Abstract
Resveratrol is a natural polyphenol compound highly found in red wine that displays several beneficial effects on the central nervous system (CNS), preventing or slowing the progression of a wide variety of neurological diseases. Its neuroprotective role is particularly associated to modulation of antioxidant and anti-inflammatory responses in glial cells in a mechanism dependent of heme oxygenase 1 (HO-1) signaling pathway. Oligodendrocyte progenitor cells (OPC), primarily known for giving rise to mature oligodendrocytes, have emerged as dynamic cells that are also important to maintain the CNS homeostasis. In this sense, we have demonstrated that resveratrol has a protective effect on oligodendroglial functionality against lipopolysaccharide (LPS)-mediated cytotoxicity and that its glioprotective mechanism involves the nuclear factor erythroid 2-related factor 2 (Nrf2) and HO-1 pathways. LPS, through toll-like receptor 4 (TLR4), affected the release of trophic factors by OPC, including transforming growth factor beta (TGF-β), brain-derived neurotrophic factor (BDNF), and glial cell-derived neurotrophic factor (GDNF), and resveratrol reestablished the trophic factor release to control levels. Additionally, resveratrol prevented the LPS-induced increase in the intracellular reactive oxygen species (ROS) as well as the decrease in glutathione (GSH) levels and in glutamate cysteine ligase (GCL) activity, through Nrf2/HO-1 signaling pathways. Resveratrol also prevented the increase of the transcriptional activities of nuclear factor κB (NFκB) and hypoxia-inducible factor 1 alpha (HIF-1α) after LPS challenge. In summary, this is the first study showing the glioprotective effect of resveratrol on oligodendroglial cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a natural polyphenol compound found in grapes, berries, and red wine [1, 2]. Resveratrol is considered a multi-target molecule that might prevent or slow the progression of a wide variety of illnesses, including cancer, cardiovascular, and neurological diseases [3–6]. In line with this, our group showed that resveratrol modulates important glial functions, particularly, those associated to antioxidant and anti-inflammatory activities [7, 8]. Although there is increasing evidence indicating the neuroprotective effect of resveratrol, the precise mechanisms of these effects are not fully understood. However, a wide amount of studies have hypothesized that resveratrol action may be due to the activation of heme oxygenase 1 (HO-1) signaling pathway [9, 10]. HO-1, an inducible endogenous enzyme modulated by the nuclear factor erythroid 2-related factor 2 (Nrf2), has been reported as a potential intracellular pathway that provides resistance against stressful conditions, such as oxidative stress and inflammation [8, 10, 11].
Oligodendrocyte progenitor cells (OPC) are a uniformly distributed population of proliferative glial cells, which represent approximately 5% of the total cells in the central nervous system (CNS) [12]. These precursor cells are well known for giving rise to mature oligodendrocytes, also known collectively as oligodendroglia, which are primarily responsible for generating and maintaining the myelin sheath in the CNS [12, 13]. Due to the critical importance of myelin, enabling rapid saltatory conduction of action potentials and maintaining axonal integrity and function, myelination has been the best well-described role of oligodendroglia [14, 15]. However, previous studies have reported the crosstalk between OPC and other cells such as neurons, astrocytes, and cerebral endothelium as an important function beyond myelination. Such crosstalk appears to involve the signaling of important trophic factors such as transforming growth factor β (TGF-β), brain-derived neurotrophic factor (BDNF), and glial cell-derived neurotrophic factor (GDNF) [16]. Changes in oligodendroglia functionality may compromise the CNS homeostasis and contribute to the pathogenesis of several neurological diseases [12, 16, 17].
In this sense, previous studies have shown that OPC are able to respond to exogenous agents. Bacterial inflammogen lipopolysaccharide (LPS) is the main component of outer membrane of gram-negative bacteria and has been widely employed as an exogenous signal to study experimental inflammatory response in the CNS [18, 19]. Moreover, OPC have also been reported as being modulated by LPS through TLR4, a member of the toll-like receptor (TLR) family responsible for recognizing LPS [20, 21]. Furthermore, there is a close relationship between inflammatory response and reactive oxygen species (ROS), by activation of several common pathways, including nuclear factor κB (NFκB), the master regulator of inflammatory, and oxidative responses [9, 22–25]. Additionally, NFκB activation has been shown to directly activate the expression of hypoxia-inducible factors (HIFs), which were first described as transcriptional factors for hypoxic adaptation in mammalian cells and, recently, have emerged as essential regulators of inflammation and oxidative stress under normoxic conditions [13, 26–30].
Considering the little information about the effects of resveratrol on oligodendroglia functionality and the role of NFκB and HIF-1α, an active and stable subunit of HIFs, in OPC maturation, that might be strongly affected by oxidative stress and inflammation, the aim of this study was to evaluate the glioprotective effect of resveratrol against LPS-induced cytotoxicity in OPC cultures. Therefore, we evaluated the cell membrane integrity, measured the trophic factor release, the inflammatory response, the oxidative stress parameters, and assessed the transcriptional activity of NFκB and HIF-1α. We also indicated that the glioprotective mechanism of resveratrol involves Nrf2 and HO-1 signaling pathways. Thus, to the best of our knowledge, this is the first study to assess the potential glioprotective role of resveratrol and its mechanism in oligodendroglia.
Materials and Methods
Reagents
Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), and other materials for cell culture were purchased from Gibco/Invitrogen (Carlsbad, CA, USA). DNase, resveratrol, 2′-7′-dichlorofluorescein diacetate (DCFH-DA), glutathione (GSH) standard, o-phthaldialdehyde, propidium iodide (PI), zinc protoporphyrin IX (ZnPP IX), fibroblast growth factor basic (bFGF), lipopolysaccharide (LPS), and pyrrolidine dithiocarbamate (PDTC) were obtained from Sigma–Aldrich (St. Louis, MO, USA). Pierce™ BCA protein assay kit was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Inhibitor for TLR4 (CLI-095) was purchased from InvivoGen (San Diego, CA, USA). Polyclonal rabbit anti-glial fibrilary acidic protein (GFAP) was purchased from Dako (catalog number Z0334), and monoclonal mouse anti-oligodendrocyte marker O4 and monoclonal mouse anti-NG2 were purchased from Sigma–Aldrich (catalog numbers O7139 and N8912, respectively). Alexa Flour® 555 goat anti-rabbit and Alexa Fluor® 488 goat anti-mouse were purchased from Invitrogen (catalog numbers A31576 and A11001, respectively). ELISA kits were purchased from PeproTech (Rocky Hill, NJ, USA) for TNF-α; R&D Systems (Minneapolis, MN, USA) for TGF-β and GDNF; eBioscience (San Diego, CA, USA) for IL-1β; Invitrogen (Carlsbad, CA, USA) for NFκB p65; and Thermo Scientific (Waltham, MA, USA) for HIF-1α and BDNF. TRIzol Reagent was obtained from Invitrogen (Carlsbad, CA, USA); Applied Biosystems™ High-Capacity cDNA Reverse Transcription Kit was purchased from Applied Biosystems (Foster City, CA, USA). All other chemicals were purchased from common commercial suppliers.
Animals
Newborn Wistar rats (1–2 days old) were obtained from our breeding colony (Department of Biochemistry, UFRGS, Brazil) and maintained under a controlled environment (12 h light/12 h dark cycle; 22 ± 1 °C; with their mother). All animal experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Federal University of Rio Grande do Sul Animal Care and Use Committee (process number 27544).
Cell Culture Obtainment and Maintenance
OPC cultures were performed based in a previously described protocol with some modifications [31]. Briefly, Wistar rats had their cerebral cortices aseptically dissected and their meninges removed in a Petri dish containing Hank’s Balanced Salt Solution (HBSS). Then, tissue was mechanically dissociated for 10 min using a Pasteur pipette before more 10 min centrifugation at 1000 rpm. Supernatant was carefully aspirate and discarded with a Pasteur pipette. Pellet was resuspended in DMEM (10% fetal bovine serum [FBS], 15 mM HEPES, 14.3 mM NaHCO3, 1% Fungizone, and 0.04% gentamicin), and tissue suspension was passed through a 70-μm nylon cell strainer placed on a 50-ml conical tube to collect the flow-through. Filtered cell suspensions were plated in a T75 poly-L-lysine flask (approximately 10 million cells per flask) and cultured at 37 °C in a 5% CO2 incubator. Twenty-four hours later, the first medium exchange was performed; after that, culture medium was replaced once every 3 days until cells reached confluence (at approximately 9th day). Once mixed glial cultures reached confluence, culture was ready to be shaken to obtain OPC. Flasks were pre-shaken on an orbital shaker for 1 h at 170 rpm at 37 °C to remove microglial cells. Sequentially, culture medium was discarded and 10 ml of fresh DMEM was added to each flask for shaking again at 170 rpm overnight at 37 °C (approximately 22 h). Then, cell suspension was collected from each flask and transferred to untreated Petri dishes that were incubated for 40 min at 37 °C in a 5% CO2 incubator. Cell suspension from each Petri dish was carefully collected, passed through a 40-μm nylon cell strainer, and then centrifuged for 10 min at 1000 rpm. The supernatant was discarded with a Pasteur pipette, and the pellet was suspended and dissociated in a small amount of the remaining medium (approximately 0.5 ml). The number of living cells was estimated using the Trypan blue exclusion assay, and cells were plated in 24-well plates pre-coated with poly-L-lysine and cultured at 37 °C in a 5% CO2 incubator. The number of cells seeded in each well was approximately 1.5 × 105 cells. These cells were maintained in DMEM 10% FBS for 3 days to proliferate before the treatments. Cell culture was characterized based on typical OPC morphology, following the positive expression of both NG2 and O4 specific markers.
Primary astrocyte cultures were performed as previously described by our group [25]. Briefly, newborn Wistar rats had their cerebral cortices aseptically dissected and meninges removed. Tissue was digested using 0.05% trypsin at 37 °C. After mechanical dissociation and centrifugation, cells were resuspended in DMEM/F12 (10% fetal bovine serum [FBS], 15 mM HEPES, 14.3 mM NaHCO3, 1% Fungizone, and 0.04% gentamicin), plated on 6- or 24-well plates pre-coated with poly-L-lysine, and cultured at 37 °C in a 5% CO2 incubator. Cells were seeded at a density of 3–5 × 105 cells/cm2. Twenty-four hours later, the first medium exchange was performed; during the first week, medium was replaced once every 2 days, and from the second week on, it was replaced once every 4 days. Experiments were performed when cell reached confluence.
Immunofluorescence Analysis
For NG2 and O4 staining, cells were incubated in a 24-well plate with antibodies anti- NG2 (1:400) or O4 (1:400) in 200 ml of DMEM 10% FSB overnight at 37 °C in a 5% CO2 incubator. Following that, cells were washed with HBSS and incubated with specific anti-mouse secondary antibody conjugated with Alexa Fluor® 488 (1:1000) in 200 ml of DMEM 10% FSB for 1 h. Again, cells were washed with HBSS and fixed with 4% paraformaldehyde for 20 min. GFAP immunocytochemistry was performed as previously described by our group [25]. In short, cell cultures were fixed with 4% paraformaldehyde for 20 min and then permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature. After blocking overnight with 4% albumin, cells were incubated overnight with anti-GFAP (1:400) at 4 °C; this was followed by PBS washes and incubation with a specific secondary anti-rabbit antibody conjugated with Alexa Fluor® 555 (1:1000) for 1 h at room temperature. For all immunostaining-negative controls, reactions were performed by omitting primary or secondary antibody. No reactivity was observed when primary or secondary antibody was excluded. Cell nuclei were stained with 2.5 μg/ml Hoescht. Cells were visualized with the Olympus IX70 inverted microscope, and images were acquired by CellM software.
Flow Cytometry Analysis
To quantify the purity of culture, cells were incubated in a 24-well plate with antibodies anti-NG2 (1:400) or O4 (1:400) in 200 μl of DMEM 10% FSB overnight at 37 °C in a 5% CO2 incubator. After that, cells were washed three times with HBSS and incubated with specific anti-mouse secondary antibody conjugated with Alexa Fluor® 488 (1:1000) for 1 h. Then again, cells were washed three times with HBSS, lifted with 0.05% trypsin for approximately 5 min, centrifuged at 1000 rpm for 10 min, and resuspended in flow cytometry buffer. For GFAP staining, cells were first lifted with 0.05% trypsin, permeabilized with 0.1% Triton X-100 in HBSS for 5 min at room temperature, and then incubated with anti-GFAP (1:50) for 1 h. After that, cells were also washed three times with HBSS and incubated with specific anti-rabbit secondary antibody conjugated with Alexa Fluor® 555 (1:1000) for 1 h. Samples were run on a BD FACSCalibur flow cytometer and analyzed using FCS Express 4 Analysis Software by De Novo Software. Each sample had 10,000 events analyzed, which means that 1 × 104 cells were obtained from each sample. The membrane integrity (propidium iodide (PI) incorporation) was also evaluated by flow cytometry. Firstly, cells were washed with HBSS, lifted with 0.05% trypsin for 5 min, centrifuged at 1000 rpm for 10 min, and then resuspended in flow cytometry buffer with 7.5 μM PI for approximately 10 min. Samples were run on a BD FACSCalibur flow cytometer and analyzed using FCS Express 4 Analysis Software (De Novo, Ontario, Canada). Each sample had 10,000 events analyzed, which means that 1 × 104 cells were obtained from each sample.
Resveratrol and LPS Treatments
To measure the glioprotective effect of resveratrol on OPC cultures under LPS exposure, cells were pre-incubated with 10 μM resveratrol for 1 h at 37 °C in atmosphere with 5% of CO2 in serum-free DMEM. Subsequently, 10 μg/ml LPS was added for 24 h in the presence or absence of resveratrol. To elucidate a possible mechanism of resveratrol on LPS-induced inflammatory response, resveratrol was co-incubated with ZnPP IX (10 μM), a specific HO-1 inhibitor. To evaluate the mechanism by which LPS induces inflammatory response, LPS was co-incubated with CLI-095 (1 μM), a specific TLR4 inhibitor. To estimate the role of NFκB activity in resveratrol glioprotective mechanism, OPC were co-incubated with PDTC (50 μM), an NFκB inhibitor. The concentrations of resveratrol, LPS, and inhibitors were chosen based on our previous studies and other recent publications [9, 23, 32].
Trophic Factor Measurement
The levels of TGF-β, GDNF, and BDNF were carried out in the extracellular medium, using ELISA kits from R&D Systems (Minneapolis, MN, USA) for TGF-β and GDNF, and from Thermo Scientific (Waltham, MA, USA) for BDNF. The results are expressed as pg/ml. The ELISA kits detect a minimum of 15.4 pg/ml for TGF-β, 31.2 pg/ml for GDNF, and 15.6 pg/ml for BDNF.
Inflammatory Response Measurement
The levels of TNF-α and IL-1β were carried out in the extracellular medium, using ELISA kits from PeproTech (Rocky Hill, NJ, USA) for TNF-α and from eBioscience (San Diego, CA, USA) for IL-1β. The results are expressed as pg/ml. The ELISA kits detect a minimum of 5.0 pg/ml for TNF-α and 12.0 pg/ml for IL-1β.
DCFH Oxidation
Intracellular ROS levels were detected using DCFH-DA. DCFH-DA was added to the medium at a concentration of 10 μM and cells were incubated for 30 min at 37 °C. Following DCFH-DA exposure, cells were scraped into phosphate-buffered saline with 0.2% Triton X-100. Fluorescence was measured in a plate reader (Spectra Max M5, Molecular Devices, USA) with excitation at 485 nm and emission at 520 nm [33]. Values were obtained as fluorescence units/mg protein and expressed as percentages relative to the control conditions.
Glutamate Cysteine Ligase Activity
GCL (EC 6.3.2.2) was assayed according to Seelig et al., with slight modifications [34]. Cell lysate, suspended in a sodium phosphate buffer containing 140 mM KCl, was diluted with 100 mM sodium phosphate buffer (pH 8.0) containing 5 mM EDTA. The enzyme activity was determined after monitoring the NADH oxidation at 340 nm in sodium phosphate/KCl (pH 8.0) containing 5 mM Na2-ATP, 2 mM phosphoenolpyruvate, 10 mM L-glutamate, 10 mM L-α-aminobutyrate, 20 mM MgCl2, 2 mM Na2-EDTA, 0.2 mM NADH, and 17 μg of pyruvate kinase/lactate dehydrogenase. The results are expressed in nmol/mg protein/min.
GSH Content
GSH content was assessed as previously described [35]. Cell lysate suspended in a sodium phosphate buffer with 140 mM KCl was diluted in 100 mM sodium phosphate buffer (pH 8.0) containing 5 mM EDTA. For measuring GSH, protein was precipitated with 1.7% meta-phosphoric acid. The supernatant was assayed with o-phthaldialdehyde (at a concentration of 1 mg/ml methanol) at 22 °C for 15 min. Fluorescence was assessed using excitation and emission wavelengths of 350 and 420 nm, respectively. A calibration curve was performed with standard GSH solutions at concentrations ranging from 0 to 500 μM. The results are expressed in nmol/mg protein.
NFκB Transcriptional Activity
Levels of NFκB p65 in the nuclear fraction, which had been isolated from lysed cells with Igepal CA-630 and centrifugation (following manufacturer’s instructions), were measured using an ELISA commercial kit from Invitrogen (Carlsbad, CA, USA). The results are expressed as pg/mg protein.
HIF-1α Levels
Levels of HIF-1α were carried out using ELISA kit for HIF-1α from Thermo Scientific (Waltham, MA, USA). The results are expressed as pg/mg protein.
RNA Extraction and Quantitative RT-PCR
Total RNA was isolated from OPC cultures using TRIzol Reagent. The concentration and purity of the RNA were determined spectrophotometrically at a ratio of 260/280. Then, 1 μg of total RNA was reverse transcribed using High-Capacity cDNA Reverse Transcription Kit in a 20-μl reaction, according to manufacturer’s instructions. mRNA quantification of HO-1 was performed using Power SYBR Green PCR Master Mix (Invitrogen), forward primer: 5′-ACTTTCAGAAGGGTCAGGTGTCC-3′ and reverse primer: 5′-TTGAGCAGGAAGGCGGTCTTAG-3′. Similarly, Nrf2 was measured using forward primer: 5′-TGAGTCGCTTGCCCTGGATAT-3′ and reverse primer: 5′-AGTCATGGCTGCCTCCAGAGA-3′. Quantitative RT-PCR was performed in duplicate using the Applied Biosystems 7500 Fast system. No-template and no-reverse transcriptase controls were included in each assay, and these produced no detectable signal during the 40 cycles of amplification. Target mRNA levels were normalized to β-actin levels as a housekeeping gene and expressed relative to the levels of control OPC cultures using the 2−ΔΔCt method [36].
Protein Determination
Protein concentration was assessed by the BCA assay Kit (Pierce) from Thermo Fisher Scientific (Waltham, MA, USA).
Statistical Analysis
Each set of results was obtained from three independent experiments performed in triplicate. Differences among groups were statistically analyzed using one- or two-way analysis of variance (ANOVA) followed by Tukey’s test. t test was applied for the evaluation of mRNA expression levels. Values of P < 0.05 were considered significant. All analyses were performed using the Statistical Package for Social Sciences (SPSS) software.
Results
OPC Cultures Presented Classical Protein Markers
To ensure that our rat OPC cultures serve as a model for studying oligodendroglial functionality, we performed several experimental paradigms to show homogeneity and classical OPC markers (Fig. 1). Flow cytometry and immunofluorescence analysis indicated that OPC showed the typical bipolar/tripolar morphology (Fig. 1a), as well as the expression of NG2 (a proteoglycan found in OPC membrane, Fig. 1b, c) and O4 (a sulfatide differentiation marker, found from 3rd day onwards in cultured OPC, Fig. 1c).
OPC cultures showed high homogeneity and positive OPC markers expression. a Light microscopy of OPC—typical bipolar or tripolar morphology, positive expression of NG2, and a merge for Hoestch staining. All images are representative fields from at least three experiments performed in duplicate. Scale bars = 20 μm. b Flow cytometry analysis—approximately 85% of cells have the same morphological profile which is indicated by the gate covering the similar points on the plot. The plot is a representative field from at least three experiments performed in triplicate with 1 × 104 events each plicate. c Marker expression of early OPC (approximately 82% of NG2), intermediary OPC (about 13% of O4), and remaining primary astrocytes (approximately 7% of GFAP) quantified by flow cytometry analysis proved the amount of nearly >95% of homogeneous oligodendrocyte precursors in OPC culture. All plots are representative fields from at least three independent experiments performed in triplicate. The data are expressed as percentages relative to the total number of cells (1 × 104 cells per plicate) and represent the mean ± SEM of these experimental determinations analyzed statistically by one-way ANOVA followed by Tukey’s test
Resveratrol Protected OPC Against LPS Cytotoxicity
To explore whether OPC cultures would be able to respond to external stimuli such as resveratrol and LPS, membrane integrity was evaluated using PI incorporation assay by flow cytometry. As shown in Fig. 2, resveratrol per se did not change OPC viability compared with the control. On the other hand, LPS displayed a cytotoxic effect increasing the number of PI-positive cells. The pretreatment with 10 μM resveratrol prevented the LPS-induced cytotoxicity in OPC.
Resveratrol (RSV) protected OPC against LPS challenge. Cells were pretreated with 10 μM resveratrol for 1 h, followed by addition of 10 μg/ml LPS for 24 h in serum-free DMEM. PI incorporation assay was performed as described in the “Materials and Methods” section. The data are expressed as percentages of positive cells relative to 1 × 104 cells per sample and represent the mean ± SEM of three independent experimental determinations performed in triplicate and analyzed statistically by one-way ANOVA followed by Tukey’s test. Values of P < 0.05 were considered significant. a indicates differences from control conditions and b differences from LPS
LPS Did Not Induce Inflammatory Response in OPC Cultures
Although LPS has induced change in membrane integrity in OPC, the release of TNF-α or IL-1β in the extracellular medium was not altered after LPS exposure (Table 1). To ensure that LPS can stimulate inflammatory response, we measured TNF-α and IL-1β release from primary astrocyte cultures and we observed a significant increase in cytokines release after LPS exposure (Table 1).
Resveratrol Modulated TGF-β, GDNF, and BDNF Release from OPC After LPS Exposure
As shown in Fig. 3a, the levels of TGF-β were significantly increased under LPS exposure by about 23%. Conversely, LPS decreased the release of GDNF (29%) and BDNF (22%) (Fig. 3b, c, respectively). Additionally, resveratrol per se increased GDNF release by 18% (Fig. 3b). To demonstrate whether the effect of resveratrol was mediated by HO-1 pathway, OPC were co-incubated in the presence of ZnPP IX, a HO-1 inhibitor. The HO-1 inhibitor abolished the effect of resveratrol on trophic factor release, indicating that resveratrol’s actions occurred through HO-1.
Resveratrol (RSV) prevented LPS-induced changes in trophic factor release from OPC through HO-1/NFκB pathway. a TGF-β, b GDNF, and c BDNF levels were measured as described in the “Materials and Methods” section. Cells were pretreated with 10 μM resveratrol and/or 10 μM ZnPP IX, 1 μM CLI-095, and 50 μM PDCT for 1 h, followed by addition of 10 μg/ml LPS for 24 h in serum-free DMEM. The data are expressed in pg/ml and represent the mean ± SEM of three independent experimental determinations performed in triplicate and analyzed statistically by two-way ANOVA followed by Tukey’s test. Values of P < 0.05 were considered significant. a indicates differences from control conditions and b differences from LPS. With (w/), without (w/o)
We also tested whether the effect of LPS in OPC culture was through TLR4 and NFκB (Fig. 3a–c). OPC was incubated in the presence of CLI-095, a TLR4 inhibitor, and our results showed that the effect of LPS on TGF-β, GDNF, and BDNF was mediated by TLR4. Additionally, we incubated OPC with PDTC, a NFκB inhibitor, and also observed that the effect of LPS on trophic factors was mediated by NFκB pathway.
Resveratrol Inhibited ROS Accumulation Induced by LPS
As shown in the Fig. 4, LPS exposure significantly increased ROS levels, which was prevented by the pretreatment with resveratrol. Furthermore, resveratrol per se reduced ROS generation compared to control conditions.
Antioxidant effect of resveratrol (RSV) in OPC cultures. Cells were pretreated with 10 μM resveratrol for 1 h, followed by addition of 10 μg/ml LPS for 24 h in serum-free DMEM. ROS levels were measured as described in the “Materials and Methods” section. The data are expressed as percentages relative to the control conditions (indicated by the line) and represent the mean ± SEM of three independent experimental determinations performed in triplicate and analyzed statistically by one-way ANOVA followed by Tukey’s test. Values of P < 0.05 were considered significant. a indicates differences from control conditions and b differences from LPS
Resveratrol Prevented LPS-Induced GSH Depletion through HO-1 Pathway
Resveratrol per se increased GCL activity (Fig. 5a) and GSH levels (Fig. 5b). The pretreatment with resveratrol also prevented the LPS-mediated GCL activity reduction and GSH level decrease. The HO-1 inhibitor completely abolished the positive effects of resveratrol in GSH biosynthesis.
Resveratrol (RSV) modulated GSH biosynthesis in OPC cultures via HO-1. a GCL activity and b GSH content were measured as described in the “Materials and Methods” section. Cells were pretreated with 10 μM resveratrol and/or 10 μM ZnPP IX for 1 h, followed by addition of 10 μg/ml LPS for 24 h in serum-free DMEM. The data are expressed in nmol/mg protein/min for GCL activity and nmol/mg protein for GSH content, and both represent the mean ± SEM of three independent experimental determinations performed in triplicate and analyzed statistically by two-way ANOVA followed by Tukey’s test. Values of P < 0.05 were considered significant. a indicates differences from control conditions and b differences from LPS. With (w/), without (w/o)
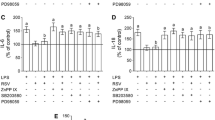
Resveratrol Prevented LPS-Mediated Increase in NFκB and HIF-1α Transcriptional Activities via HO-1 Pathway
LPS increased the transcriptional activity of NFκB p65 (Fig. 6a) and HIF-1α (Fig. 6b), both by about 65% in OPC cultures. The pretreatment with resveratrol was able to prevent these effects. The presence of HO-1 inhibitor abolished the effects of resveratrol on NFκB and HIF-1α, which were not modulated by resveratrol per se.
Resveratrol (RSV) modulated NFκB and HIF-1α transcriptional activities via HO-1. a NFκB and b HIF-1α levels were measured as described in the “Materials and Methods” section. Cells were pretreated with 10 μM resveratrol and/or 10 μM ZnPP IX for 1 h, followed by addition of 10 μg/ml LPS for 24 h in serum-free DMEM. The data are expressed in pg/mg protein and represent the mean ± SEM of three independent experimental determinations performed in triplicate and analyzed statistically by two-way ANOVA followed by Tukey’s test. Values of P < 0.05 were considered significant. a indicates differences from control conditions and b differences from LPS. With (w/), without (w/o)
Moreover, the transcriptional activity of NFkB was blocked by PDTC, and interestingly, resveratrol was able to potentiate the effect of the inhibitor on NFkB p65 levels (Table 2).
The Glioprotective Effect of Resveratrol Involved Nrf2 and HO-1 Pathways
To confirm the mechanism of resveratrol on OPC cultures, we evaluated the mRNA expression levels of Nrf2 and HO-1. As expected, Fig. 7 shows that resveratrol increased the expression of Nrf2 (29%) and HO-1 (45%).
Resveratrol (RSV) increased Nrf2 and HO-1 mRNA expression levels. Cells were treated with 10 μM resveratrol for 25 h in serum-free DMEM. The data represent the mean ± SEM of two independent experimental determinations performed in triplicate and analyzed statistically by t test. Values of P < 0.05 were considered significant. a indicates differences from control conditions
Discussion
Resveratrol, a multi-target molecule that displays several beneficial effects on the CNS, was explored in the present study as a glioprotective agent against LPS-mediated cytotoxicity. As monotypic CNS cell cultures provide a useful basis for in vitro studies, contributing to the understanding of physiological and pathological functions of the brain, we used high purity OPC cultures to evaluate the protective effect of resveratrol on oligodendroglial functionality. In this sense, our results showed that cytotoxic effects stimulated by LPS were prevented by resveratrol through Nrf2/HO-1 signaling pathway (Fig. 8). Thus, this is the first study to point out the glioprotective effect of resveratrol in oligodendroglial cells.
Schematic illustration of the signaling mechanisms involved in resveratrol glioprotective effects against LPS-mediated cytotoxicity in OPC culture. LPS modulates through TLR4/NFκB the oxidative response and the release of GDNF, BDNF, and TGF-β. Resveratrol (RSV) activates Nrf2/HO-1 pathways, modulating GSH biosynthesis and trophic factors release, reducing ROS production, and preventing the increase of the transcriptional activity of NFκB and HIF-1α
Although the pivotal role as myelinating cells has usually been more explored, oligodendroglia can also act as a critical source of trophic factors [37, 38]. In line with this, LPS affected the release of TGF-β, BDNF, and GDNF from OPC and resveratrol reestablished the values near control conditions. Concerning TGF-β, it is an important growth factor related to many signaling pathways in the CNS, including migration, proliferation, and differentiation of OPC [39–41]. In turn, OPC have been reported to release TGF-β in vitro cell culture systems, which appears to help the maintenance of the blood-brain barrier (BBB) integrity during development [42]. Additionally, previous studies have reported that TGF-β release increases in pathological conditions associated to neuroinflammation, such as Alzheimer and amyotrophic lateral sclerosis (ALS) diseases [43, 44]. Thus, our results showed that LPS increased TGF-β release and resveratrol was able to prevent this change through HO-1. Therefore, as TGF-β may have a dual role, its release from OPC depends of patho- or physiological conditions [45].
Several previous in vivo and in vitro studies have detected the expression of BDNF and GDNF in oligodendroglia [17, 37]. Moreover, increased levels of BDNF and GDNF have been correlated with neuroprotection against metabolic and excitotoxic insults [46, 47], and recent reports have also shown that such expression by glial cells appears to be modulated by HO-1 [48]. BDNF plays an important role on OPC fate, stimulating their proliferation and differentiation into mature oligodendrocytes [38]. Furthermore, BDNF is well known to act on neurons, supporting their survival, growth, and differentiation, as well as helping to promote synaptogenesis [49, 50]. Our findings demonstrated that resveratrol prevented LPS-mediated BDNF decrease in OPC via HO-1 pathway. In this sense, resveratrol should be taken as a promising glioprotective compound, which might support CNS development and functionality under cell injury.
GDNF, in turn, is known for their role of promoting neuronal survival and axonal outgrowth, and its release is primarily done by glial cells such as astrocytes and oligodendrocytes [16]. As well as BDNF, GDNF secretion was decreased by LPS challenge, which was prevented by resveratrol via HO-1 pathway. Furthermore, resveratrol per se was able to enhance GDNF release from OPC, in accordance with other glial cell cultures [51]. Since the modulation of GDNF has been described as a potential therapeutic agent against a variety of pathological events, including ALS and Parkinson’s disease [52, 53], our findings reinforce the protective role of resveratrol against neurological disorders via GDNF release mechanism.
Our results, using CLI-095, a specific TLR4 inhibitor, confirmed that OPC cultures appear to express a functional TLR4 in vitro by which LPS may modulate several signaling pathways. Although TLR4 activation by LPS is well known to induce proinflammatory cytokine release in a wide variety of cells, including astrocytes, our results corroborated previous findings, which showed that OPC cultures did not release TNF-α or IL-1β in vitro after LPS stimulation [54]. Additionally, NFκB is closely associated to LPS cytotoxic effects, and we demonstrated that the release of TGF-β, BDNF, and GDNF is dependent of NFκB pathway. Thus, because LPS/TLR4/NFκB activation is commonly associated to redox imbalance, which most likely contributes to changes in cell viability, we also explore the possible oxidative responses in OPC culture under LPS challenge [22]. Our results strengthen that the cytotoxicity mediated by LPS was connected with an increase in ROS generation, which was associated with a decrease in the levels of GSH, the major antioxidant molecule of the brain [55]. Additionally, LPS induced a decrease in the activity of GCL, the rate-limiting enzyme required for GSH biosynthesis [56]. Moreover, these changes in redox homeostasis may contribute to disruption in OPC cell membrane permeability. Interestingly, resveratrol per se counteracted ROS production and also modulated GSH biosynthesis, reinforcing its antioxidant effect, as well as its glioprotective effect associated to HO-1 pathway.
Oxidative responses stimulated by LPS are often correlated with NFκB and HIF-1α signaling pathways [9, 23, 24]. In this sense, HO-1 is an upstream signal of NFκB [57]. Our results demonstrated that resveratrol completely prevented the increase in the transcriptional activity of both NFκB and HIF-1α, protecting OPC against LPS challenge in a mechanism dependent of HO-1. As previously mentioned, HIF-1α activity inhibits the formation of myelin sheath through OPC differentiation arrest [30]. Moreover, resveratrol has been reported as a pro-myelinative compound, preserving myelination and preventing neuronal loss in chronic demyelinating disease models [58–60]. We also demonstrated that decreasing NFkB transcriptional activity has the same protective effect of resveratrol on OPC culture challenged with LPS, as well as resveratrol potentiated the NFkB inhibitor effect. In this sense, we reinforced that HO-1/NFκB/HIF-1α pathways might be putative mechanisms by which resveratrol prevents OPC maturation arrest during brain injury.
To the best of our knowledge, this study provides the first demonstration of the role of resveratrol in oligodendroglial cells, particularly indicating resveratrol as a potent glioprotective molecule against LPS-mediated cytotoxicity via TLR4 in OPC. In this sense, understanding the influence of resveratrol in oligodendroglial functionality is critical for elucidating its cellular and molecular protective mechanisms in the CNS. Thus, the main conclusions of this study demonstrate that cytotoxicity, oxidative stress, and changes in the trophic factors release induced by LPS are prevented by resveratrol through Nrf2/HO-1 signaling pathway and involved the transcriptional activity of NFκB p65 and HIF-1α. Overall, these observations reinforce the glioprotective effect of resveratrol, which may potentially be used in brain pathological conditions in which oligodendroglia appears to be involved.
References
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5(6):493–506. doi:10.1038/nrd2060
Catalgol B, Batirel S, Taga Y, Ozer NK (2012) Resveratrol: French paradox revisited. Front Pharmacol 3:141. doi:10.3389/fphar.2012.00141
Dolinsky VW, Dyck JR (2011) Calorie restriction and resveratrol in cardiovascular health and disease. Biochim Biophys Acta 1812(11):1477–1489. doi:10.1016/j.bbadis.2011.06.010
Kundu JK, Surh YJ (2008) Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett 269(2):243–261. doi:10.1016/j.canlet.2008.03.057
Pasinetti GM, Wang J, Ho L, Zhao W, Dubner L (2015) Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim Biophys Acta 1852(6):1202–1208. doi:10.1016/j.bbadis.2014.10.006
Sun AY, Wang Q, Simonyi A, Sun GY (2010) Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol 41(2–3):375–383. doi:10.1007/s12035-010-8111-y
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2014) Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol in Vitro 28(4):479–484. doi:10.1016/j.tiv.2014.01.006
Quincozes-Santos A, Bobermin LD, Latini A, Wajner M, Souza DO, Goncalves CA, Gottfried C (2013) Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS One 8(5):e64372. doi:10.1371/journal.pone.0064372
Bellaver B, Souza DG, Bobermin LD, Souza DO, Goncalves CA, Quincozes-Santos A (2015) Resveratrol protects hippocampal astrocytes against LPS-induced neurotoxicity through HO-1, p38 and ERK pathways. Neurochem Res 40(8):1600–1608. doi:10.1007/s11064-015-1636-8
Sakata Y, Zhuang H, Kwansa H, Koehler RC, Dore S (2010) Resveratrol protects against experimental stroke: putative neuroprotective role of heme oxygenase 1. Exp Neurol 224(1):325–329. doi:10.1016/j.expneurol.2010.03.032
Son Y, Lee JH, Chung HT, Pae HO (2013) Therapeutic roles of heme oxygenase-1 in metabolic diseases: curcumin and resveratrol analogues as possible inducers of heme oxygenase-1. Oxidative Med Cell Longev 2013:639541. doi:10.1155/2013/639541
Fernandez-Castaneda A, Gaultier A (2016) Adult oligodendrocyte progenitor cells—multifaceted regulators of the CNS in health and disease. Brain Behav Immun. doi:10.1016/j.bbi.2016.01.005
Naruse M, Ishizaki Y, Ikenaka K, Tanaka A, Hitoshi S (2016) Origin of oligodendrocytes in mammalian forebrains: a revised perspective. J Physiol Sci. doi:10.1007/s12576-016-0479-7
Aggarwal S, Yurlova L, Simons M (2011) Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol 21(10):585–593. doi:10.1016/j.tcb.2011.06.004
Lopez Juarez A, He D, Richard Lu Q (2016) Oligodendrocyte progenitor programming and reprogramming: toward myelin regeneration. Brain Res 1638(Pt B):209–220. doi:10.1016/j.brainres.2015.10.051
Bankston AN, Mandler MD, Feng Y (2013) Oligodendroglia and neurotrophic factors in neurodegeneration. Neurosci Bull 29(2):216–228. doi:10.1007/s12264-013-1321-3
Wilkins A, Majed H, Layfield R, Compston A, Chandran S (2003) Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci 23(12):4967–4974 doi:23/12/4967
Hanke ML, Kielian T (2011) Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci (Lond) 121(9):367–387. doi:10.1042/CS20110164
Carpentier PA, Duncan DS, Miller SD (2008) Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav Immun 22(2):140–147. doi:10.1016/j.bbi.2007.08.011
Li Y, Du XL, He BP (2016) Lipopolysaccharide upregulates the expression of CINC-3 and LIX in primary NG2 cells. Neurochem Res 41(6):1448–1457. doi:10.1007/s11064-016-1856-6
Yao SY, Natarajan C, Sriram S (2012) nNOS mediated mitochondrial injury in LPS stimulated oligodendrocytes. Mitochondrion 12(2):336–344. doi:10.1016/j.mito.2012.01.002
Bellaver B, Souza DG, Bobermin LD, Goncalves CA, Souza DO, Quincozes-Santos A (2015) Guanosine inhibits LPS-induced pro-inflammatory response and oxidative stress in hippocampal astrocytes through the heme oxygenase-1 pathway. Purinergic Signal. doi:10.1007/s11302-015-9475-2
Guerra MC, Tortorelli LS, Galland F, Da Re C, Negri E, Engelke DS, Rodrigues L, Leite MC et al (2011) Lipopolysaccharide modulates astrocytic S100B secretion: a study in cerebrospinal fluid and astrocyte cultures from rats. J Neuroinflammation 8:128. doi:10.1186/1742-2094-8-128
Lu X, Ma L, Ruan L, Kong Y, Mou H, Zhang Z, Wang Z, Wang JM et al (2010) Resveratrol differentially modulates inflammatory responses of microglia and astrocytes. J Neuroinflammation 7:46. doi:10.1186/1742-2094-7-46
Souza DG, Bellaver B, Souza DO, Quincozes-Santos A (2013) Characterization of adult rat astrocyte cultures. PLoS One 8(3):e60282. doi:10.1371/journal.pone.0060282
Bandarra D, Biddlestone J, Mudie S, Muller HA, Rocha S (2015) HIF-1alpha restricts NF-kappaB-dependent gene expression to control innate immunity signals. Dis Model Mech 8(2):169–181. doi:10.1242/dmm.017285
Fabian Z, Taylor CT, Nguyen LK (2016) Understanding complexity in the HIF signaling pathway using systems biology and mathematical modeling. J Mol Med (Berl) 94(4):377–390. doi:10.1007/s00109-016-1383-6
Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA et al (2010) Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest 120(8):2699–2714. doi:10.1172/JCI39506
van Uden P, Kenneth NS, Rocha S (2008) Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J 412(3):477–484. doi:10.1042/BJ20080476
Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SP, Zahed H, Maltepe E et al (2014) Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 158(2):383–396. doi:10.1016/j.cell.2014.04.052
Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, Lu QR (2007) Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc 2(5):1044–1051. doi:10.1038/nprot.2007.149
de Souza DF, Leite MC, Quincozes-Santos A, Nardin P, Tortorelli LS, Rigo MM, Gottfried C, Leal RB et al (2009) S100B secretion is stimulated by IL-1beta in glial cultures and hippocampal slices of rats: Likely involvement of MAPK pathway. J Neuroimmunol 206(1–2):52–57. doi:10.1016/j.jneuroim.2008.10.012
Quincozes-Santos A, Bobermin LD, Kleinkauf-Rocha J, Souza DO, Riesgo R, Goncalves CA, Gottfried C (2009) Atypical neuroleptic risperidone modulates glial functions in C6 astroglial cells. Prog Neuro-Psychopharmacol Biol Psychiatry 33(1):11–15. doi:10.1016/j.pnpbp.2008.08.023
Seelig GF, Simondsen RP, Meister A (1984) Reversible dissociation of gamma-glutamylcysteine synthetase into two subunits. J Biol Chem 259(15):9345–9347
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. Methods Mol Biol 108:347–352. doi:10.1385/0-89603-472-0:347
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Dai X, Lercher LD, Clinton PM, Du Y, Livingston DL, Vieira C, Yang L, Shen MM et al (2003) The trophic role of oligodendrocytes in the basal forebrain. J Neurosci 23(13):5846–5853 doi:23/13/5846
Miyamoto N, Pham LD, Seo JH, Kim KW, Lo EH, Arai K (2014) Crosstalk between cerebral endothelium and oligodendrocyte. Cell Mol Life Sci 71(6):1055–1066. doi:10.1007/s00018-013-1488-9
Choe Y, Huynh T, Pleasure SJ (2014) Migration of oligodendrocyte progenitor cells is controlled by transforming growth factor beta family proteins during corticogenesis. J Neurosci 34(45):14973–14983. doi:10.1523/JNEUROSCI.1156-14.2014
Palazuelos J, Klingener M, Aguirre A (2014) TGFbeta signaling regulates the timing of CNS myelination by modulating oligodendrocyte progenitor cell cycle exit through SMAD3/4/FoxO1/Sp1. J Neurosci 34(23):7917–7930. doi:10.1523/JNEUROSCI.0363-14.2014
Zhang Y, Zhang J, Navrazhina K, Argaw AT, Zameer A, Gurfein BT, Brosnan CF, John GR (2010) TGFbeta1 induces Jagged1 expression in astrocytes via ALK5 and Smad3 and regulates the balance between oligodendrocyte progenitor proliferation and differentiation. Glia 58(8):964–974. doi:10.1002/glia.20978
Seo JH, Maki T, Maeda M, Miyamoto N, Liang AC, Hayakawa K, Pham LD, Suwa F et al (2014) Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-beta signaling. PLoS One 9(7):e103174. doi:10.1371/journal.pone.0103174
Endo F, Komine O, Fujimori-Tonou N, Katsuno M, Jin S, Watanabe S, Sobue G, Dezawa M et al (2015) Astrocyte-derived TGF-beta1 accelerates disease progression in ALS mice by interfering with the neuroprotective functions of microglia and T cells. Cell Rep 11(4):592–604. doi:10.1016/j.celrep.2015.03.053
Wyss-Coray T, Lin C, Sanan DA, Mucke L, Masliah E (2000) Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. Am J Pathol 156(1):139–150
Morikawa M, Derynck R, Miyazono K (2016) TGF-beta and the TGF-beta family: context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol 8(5). doi:10.1101/cshperspect.a021873
Cohen AD, Zigmond MJ, Smith AD (2011) Effects of intrastriatal GDNF on the response of dopamine neurons to 6-hydroxydopamine: time course of protection and neurorestoration. Brain Res 1370:80–88. doi:10.1016/j.brainres.2010.11.006
Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A et al (2009) Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med 15(3):331–337. doi:10.1038/nm.1912
Hung SY, Liou HC, Fu WM (2010) The mechanism of heme oxygenase-1 action involved in the enhancement of neurotrophic factor expression. Neuropharmacology 58(2):321–329. doi:10.1016/j.neuropharm.2009.11.003
Lu B, Nagappan G, Guan X, Nathan PJ, Wren P (2013) BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci 14(6):401–416. doi:10.1038/nrn3505
Nagahara AH, Tuszynski MH (2011) Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov 10(3):209–219. doi:10.1038/nrd3366
Zhang F, Lu YF, Wu Q, Liu J, Shi JS (2012) Resveratrol promotes neurotrophic factor release from astroglia. Exp Biol Med (Maywood) 237(8):943–948. doi:10.1258/ebm.2012.012044
Hoban DB, Howard L, Dowd E (2015) GDNF-secreting mesenchymal stem cells provide localized neuroprotection in an inflammation-driven rat model of Parkinson’s disease. Neuroscience 303:402–411. doi:10.1016/j.neuroscience.2015.07.014
Ibanez CF, Andressoo JO (2016) Biology of GDNF and its receptors—relevance for disorders of the central nervous system. Neurobiol Dis. doi:10.1016/j.nbd.2016.01.021
Taylor DL, Pirianov G, Holland S, McGinnity CJ, Norman AL, Reali C, Diemel LT, Gveric D et al (2010) Attenuation of proliferation in oligodendrocyte precursor cells by activated microglia. J Neurosci Res 88(8):1632–1644. doi:10.1002/jnr.22335
Rahman K (2007) Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2(2):219–236
Iles KE, Liu RM (2005) Mechanisms of glutamate cysteine ligase (GCL) induction by 4-hydroxynonenal. Free Radic Biol Med 38(5):547–556. doi:10.1016/j.freeradbiomed.2004.11.012
Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW (2010) When NRF2 talks, who’s listening? Antioxid Redox Signal 13(11):1649–1663. doi:10.1089/ars.2010.3216
Arteaga O, Revuelta M, Uriguen L, Alvarez A, Montalvo H, Hilario E (2015) Pretreatment with resveratrol prevents neuronal injury and cognitive deficits induced by perinatal hypoxia-ischemia in rats. PLoS One 10(11):e0142424. doi:10.1371/journal.pone.0142424
Ghaiad HR, Nooh MM, El-Sawalhi MM, Shaheen AA (2016) Resveratrol promotes remyelination in cuprizone model of multiple sclerosis: biochemical and histological study. Mol Neurobiol. doi:10.1007/s12035-016-9891-5
Stettner M, Wolffram K, Mausberg AK, Albrecht P, Derksen A, Methner A, Dehmel T, Hartung HP et al (2013) Promoting myelination in an in vitro mouse model of the peripheral nervous system: the effect of wine ingredients [corrected]. PLoS One 8(6):e66079. doi:10.1371/journal.pone.0066079
Acknowledgments
We would like to thank the contributions of Bruna Bellaver. This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Financiadora de Estudos e Projetos (FINEP)–Instituto Brasileiro de Neurociências (IBN Net) 01.06.0842-00, Federal University of Rio Grande do Sul (UFRGS), and Instituto Nacional de Ciência e Tecnologia para Excitotoxicidade e Neuroproteçãao (INCTEN/CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosa, P.M., Martins, L.A.M., Souza, D.O. et al. Glioprotective Effect of Resveratrol: an Emerging Therapeutic Role for Oligodendroglial Cells. Mol Neurobiol 55, 2967–2978 (2018). https://doi.org/10.1007/s12035-017-0510-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0510-x