz), 6.47 (br, 1H), 4.34–4.21 (m, 1H), 4.08–4.01 (m, 1H), 3.68 (s, 3H), 3.61–3.50 (m, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ 178.39, 164.07, 162.72, 159.58, 152.94, 151.54, 147.10, 139.62, 134.14, 131.91, 129.84, 124.27, 123.73, 123.10, 120.47, 116.79, 116.20, 114.33, 114.25, 112.62, 102.52, 55.21, 48.27, 35.22, 29.71 ppm; MS (EI, 70 eV) m/z (%) = 485 (M+, 100), 135 (28), 121 (38), 95 (48), 43 (39); FT-IR (KBr, cm−1): 3430, 2921, 1698, 1610, 1466, 1249, 1176, 1028, 761.
5-(Hydroxy(phenyl)methylene)-4-(thiophene-2-carbonyl)-1,2-dihydro-5H,6H-chromeno[3,4-e]thiazolo[3,2-a]pyridin-6-one (7{6, 1})
Yellow solid; m.p. 154–156 °C (yield = 85%). 1H NMR (300 MHz, CDCl3): δ 10.42 (br, 1 H), 7.80 (d, 1H, J = 8.1 Hz), 7.68 (s, 1H), 7.5 (d, 2H, J = 3 Hz), 7.26–7.22 (m, 2H), 7.05 (br, 4H), 6.04 (d, 2H, J = 9.6 Hz), 4.23–4.17 (m, 1H), 3.89–3.83 (m, 1H), 345–3.39 (m, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ 184.75, 164.66, 162.21, 153.02, 142.56, 139.29, 135.51, 135.05, 134.52, 131.91, 130.95, 128.76, 128.41, 128.08, 124.40, 123.65, 118.11, 117.07, 116.16, 113.90, 102.59, 48.50, 35.39, 29.71 ppm; MS (EI, 70 eV) m/z (%) = 471 (M+, 16), 111 (77), 43 (100); FT-IR (KBr, cm−1): 3430, 2932, 1702, 1609, 1495, 1415, 1248, 764.
5-(Hydroxy(4-methoxyphenyl)methylene)-4-(thiophene-2-carbonyl)-1,2-dihydro-5H,6H-chromeno[3,4-e]thiazolo[3,2-a]pyridin-6-one (7{6, 2})
Yellow solid; m.p. 142–144 °C (yield = 78%). 1H NMR (300 MHz, CDCl3): δ 10.51 (br, 1H), 7.80 (d, 1H, J = 7.8 Hz), 7.67 (d, 1H, J = 3.6 Hz), 7.48 (d, 1H, J = 4.8 Hz), 7.25 (t, 1H, J = 15.9 Hz), 7.06–6.90 (m, 5H), 6.59 (d, 2H, J = 8.4 Hz), 4.22–4.13 (m, 1H), 3.89–3.81 (m, 1H), 3.53 (s, 3H), 3.46–3.35 (m,2H) ppm; 13C NMR (75 MHz, CDCl3): δ 184.73, 164.62, 162.24, 159.59, 153.01, 142.60, 138.93, 135.44, 134.95, 134.43, 131.85, 129.76, 128.05, 124.39, 123.62, 123.29, 118.02, 117.12, 116.15, 114.26, 102.71, 55.21, 48.35, 35.36, 29.70 ppm; MS (EI, 70 eV) m/z (%) = 501 (M+, 19), 121 (77), 111 (100), 65 (22); FT-IR (KBr, cm−1): 3430, 3075, 2920, 1694, 1608, 1483, 1416, 758.
4-(4-Bromobenzoyl)-5-(hydroxy(phenyl)methylene)-1,2-dihydro-5H,6H-chromeno[3,4-e]thiazolo[3,2-a]pyridin-6-one (7{2, 1})
Yellow solid; m.p. 188–190 °C (yield = 95%). 1H NMR (300 MHz, CDCl3): δ 7.97 (d, 1H, J = 7.8 Hz), 7.68–7.65 (m, 2H), 7.55–7.52 (m, 3H), 7.38–7.22 (m, 8H), 4.44–4.38 (m, 1H), 4.20–4.12 (m, 1H), 3.70–3.60 (m, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ 191.69, 163.68, 162.47, 152.96, 142.03, 136.80, 134.37, 132.10, 131.88, 131.68, 130.75, 130.56, 128.88, 128.62, 128.41, 127.53, 124.07, 123.82, 117.40, 116.31,113.37, 102.06, 48.51, 35.24, 29,71 ppm; MS (EI, 70 eV) m/z (%) = 545 (M+, 9), 185 (51), 105 (46), 43 (100); FT-IR (KBr, cm−1): 3434, 2925, 1721, 1099.
4-(4-Chlorobenzoyl)-5-(hydroxy(phenyl)methylene)-1,2-dihydro-5H,6H-chromeno[3,4-e]thiazolo[3,2-a]pyridin-6-one (7{3, 1})
Yellow solid; m.p. 158–160 °C (yield = 92%). 1H NMR (300 MHz, CDCl3): δ 9.57 (br, 1H), 7.97 (d, 1H, J = 9 Hz), 7.76 (d, 2H, J = 9 Hz), 7.54–7.49 (m, 2H), 7.40–7.21 (m, 9H), 4.46–4.37 (m, 1H), 4.22–4.11 (m, 1H), 3.65 (t, 2H, J = 14.4 Hz) ppm; 13C NMR (75 MHz, CDCl3): δ 191.52, 163.53, 162.17, 152.99, 141.79, 138.92, 136.38, 134.34, 132.29, 132.09, 130.69, 130.60, 128.85, 128.70, 128.58, 128.42, 124.06, 123.73, 117.48, 116.26,113.46, 102.14, 48.50, 35.24, 29,70 ppm; MS (EI, 70 eV) m/z (%) = 499 (M+, 11), 139 (94), 43 (100); FT-IR (KBr, cm−1): 3434, 2923, 2925, 1621, 1100.
General procedure for the synthesis of thiazolonaphthyridines analogues (9)
A round-bottom flask was charged with the appropriate α-enolicdithioesters (1 mmol), cysteamine (1 mmol) and they were stirred at 90 °C over a pre-heated oil bath under solvent-free conditions. When conjugated thiazole was formed (monitored by TLC), arylglyoxal monohydrate (1 mmol) and 4-hydroxy-1-methyl-2(1H)-quinolone (1 mmol) were added to reaction mixture and stirring continued for an appropriate period of time. The completion of reaction was monitored by TLC. Then, the yellow solid product was solved in ethanol and quenched with distilled water. The obtained crude was dried and crystallized with H2O–EtOH to produce the pure product.
4-(Furan-2-carbonyl)-5-(hydroxyl(phenyl)methylene)-7-methyl-1,2,5,7-tetrahydro-6H-benzo[h]thiazolo[3,2-a] [1,6]naphthyridin-6-one (9{5, 1})
Yellow solid; m.p. 180–182 °C (yield = 83%). 1H NMR (300 MHz, CDCl3): δ 8.57 (br, 1H), 8.14 (d, 1H, J = 7.5 Hz), 7.58–7.50 (m, 3H), 7.34–7.21 (m, 7H), 6.49–6.48 (m, 1H), 4.47–4.38 (m, 1H), 4.25–4.16 (m, 1H), 3.71–3.66 (m, 2H), 3.54 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 178.02, 162.82, 159.49, 151.98, 146.34, 139.48, 133.50, 131.17, 130.83, 128.56, 128.39, 127.90, 124.59, 124.03, 121.43, 119.42, 117.26, 116.77, 113.62, 112.26, 108.08, 48.43, 35.17, 29.69, 29.37 ppm; MS (EI, 70 eV) m/z (%) = 468 (M+, 33), 95 (100), 77 (33); FT-IR (KBr, cm−1): 3410, 2924, 1735, 1627, 1467, 755.
4-(Furan-2-carbonyl)-5-(hydroxyl(4-nitrophenyl)methylene)-7-methyl-1,2,5,7-tetrahydro-6H-benzo[h]thiazolo[3,2-a][1,6]naphthyridin-6-one (9{5, 3})
Orange solid; m.p. 200–202 °C (yield = 95%). 1H NMR (300 MHz, DMSO): δ 8.16 (d, 1H, J = 9 Hz), 7.90 (d, 1H, J = 6 Hz), 7.64–7.58 (m, 4 H), 7.50–7.43 (m, 2H), 7.28–7.17 (m, 2H), 7.01 (d, 1H, J = 3 Hz), 6.48–6.49 (m, 1H), 4.42–4.33 (m, 2H), 3.87–3.78 (m, 2H) ppm; 13C NMR (75 MHz, DMSO): δ 176.29, 162.96, 161.48, 152.28, 146.48, 146.42, 141.99, 140.43, 139.52, 138.54, 131.79, 129.92, 129.29, 124.07, 123.55, 121.66, 118.94, 117.14, 114.99, 112.19, 98.38, 48.99, 35.05, 29.50, 28.91 ppm; MS (EI, 70 eV) m/z (%) = 513 (M+, 0.01), 316 (13), 175 (100), 146 (40), 132 (48), 77 (19); FT-IR (KBr, cm−1): 3430, 2921, 1596, 1564, 1338, 1240, 1113, 753.
4-(Furan-2-carbonyl)-5-(hydroxyl(4-methoxyphenyl)methylene)-7-methyl-1,2,5,7-tetrahydro-6H-benzo[h]thiazolo[3,2-a][1,6]naphthyridin-6-one (9{5, 2})
Yellow solid; m.p. 178–180 °C (yield = 83%). 1H NMR (300 MHz, CDCl3): δ 8.067 (br, 1H), 7.86 (d, 1H, J = 7.8 Hz), 7.31–7.26 (m, 2H), 7.06–6.97 (m, 5H), 6.55 (d, 2H, J = 8.4 Hz), 6.25–6.24 (m, 1H), 4.15–4.06 (m,1H), 3.99–3.87 (m, 1H), 3.51 (s, 3H), 3.48–3.39 (m, 2H), 3.29 (s 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 177.90, 162.66, 159.24, 159.02, 152.08, 146.18, 139.52, 133.26, 130.72, 129.72, 124.53, 123.60, 121.28, 119.14, 117.11, 116.66, 114.70, 114.05, 113.54, 112.23, 108.20, 55.19, 48.28, 35.15, 29.72, 29.66 ppm; MS (EI, 70 eV) m/z (%) = 498 (M+, 21), 95 (100), 77 (20); FT-IR (KBr, cm−1): 3430, 2924, 1745, 1610, 1464, 1249, 1173, 1028, 757.
5-(Hydroxyl(phenyl)methylene)-7-methyl-4-(thiophene-2-carbonyl)-1,2,5,7-tetrahydro-6H-benzo[h]thiazolo[3,2-a][1,6]naphthyridin-6-one (9{6, 1})
Yellow solid; m.p. 190–192 °C (yield = 92%). 1H NMR (300 MHz, DMSO): δ 8.75–8.73 (m, 1H), 7.79–7.72 (m, 1H), 7.54–7.49 (m, 1H), 7.33–7.32 (m, 2H), 7.19–6.95 (m, 7H), 6.76–6.69 (m, 1H), 4.13–4.04 (m, 2H), 3.54–3.44 (m, 2H), 3.35 (s, 3H) ppm; 13C NMR (75 MHz, DMSO): δ 211.55, 187.14, 167.82, 163.04, 148.61, 144.08, 137.09, 136.73, 136.07, 135.39, 133.29, 133.14, 132.43, 131.70, 128.81, 125.87, 122.18, 121.00, 120.58, 118.39, 111.34, 53.21,, 39.73, 35.70, 34.25 ppm; MS (EI, 70 eV) m/z (%) = 484 (M+, 13), 111 (100), 77 (33); FT-IR (KBr, cm−1): 3430, 3077, 2924, 1607, 1579, 1415, 1238, 756.
5-(Hydroxyl(4-methoxyphenyl)methylene)-7-methyl-4-(thiophene-2-carbonyl)-1,2,5,7-tetrahydro-6H-benzo[h]thiazolo[3,2-a][1,6]naphthyridin-6-one (9{6, 2})
Yellow solid; m.p. 206–208 °C (yield = 89%). 1H NMR (300 MHz, CDCl3): δ 7.90 (d, 1H, J = 9 Hz), 7.57 (d, 1H, J = 3 Hz), 7.37 (d, 1H,J = 3 Hz), 7.29 (t, 1H, J = 15 Hz), 7.01–6.96 (m, 4H), 6.79 (t, 1H, J = 4 Hz), 6.56 (d, 2H, J = 9 Hz), 4.20–4.11 (m, 1H), 3.95–3.87 (m, 1H), 3.51 (s, 3H), 3.45–3.38 (m, 2H), 3.26 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 207.04, 183.95, 162.63, 159.75, 159.18, 143.28, 139.47, 138.67, 134.41, 133.81, 130.78, 129.65, 127.55, 124.62, 123.68, 121.35, 116.91, 114.80, 114.02, 113.58, 108.17, 55.18, 48.36, 35.27, 30.96, 29.77 ppm; MS (EI, 70 eV) m/z (%) = 514 (M+, 2), 135 (100), 111 (73), 77 (29); FT-IR (KBr, cm−1): 3435, 2927, 1632, 1511, 1414, 1249, 1174, 1107, 755.
4-(4-Bromobenzoyl)-5-(hydroxyl(phenyl)methylene)-7-methyl-1,2,5,7-tetrahydro-6H-benzo[h]thiazolo[3,2-a][1,6]naphthyridin-6-one (9{2, 1})
Yellow solid; m.p. 220–222 °C (yield = 90%). 1H NMR (300 MHz, CDCl3): δ 7.95 (d, 1H, J = 9 Hz), 7.58–7.53 (m, 2H), 7.45 (d, 2H, J = 9 Hz), 7.32–7.19 (m, 9H), 4.33–4.28 (m, 2H), 3.74–3.66 (m, 2H), 3.50 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 190.72, 162.40, 157.93, 142.57, 139.40, 137.94, 132.93, 131.11, 130.67, 129.80, 128.73, 128.34, 128.15, 125.62, 124.05, 121.46, 116.86, 115.57, 114.70, 113.73, 106.87, 48.44, 34.94, 29.72, 29.54 ppm; MS (EI, 70 eV) m/z (%) = 558 (M+, 15), 293 (20), 184 (45), 105 (100), 43 (89); FT-IR (KBr, cm−1): 3443, 2925, 1627, 1076.
4-(4-Chlorobenzoyl)-5-(hydroxyl(phenyl)methylene)-7-methyl-1,2,5,7-tetrahydro-6H-benzo[h]thiazolo[3,2-a][1,6]naphthyridin-6-one (9{3, 1})
Yellow solid; m.p. 176–178 °C (yield = 89%). 1H NMR (300 MHz, CDCl3): δ 7.96 (d, 1H, J = 9 Hz), 7.55 (d, 4H, J = 9 Hz), 7.32–7.19 (m, 7H), 7.12 (d, 2H, J = 8.1 Hz), 4.31–4.29 (m, 2H), 3.72 (t, 2H, J = 7.2 Hz), 3.50 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 190.68, 162.43, 158.08, 142.39, 139.40, 137.41, 133.01, 131.07, 130.74, 129.79,129.34, 128.71, 128.35, 127.77, 124.10, 121.46, 116.98, 115.67, 114.76, 113.67, 106.96, 48.46, 34.97, 29.71, 29.55 ppm; MS (EI, 70 eV) m/z (%) = 512 (M+, 25), 238 (30), 139 (100), 77 (47); FT-IR (KBr, cm−1): 3432, 2925, 1629, 1466, 1091.
5-(Hydroxyl(phenyl)methylene)-4-(4-methoxybenzoyl)-7-methyl-1,2,5,7-tetrahydro-6H-benzo[h]thiazolo[3,2-a][1,6]naphthyridin-6-one (9{4, 1})
Yellow solid; m.p. 180–182 °C (yield = 92%). 1H NMR (300 MHz, CDCl3): δ 8.13 (d, 1H, J = 7.8 Hz), 7.81 (d, 2H, J = 8.7 Hz), 7.63–7.52 (m, 2H), 7.31–7.23 (m, 7H), 6.83 (d, 2H, J = 9 Hz), 4.44–4.38 (m, 1H), 4.25–4.11 (m, 1H), 3.82 (s, 3H), 3.79–3.63 (m, 2H), 3.48 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 190.34, 162.97, 159.36, 142.14, 139.49, 137.34, 133.26, 131.72, 130.94, 130.71, 128.55, 128.30, 127.79, 126.28, 124.54, 121.25, 116.80, 115.52, 114.66, 113.25, 106.39,55.32, 48.47, 35.21, 29.71, 29.61 ppm; MS (EI, 70 eV) m/z (%) = 508 (M+, 0.1), 149 (13), 83 (20), 57 (51), 43 (100); FT-IR (KBr, cm−1): 3430, 2925, 1629, 1107.
References
M.B. Gawande, V.D.B. Bonifa, R. Luque, P.S. Branco, R.S. Varma, Chem. Soc. Rev. 42, 5522 (2013)
M. Gawande, V. Bonifacio, R. Luque, P. Branco, R. Varma, ChemSusChem 7, 24 (2014)
C.-J. Li, B. Trost, PNAS 105, 13197 (2008)
S. Vidyacharan, A. Shinde, B. Satpathi, D. Sharada, Green Chem. 16, 1168 (2014)
Y. Moglie, M. González-Soria, I. Martín-García, G. Radivoy, F. Alonso, Green Chem. 18, 4896 (2016)
D. Das, ChemistrySelect 1, 1959 (2016)
M.-Y. Wu, W.-W. He, X.-Y. Liu, B. Tan, Angew. Chem. 127, 9541 (2015)
T. Zarganes-Tzitzikas, A. Chandgude, A. Dömling, Chem. Rec. 15, 981 (2015)
X. Huang, Z. Liu, J. Org. Chem. 67, 6731 (2002)
M. Dinakaran, P. Senthilkumar, P. Yogeeswari, A. China, V. Nagaraja, D. Sriram, Med. Chem. 4, 482 (2008)
A. Klásek, V. Mrkvička, A. Pevec, J. Kosmrlj, J. Org. Chem. 69, 5646 (2004)
D. Chu, P. Fernandes, A. Pernet, Med. Chem. 29, 1531 (1986)
T.I. El-Emary, A. Khalil, G.A.M. El-Hag Ali, A.A.A.M. El-Adasy, Phosphorus Sulfur Silicon 180, 19 (2005)
A.A. Altaf, A. Shahzad, Z. Gul, N. Rasool, A. Badshah, B. Lal, E. Khan, JDDMC 1, 1 (2015)
F. Shi, C. Li, M. Xia, K. Miao, Y. Zhao, S. Tu, G. Zhang, N. Ma, W. Zheng, Bioorg. Med. Chem. Lett. 19, 5565 (2013)
R.-Z. Jin, Y.-L. Li, X.-S. Wang, Heterocycl. Commun. 21, 377 (2015)
B. Singh, E.R. Bacon, G.Y. Lesher, S. Robinson, P.O. Pennock, D.C. Bode, E.D. Pagani, R.G. Bentley, M.J. Connell, L.T. Hamel. J. Med. Chem. 38, 2546 (1995)
W.-Q. Lu, R. Zhuang, D. Chen, X.-S. Wang, Polycycl. Aromat. Comp. 34, 606 (2014)
K. Hodgetts, M. Kershaw, Org. Lett. 5, 2911 (2003)
H.R. Kokatla, E. Yoo, D. Salunke, D. Sil, C. Ng, R. Balakrishna, S. Malladi, L. Fox, S. David, Org. Biomol. Chem. 11, 1179 (2013)
A. Nagaraju, G.K. Verma, B.J. Ramulu, G. Shukla, K. Raghuvanshi, A. Srivastava, M.S. Singh, Green Chem. 17, 950 (2015)
A. Krauze, J. Popelis, G. Duburs, Tetrahedron 54, 9161 (1998)
G. Singh, S.S. Bhattacharjee, H. Ila, H. Junjappa, Synthesis 1982, 693 (1982)
P. Mathew, C.V. Asokan, Tetrahedron 62, 1708 (2006)
Z. Arabpoor, H.R. Shaterian, RSC Adv. 6, 44459 (2016)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arabpoor, Z., Shaterian, H.R. Applying green and highly efficient approach for a facile synthesis of new thiazoloquinoline, thiazolopyridine, and thiazolonaphthyridine derivatives. J IRAN CHEM SOC 16, 1091–1103 (2019). https://doi.org/10.1007/s13738-018-01579-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-01579-x