Abstract
Astrocytes are dynamic glial cells associated to neurotransmitter systems, metabolic functions, antioxidant defense, and inflammatory response, maintaining the brain homeostasis. Elevated concentrations of homocysteine (Hcy) are involved in the pathogenesis of age-related neurodegenerative disorders, such as Parkinson and Alzheimer diseases. In line with this, our hypothesis was that Hcy could promote glial reactivity in a model of cortical primary astrocyte cultures from adult Wistar rats. Thus, cortical astrocytes were incubated with different concentrations of Hcy (10, 30, and 100 μM) during 24 h. After the treatment, we analyzed cell viability, morphological parameters, antioxidant defenses, and inflammatory response. Hcy did not induce any alteration in cell viability; however, it was able to induce cytoskeleton rearrangement. The treatment with Hcy also promoted a significant decrease in the activities of Na+, K+ ATPase, superoxide dismutase (SOD), and glutathione peroxidase (GPx), as well as in the glutathione (GSH) content. Additionally, Hcy induced an increase in the pro-inflammatory cytokine release. In an attempt to elucidate the putative mechanisms involved in the Hcy-induced glial reactivity, we measured the nuclear factor kappa B (NFκB) transcriptional activity and heme oxygenase 1 (HO-1) expression, which were activated and inhibited by Hcy, respectively. In summary, our findings provide important evidences that Hcy modulates critical astrocyte parameters from adult rats, which might be associated to the aging process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Homocysteine (Hcy) is an amino acid sulfur and non-proteinogenic that is formed in unequal quantities in the metabolism of methionine, an essential amino acid. Hcy levels are controlled through two regulatory mechanisms: (a) remethylation, forming methionine and getting a methyl group from 5-methyltetrahydrofolate or betaine, and (b) transsulfuration, when it undergoes condensation with serine, producing cystathionine, via reaction catalyzed by cystathionine-β-synthase, this product after being cleaved to cysteine [1]. Moreover, Hcy metabolism requires coenzymes such as vitamins B6 and B12 and folic acid. Deficiencies in these cofactors are associated with hyperhomocysteinemia (HHcy) that is an abnormal high level of Hcy in the blood, commonly associated to cytotoxicity. In addition, mild levels of Hcy (>30 μM) have been reported as an independent risk factor for cognitive dysfunction [2] and neurodegenerative disorders [3]. According to previous studies from our group, mild HHcy induces oxidative stress and neuroinflammation in the cerebral cortex of rats [4, 5]. Recently, we have also demonstrated that Hcy (30 μM) altered mitochondrial functionality and induced oxidative stress and neuronal death in slices from the cerebral cortex of rats [6].
Astrocytes correspond to 50% of the total number of cells in the central nervous system (CNS), being the most versatile cells in the brain [7]. These cells have a variety of functions, including the control of neurotransmitter systems and ionic homeostasis, the regulation of metabolic functions, antioxidant defenses, and inflammatory response [8]. Astrocytes possess the main antioxidant enzymatic defenses, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). In this sense, GPx is extremely important for the biosynthesis of glutathione (GSH), which is the major non-enzymatic antioxidant defense in the CNS [9]. Additionally, astrocytes express the enzyme Na+, K+ ATPase, which is crucial for maintaining the membrane potential through the active transport of Na+ and K+ ions in the CNS. Because astrocytes are involved in the regulation of ionic homeostasis and in the glutamate transport (highly dependent of Na+ ion), there is a close relationship between Na+, K+ ATPase activity and astrocyte functionality [10]. Concerning inflammatory response, astrocytes release pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin 1-beta (IL-1β), and interleukin-6 (IL-6), which might be regulated by the transcription factor nuclear factor kappa B (NFκB), the master regulator of oxidative stress and inflammation [11].
Recently, our group established a routine culture protocol of astrocyte from cortical adult Wistar rats, which presents connections more organized and well established than astrocytes derived from newborn animals, being more reliable to study brain aging, as well as age-related neurological diseases [12]. Our culture model showed classical astrocytic markers, such as glial fibrillary acidic protein (GFAP), and actively responded to external stimuli that could change metabolic functions and antioxidant and inflammatory activities [12].
Heme oxygenase 1 (HO-1) is the inducible isoform of heme oxygenase regulated by the transcription factor nuclear factor erythroid 2 (Nrf2)-regulated gene that plays a critical role in the prevention of oxidative stress and inflammation [13]. The potential link between inflammation and HO-1 was initially demonstrated in animal models, which the upregulation of HO-1 attenuated the activation of pro-inflammatory pathways [14]. This effect might be attributed to the ability of HO-1 to inhibit the translocation of NFκB from the cytoplasm to the nucleus [13]. Additionally, HO-1 can act as a sequestering nitric oxide (NO), contributing to the control of oxidative/nitrosative stress [15].
Using astrocyte primary cultures prepared from newborn animals, we have shown that Hcy induced oxidative stress and cytoskeletal reorganization, which were prevented by antioxidants, suggesting that the oxidative stress is associated with deleterious effects of Hcy [16]. Since Hcy is a risk factor to neurodegenerative diseases [17–20], and adult astrocytes are a relevant model to study the mechanisms of brain aging, the hypothesis of this study was that Hcy could promote astrocytic dysfunction via NFκB/HO-1 signaling pathways in cortical primary astrocyte cultures from adult rats. Therefore, we treated astrocyte cultures with different concentrations of Hcy and evaluated cytoskeleton proteins and antioxidant and inflammatory responses, as well as the putative mechanisms involved in these effects.
Material and Methods
Ethics Statement
Our work has followed the National Institute of Health Guide for the Care and Use of Laboratory Animals “Guide for the Care and Use of Laboratory Animals” (NIH publication No. 80-23, revised 1996), and experiments were approved by the local Ethics Commission (CEUA/UFRGS), under the project number 26073.
Animal
Male Wistar rats (90 days old) were obtained from our breeding colony at the Central Animal House of the Department of Biochemistry. They were held under a normal light-dark cycle (lights on 7–19 h) at room temperature (22 ± 1 °C), with water and commercial food pellets available ad libitum. These conditions were kept constant throughout the experiments.
Cortical Primary Astrocyte Cultures from Wistar Adult Rats
Cerebral cortex was dissected aseptically and the meninges removed. During the dissection, the structures were maintained in Hank’s Balanced Salt Solution (HBSS) containing 0.05% trypsin and 0.003% DNAse and maintained at 37 °C for 15 min. The tissues were mechanically dissociated, using a Pasteur pipette, and centrifuged at 400×g for 5 min. The pellets were resuspended in HBSS solution containing 40 U of papain per milliliter, cysteine 0.02%, and 0.003% DNAse and again gently mechanically dissociated with a Pasteur pipette. After another centrifugation step (400×g, 5 min), cells were resuspended in HBSS containing only DNAse (0.003%) and after naturally decanted for 30–40 min. The supernatant was collected and centrifuged for 7 min (400×g). The cells from the supernatant were resuspended in DMEM/F12 10% fetal bovine serum (FBS), 15 mM HEPES, 14.3 mM NaHCO3, 1% fungizone, and 0.04% gentamicin and then plated in 6- or 24-well plates pre-coated with poly-l-lysine and cultured at 37 °C and 5% CO2 [12, 21].
Maintenance of Cell Culture
The first change of medium was performed after 24 h of culture. During the first week, the medium change occurred once every 2 days and, from the second week, once every 4 days. From the third week onwards, the cells received medium supplemented with 20% FBS. Around the third to fourth weeks, cells reached confluence and were used for the experiments.
Treatments
After astrocytes reached the confluence, we studied the response of these cells on Hcy. The culture medium was removed, and the cells were incubated with different concentrations of Hcy (10, 30, and 100 μM) in DMEM/F12 with 1% FBS for 24 h at 37 °C in an atmosphere with 5% CO2.
Cellular Viability—MTT Reduction Assay
After incubation of Hcy, MTT was added (50 μg/mL) and cells were incubated for 30 min at 37 °C. Subsequently, the medium was removed and the MTT crystals were dissolved in dimethyl sulfoxide (DMSO). Absorbance values were measured at 560 and 650 nm. The results are expressed as percentages of the control value.
Cellular Membrane Integrity—Propidium Iodide Incorporation Assay
Cells were treated simultaneously with 7.5 μM PI and incubated for up 24 h at 37 °C and 5% CO2. The optical density of fluorescent nuclei (labeled with PI), indicative of loss membrane integrity, was determined with OptiQuant software (Packard Instrument Company). Density values obtained are expressed as a percentage of the control value.
Immunofluorescence
As previously described by our group [22], immunofluorescence was performed by fixing the cell cultures with 4% paraformaldehyde for 20 min and permeabilizing with 0.1% Triton X-100 in PBS for 5 min at room temperature. After blocking overnight with 4% albumin, cells were incubated again overnight with anti-glial fibrillary acidic protein (GFAP, 1:400), at 4 °C, followed by washing with PBS and incubation with specific secondary antibody conjugated to Alexa Fluor® 488 (green color) for 1 h at room temperature. For actin-labeling analyses, the cells were incubated with 10 mg/mL rhodamine-labeled phalloidin in PBS for 45 min and two washes with PBS. Cell nuclei were stained with 0.2 mg/mL of 4′,6′-diamino-2-phenylindole (DAPI). Astrocytes were analyzed and photographed with a Nikon microscope and a TE-FM Epi-Fluorescence accessory.
Determination of the Activity of the Na+, K+ ATPase
The reaction mixture for determining the activity of the Na+, K+ ATPase contains 5.0 mM MgCl2, 80.0 mM NaCl, 20.0 mM KCl, and 40.0 mM Tris-HCl, pH 7.4, in a final volume of 200 μL. After 10 min pre-incubation at 37 °C, the reaction was initiated by addition of ATP to a final concentration of 3.0 mM and incubated for 20 min. The controls were performed under the same conditions, with addition of 1.0 mM ouabain. Its activity was calculated as the difference between the two assays [23]. The free inorganic phosphate (Pi) was measured by method [24], and specific activity of the enzyme was expressed by free nanomoles of Pi per minute per milligram of protein.
Evaluation of Antioxidant Enzyme Activities
The activity of SOD was determined by the method based on the ability to oxidize pyrogallol, a process dependent of superoxide. This inhibition of auto-oxidation occurs in the presence of SOD, and its activity can be analyzed by a spectrophotometer at 420 nm [25]. The activity of CAT was analyzed according to the method of Aebi [26], which is based on the disappearance of H2O2 at 240 nm. A unit of CAT was defined as 1 μmol consumed hydrogen peroxide per minute, and the CAT activity was calculated by units per milligram of protein. Activity of GPx was measured using tert-butyl hydroperoxide as substrate. The disappearance of NADPH was monitored at 340 nm. One unit of GPx is defined as 1 mmol of NADPH consumed per minute, and the activity is represented in units per milligram of protein [27].
Glutathione Content
The levels of GSH were evaluated as described by Souza et al. [12]. Homogenate astrocytes were diluted in 100 mM sodium phosphate buffer (pH 8.0) containing 5 mM EDTA, and the protein was precipitated with 1.7% meta-phosphoric acid. The supernatant was analyzed with o-phthaldialdehyde (1 mg/mL methanol) at room temperature for 15 min. Fluorescence was measured using wavelengths of excitation and emission at 350 and 420 nm, respectively. A calibration curve was performed with standard GSH solutions at concentrations ranging from 0 to 500 μM. The calculated results are expressed in nanomoles per milligram of protein.
Inflammatory Response
TNF-α levels in the extracellular medium were assayed using ELISA for TNF-α from PeproTech. The levels of IL-1β and IL-6 in the extracellular medium were measured using ELISA kits from eBioscience. The results are expressed in nanograms per milliliter. The average minimum sensitivity of the ELISA kit detection is 0.4 ng/mL of cytokines [21].
NFκB Levels
The levels of NFκB p65 in the nuclear fraction, which had been isolated from lysed cells by centrifugation, were measured using an ELISA commercial kit from Invitrogen (USA). The results are expressed as percentages relative to the control levels. The ELISA kit detects a minimum of 50.0 pg/mL [21].
Western Blot Analysis
According to the method of Souza et al. [12] with modifications, cells were homogenized using a lysis buffer solution with 4% SDS, 2 mM EDTA, and 50 mM Tris–HCl, pH 6.8. Equal amounts of proteins (45 μg) from each sample were boiled in a sample buffer [62.5 mM Tris–HCl, pH 6.8, 2% (w/v) SDS, 5% β-mercaptoethanol, 12% (v/v) glycerol, 0.002% (w/v) bromophenol blue] and submitted to electrophoresis in 10% (w/v) SDS–polyacrylamide gel. The separated proteins were blotted onto a nitrocellulose membrane. Equal loading of each sample was confirmed with Ponceau S staining (Sigma-Aldrich). The membranes were incubated with polyclonal anti-HO1 (1:200). GAPDH was used as a loading control. After incubating overnight with the primary antibody at 4 °C, the membrane was washed and incubated with peroxidase-conjugated anti-rabbit immunoglobulin (IgG) at a dilution of 1:5000 for 2 h at room temperature. The chemiluminescence signal was detected using an ECL kit (Amersham), and after the films were scanned, bands were quantified using the ImageJ software (1.48v, National Institutes of Health, USA).
Statistical Analyses
Data are expressed as the mean ± S.E.M. All analyses were performed using the Statistical Package for the Social Sciences (SPSS 16.0, Chicago, IL, USA) software. Differences among groups were analyzed using one-way ANOVA followed by Tukey’s post hoc test, P < 0.05.
Results
Effects of Hcy on Cell Viability, Morphology, and Cytoskeleton Proteins
First, we performed the MTT assay and PI incorporation to analyze the cell viability/integrity of cortical astrocytes treated during 24 h with different concentrations of Hcy (10, 30, and 100 μM). Figure 1 shows that Hcy did not affect the membrane integrity (1A) or the cell viability (1B). We also conduct the same analysis at different times (4, 8, and 12 h) and did not observe any significant change (data not shown).
Effects of Hcy on cell viability. Membrane integrity (a) and cell viability (b) of cortical astrocytes treated during 24 h with different concentrations of Hcy were measured as described in the “Material and Methods” section. The results are expressed as percentage of control and represent the mean ± S.E.M. The data were analyzed statistically using a one-way ANOVA followed by Tukey’s test
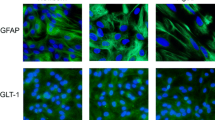
Next, we evaluated the cellular morphology of astrocytes treated with Hcy for 24 h. Figure 2 displays that basal-cultured adult astrocytes showed a polygonal to fusiform and flat morphology, while Hcy induced few differential morphological alterations with process-bearing cells, mainly at 100 μM Hcy. We also performed immunofluorescence analysis using anti-GFAP and phalloidin (for actin) to verify the effect of Hcy on the cytoskeletal structure (Fig. 2). Under control conditions, adult astrocytes presented an intense immunostaining for GFAP, attesting the astrocytic phenotype, and actin. Hcy induced stress fiber reorganization as well as rearrangement of the GFAP filament distributed along the cytoplasm and processes. Therefore, an altered organization of GFAP filaments is supported by the reorganization of the actin cytoskeleton. This effect probably is associated with the time of treatment. No change in nuclear morphology was observed.
Effects of Hcy on cellular morphology and classical cytoskeleton astrocyte markers. Representative cell morphology (phase contrast), immunofluorescence of GFAP (green) and actin (red), and merged images with DAPI (blue) in cortical astrocytes after 24 h of exposure to different concentrations of Hcy. The data are three independent experiments. Scale bar 50 μm (color figure online)
Hcy Treatment Impaired Na+, K+ ATPase Activity and Antioxidant Defenses in Adult Cortical Astrocytes
Figure 3 shows that Hcy (10, 30, and 100 μM) decreased the activity of the enzyme Na+, K+ ATPase in adult cortical astrocytes (P < 0.01). Interestingly, we did not observe differences between different doses of Hcy.
Effects of Hcy in Na+, K+ ATPase activity. Cells were treated during 24 h with different concentrations of Hcy. The results are expressed as nanomoles of Pi per minute per milligram of protein and represent the mean ± S.E.M. The data were analyzed statistically using a one-way ANOVA followed by Tukey’s test. Asterisk indicates significant differences from the basal group. **P < 0.01
Additionally, we measured the main non-enzymatic antioxidant defense, GSH, in cultured adult astrocytes and observed a significant decrease in GSH content after the exposure of all doses of Hcy (Fig. 4, P < 0.05).
Effects of Hcy on GSH levels. Cells were treated with different concentrations of Hcy during 24 h. The results are expressed as nanomoles per milligram of protein and represent the mean ± S.E.M. The data were analyzed statistically using a one-way ANOVA followed by Tukey’s test. Asterisk indicates significant differences from basal group. **P < 0.05
We also evaluated the antioxidant enzymatic defenses in adult astrocytes and observed that Hcy promoted a decrease in SOD (P < 0.05 to 10 μM and P < 0.001 to 30 and 100 μM, Fig. 5a) and GPx activities (P < 0.05 to 10 and 30 μM and P < 0.01 to 100 μM, Fig. 5c). Hcy did not alter CAT activity (Fig. 5b).
Effects of Hcy on enzymatic antioxidant defenses. Cells were treated with different concentrations of Hcy during 24 h. SOD (a), CAT (b), and GPx (c) activities were measured as described in the “Material and Methods” section. All results are expressed as units per milligram of protein and represent the mean ± S.E.M. The data were analyzed statistically using a one-way ANOVA followed by Tukey’s test. Asterisk indicates significant differences from the basal group. *P < 0.05, **P < 0.01, and ***P < 0.001
Hcy-Induced Inflammatory Response
Changes in redox homeostasis are closely associated with inflammatory response. In this sense, the levels of classical pro-inflammatory cytokines, TNF-α, IL-1β, and IL-6 were measured (Fig. 6). Hcy increased the release of these cytokines, mainly at 100 μM.
Effects of Hcy on pro-inflammatory cytokines release. Cells were treated with different concentrations of Hcy during 24 h. TNF-α (a), IL-1β (b), and IL-6 (c) levels were measured as described in the “Material and Methods” section. All results are expressed as percentage of control and represent the mean ± S.E.M. The data were analyzed statistically using a one-way ANOVA followed by Tukey’s test. Asterisk indicates significant differences from basal group. *P < 0.05 and **P < 0.01
Hcy Augmented NFκB Transcriptional Activity and Reduced HO-1 Expression Protein
To investigate the putative mechanisms of Hcy neurotoxicity, we measured the transcriptional activity of NFκB, the master regulator of oxidative and inflammatory responses, and the expression levels of its regulator, HO-1, a key enzyme to maintain redox homeostasis.
Hcy in a dose-dependent manner increased NFκB transcriptional activity (P < 0.05, Fig. 7a) and decreased HO-1 expression levels (P < 0.05, Fig. 7b) in adult astrocytes.
Signaling pathways involved in Hcy-induced astrocyte dysfunction. NFκB p65 transcriptional activity (a) and HO-1 expression (b). All results are expressed as percentage of control and represent the mean ± S.E.M. of four independent experimental determinations, performed in triplicate. The data were analyzed statistically using a one-way ANOVA followed by Tukey’s test. Asterisk indicates significant differences from the basal group. *P < 0.05 and **P < 0.01
Discussion
The detrimental effects of Hcy in the CNS are well documented, but the cellular mechanisms underlying Hcy-induced glial reactivity needs to be better elucidated. In this sense, in the present study, for the first time in our knowledge, we demonstrated that Hcy induced in adult astrocytes changes in (i) cellular morphology/cytoskeleton proteins, (ii) pro-inflammatory cytokine release, (iii) cellular antioxidant defenses, (iv) NFκB transcriptional activity, and (v) HO-1 expression levels.
Recently, we demonstrated that Hcy (30 μM) promoted a decrease in the enzymatic antioxidant activities (SOD and GPx) and an increase in neuronal death in cerebral cortex slices from rats, providing some evidences that CNS is susceptible to damage caused by Hcy [6]. There are several hypotheses relating that the neurotoxic effects of Hcy are associated to oxidative stress, due to Hcy auto-oxidation [28]. Our present findings reinforce this hypothesis, indicating that Hcy causes a significant decrease in SOD and GPx activities. SOD catalyzes the dismutation of the superoxide radical (O2 −), forming hydrogen peroxide (H2O2), a reactive species less harmful that is degraded by other enzymes such GPx and CAT [29]. The decrease in enzymatic activity of SOD and GPx may be caused by accumulation of reactive species, such as O2 − and H2O2, which might be toxic inducing cellular biomolecule damage and activating signaling pathways, such as NFκB, which may lead to oxidative stress, inflammation, energetic dysfunction, and finally, cell death.
A proper operation of the enzyme Na+, K+ ATPase is very important for the cellular physiological process and is dependent on concentrations of Na+ and K+. In astroglial cells, this process is crucial to glutamate uptake, and increases in oxidative/nitrosative stress generation might lead to an inappropriate functioning of the Na+, K+ ATPase [30, 31]. In the present study, Hcy (30 μM) exposure induced a decrease in Na+, K+ ATPase activity, which was consistent with previous report [32]. We cannot exclude the idea that the Na+, K+ ATPase acts as a potent regulator of astrocytic functionality, due to its essential role in brain excitability [33] and metabolic energy production [34] and classical glial functions.
GSH is an important regulator of intracellular redox state, and its production might be regulated by HO-1, a signaling pathway that regulates antioxidant defenses [35, 36]. In our study, Hcy decreased GSH levels and HO-1 immunocontent in the cerebral cortex, both in a dose-dependent manner, which suggests that Hcy impairs cellular defense against oxidative/nitrosative damage. Furthermore, the inducible HO-1 is a phase 2 enzyme upregulated in response to oxidative stress, inflammation, and cellular injury [37]. To our knowledge, this study is the first to show the effects of Hcy on the HO-1 pathway in astrocytes. Other studies in peripheral tissues demonstrated that Hcy promotes a downregulation in HO-1 expression [38, 39]. This finding might indicate reduced capacity in protective mechanisms, inducing cellular damage in lipids, proteins, and nucleic acids [40]. Interestingly, GSH depletion and HO-1 reduced levels can lead to neuroinflammation, commonly associated to age-related neurodegenerative disorders.
In line with this, Hcy-induced inflammatory response seems to be associated with vascular dementia [41, 42]. In this sense, our data showed that Hcy promoted a dose-dependent release of TNF-α, IL-1β, and IL-6 from adult astrocytes. In accordance with our previous results, Scherer et al. [4] demonstrated that an in vivo experimental model of mild HHcy in rats promoted an increase in TNF-α, IL-1β, and IL-6 in the cerebral cortex. Similarly, another study using an acute Hcy administration model in rats demonstrated an increase in the same inflammatory mediators in the cerebral cortex [43]. Several studies have shown that astrocytes can respond to different stimuli, such as lipopolysaccharide, ammonia, glutamate, and oxidizing agents, releasing pro-inflammatory cytokines, probably by the NFκB signaling pathway [44–46].
NFκB is a transcription factor responsible for the activation of a number of genes and damaged responses in the CNS [47]. In the cytoplasm, the NFκB p50/p65 heterodimer is inactivated through binding to IkB proteins, and the cytokines exert the opposite effect by activating the phosphorylation of inhibitory proteins by IKKs, allowing the translocation of NFκB into the nucleus [48]. According to our results of inflammatory mediators, NFκB transcriptional activity was increased by Hcy treatment in astrocyte cultures, in a dose-dependent manner, indicating that Hcy may trigger cellular damage through the NFκB pathway, which may also be associated with a decrease in enzymatic and non-enzymatic antioxidant defenses as well as HO-1 expression [49–51]. In this sense, HO-1 is able to inhibit NFκB translocation from the cytoplasm to the nucleus and, consequently, the inflammatory response associated to NFκB translocation [15]. Additionally, there is a close relationship between Nrf2, the regulatory transcription factor of HO-1, and energy metabolism and therefore with Na+, K+ ATPase activity [15]. Moreover, in a large clinical study, Gori et al. [52] analyzed more than 1000 subjects in two small towns near Florence, Italy, and demonstrated that high circulating concentrations of cytokines and NFκB correlate with HHcy.
Despite many advances in neuroscience, little is known about the effects of Hcy in glial functionality. Figure 8 depicts the main conclusions of this study, which demonstrates Hcy-induced glial reactivity in adult astrocytes. These cells may represent an important new tool for understanding the mechanisms involved in the mature brain and might contribute to the comprehension of the neurochemical and physiological effects of Hcy in the CNS. In summary, our results may indicate astrocytes as target for HHcy treatments and as possible mechanisms to be explored in the search for therapeutic agents.
Schematic illustration of the signaling mechanisms involved in Hcy-induced glial reactivity in adult cortical astrocyte cultures. Hcy leads to a cellular redox imbalance, reducing antioxidant defenses, promoting pro-inflammatory cytokine release, decreasing HO-1 expression levels, and activating NFκB transcriptional activity
References
Kruger WD, Gupta S (2016) The effect of dietary modulation of sulfur amino acids on cystathionine beta synthase-deficient mice. Ann N Y Acad Sci 1363:80–90. doi:10.1111/nyas.12967
Jin Y, Brennan L (2008) Effects of homocysteine on metabolic pathways in cultured astrocytes. Neurochem Int 52(8):1410–1415. doi:10.1016/j.neuint.2008.03.001
Bonetti F, Brombo G, Zuliani G (2016) The relationship between hyperhomocysteinemia and neurodegeneration. Neurodegenerative disease management 6(2):133–145. doi:10.2217/nmt-2015-0008
Scherer EB, Loureiro SO, Vuaden FC, da Cunha AA, Schmitz F, Kolling J, Savio LE, Bogo MR et al (2014) Mild hyperhomocysteinemia increases brain acetylcholinesterase and proinflammatory cytokine levels in different tissues. Mol Neurobiol 50(2):589–596. doi:10.1007/s12035-014-8660-6
Scherer EB, Cunha AA, Kolling J, da Cunha MJ, Schmitz F, Sitta A, Lima DD, Magro DD et al (2011) Chronic mild hyperhomocysteinemia induces oxidative damage in cerebral cortex of rats. J Inherit Metab Dis 34:S113–S113
Longoni A, Kolling J, Dos Santos TM, Dos Santos JP, da Silva JS, Pettenuzzo L, Goncalves CA, de Assis AM et al (2016) 1,25-Dihydroxyvitamin D3 exerts neuroprotective effects in an ex vivo model of mild hyperhomocysteinemia. Int J Dev Neurosci 48:71–79. doi:10.1016/j.ijdevneu.2015.11.005
Verkhratsky A, Nedergaard M, Hertz L (2015) Why are astrocytes important? Neurochem Res 40(2):389–401. doi:10.1007/s11064-014-1403-2
Sofroniew MV (2015) Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 16(5):249–263. doi:10.1038/nrn3898
Wilson JX (1997) Antioxidant defense of the brain: a role for astrocytes. Can J Physiol Pharmacol 75(10–11):1149–1163
Illarionova NB, Brismar H, Aperia A, Gunnarson E (2014) Role of Na,K-ATPase alpha1 and alpha2 isoforms in the support of astrocyte glutamate uptake. PLoS One 9(6):e98469. doi:10.1371/journal.pone.0098469
Arvin B, Neville LF, Barone FC, Feuerstein GZ (1996) The role of inflammation and cytokines in brain injury. Neurosci Biobehav Rev 20(3):445–452
Souza DG, Bellaver B, Souza DO, Quincozes-Santos A (2013) Characterization of adult rat astrocyte cultures. PLoS One 8(3):e60282. doi:10.1371/journal.pone.0060282
Araujo JA, Zhang M, Yin F (2012) Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol 3:119. doi:10.3389/fphar.2012.00119
Willis D, Moore AR, Frederick R, Willoughby DA (1996) Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med 2(1):87–90
Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW (2010) When NRF2 talks, who’s listening? Antioxid Redox Signal 13(11):1649–1663. doi:10.1089/ars.2010.3216
Loureiro SO, Romao L, Alves T, Fonseca A, Heimfarth L, Moura Neto V, Wyse AT, Pessoa-Pureur R (2010) Homocysteine induces cytoskeletal remodeling and production of reactive oxygen species in cultured cortical astrocytes. Brain Res 1355:151–164. doi:10.1016/j.brainres.2010.07.071
Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C (2002) The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci 202(1–2):13–23
Herrmann W, Obeid R (2011) Homocysteine: a biomarker in neurodegenerative diseases. Clin Chem Lab Med 49(3):435–441. doi:10.1515/CCLM.2011.084
Obeid R, Herrmann W (2006) Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett 580(13):2994–3005. doi:10.1016/j.febslet.2006.04.088
Szadejko K, Szabat K, Ludwichowska A, Slawek J (2013) Homocysteine and its role in pathogenesis of Parkinson’s disease and other neurodegenerative disorders. Przegl Lek 70(7):443–447
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2016) Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Mol Neurobiol. doi:10.1007/s12035-016-9880-8
Quincozes-Santos A, Nardin P, de Souza DF, Gelain DP, Moreira JC, Latini A, Goncalves CA, Gottfried C (2009) The janus face of resveratrol in astroglial cells. Neurotox Res 16(1):30–41. doi:10.1007/s12640-009-9042-0
Wyse AT, Streck EL, Barros SV, Brusque AM, Zugno AI, Wajner M (2000) Methylmalonate administration decreases Na+,K+-ATPase activity in cerebral cortex of rats. Neuroreport 11(10):2331–2334
Chan KM, Delfert D, Junger KD (1986) A direct colorimetric assay for Ca2+ −stimulated ATPase activity. Anal Biochem 157(2):375–380
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3):469–474
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Hogg N (1999) The effect of cyst(e)ine on the auto-oxidation of homocysteine. Free Radic Biol Med 27(1–2):28–33
Halliwell B (2011) Free radicals and antioxidants—quo vadis? Trends Pharmacol Sci 32(3):125–130. doi:10.1016/j.tips.2010.12.002
Grisar T, Guillaume D, Delgado-Escueta AV (1992) Contribution of Na+,K(+)-ATPase to focal epilepsy: a brief review. Epilepsy Res 12(2):141–149
Quincozes-Santos A, Bobermin LD, Tramontina AC, Wartchow KM, Tagliari B, Souza DO, Wyse AT, Goncalves CA (2014) Oxidative stress mediated by NMDA, AMPA/KA channels in acute hippocampal slices: neuroprotective effect of resveratrol. Toxicol in Vitro 28(4):544–551. doi:10.1016/j.tiv.2013.12.021
Scherer EB, Loureiro SO, Vuaden FC, Schmitz F, Kolling J, Siebert C, Savio LE, Schweinberger BM et al (2013) Mild hyperhomocysteinemia reduces the activity and immunocontent, but does not alter the gene expression, of catalytic alpha subunits of cerebral Na+,K+-ATPase. Mol Cell Biochem 378(1–2):91–97. doi:10.1007/s11010-013-1598-6
Sastry BS, Phillis JW (1977) Antagonism of biogenic amine-induced depression of cerebral cortical neurones by Na+, K+-ATPase in inhibitors. Can J Physiol Pharmacol 55(2):170–179
Mata M, Fink DJ, Gainer H, Smith CB, Davidsen L, Savaki H, Schwartz WJ, Sokoloff L (1980) Activity-dependent energy metabolism in rat posterior pituitary primarily reflects sodium pump activity. J Neurochem 34(1):213–215
Brennan MS, Matos MF, Li B, Hronowski X, Gao B, Juhasz P, Rhodes KJ, Scannevin RH (2015) Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro. PLoS One 10(3):e0120254. doi:10.1371/journal.pone.0120254
Steele ML, Fuller S, Patel M, Kersaitis C, Ooi L, Munch G (2013) Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells. Redox Biol 1:441–445. doi:10.1016/j.redox.2013.08.006
Syapin PJ (2008) Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br J Pharmacol 155(5):623–640. doi:10.1038/bjp.2008.342
Luo X, Xiao L, Yang H, Zhang R, Jiang M, Ni J, Lei T, Wang N (2014) Homocysteine downregulates gene expression of heme oxygenase-1 in hepatocytes. Nutr Metab (Lond) 11(1):55. doi:10.1186/1743-7075-11-55
Tan M, Ouyang Y, Jin M, Chen M, Liu P, Chao X, Chen Z, Chen X et al (2013) Downregulation of Nrf2/HO-1 pathway and activation of JNK/c-Jun pathway are involved in homocysteic acid-induced cytotoxicity in HT-22 cells. Toxicol Lett 223(1):1–8. doi:10.1016/j.toxlet.2013.08.011
Takahashi T, Morita K, Akagi R, Sassa S (2004) Heme oxygenase-1: a novel therapeutic target in oxidative tissue injuries. Curr Med Chem 11(12):1545–1561
Sudduth TL, Powell DK, Smith CD, Greenstein A, Wilcock DM (2013) Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J Cereb Blood Flow Metab 33(5):708–715. doi:10.1038/jcbfm.2013.1
Lazzerini PE, Capecchi PL, Selvi E, Lorenzini S, Bisogno S, Galeazzi M, Laghi Pasini F (2007) Hyperhomocysteinemia, inflammation and autoimmunity. Autoimmun Rev 6(7):503–509. doi:10.1016/j.autrev.2007.03.008
da Cunha AA, Ferreira AG, Wyse AT (2010) Increased inflammatory markers in brain and blood of rats subjected to acute homocysteine administration. Metab Brain Dis 25(2):199–206. doi:10.1007/s11011-010-9188-8
Santos CL, Bobermin LD, Souza DG, Bellaver B, Bellaver G, Arus BA, Souza DO, Goncalves CA et al (2015) Lipoic acid and N-acetylcysteine prevent ammonia-induced inflammatory response in C6 astroglial cells: the putative role of ERK and HO1 signaling pathways. Toxicol in Vitro 29(7):1350–1357. doi:10.1016/j.tiv.2015.05.023
Efremova L, Chovancova P, Adam M, Gutbier S, Schildknecht S, Leist M (2016) Switching from astrocytic neuroprotection to neurodegeneration by cytokine stimulation. Arch Toxicol. doi:10.1007/s00204-016-1702-2
Soliman ML, Combs CK, Rosenberger TA (2013) Modulation of inflammatory cytokines and mitogen-activated protein kinases by acetate in primary astrocytes. J NeuroImmune Pharmacol 8(1):287–300. doi:10.1007/s11481-012-9426-4
Jones KA, Thomsen C (2013) The role of the innate immune system in psychiatric disorders. Mol Cell Neurosci 53:52–62. doi:10.1016/j.mcn.2012.10.002
Abraham E (2000) NF-kappaB activation. Crit Care Med 28(4 Suppl):N100–N104
Alcaraz MJ, Vicente AM, Araico A, Dominguez JN, Terencio MC, Ferrandiz ML (2004) Role of nuclear factor-kappaB and heme oxygenase-1 in the mechanism of action of an anti-inflammatory chalcone derivative in RAW 264.7 cells. Br J Pharmacol 142(7):1191–1199. doi:10.1038/sj.bjp.0705821
Cuadrado A, Martin-Moldes Z, Ye J, Lastres-Becker I (2014) Transcription factors NRF2 and NF-kappaB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J Biol Chem 289(22):15244–15258. doi:10.1074/jbc.M113.540633
Bellezza I, Tucci A, Galli F, Grottelli S, Mierla AL, Pilolli F, Minelli A (2012) Inhibition of NF-kappaB nuclear translocation via HO-1 activation underlies alpha-tocopheryl succinate toxicity. J Nutr Biochem 23(12):1583–1591. doi:10.1016/j.jnutbio.2011.10.012
Gori AM, Corsi AM, Fedi S, Gazzini A, Sofi F, Bartali B, Bandinelli S, Gensini GF et al (2005) A proinflammatory state is associated with hyperhomocysteinemia in the elderly. Am J Clin Nutr 82(2):335–341
Acknowledgements
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Financiadora de Estudos e Projetos (FINEP)—Instituto Brasileiro de Neurociências (IBN Net) 01.06.0842-00, Federal University of Rio Grande do Sul (UFRGS), and Instituto Nacional de Ciência e Tecnologia para Excitotoxicidade e Neuroproteçãao (INCTEN/CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Our work has followed the National Institute of Health Guide for the Care and Use of Laboratory Animals “Guide for the Care and Use of Laboratory Animals” (NIH publication No. 80-23, revised 1996), and experiments were approved by the local Ethics Commission (CEUA/UFRGS), under the project number 26073.
Rights and permissions
About this article
Cite this article
Longoni, A., Bellaver, B., Bobermin, L.D. et al. Homocysteine Induces Glial Reactivity in Adult Rat Astrocyte Cultures. Mol Neurobiol 55, 1966–1976 (2018). https://doi.org/10.1007/s12035-017-0463-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0463-0