Abstract
Astrocytes may undergo a functional remodeling with aging, acquiring a pro-inflammatory state. In line with this, resveratrol represents an interesting strategy for a healthier brain aging since it can improve glial functions. In the present study, we investigated the glioprotective role of resveratrol against lipopolysaccharide (LPS)-induced gliotoxicity in hippocampal aged astrocytes. Astrocyte cultures were obtained from aged rats (365 days old) and challenged in vitro with LPS in the presence of resveratrol. Cultured astrocytes from newborn rats were used as an age comparative for evaluating LPS gliotoxicity. In addition, aged rats were submitted to an acute systemic inflammation with LPS. Hippocampal astrocyte cultures were also obtained from these LPS-stimulated aged animals to further investigate the glioprotective effects of resveratrol in vitro. Overall, our results show that LPS induced a higher inflammatory response in aged astrocytes, compared to newborn astrocytes. Several inflammatory and gene expression alterations promoted by LPS in aged astrocyte cultures were similar in hippocampal tissue from aged animals submitted to in vivo LPS injection, corroborating our in vitro findings. Resveratrol, in turn, presented anti-inflammatory effects in aged astrocyte cultures, which were associated with downregulation of p21 and pro-inflammatory cytokines, Toll-like receptors (TLRs), and nuclear factor κB (NFκB). Resveratrol also improved astroglial functions. Upregulation of sirtuin 1 (SIRT1), nuclear factor erythroid 2-related factor 2 (Nrf2), and heme oxygenase 1 (HO-1) represent potential molecular mechanisms associated with resveratrol-mediated glioprotection. In summary, our data show that resveratrol can prime aged astrocytes against gliotoxic stimuli, contributing to a healthier brain aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is a complex process characterized by an intrinsic physiological and functional decline of an organism [1, 2], with increased risk for several diseases. In the central nervous system (CNS), cognitive decline is an important feature of aging [3, 4]. Hippocampus is a key structure involved in learning and memory functions, and represents a vulnerable region for age-related functional alterations [3, 5]. Astrocytes are versatile cells of the CNS that become important candidates for age-related hippocampal alterations, since they display several roles for physiological maintenance of the CNS. They participate in neurotransmitter homeostasis, blood–brain barrier, and neural remodeling [6,7,8]. In addition, they provide energy metabolites, trophic factors, and antioxidant defenses, including reduced glutathione (GSH), to neurons [6, 9, 10].
Astrocytes also present immune functions since they are able to evoke inflammatory responses in the presence of damage molecules and pathogens [11, 12]. Considering that astrocytes express Toll-like receptor 4 (TLR4), they can be stimulated by the inflammogen lipopolysaccharide (LPS), which leads to nuclear factor κB (NFκB) activation and production of several pro-inflammatory cytokines [13, 14]. Moreover, astrocytes from different brain areas show an age-dependent functional remodeling [15]. In this context, previous studies from our group have demonstrated that astrocytes acquire a pro-inflammatory profile consistent with the inflammaging process, which is characterized by increased expression and/or release of inflammatory mediators, such as tumor necrosis factor alpha (TNF-α), interleukins 1β (IL-1β) and 6 (IL-6), NFκB p65 subunit, and cyclooxygenase 2 (COX-2) [16,17,18,19,20].
The modulation of inflammaging can represent an interesting strategy for a healthier aging [21,22,23]. Resveratrol, a natural polyphenolic compound, has received increasingly attention in this research area. In the CNS, the beneficial action of resveratrol can be related to its glioprotective effects [13, 24,25,26,27]. Resveratrol is an important activator of sirtuin 1 (SIRT1), which belongs a family of histone and protein deacetylases with several cellular functions [28,29,30,31]. SIRT1 can regulate inflammation and aging, whereas decreased SIRT1 levels are correlated with inflammaging and inflammatory-related diseases [28, 32,33,34]. Besides SIRT1, resveratrol can modulate several signaling molecules, including nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase 1 (HO-1), adenosine receptors, and mitogen activated protein kinase p38 (p38 MAPK) [13, 25,26,27, 35,36,37].
The purpose of the present study was to expand the knowledge about the glioprotective potential of resveratrol during aging and inflammatory conditions, by investigating the multi-target effects of resveratrol on LPS-induced gliotoxicity in hippocampal astrocyte cultures obtained from aged rats. For this, different experimental approaches were used: (i) we initially evaluated age-related effects of LPS and resveratrol in primary astrocyte cultures obtained from newborn and aged rats; (ii) after, we exposed astrocyte cultures obtained from aged rats to LPS and resveratrol (in vitro experimental model) to evaluate inflammatory, oxidative, and glial parameters, as well as potential mechanisms associated with resveratrol-mediated glioprotection, particularly SIRT1, Nrf2 and HO-1; (iii) finally, to corroborate our findings related to the in vitro experimental model with LPS, we induced an in vivo acute systemic inflammation in aged rats with LPS and evaluated biochemical, inflammatory, and gene expression parameters in blood serum, cerebrospinal fluid (CSF), and/or hippocampal tissue, as well as astrocyte cultures were obtained from aged rats stimulated in vivo with LPS to further investigate the glioprotective roles of resveratrol in vitro. To our knowledge, the present study provides the first demonstration that resveratrol can act on astrocytes derived from aged rats by improving their function and preventing LPS-induced gliotoxicity.
Materials and Methods
Animals
Male Wistar rats (1–2 and 365 days old) were obtained from the breeding colony of Department of Biochemistry (UFRGS, Porto Alegre, Brazil), and maintained under controlled environment (12-h light/12-h dark cycle; 22 ± 1 °C; ad libitum access to food and water). All animal experiments were performed in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals and Brazilian Society for Neuroscience and Behavior recommendations for animal care. The experimental protocols were approved by the Federal University of Rio Grande do Sul Animal Care and Use Committee (process number: 21215).
Hippocampal Primary Astrocyte Cultures
Newborn (1–2 days old) and aged (365 days old) Wistar rats had their hippocampi aseptically dissected from cerebral hemispheres, followed by meninges removal. The tissues were digested in Hank’s balanced salt solution (HBSS) containing 0.003% DNase using trypsin (0.05%) as previously described [17]. After mechanical dissociation and centrifugation, the cells were resuspended in DMEM/F12 (Gibco) [10% fetal bovine serum (FBS), 15 mM HEPES, 14.3 mM NaHCO3, 2.5 μg/mL amphotericin B, and 0.05 mg/mL gentamicin], plated on 6- or 24-well plates pre-coated with poly-L-lysine at a density of 3–5 × 105 cells/cm2. Astrocytes were cultured at 37 °C in a 5% CO2 incubator. After 24 h, the culture medium was exchanged; during the first week, the medium was replaced once every 2 days, and from the second week on, once every 4 days. From the third week on, astrocytes received medium supplemented with 20% FBS until they reached confluence (around at third week for newborn astrocytes and at approximately the fourth week for mature astrocytes), when were used for the experiments.
In vitro LPS Challenge and Resveratrol Treatment
When the primary astrocyte cultures presented the same number of adherent cells in culture plates (confluence), the culture medium was exchanged with DMEM/F12 1% FBS in the presence or absence of resveratrol (10 μM, Sigma-Aldrich) for 1 h, in accordance with our previous studies [13, 27, 38]. Then, LPS (1 μg/ml, Sigma-Aldrich) was added to the incubation medium for 24 h [13], and resveratrol was maintained. A higher dose of LPS (10 μg/ml) was also tested in the first set of experiments to investigate cell viability and integrity. Alternatively, we co-incubated aged astrocytes with HO-1 inhibitor ZnPP IX (10 μM) [26] or p38 MAPK inhibitor SB203580 (10 μM) [39] to test the roles of these pathways in the modulation of specific parameters by resveratrol and/or LPS.
In vivo Intraperitoneal LPS Injection
Aged Wistar rats (365 days old) received an intraperitoneal injection of 250 μg/kg LPS (20 animals) [40] or phosphate-buffered saline (control condition – sham; 20 animals). After 24 h, the animals were anesthetized with ketamine/xylazine (75 and 10 mg/ kg, respectively) and placed in a stereotaxic apparatus for CSF collection from the cisterna magna. Blood samples were obtained by intracardiac puncture. Hippocampi were aseptically dissected from animals and used for hippocampal tissue analyses or preparation of primary astrocyte cultures. Blood, CSF, and hippocampal tissue samples were frozen at − 80 °C until further analyses. Primary astrocyte cultures were immediately prepared as described above.
In vitro resveratrol treatment of primary astrocyte cultures derived from LPS-injected rats
At confluence, primary astrocyte cultures obtained from LPS-injected and control rats had their culture medium exchanged with DMEM/F12 1% FBS and were incubated in the presence or absence of resveratrol (10 μM) for 24 h.
MTT Reduction Assay
MTT (methylthiazolyldiphenyl-tetrazolium bromide, Sigma-Aldrich) was added to the culture medium at a concentration of 50 μg/mL and cells were incubated for 3 h at 37 °C in an atmosphere of 5% CO2. Subsequently, the medium was removed and the MTT crystals were dissolved in dimethyl-sulfoxide. Absorbance values were measured at 560 nm and 650 nm [39]. The results are expressed as percentages relative to the control conditions.
Propidium Iodide Incorporation Assay
Astrocyte cultures were incubated with 7.5 μM PI for 30 min in 5% CO2 at 37 °C. The optical density of fluorescent nuclei (labeled with PI), indicative of cell death, was determined with Image J software. Density values obtained are expressed as a percentage of the control value.
Lactate Dehydrogenase (LDH) Assay
The release of the enzyme lactate dehydrogenase (LDH) was assessed measuring its activity in the culture medium (100 μL) of astrocytes using a commercial UV assay (Bioclin, Brazil). Results are expressed as percentages of the control value.
Actin-Labeling Analysis
For actin-labeling analysis, cells were fixed for 20 min with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), rinsed with PBS, and then permeabilized for 10 min in PBS containing 0.2% Triton X-100. After, an incubation with 10 μg/mL rhodamine-labeled phalloidin in PBS for 20 min was performed, followed by two washes with PBS. Cell nuclei were stained with 0.2 μg/mL of 4′, 6′-diamidino-2-phenylindole (DAPI) for further 20 min. Astrocyte cultures were analyzed using a Nikon microscope and photographed with a digital camera DXM1200C and TE-FM epi-fluorescence accessory.
Inflammatory Response Measurement
The levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), and monocyte chemoattractant protein-1 (MCP-1/CCL2) were measured in the culture medium of astrocytes, serum, CSF, or hippocampal homogenates using ELISA commercial kits. The assay ranges for the kits are the following: 16 to 2000 pg/ml for TNF-α (Invitrogen, catalog #88–7340-22); 31.3 to 2000 pg/ml for IL-1β (Invitrogen, catalog #BMS630); 31.3 to 2000 pg/ml for IL-6 (Invitrogen, catalog #BMS625); 15.6 to 1000 pg/ml for IL-10 (Invitrogen, catalog #BMS629); and 7.8–500 pg/ml for MCP-1 (Invitrogen, catalog #BMS631INST). The results are expressed in pg/ml for culture medium of astrocytes, serum, and CSF samples. For hippocampal tissue homogenates, the results were calculated as pg/ml and then corrected by the total amount of protein, thus they are expressed as pg/mg protein.
RNA Extraction and Quantitative RT-PCR
Total RNA was isolated from astrocyte cultures and hippocampal tissue using TRIzol Reagent (Invitrogen). The concentration and purity of the RNA were determined spectrophotometrically at a ratio of 260:280. Then, 1 μg of total RNA was reverse transcribed using Applied Biosystems™ HighCapacity complementary DNA (cDNA) Reverse Transcription Kit (Applied Biosystems) in a 20-μL reaction according to manufacturer’s instructions. The messenger RNA (mRNA) encoding TNF-α (#Rn99999017_m1), IL-1β (#Rn00580432_m1), TLR2 (#Rn02133647_s1), TLR4 (#Rn00569848_m1), p65 NFκB (#Rn01399572_m1), COX-2 (#Rn01483828_m1), inducible nitric oxide synthase (iNOS; #Rn00561646_m1), Nrf2 (#Rn00582415_m1), HO-1 (#Rn01536933_m1), glutamate cysteine ligase (GCL; #Rn00689046_m1), SIRT1 (#Rn01428096_m1), p21 (#Rn 00589996_m1), glutamate/aspartate transporter (GLAST; #Rn00570130_m1), glutamate transporter-1 (GLT-1; #Rn00691548_m1), glutamine synthetase (GS; #Rn01483107_m1), and β-actin (#Rn00667869_m1) was quantified using the TaqMan real-time RT-PCR system using inventory primers and probes purchased from Applied Biosystems. Quantitative RT-PCR was performed using the Applied Biosystems 7500 Fast system during 40 cycles of amplification. Target mRNA levels were normalized to β-actin levels and expressed relative to the levels in control astrocytes using the 2−ΔΔCt method [41].
p38 MAPK Levels
The p38 MAPK levels were detected using the rat p38 MAPK ELISA commercial kit (Sigma-Aldrich, catalog #PM0100). These levels were evaluated in the cell lysate suspended in a specific buffer from the ELISA kit. The assay range of the ELISA kit is 31.2 to 2000 pg/ml. The results are expressed as percentages relative to the control levels.
NFκB Transcriptional Activity
Nuclear content was obtained treating lysed cells with 1% Igepal CA-630, followed by a vortex for 10 s and centrifugation at 400 × g for 5 min. After centrifugation, nuclei pellets were resuspended in specific buffer (provided by ELISA kit manufacturer) and assayed for p65 NFκB using a commercial ELISA kit with some modifications (Invitrogen, catalog #85–86,081). The results are expressed as percentages relative to the control levels.
DCFH Oxidation
Intracellular ROS levels were detected using DCFH-DA (Sigma-Aldrich). DCFH-DA was added to the medium at a concentration of 10 μM and astrocytes were incubated for 30 min at 37 °C. Following DCFH-DA exposure, the cells were scraped into phosphate-buffered saline with 0.2% Triton X-100. The fluorescence was measured with excitation at 485 nm and emission at 520 nm [42]. The results are expressed as percentages relative to the control conditions.
JC-1 Assay
To determine the mitochondrial membrane potential (ΔΨm), cells were incubated for 30 min with JC-1 (Invitrogen, 2 μg/mL) [43]. Then, cells were washed once with Hank’s balanced salt solution (HBSS) and the fluorescence was immediately read using an excitation wavelength of 485 nm and emission wavelengths of 540 and 590 nm. The ΔΨm was calculated using the ratio of 595 nm (red fluorescent J-aggregates) to 535 nm (green monomers). The results are expressed as percentages relative to the control conditions.
RNA Oxidation
Total RNA was isolated from astrocyte cultures using TRIzol Reagent (Invitrogen). The concentration and purity of RNA were determined spectrophotometrically at a ratio of 260:280. The measurement of RNA oxidation was carried out using a rat ELISA kit from Cayman Chemical specific for 8-hydroxyguanosine. The results are expressed as fold relative to control.
Glutathione Levels
Glutathione (GSH) levels were assessed as previously described [44]. Astrocyte lysate suspended in a sodium phosphate buffer with 140 mM KCl was diluted with a 100 mM sodium phosphate buffer (pH 8.0) containing 5 mM EDTA, and the protein was precipitated with 1.7% meta-phosphoric acid. The supernatant was assayed with o-phthaldialdehyde (at a concentration of 1 mg/mL methanol) at 22 °C for 15 min. Fluorescence was measured using excitation and emission wavelengths of 350 and 420 nm, respectively. A calibration curve was performed with standard GSH solutions at concentrations ranging from 0 to 500 μM. The results are expressed in nmol/mg protein.
Glutamate Cysteine Ligase Activity
Glutamate cysteine ligase (GCL) was assayed according to Seelig et al., with slight modifications [45]. Cell lysate, suspended in a sodium phosphate buffer containing 140 mM KCl, was diluted with 100 mM sodium phosphate buffer (pH 8.0) containing 5 mM EDTA. The enzyme activity was determined after monitoring the NADH oxidation at 340 nm in sodium phosphate/KCl (pH 8.0) containing 5 mM ATP-Na2, 2 mM phosphoenolpyruvate, 10 mM L-glutamate, 10 mM L-α-amino-butyrate, 20 mM MgCl2, 2 mM EDTA-Na2, 0.2 mM NADH, and 17 μg of pyruvate kinase/lactate dehydrogenase. The results are expressed in nmol/mg protein/min.
Glutathione Peroxidase (GPx) Activity
Glutathione peroxidase (GPx) activity was measured using the RANSEL kit from Randox (Autrim, UK). The concentration of GPx in lysed cells is assessed by measuring the absorption of NADPH at 340 nm. The results are expressed as U/mg protein.
Glutamate Uptake
The glutamate uptake assay was performed using H3-labelled glutamate [18]. Briefly, astrocytes were rinsed once with PBS and were incubated at 37 °C in HBSS containing the following components (in mM): 137 NaCl, 5.36 KCl, 1.26 CaCl2, 0.41 MgSO4, 0.49 MgCl2, 0.63 Na2HPO4, 0.44 KH2PO4, 4.17 NaHCO3, and 5.6 glucose, adjusted to pH 7.4. Subsequently, 0.1 mM L-glutamate and 0.33 μCi/ml L-[3,4-3H] glutamate were added to initiate the assay. After 7 min, the incubation was stopped by removal of the medium and rinsing the cells twice with ice-cold HBSS. The cells were then lysed in a 0.5 M NaOH solution and incorporated radioactivity was measured using a scintillation counter. Sodium-independent uptake was determined using ice-cold N-methyl-D-glucamine instead of sodium chloride. Sodium-dependent glutamate uptake was obtained by subtracting the sodium-independent uptake from the total uptake. The results are expressed as nmol/mg protein/min.
Glutamine Synthetase (GS) Activity
The activity of glutamine synthetase (GS) was determined as previously described [18]. Briefly, the cell homogenate was added to a reaction mixture containing 10 mM MgCl2, 50 mM L-glutamate, 100 mM imidazole–HCl buffer (pH 7.4), 10 mM 2-mercaptoethanol, and 50 mM hydroxylamine–HCl. The addition of 10 mM ATP started the reaction, which was continued for 15 min at 37 °C. A solution containing 370 mM ferric chloride, 670 mM HCl, and 200 mM trichloroacetic acid was then added to stop the reaction. After centrifugation, the absorbance of the supernatant was measured at 530 nm. A calibration curve was prepared using γ-glutamyl hydroxamate and treated with ferric chloride reagent. The results are expressed in μmol/mg protein/h.
BDNF and GDNF Measurements
Extracellular levels of BDNF and GDNF were measured in the culture medium of primary astrocyte cultures using ELISA kits. The assay ranges are 12.3 to 3000 pg/ml for BDNF (Invitrogen, catalog #ERBDNF) and 31.2 to 2000 pg/ml for GDNF (Abcam; catalog #ab213901). The results are expressed as percentages relative to the control conditions.
Protein Assay
Protein content was measured using Lowry’s method with bovine serum albumin as a standard [46].
Statistical Analyses
Differences among groups were statistically analyzed using one-way or two-way analysis of variance (ANOVA) followed by Tukey’s test. Student’s t test was used for comparisons between control and LPS in vivo injection in aged Wistar rats. Correlations were analyzed by Pearson correlation coefficient. All analyses were performed using the GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA). Values of P < 0.05 were considered significant (a refers to statistically significant differences from the control; b refers to statistically significant differences from LPS challenge). Outliers were removed because some astrocyte cultures did not reach the confluence at the same time.
Results
How Do LPS and Resveratrol Affect Cellular Viability, Integrity, and Morphology of Hippocampal Astrocyte Cultures?
We firstly investigated the potential cytotoxic effect of LPS (1 and 10 μg/mL) exposure for 24 h on hippocampal astrocyte cultures obtained from newborn and aged (365 days old) rats. Only at 10 μg/mL, LPS decreased MTT reduction (P < 0.0001), indicating an impairment of mitochondrial activity, and consequently, cellular viability (Fig. 1B). In addition, the same dose of LPS caused a loss of membrane integrity that was evaluated by PI incorporation (Fig. 1C; P < 0.0001) and extracellular LDH activity (Fig. 1D; P < 0.0001) assays. However, both doses of LPS at 24 h did not affect viability and integrity of astrocyte cultures from newborn rats (Fig. 1B–D). Thus, we chose 1 μg/mL LPS as the dose to challenge our cultured astrocytes.
The effects of LPS and resveratrol on cellular viability, integrity, and actin cytoskeleton of hippocampal astrocyte cultures. Primary hippocampal astrocyte cultures from newborn and aged rats were incubated with LPS (1 or 10 μg/ml) for 24 h in the presence or absence of resveratrol (10 μM), as depicted (A). MTT reduction (B), PI incorporation (C), and LDH extracellular activity (D) were measured. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using two-way analysis of variance (ANOVA), followed by Tukey’s test (n = 4–8 independent cultures and, at least, duplicate of treatments). Values of P < 0.05 were considered significant. a refers to statistically significant differences from the control conditions; b refers to statistically significant differences from LPS challenge. For actin cytoskeleton analysis (E), cultured astrocytes were incubated with LPS (1 μg/ml) for 24 h in the presence or absence of resveratrol (10 μM). E Representative images of the rhodamine-phalloidin labeling merged with the DAPI staining of newborn and aged astrocyte cultures. RSV, resveratrol
We also confirmed that 10 μM resveratrol did not present cytotoxic effects in hippocampal astrocytes derived from newborn and aged rats (Fig. 1B–D). Moreover, resveratrol was able to attenuate the effects of LPS on MTT reduction and extracellular LDH activity in aged astrocyte cultures.
Newborn and aged astrocytes showed significant staining for actin, the major determinant of the cell morphology, with parallel arrangement of stress fiber organization (Fig. 1E). LPS (1 μg/mL) caused a slight actin reorganization, particularly in aged astrocytes, which did not occur in the presence of resveratrol.
How Do LPS and Resveratrol Modulate the Inflammatory Response and the Senescence Marker p21 in Cultured Hippocampal Astrocytes from Newborn and Aged Animals?
LPS (1 μg/mL) caused a markedly increase in the release of TNF-α (Fig. 2A; P < 0.0001) and IL-1β (Fig. 2B; P < 0.0001) in aged astrocytes. As a comparative, we also exposed newborn astrocyte cultures with LPS 1 μg/mL and as expected, it was observed an increase in the release of TNF-α and IL-1β (Fig. 2A, B; P < 0.0001 and P = 0.0014, respectively), but it was weaker than in aged astrocytes (P < 0.0001). Additionally, the mRNA expression of both cytokines was upregulated in aged astrocyte cultures (Fig. 2C, D; P < 0.0001), but not in newborn astrocytes (Fig. 2C, D). Notably, aged astrocytes showed increased release and expression of TNF-α and IL-1β compared to newborn astrocytes at control conditions.
LPS and resveratrol modulate inflammatory response and senescence marker p21 in cultured hippocampal astrocytes from newborn and aged animals. Primary hippocampal astrocyte cultures from newborn and aged rats were incubated with LPS (1 μg/ml) for 24 h in the presence or absence of resveratrol (10 μM)–experimental design depicted in Fig. 1A. The extracellular levels of TNF-α (A) and IL-1β (B), and the mRNA expressions of TNF-α (C), IL-1β (D), and p21 (E) were evaluated. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using two-way analysis of variance (ANOVA), followed by Tukey’s test (n = 7–8 independent cultures and, at least, duplicate of treatments). Values of P < 0.05 were considered significant. a refers to statistically significant differences from the control conditions; b refers to statistically significant differences from LPS challenge. The lines indicate differences between ages. RSV, resveratrol
We then investigated the glial anti-inflammatory effect of resveratrol on newborn and aged astrocytes. Resveratrol prevented LPS-induced inflammation (both expression and release of cytokines), as well as decreased extracellular levels of TNF-α (P = 0.0021) in unstimulated aged astrocytes (Fig. 2C, D).
In addition, we confirmed that aged astrocytes express increased mRNA levels of p21 (90%, P = 0.0002), a cellular senescence marker, compared to newborn cultures (Fig. 2E). Moreover, although LPS induced a non-statistically significant increase in p21 mRNA expression in newborn astrocytes (Fig. 2E; P = 0.0806), it markedly upregulated p21 in aged astrocytes (P < 0.0001), threefold higher than in neonatal cultures. Interestingly, resveratrol not only prevented the LPS-induced increase in p21, as maintained its expression similar to newborn levels in unstimulated aged astrocytes (Fig. 2E).
This first set of experiments was performed to ensure that cultured astrocytes obtained from aged (365 days old) rats represent a model for studying aging, as they present an exacerbation of inflammatory response and senescence marker when compared to newborn cultures. Therefore, to study the glioprotective properties of resveratrol, the subsequent experiments were performed only in hippocampal astrocytes obtained from aged animals.
Resveratrol Exerts Additional Glial Anti-inflammatory Effects on Aged Astrocytes
To further investigate the effect of resveratrol on inflammatory response, we analyzed the extracellular levels of IL-6, MCP-1, and IL-10 released by aged astrocytes. Resveratrol prevented the increase in IL-6 (Fig. 3B) and MCP-1 (Fig. 3C) levels induced by LPS (P < 0.0001, LPS versus control conditions). Moreover, resveratrol per se decreased the release of MCP-1 (P = 0.0371). However, LPS increased IL-10 release (Fig. 3D; P < 0.0001), and resveratrol did not change this effect. Moreover, in unstimulated astrocytes, resveratrol also promoted an increase in IL-10 levels (Fig. 3C; P = 0.0025). To investigate the putative role of HO-1 on anti-inflammatory activity of resveratrol, aged astrocytes were co-incubated with ZnPP IX; in the presence of this HO-1 inhibitor, resveratrol did not show its anti-inflammatory effect per se, as well as did not prevent LPS-induced inflammation (Fig. S1).
The effects of LPS and resveratrol on the release of IL-6, MCP-1, and IL-10 in aged astrocytes. Primary hippocampal astrocyte cultures from aged rats were incubated with LPS (1 μg/ml) for 24 h in the presence or absence of resveratrol (10 μM), as depicted in (A). The extracellular levels of IL-6 (B), MCP-1 (C), and IL-10 (D) were evaluated. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s test (n = 8 independent cultures and, at least, duplicate of treatments). Values of P < 0.05 were considered significant. a refers to statistically significant differences from the control conditions; b refers to statistically significant differences from LPS challenge. RSV, resveratrol
Resveratrol Modulates Inflammatory Signaling in Aged Astrocytes
We further investigated inflammatory pathways associated with LPS stimulation as potential targets for resveratrol-mediated glioprotection in hippocampal aged astrocytes. The expression of TLR4 (Fig. 4A) and TLR2 (Fig. 4B) was markedly increased by LPS (P < 0.0001), and resveratrol prevented this upregulation.
Resveratrol modulates LPS-induced inflammatory signaling in aged astrocytes. Primary hippocampal astrocyte cultures from aged rats were incubated with LPS (1 μg/ml) for 24 h in the presence or absence of resveratrol (10 μM) – experimental design depicted in Fig. 3A. mRNA expressions of TLR4 (A) and TLR2 (B), and levels of p38 MAPK (C), nuclear content of p65 NFκB (D), mRNA expressions of p65 NFκB (E), and COX-2 (F) were evaluated. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s test (n = 5–7 independent cultures and, at least, duplicate of treatments). Values of P < 0.05 were considered significant. a refers to statistically significant differences from the control conditions; b refers to statistically significant differences from LPS challenge. RSV, resveratrol
Activation of TLRs is closely related to p38 MAPK/NFκB signaling pathway [14]. In agreement with that, we found increased levels of p38 MAPK (Fig. 4C) and nuclear p65 NFκB content (Fig. 4D) in LPS-stimulated astrocytes (P < 0.0001). Moreover, LPS also increased mRNA expression of p65 NFκB (Fig. 4E; P < 0.0001). In the presence of resveratrol, however, p38 MAPK- and NFκB-related pro-inflammatory effects were not observed.
In addition, the mRNA expression of the COX-2, a possible target of NFκB, (Fig. 4F), was also upregulated in aged astrocytes after LPS exposure (P < 0.0001). Resveratrol also prevented this effect.
Resveratrol Prevents LPS-Induced Redox Imbalance in Aged Astrocytes
Although the mRNA levels of iNOS (Table 1) were not affected neither by LPS or resveratrol, LPS increased DCFH oxidation (Table 1; P < 0.0001), which was used to evaluate ROS/RNS production. Resveratrol did not present an antioxidant activity per se, but it partially prevented LPS-induced increase in DCFH oxidation. Alterations in mitochondrial function can be associated with redox imbalance; however, we did not observe any change in the ΔΨm evaluated by the JC-1 assay (Table 1).
Moreover, inflammation and imbalance in ROS/RNS levels can induce damage to biomolecules. Here, we found that RNA oxidation was markedly increased in astrocytes challenged with LPS (Table 1; P < 0.0001), and resveratrol totally prevented this effect.
With regard to antioxidant defenses, LPS had no effect on GSH levels (Table 1), but resveratrol per se improved the astrocytic content of GSH (P = 0.0004), in addition to maintain higher GSH levels in LPS-stimulated astrocytes (P = 0.0011). The activity of GCL, a key enzyme in GSH biosynthesis, was impaired by LPS challenge (Table 1; P = 0.0014), while resveratrol was able to prevent this effect. Similarly, LPS decreased mRNA expression of GCL (Table 1; P = 0.0099), but not in the presence of resveratrol. In unstimulated astrocytes, resveratrol upregulated mRNA levels of GCL (P = 0.0225), but this result was not accompanied by a significant increase in the enzymatic activity.
GSH also participates in the enzymatic reaction of GPx. Cultured aged astrocytes challenged with LPS presented a higher activity of GPx (Table 1; P = 0.0261). Resveratrol, in turn, re-established GPx activity to control levels.
Resveratrol and LPS Modulate Glutamate Metabolism in Aged Astrocytes
Afterwards, we investigated the effects of LPS and resveratrol on glutamate metabolism. Table 1 displays that LPS decreased glutamate uptake (P = 0.0139) and that resveratrol prevented this effect. Moreover, resveratrol per se was able to increase glutamate uptake (P = 0.0024). Alternatively, we co-incubated aged astrocytes with HO-1 inhibitor ZnPP IX or p38 MAPK inhibitor SB203580 to test the roles of these pathways in the modulation of glutamate uptake. Inhibition of HO-1 abolished the effect of resveratrol (Fig. S2A), while the inhibition of p38 MAPK affects the action of LPS on glutamate uptake (Fig. S2B).
LPS also induced a decrease in mRNA levels of glutamate transporters GLAST (50%, P = 0.0009) and GLT-1 (55%, P < 0.0001). Resveratrol prevented only the effects on GLT-1 expression, as well as increased per se the mRNA levels of GLT-1 (Table 1; P = 0.0007). Moreover, we found that glutamate uptake was positively correlated with the mRNA expression of both GLAST (r = 0.4714 and P = 0.02; data not shown) and GLT-1 (r = 0.7724 and P < 0.0001; data not shown).
After taken up by astrocytes, glutamate might be converted into glutamine by the enzyme GS. In LPS-stimulated astrocytes (Table 1; P = 0.0170 and P = 0.0001, respectively), GS activity and GS expression were decreased, and resveratrol totally prevented these effects. Resveratrol also increased mRNA levels of GS (P = 0.0017). GS activity and expression were also positively correlated (r = 0.8600 and P < 0.0001; data not shown).
Resveratrol Prevents LPS-Induced Decrease in Trophic Factor Release
We also evaluated the release of BDNF and GDNF (Table 1) after LPS challenge. LPS decreased the levels of BDNF (P < 0.0001) and GDNF (P < 0.0001). Resveratrol prevented both effects induced by LPS on trophic factors. Moreover, resveratrol per se increased the release of BDNF and GDNF (P < 0.0001).
Putative Signaling Pathways Associated with Resveratrol-Mediated Glioprotection Against LPS-Induced Gliotoxicity in Aged Astrocytes
SIRT1, Nrf2, and HO-1 are important mechanisms involved in the glioprotective effects of resveratrol [13, 31]. Aged astrocytes had mRNA levels of SIRT1 (Fig. 5A), Nrf2 (Fig. 5B), and HO-1 (Fig. 5C) decreased after LPS challenge (P = 0.0428 for SIRT1; P = 0.037 for Nrf2; P = 0.0013 for HO-1). Besides to totally prevent these effects, resveratrol maintained SIRT1 expression higher than control levels in LPS-stimulated astrocytes (P = 0.0118). Importantly, resveratrol significantly upregulated mRNA expression of SIRT1 (P < 0.0001), Nrf2 (P = 0.0003), and HO-1 (P = 0.0433) in unstimulated aged astrocytes. This data regarding HO-1 expression is in accordance with the results incubating aged astrocytes with HO-1 inhibitor, reinforcing the role of Nrf2/HO-1 in resveratrol-mediated glioprotection.
SIRT1, Nrf2, and HO-1 signaling pathways are associated with resveratrol-mediated glioprotection. Primary hippocampal astrocyte cultures from aged rats were incubated with LPS (1 μg/ml) for 24 h in the presence or absence of resveratrol (10 μM) – experimental design depicted in Fig. 3A. The mRNA expressions of SIRT1 (A), Nrf2 (B), and HO-1 (C) were evaluated. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s test (n = 6 independent cultures and, at least, duplicate of treatments). Values of P < 0.05 were considered significant. a refers to statistically significant differences from the control conditions; b refers to statistically significant differences from LPS challenge. RSV, resveratrol
LPS Challenge In vivo Induces Peripheral and Central Inflammatory Responses and Alters Gene Expression in Hippocampus
To confirm that our in vitro experimental model of inflammation may reproduce the main observations of an in vivo acute systemic inflammation, we challenged aged rats (365 days old) with LPS (intraperitoneal) and evaluated biochemical, inflammatory, and glial parameters in blood serum, CSF, or hippocampal tissue 24 h after LPS administration (Fig. 6A). LPS induced an increase in the levels of TNF-α, IL-1β, IL-6, and MCP-1, but decreased IL-10, in all samples analyzed (Table 2 and Fig. 6B–F). The biochemical parameters such as glucose, cholesterol, and triglycerides were measured in the blood and were not altered by LPS administration (data not shown). Additionally, we found that LPS increased the mRNA levels of TNF-α (P < 0.0001; Fig. 6G), IL-1β (P < 0.0001; Fig. 6H), TLR4 (P < 0.0001; Fig. 6J); TLR2 (P < 0.0001; Fig. 6K), p65 NFκB (P < 0.0001; Fig. 6L), and Nrf2 (P < 0.0001; Fig. 6N), and decreased HO-1 (P < 0.0001; Fig. 6O) in hippocampal tissue. The expression of SIRT1 (Fig. 6M) and p21 (Fig. 6I) did not change in hippocampus after acute systemic inflammation.
In vivo injection of LPS changes gene expression in hippocampal tissue of aged rats. Aged (365 days old) Wistar rats were intraperitonially injected with LPS or saline (control). A How blood serum, CSF, and hippocampal tissue were collected after 24 h for further analyses, and astrocyte cultures were prepared. The levels of TNF-α (B), IL-1β (C), IL-6 (D), MCP-1 (E), and IL-10 (F), and the mRNA expressions of TNF-α (G), IL-1β (H), p21 (I), TLR4 (J), TLR2 (K), p65 NFκB (L), SIRT1 (M), Nrf2 (N), and HO-1 (O) were evaluated in hippocampal tissue. Data are presented as mean ± S.D. and differences between control and LPS groups were statistically analyzed using Student’s t test (n = 5–8 animals per group and, at least, duplicate of samples). Values of P < 0.05 were considered significant. The asterisk indicates statistically significant differences. RSV, resveratrol
Glioprotective Effects of Resveratrol In vitro After LPS Challenge In vivo in Aged Astrocytes
To further characterize the glioprotective role of resveratrol, we prepared astrocyte cultures from these aged rats stimulated in vivo with LPS. After cellular confluence, we treated these cells with 10 μM resveratrol for 24 h. It is important to note that we did not observe any change in cellular viability (data not shown). To determine the glial anti-inflammatory effect of resveratrol, we then evaluated the levels of TNF-α, IL-1β, IL-6, MCP-1, and IL-10, as well as the mRNA expression of TNF-α and IL-1β. We observed an enhanced inflammatory response in astrocytes derived from LPS-stimulated rats compared to those obtained from control rats, as indicated by the increase in extracellular content and mRNA levels of TNF-α (P < 0.0001 and P = 0.0003; Fig. 7A and B, respectively) and IL-1β (P < 0.0001 and P = 0.0005; Fig. 7C and D, respectively). In addition, the release of IL-6 (P < 0.0001; Fig. 7E) and MCP-1 (P < 0.0001; Fig. 7F) was increased, while IL-10 levels were decreased (P < 0.0001; Fig. 7G). In vitro treatment with resveratrol re-established the extracellular content of pro-inflammatory cytokines near to control values, except for IL-10. Resveratrol did not also reduce the mRNA expression of TNF-α and IL-1β. Regarding cultured astrocytes obtained from control rats, the effects of resveratrol followed a similar profile as described for unstimulated astrocytes in the previous sections.
Glioprotective effects of resveratrol in vitro after LPS challenge in vivo in aged rats. Aged (365 days old) Wistar rats were intraperitonially injected with LPS or saline (control). After 24 h, astrocyte cultures were prepared and treated with resveratrol (10 μM; 24 h) at confluence, according to the experimental design depicted in Fig. 6A. The extracellular levels of TNF-α (A), IL-1β (C), IL-6 (E), MCP-1 (F), and IL-10 (G), and the mRNA expressions of TNF-α (B) and IL-1β (D) were evaluated. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using two-way analysis of variance (ANOVA), followed by Tukey’s test (n = 6–7 independent cultures and, at least, duplicate of treatments). Values of P < 0.05 were considered significant. a refers to statistically significant differences from the control conditions; b refers to statistically significant differences from LPS challenge. RSV, resveratrol
To demonstrate the potential signaling pathways associated with the resveratrol-mediated glioprotection, we measured the mRNA levels of p21, TLR4, TLR2, p65 NFκB, SIRT1, Nrf2, and HO-1. Figure 8 displays that astrocyte cultures from LPS-stimulated rats presented an increase in the mRNA expression of p21 (Fig. 8A, P < 0.0001), TLR2 (Fig. 8C, P = 0.0027), p65 NFκB (Fig. 8D, P = 0.0027), and HO-1 (Fig. 8G, P < 0.0001) compared to those derived from control animals, while the expression of SIRT1 was decreased (8E, P = 0.0129). Nrf2 (Fig. 8F) and TLR4 mRNA levels (Fig. 8B) were not changed. The treatment with resveratrol in this experimental model re-established the alterations on NFκB and SIRT1, and increased Nrf2 and HO-1. However, resveratrol did not change p21 and TLR2 expression. These findings support Nrf2, HO-1, and SIRT1 as classical pathways associated with glioprotective role of resveratrol.
Resveratrol changes gene expression of cultured astrocytes obtained from in vivo LPS-injected aged rats. Aged (365 days old) Wistar rats were intraperitonially injected with LPS or saline (control). After 24 h, astrocyte cultures were prepared and treated with resveratrol (10 μM; 24 h) at confluence, according to the experimental design depicted in Fig. 6A. The mRNA expressions of p21 (A), TLR4 (B), TLR2 (C), p65 NFκB (D), SIRT1 (E), Nrf2 (F), and HO-1 (G) were evaluated. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using two-way analysis of variance (ANOVA), followed by Tukey’s test (n = 6 independent cultures and, at least, duplicate of treatments). Values of P < 0.05 were considered significant. a refers to statistically significant differences from the control conditions; b refers to statistically significant differences from LPS challenge. RSV, resveratrol
Discussion
Increasing evidence has pointed astrocytes as a significant source of the pro-inflammatory mediators involved in neuro-inflammaging [15, 47, 48]. The phenomenon of inflammaging is defined as the low-grade inflammation typical of aging, as a result of higher levels of pro-inflammatory markers in cells and tissues of older organisms, which becomes potentially detrimental and acts as a key element for the pathogenesis of neurodegenerative diseases [49, 50]. Importantly, in addition to induce a chronic inflammation, aging can reduce the ability of the cells to adapt to environmental changes and challenging stimuli [17, 20, 48, 51, 52]. Although a previous study from our research group has showed that resveratrol improved some functional parameters and attenuated the release of inflammatory cytokines in mature astrocytes (from 90 and 180 days old Wistar rats) at basal conditions [38], here we studied the potential glioprotective role of resveratrol in chronically inflamed astrocytes from older animals challenged with LPS. For this, primary astrocyte cultures obtained from hippocampus of aged Wistar rats (1-year old) were exposed to LPS (in vitro experimental model), as well as we prepared hippocampal astrocyte cultures from aged rats that received an in vivo LPS injection. Collectively, we demonstrated that resveratrol was able not only to prevent LPS-induced gliotoxicity in vitro, but also to reverse an inflammatory hallmark left in astrocytes by an in vivo LPS injection.
Our previous studies have demonstrated that cultured astrocytes derived from different brain regions (hippocampus, cortex, and hypothalamus) of rats at adult and post-reproductive ages express and/or release increased amounts of pro-inflammatory mediators [17, 18, 39, 51, 54]. Here, we confirm that cultured astrocytes from aged (365 days old) rats in fact presented a marked pro-inflammatory status compared to cultured astrocytes obtained from newborn animals, in addition to have an increased expression of p21 senescence marker, supporting the glial-inflammaging process at basal conditions. Moreover, the acute in vitro inflammatory stimulus with LPS produces a powerful inflammatory response in aged astrocytes compared to newborn astrocytes.
Since endogenous protective mechanisms of astrocytes may be insufficient to counteract age-related and exogenously induced inflammation, glioprotective molecules — namely molecules with ability to induce protective responses in glial cells — can represent important strategies to promote a healthier brain aging. In this context, the glioprotective potential of resveratrol in different conditions has received increasingly attention [13, 25, 26, 54, 55]. Here, we observed that resveratrol prevented LPS-induced expression and release of classical cytokines and inflammatory signaling molecules in newborn and aged astrocytes, but especially in aged astrocytes. It is important to note that the upregulation of gene expression can be associated with a further persistent inflammatory response [56, 57]. Reinforcing its glioprotective role, resveratrol was able to prevent the upregulation of p21 in cultured astrocytes stimulated with LPS in vitro, in addition to decrease the expression of p21 by aged astrocytes at basal (unstimulated) conditions. Particularly in the hippocampus, neuroinflammation and cellular senescence can promote several functional alterations that affect neural plasticity, thus contributing to the cognitive decline observed with aging [58, 59]. Thus, these protective actions of resveratrol in aged astrocytes can represent an important strategy to maintain hippocampal functionality.
ROS overproduction and biomolecule damage are processes that can induce cellular dysfunctions and are frequently associated with both aging and inflammatory responses [2, 60]. Resveratrol also displayed an antioxidant effect in aged astrocytes against LPS in vitro exposure by preventing the increase of ROS production and RNA oxidation. The close proximity of RNA to mitochondria in the cytosol and its single-stranded structure make RNA vulnerable to oxidation, which can impact protein synthesis and structure, as well as the functions of non-coding RNAs in the brain, and have been linked to neurodegenerative diseases [61]. Moreover, the levels of GSH decrease in astrocyte cultures and in brain tissue with age [17, 51, 62]. Although LPS had no effect, resveratrol could improve GSH levels in aged astrocytes due to the stimulation of GSH synthesis, targeting GCL expression and/or activity, potentially increasing the endogenous ability of these cells to maintain redox homeostasis and respond to oxidative challenges. It is important to note that our group has showed the positive effect of resveratrol on GSH metabolism [25, 35, 38, 63].
Regulation of neurotransmitter systems and trophic signaling are other important functions mediated by astrocytes. Although glutamate is a critical neurotransmitter involved in hippocampal functions such as memory and learning, its excitatory neurotransmission must be tightly regulated to achieve efficient synaptic communication and avoid excitotoxicity [64,65,66]. In this regard, glutamate transporter downregulation and/or hypofunction in astrocytes impair glutamate uptake and has been naturally observed with aging [17, 51, 64, 67]. In our study, resveratrol improved glutamate uptake, which was correlated with increases in the expression of GLAST and GLT-1 in aged astrocytes in both control and inflammatory conditions. Of note, glutamate uptake can be regulated by several mechanisms, which include gene expression, post-translational modifications, and trafficking to and from the plasma membrane of glutamate transporters [68, 69]. Downregulation of glutamate transporters can be induced by p38 MAPK signaling [70] and may represent a mechanism by which LPS compromises glutamate uptake. Since the activity of glutamate transporters is highly susceptible to oxidation [71], we may also hypothesize that resveratrol improved glutamate uptake due to its antioxidant properties and related pathways, including HO-1 [26]. However, although our study has indicated changes in both mRNA expression and activity of glutamate transporters, further studies are needed to deeply investigate the mechanisms involved in LPS- and resveratrol-mediated effects on glutamate uptake. The expression and activity of GS, an astrocytic enzyme responsible for conversion of glutamate to glutamine that is highly sensitive to oxidative stress [72], was likewise positively modulated by resveratrol. Trophic support mediated by astrocytes is also essential for hippocampal function and plasticity, but can be compromised by aging and inflammation [13, 17, 73,74,75]. Similarly to the effects on glutamate metabolism, resveratrol enhanced the release of BDNF and GDNF by aged astrocytes, reinforcing its glioprotective action.
Resveratrol-mediated glioprotection may be associated with several signaling pathways. Nrf2 is a transcription factor that induces the expression of genes associated with antioxidant, anti-inflammatory, and cytoprotective responses, including GCL and HO-1. Nrf-2/HO-1 signaling has been increasingly proposed as an important mechanism underlying the protective roles of resveratrol [25, 26, 63, 76] and may be implicated in the attempt of astrocytes to combat oxidative and inflammatory responses within themselves and neighboring neural cells [77, 78]. Moreover, resveratrol classically activates SIRT1, which can also downregulate inflammatory genes by targeting NFκB transcriptional activity [79]. Importantly, resveratrol prevented the decrease of the expression of Nrf2, HO-1 and SIRT1 in aged astrocytes exposed to LPS in vitro, in addition to upregulate these genes at basal conditions. Importantly, the effect of resveratrol in promoting not only the direct activation of these proteins, as typically described, but also their gene expression may indicate the induction of an adaptive reprogramming of signaling pathways in astrocytes.
Cultured astrocytes obtained from aged rats can represent a relevant tool for studying cellular, neurochemical, and molecular alterations underlying the aging process in astrocytes, as well as their response to external inflammatory stimuli and potential glioprotective strategies. Importantly, this in vitro model could reproduce major molecular changes observed during an in vivo acute systemic inflammation. In this regard, the expression of pro-inflammatory genes (e.g., cytokines, TLR, p65 NFκB, and p21) was similarly increased in aged astrocytes exposed to LPS in vitro and in astrocytes obtained from aged rats stimulated with LPS in vivo. Pro-inflammatory markers were also similarly increased in the hippocampal tissue of LPS-injected animals, as well as in their CSF and blood serum. However, it is important to note that the expression of SIRT1 and p21 did not change in hippocampus after LPS challenge. Of note, cellular response to inflammatory stimuli may vary dependent on the presence or absence of other cell types, such as microglial cells [80], as observed with the LPS stimulation in vitro or in vivo and the results from astrocyte cultures and hippocampal tissue. These data are in agreement with a previous study from our group that showed an overexpression of inflammatory mediators in cultured astrocytes obtained from animals submitted to a severe systemic inflammation model induced by cecal ligation and perforation [80].
Resveratrol, in turn, also had some different effects depending on the inflammatory experimental model used. In aged astrocytes exposed to LPS in vitro, resveratrol was incubated for 1 h prior the addition of LPS and totally prevented its pro-inflammatory effects. However, in cultured astrocytes obtained from aged animals previously subjected to an in vivo LPS injection, resveratrol was not able to completely inhibit the pro-inflammatory status presented by astrocytes in culture, particularly regarding to gene expression of TNF-α, IL-1β, TLR2, and p21, which can indicate a chronic inflammation. These results suggest that resveratrol can act more effectively in preventing rather than reversing inflammatory responses. In addition, anti-inflammatory and cytoprotective molecules (IL-10, Nrf2 and HO-1) were differently modulated in cultured astrocytes exposed to LPS in vitro and in astrocytes obtained from animals stimulated with LPS in vivo, suggesting different cellular adaptive responses. Interestingly, resveratrol was able to recover the downregulation of SIRT1 in astrocytes obtained from LPS-animals, in addition to potentiate the upregulation of Nrf2 and HO-1, reinforcing its ability to enhance cytoprotective pathways in glial cells. Particularly regarding IL-10, resveratrol increased the release of this cytokine at basal conditions, but it did not modulate the different effects of LPS (increase of IL-10 in the in vitro LPS stimulation and decrease of IL-10 in the in vivo LPS stimulation). It is possible to conceive a complex interaction of signaling pathways simultaneously activated in the presence of LPS, resveratrol or both stimuli in the cells, which culminates in a specific astrocyte response. While LPS may stimulate IL-10 expression through NFκB induction [81], probably as a compensatory mechanism for the acute inflammation, resveratrol may increase IL-10 in a mechanism dependent on HO-1 signaling, as suggested by our data and previous reports [82]. Moreover, it is important to note that the inflammatory response produced by LPS injection in vivo can leave inflammatory marks and produce cellular adaptations that could be later observed in astrocytes in culture, in contrast to the in vitro stimulation of isolated astrocytes [40]. Astrocytes derived from animals challenged with LPS may also produce and release a different profile of signaling molecules throughout the culture period compared to astrocytes derived from control or naive animals, which we can speculate that impact in the astrocyte gene expression and functionality [83, 84].
Considering that astrocytes have been emerged as potential targets for protection during aging process, with this study we intended to address the effects of resveratrol in the major pathomechanisms associated with aging (Fig. 9). In summary, our data showed that aging can make astrocytes more responsive to inflammation, contributing to the impairment of astrocyte ability to promote neuroprotection/glioprotection, which may exacerbate neuronal injury and trigger neurodegenerative processes. On the contrary, resveratrol can exert an important glioprotective role, preparing aged astrocytes to better respond to external challenging stimuli and thus contributing to a healthier brain aging.
Schematic illustration of the effects of LPS and resveratrol in primary astrocyte cultures obtained from aged Wistar rats. LPS can induce gliotoxicity (represented by the balance at left with reactive/dysfunctional astrocyte), while resveratrol can promote glioprotection (represented by the balance at right with ramified/functional astrocyte) by several cellular and molecular mechanisms, which contribute to maintenance of astrocyte functionality. RSV, resveratrol
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Partridge L, Deelen J, Slagboom PE (2018) Facing up to the global challenges of ageing. Nature 561:45–56. https://doi.org/10.1038/s41586-018-0457-8
López-Otín C, Blasco MA, Partridge L et al (2013) The Hallmarks of aging. Cell 153:1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Shivarama Shetty M, Sajikumar S (2017) ‘Tagging’ along memories in aging: synaptic tagging and capture mechanisms in the aged hippocampus. Ageing Res Rev 35:22–35. https://doi.org/10.1016/j.arr.2016.12.008
Burke SN, Barnes CA (2006) Neural plasticity in the ageing brain. Nat Rev Neurosci 7:30–40. https://doi.org/10.1038/nrn1809
Rosenzweig ES, Barnes CA (2003) Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol 69:143–179. https://doi.org/10.1016/S0301-0082(02)00126-0
Valori CF, Guidotti G, Brambilla L, Rossi D (2019) Astrocytes: emerging therapeutic targets in neurological disorders. Trends Mol Med 25:750–759. https://doi.org/10.1016/j.molmed.2019.04.010
Mederos S, González-Arias C, Perea G (2018) Astrocyte–neuron networks: a multilane highway of signaling for homeostatic brain function. Front Synaptic Neurosci 10:45. https://doi.org/10.3389/fnsyn.2018.00045
Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431. https://doi.org/10.1016/j.tins.2009.05.001
Bolaños JP (2016) Bioenergetics and redox adaptations of astrocytes to neuronal activity. J Neurochem 139:115–125. https://doi.org/10.1111/jnc.13486
Gonçalves C-A, Rodrigues L, Bobermin LD et al (2018) Glycolysis-Derived compounds from astrocytes that modulate synaptic communication. Front Neurosci 12:1035. https://doi.org/10.3389/fnins.2018.01035
Colombo E, Farina C (2016) Astrocytes: key regulators of neuroinflammation. Trends Immunol 37:608–620. https://doi.org/10.1016/j.it.2016.06.006
Jensen CJ, Massie A, De Keyser J (2013) Immune players in the CNS: the astrocyte. J Neuroimmune Pharmacol 8:824–839. https://doi.org/10.1007/s11481-013-9480-6
Bobermin LD, Roppa RHA, Quincozes-Santos A (2019) Adenosine receptors as a new target for resveratrol-mediated glioprotection. Biochim Biophys Acta Mol Basis Dis 1865:634–647. https://doi.org/10.1016/j.bbadis.2019.01.004
Gorina R, Font-Nieves M, Márquez-Kisinousky L et al (2011) Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia 59:242–255. https://doi.org/10.1002/glia.21094
Palmer AL, Ousman SS (2018) Astrocytes and aging Front Aging Neurosci 10:337. https://doi.org/10.3389/fnagi.2018.00337
Souza DG, Bellaver B, Souza DO, Quincozes-Santos A (2013) Characterization of adult rat astrocyte cultures. PLoS ONE 8:e60282. https://doi.org/10.1371/journal.pone.0060282
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2017) Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Mol Neurobiol 54:2969–2985. https://doi.org/10.1007/s12035-016-9880-8
Santos CL, Roppa PHA, Truccolo P et al (2018) Age-dependent neurochemical remodeling of hypothalamic astrocytes. Mol Neurobiol 55:5565–5579. https://doi.org/10.1007/s12035-017-0786-x
Longoni A, Bellaver B, Bobermin LD et al (2018) Homocysteine induces glial reactivity in adult rat astrocyte cultures. Mol Neurobiol 55:1966–1976. https://doi.org/10.1007/s12035-017-0463-0
Santos CL, Bobermin LD, Souza DO, Quincozes-Santos A (2018) Leptin stimulates the release of pro-inflammatory cytokines in hypothalamic astrocyte cultures from adult and aged rats. Metab Brain Dis 33:2059–2063. https://doi.org/10.1007/s11011-018-0311-6
Wyss-Coray T (2016) Ageing, neurodegeneration and brain rejuvenation. Nature 539:180–186. https://doi.org/10.1038/nature20411
Davinelli S, Maes M, Corbi G et al (2016) Dietary phytochemicals and neuro-inflammaging: from mechanistic insights to translational challenges. Immun Ageing 13:16. https://doi.org/10.1186/s12979-016-0070-3
Franceschi C (2007) Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev 65:S173-176. https://doi.org/10.1111/j.1753-4887.2007.tb00358.x
Quincozes-Santos A, Gottfried C (2011) Resveratrol modulates astroglial functions: neuroprotective hypothesis: resveratrol modulates astroglial functions. Ann N Y Acad Sci 1215:72–78. https://doi.org/10.1111/j.1749-6632.2010.05857.x
Quincozes-Santos A, Bobermin LD, Latini A et al (2013) Resveratrol Protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS ONE 8:e64372. https://doi.org/10.1371/journal.pone.0064372
Bellaver B, Bobermin LD, Souza DG et al (2016) Signaling mechanisms underlying the glioprotective effects of resveratrol against mitochondrial dysfunction. Biochim Biophys Acta BBA - Mol Basis Dis 1862:1827–1838. https://doi.org/10.1016/j.bbadis.2016.06.018
Rosa PM, Martins LAM, Souza DO, Quincozes-Santos A (2018) Glioprotective effect of resveratrol: an emerging therapeutic role for oligodendroglial cells. Mol Neurobiol 55:2967–2978. https://doi.org/10.1007/s12035-017-0510-x
Bonkowski MS, Sinclair DA (2016) Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol 17:679–690. https://doi.org/10.1038/nrm.2016.93
Bhullar KS, Hubbard BP (2015) Lifespan and healthspan extension by resveratrol. Biochim Biophys Acta BBA - Mol Basis Dis 1852:1209–1218. https://doi.org/10.1016/j.bbadis.2015.01.012
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5:493–506. https://doi.org/10.1038/nrd2060
Price NL, Gomes AP, Ling AJY et al (2012) SIRT1 Is Required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15:675–690. https://doi.org/10.1016/j.cmet.2012.04.003
Hwang J, Yao H, Caito S et al (2013) Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med 61:95–110. https://doi.org/10.1016/j.freeradbiomed.2013.03.015
Lee S-H, Lee J-H, Lee H-Y, Min K-J (2019) Sirtuin signaling in cellular senescence and aging. BMB Rep 52:24–34. https://doi.org/10.5483/BMBRep.2019.52.1.290
Sarubbo F, Esteban S, Miralles A, Moranta D (2018) Effects of resveratrol and other polyphenols on Sirt1: relevance to brain function during aging.Curr Neuropharmacol 16https://doi.org/10.2174/1570159X15666170703113212
Bellaver B, Souza DG, Bobermin LD et al (2015) Resveratrol Protects hippocampal astrocytes against lps-induced neurotoxicity through HO-1, p38 and erk pathways. Neurochem Res 40:1600–1608. https://doi.org/10.1007/s11064-015-1636-8
Bobermin LD, Quincozes-Santos A, Guerra MC et al (2012) Resveratrol prevents ammonia toxicity in astroglial cells. PLoS ONE 7:e52164. https://doi.org/10.1371/journal.pone.0052164
Allen EN, Potdar S, Tapias V et al (2018) Resveratrol and pinostilbene confer neuroprotection against aging-related deficits through an ERK1/2-dependent mechanism. J Nutr Biochem 54:77–86. https://doi.org/10.1016/j.jnutbio.2017.10.015
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2014) Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol Vitro Int J Publ Assoc BIBRA 28:479–484. https://doi.org/10.1016/j.tiv.2014.01.006
Bobermin LD, Roppa RHA, Gonçalves C-A, Quincozes-Santos A (2020) ammonia-induced glial-inflammaging. Mol Neurobiol 57:3552–3567. https://doi.org/10.1007/s12035-020-01985-4
Guerra M, Tortorelli LS, Galland F et al (2011) Lipopolysaccharide modulates astrocytic S100B secretion: a study in cerebrospinal fluid and astrocyte cultures from rats. J Neuroinflammation 8:128. https://doi.org/10.1186/1742-2094-8-128
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif 25:402–408. https://doi.org/10.1006/meth.2001.1262
Quincozes-Santos A, Nardin P, de Souza DF et al (2009) The janus face of resveratrol in astroglial cells. Neurotox Res 16:30–41. https://doi.org/10.1007/s12640-009-9042-0
Reers M, Smiley ST, Mottola-Hartshorn C et al (1995) Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol 260:406–417. https://doi.org/10.1016/0076-6879(95)60154-6
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. free radical and antioxidant protocols. Humana Press, New Jersey, pp 347–352
Seelig GF, Meister A (1985) Glutathione biosynthesis; gamma-glutamylcysteine synthetase from rat kidney. Methods Enzymol 113:379–390. https://doi.org/10.1016/s0076-6879(85)13050-8
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Cohen J, Torres C (2019) Astrocyte senescence: evidence and significance. Aging Cell 18:e12937. https://doi.org/10.1111/acel.12937
Clarke LE, Liddelow SA, Chakraborty C et al (2018) Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci 115:E1896–E1905. https://doi.org/10.1073/pnas.1800165115
Franceschi C, Garagnani P, Parini P et al (2018) Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14:576–590. https://doi.org/10.1038/s41574-018-0059-4
Calabrese V, Santoro A, Monti D et al (2018) Aging and Parkinson’s Disease: inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic Biol Med 115:80–91. https://doi.org/10.1016/j.freeradbiomed.2017.10.379
Souza DG, Bellaver B, Bobermin LD et al (2016) Anti-aging effects of guanosine in glial cells. Purinergic Signal 12:697–706. https://doi.org/10.1007/s11302-016-9533-4
Souza DG, Bellaver B, Raupp GS et al (2015) Astrocytes from adult Wistar rats aged in vitro show changes in glial functions. Neurochem Int 90:93–97. https://doi.org/10.1016/j.neuint.2015.07.016
Wyse AT, Siebert C, Bobermin LD et al (2020) Changes in inflammatory response, redox status and Na+, K+-ATPase activity in primary astrocyte cultures from female wistar rats subject to ovariectomy. Neurotox Res 37:445–454. https://doi.org/10.1007/s12640-019-00128-5
Kodali M, Parihar VK, Hattiangady B et al (2015) Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature and reduced glial activation. Sci Rep 5:8075. https://doi.org/10.1038/srep08075
Lalo U, Pankratov Y (2021) Astrocytes as perspective targets of exercise- and caloric restriction-mimetics. Neurochem Res. https://doi.org/10.1007/s11064-021-03277-2
Zhu X, Chen Z, Shen W et al (2021) Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: the regulation and intervention. Signal Transduct Target Ther 6:245. https://doi.org/10.1038/s41392-021-00646-9
Ahmed A, Williams B, Hannigan G (2015) Transcriptional activation of inflammatory genes: mechanistic insight into selectivity and diversity. Biomolecules 5:3087–3111. https://doi.org/10.3390/biom5043087
Bettio LEB, Rajendran L, Gil-Mohapel J (2017) The effects of aging in the hippocampus and cognitive decline. Neurosci Biobehav Rev 79:66–86. https://doi.org/10.1016/j.neubiorev.2017.04.030
Fan X, Wheatley EG, Villeda SA (2017) Mechanisms of hippocampal aging and the potential for rejuvenation. Annu Rev Neurosci 40:251–272. https://doi.org/10.1146/annurev-neuro-072116-031357
van Horssen J, van Schaik P, Witte M (2019) Inflammation and mitochondrial dysfunction: a vicious circle in neurodegenerative disorders? Neurosci Lett 710:132931. https://doi.org/10.1016/j.neulet.2017.06.050
Cobley JN, Fiorello ML, Bailey DM (2018) 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 15:490–503. https://doi.org/10.1016/j.redox.2018.01.008
López-Navarro ME, Jarquín-Martínez M, Sánchez-Labastida LA et al (2020) Decoding aging: understanding the complex relationship among aging, free radicals, and GSH. Oxid Med Cell Longev 2020:1–11. https://doi.org/10.1155/2020/3970860
Arús BA, Souza DG, Bellaver B et al (2017) Resveratrol modulates GSH system in C6 astroglial cells through heme oxygenase 1 pathway. Mol Cell Biochem 428:67–77. https://doi.org/10.1007/s11010-016-2917-5
Potier B, Billard J-M, Rivière S et al (2010) Reduction in glutamate uptake is associated with extrasynaptic NMDA and metabotropic glutamate receptor activation at the hippocampal CA1 synapse of aged rats: synaptic effects of reduced glutamate uptake in the aged rat hippocampus. Aging Cell 9:722–735. https://doi.org/10.1111/j.1474-9726.2010.00593.x
Todd AC, Hardingham GE (2020) The regulation of astrocytic glutamate transporters in health and neurodegenerative diseases. Int J Mol Sci 21:9607. https://doi.org/10.3390/ijms21249607
Barnes JR, Mukherjee B, Rogers BC et al (2020) The relationship between glutamate dynamics and activity-dependent synaptic plasticity. J Neurosci 40:2793–2807. https://doi.org/10.1523/JNEUROSCI.1655-19.2020
Zhang Y, Sloan SA, Clarke LE et al (2016) Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89:37–53. https://doi.org/10.1016/j.neuron.2015.11.013
Tian G, Lai L, Guo H et al (2007) Translational control of glial glutamate transporter EAAT2 expression. J Biol Chem 282:1727–1737. https://doi.org/10.1074/jbc.M609822200
Ryan RM, Ingram SL, Scimemi A (2021) Regulation of glutamate, GABA and dopamine transporter uptake, surface mobility and expression. Front Cell Neurosci 15:670346. https://doi.org/10.3389/fncel.2021.670346
Piao C, Ranaivo HR, Rusie A et al (2015) Thrombin decreases expression of the glutamate transporter GLAST and inhibits glutamate uptake in primary cortical astrocytes via the Rho kinase pathway. Exp Neurol 273:288–300. https://doi.org/10.1016/j.expneurol.2015.09.009
Miralles VJ, Martínez-López I, Zaragozá R et al (2001) Na+ dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) in primary astrocyte cultures: effect of oxidative stress. Brain Res 922:21–29. https://doi.org/10.1016/s0006-8993(01)03124-9
Hertz L (2003) Astrocytic amino acid metabolism under control conditions and during oxygen and/or glucose deprivation. Neurochem Res 28:243–258. https://doi.org/10.1023/A:1022377100379
Tong L, Balazs R, Soiampornkul R et al (2008) Interleukin-1β impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol Aging 29:1380–1393. https://doi.org/10.1016/j.neurobiolaging.2007.02.027
Lima Giacobbo B, Doorduin J, Klein HC et al (2019) Brain-Derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol Neurobiol 56:3295–3312. https://doi.org/10.1007/s12035-018-1283-6
Budni J, Bellettini-Santos T, Mina F, et al (2015) The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis 6:331. https://doi.org/10.14336/AD.2015.0825
Sakata Y, Zhuang H, Kwansa H et al (2010) Resveratrol protects against experimental stroke: putative neuroprotective role of heme oxygenase 1. Exp Neurol 224:325–329. https://doi.org/10.1016/j.expneurol.2010.03.032
Liddell J (2017) Are Astrocytes the predominant cell type for activation of nrf2 in Aging and neurodegeneration? Antioxidants 6:65. https://doi.org/10.3390/antiox6030065
Ahmed SMU, Luo L, Namani A et al (2017) Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta BBA - Mol Basis Dis 1863:585–597. https://doi.org/10.1016/j.bbadis.2016.11.005
Kauppinen A, Suuronen T, Ojala J et al (2013) Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25:1939–1948. https://doi.org/10.1016/j.cellsig.2013.06.007
Bellaver B, Dos Santos JP, Leffa DT et al (2018) Systemic inflammation as a driver of brain injury: the astrocyte as an emerging player. Mol Neurobiol 55:2685–2695. https://doi.org/10.1007/s12035-017-0526-2
Cian RE, López-Posadas R, Drago SR et al (2012) A Porphyra columbina hydrolysate upregulates IL-10 production in rat macrophages and lymphocytes through an NF-κB, and p38 and JNK dependent mechanism. Food Chem 134:1982–1990. https://doi.org/10.1016/j.foodchem.2012.03.134
Xu X, Guo Y, Zhao J et al (2017) Punicalagin, a PTP1B inhibitor, induces M2c phenotype polarization via up-regulation of HO-1 in murine macrophages. Free Radic Biol Med 110:408–420. https://doi.org/10.1016/j.freeradbiomed.2017.06.014
Rodgers KR, Lin Y, Langan TJ et al (2020) Innate Immune functions of astrocytes are dependent upon tumor necrosis factor-alpha. Sci Rep 10:7047. https://doi.org/10.1038/s41598-020-63766-2
Villarreal A, Seoane R, González Torres A et al (2014) S100B protein activates a RAGE-dependent autocrine loop in astrocytes: implications for its role in the propagation of reactive gliosis. J Neurochem 131:190–205. https://doi.org/10.1111/jnc.12790
Funding
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Universidade Federal do Rio Grande do Sul and Instituto Nacional de Ciência e Tecnologia para Excitotoxicidade e Neuroproteção (INCTEN/CNPq).
Author information
Authors and Affiliations
Contributions
AQS and LDB conceptualized the study. LDB, RRSA, FBW, LSM, and LM performed the experiments. LDB and AQS performed statistical analysis and written the original draft of the manuscript. AQS, ATSW, and CAG provided resources and materials/chemicals. All authors revised, edited, and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All animal experiments were performed in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals and Brazilian Society for Neuroscience and Behavior recommendations for animal care. The experimental protocols were approved by the Federal University of Rio Grande do Sul Animal Care and Use Committee (process number: 21215).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Figure S1.
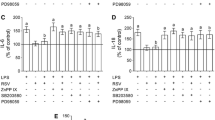
Effects of HO-1 inhibitor on cytokine release in aged astrocytes. Primary hippocampal astrocyte cultures from aged rats were incubated with LPS (1 μg/ml) for 24 h in the presence or absence of resveratrol (10 μM) and HO-1 inhibitor ZnPP IX (10 μM). The extracellular levels of TNF-α (A), IL-1β (B), IL-6 (C), MCP-1 (D), and IL-10 (E) were evaluated. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s test (n = 6 independent cultures and, at least, duplicate of treatments). Values of P < 0.05 were considered significant. a refers to statistically significant differences from the control conditions; b refers to statistically significant differences from LPS challenge. RSV, resveratrol. (PNG 14239 kb)

Figure S2.
Effects of HO-1 and p38 MAPK inhibitors on glutamate uptake in aged astrocytes. Primary hippocampal astrocyte cultures from aged rats were incubated with LPS (1 μg/ml) for 24 h in the presence or absence of resveratrol (10 μM) and HO-1 inhibitor ZnPP IX (10 μM) or p38 MAPK inhibitor SB203580 (10 μM), A and B, respectively. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s test (n = 6 independent cultures and, at least, duplicate of treatments). Values of P < 0.05 were considered significant. a refers to statistically significant differences from the control conditions; b refers to statistically significant differences from LPS challenge. RSV, resveratrol. (PNG 8928 kb)
Rights and permissions
About this article
Cite this article
Bobermin, L.D., de Souza Almeida, R.R., Weber, F.B. et al. Lipopolysaccharide Induces Gliotoxicity in Hippocampal Astrocytes from Aged Rats: Insights About the Glioprotective Roles of Resveratrol. Mol Neurobiol 59, 1419–1439 (2022). https://doi.org/10.1007/s12035-021-02664-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02664-8