Abstract
Increased expression of brain-derived neurotrophic factor (BDNF) has been associated with memory-enhancing and neuroprotective properties of some drugs under chronic cerebral hypoperfusion (CCH) condition. Ginsenoside Rd (GSRd), one of the main active ingredients in Panax ginseng, is widely used for brain protection. However, it is poorly understood whether epigenetic mechanisms implied in the BDNF modulation after GSRd treatment for CCH remain elusive. Here, we investigated the neuroprotective effects of GSRd and the involved mechanisms. We demonstrated that GSRd administration ameliorated CCH-induced impairment of learning and memory behaviors, evidenced by decreased escape latency and increased number of crossing the platform in Morris water maze test. This improvement was associated with promoted neuron survival and increased BDNF expression in the hippocampus and prefrontal cortex of CCH mice. GSRd improved neuron survival and decreased neuron apoptosis and the level of caspase-3 under oxygen–glucose deprivation/reoxygenation (OGD/R) by upregulation of BDNF as well as in vitro. The levels of acetylated histone H3 (Ac-H3) and histone deacetylase (histone deacetylase 2 (HDAC2)) were altered under OGD/R in a time-dependent manner, and GSRd reestablished the balance between Ac-H3 and HDAC2 which resulted in upregulation of BDNF and increased neuron survival. MS-275, an inhibitor of class I HDACs, abolished the levels of Ac-H3 at the bdnf promoters and enhanced upregulation of BDNF after GSRd administration, suggesting a synergistic effect between GSRd and MS-275. All the data suggested that GSRd provided neuroprotection by epigenetic modulation which accounted for the regulation of BDNF in CCH mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic cerebral hypoperfusion (CCH), also called as chronic cerebral ischemia (CCI), is a chronic ischemic neurological damage and contributes to a persistent or progressive cognitive and neurological dysfunction [1–3]. Because of the complexity of CCH causes and the harmful consequences caused by CCH, there is no ideal prevention and treatment for CCH. Ginseng, the root of Panax Ginseng C.A. Meyer (Araliaceae), is a traditional Chinese herbal medicine widely used for over 2000 years. Extensive studies proved that ginsenoside Rd (GSRd), one of the main active components of ginsenosides, has a broad effect on the central nervous system (CNS), including increasing neural stem cell proliferation [4], maintaining neurogenesis after neural injury [5], protection of nerve survival, promotion of neurite growth, and protection of neurons from death in vivo and in vitro [6]. GSRd protected the nerve degeneration caused by the overload of Ca2+, decreasing reactive oxygen species (ROS) formation and oxidative stress [7], suggesting that GSRd has potential roles in preventing and treating ischemic disease. However, the molecular mechanism of the neuroprotective effects of GSRd in CCH remains unclear.
Brain-derived neurotrophic factor (BDNF), a most abundant and widely distributed neurotrophin in the brain, plays critical roles in neuronal development, function, and survival and modulates neural and behavioral plasticity to injury [8], as well as response to drug treatment [9]. Reduced levels of BDNF mRNA and protein were shown in the brains of Alzheimer’s disease patients [10] and Parkinson’s disease patients [11] as well as in depression [9]. BDNF expression was upregulated by antidepressant treatment [9, 12], suggesting that BDNF contributes to therapeutic action. Hippocampus is a key region of the brain involved in memory formation, organization, and storage. Some studies have revealed that BDNF is highly expressed in the granule cell layer (GCL) of the dentate gyrus, indicating its involvement in synaptic plasticity and neuronal development through TrkB receptors. Moreover, BDNF expression and release might also be altered by genetic and epigenetic variations, including histone posttranslational modifications [12, 13]. Histone acetylation is a highly dynamic process regulated by two classes of enzyme: histone acetyltransferases (HATs) and histone deacetylases (HDACs). Histone acetylation, modulated by the opposite activity of HATs and HDACs, is generally associated with active gene transcription. p300/CREB-binding protein (CBP) is one of the categorized three families of HATs. CBP is a transcriptional co-activator with HAT activity that was shown to be important for long-term memory processes, which depended on de novo gene expression [14, 15], and the expression of BDNF was regulated by HAT activity of p300/CBP [16]. Evidence demonstrated that not only environmental manipulations can induce long-lasting epigenetic changes at the bdnf promoters [17], but also the medication treatment; for example, imipramine enhances H3 acetylation at bdnf P4 and P6 through downregulation of HDAC5 [12]. However, it is still unknown whether these rapid epigenetic changes are involved in the modulation of BDNF transcript expression after GSRd administration in CCH.
In this study, we showed that GSRd administration ameliorated CCH-induced impairment of learning and memory behaviors in mice, and this improvement was associated with promoted neuron survival and increased BDNF expression through reestablishing the balance between Ac-H3 and histone deacetylase 2 (HDAC2).
Materials and Methods
Materials
Neurobasal medium, B27, glutamine, and fetal bovine serum (FBS) were provided from Invitrogen (Carlsbad, CA, USA). Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12) was purchased from Hyclone (Logan, UT, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), poly-d-lysine, trypsin, anti-BDNF, anti-β-tubulin III, and anti-β-actin antibodies were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibody to caspase-3 was purchased from Chemicon (Temecula, CA, USA), antibodies to acetyl-histone H3 (H3Ac), HDAC2, and p300 were from Cell Signaling Technology (Danvers, MA, USA). All secondary antibodies conjugated with horseradish peroxidase (HRP) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Microcoil was purchased from Sawane Spring Co. (Shizuoka, Japan). GSRd with a purity of 98 % was obtained from Tai-He Biopharmaceutical Co. Ltd. (Guangzhou, China). GSRd stock solutions were prepared in saline containing 10 % 1,3-propanediol (v/v). BDNF Emax ImmunoAssay System was obtained from Promega (Madison, WI, USA). Polyvinylidene difluoride (PVDF) membrane and lactate dehydrogenase (LDH) assay kit were purchased from Roche Molecular Biochemicals (Mannheim, Germany).
Bilateral Carotid Artery Stenosis and Drug Treatment
Five- to 7-week-old male C57BL/6J mice (body weight 20–25 g) were purchased from the Experimental Animals Center of the Fourth Military Medical University (certificate No. 201000082, Grade II) and were maintained in a standard 12 h light–dark cycle, temperature-controlled room (22 ± 1 °C), with access to food and water ad libitum. The Animal Research Committee of the Fourth Military Medical University in China approved the protocol, and surgery was prepared for bilateral carotid artery stenosis (BCAS) as described previously [18]. In brief, the mice were anesthetized with sodium pentobarbital intraperitoneally, and both common carotid arteries (CCAs) were exposed by a midline cervical incision and freed from their sheaths. The microcoil, which was made of piano wire with an inner diameter of 0.18 mm, was twined by rotating around the right CCA, and another microcoil was twined around the left CCA 30 min later. For the sham operation, the CCAs of the animals were exposed, but the microcoils were not twined. Fifteen days after the BCAS or sham operation, the mice received vehicle (saline, 10 ml/kg) or GSRd (10 or 30 mg/kg, respectively) intraperitoneally once daily for 21 consecutive days; then, learning and memory behaviors were examined. The brain tissues were harvested for hematoxylin and eosin staining, enzyme-linked immunosorbent assay (ELISA) assay, and Western blot analysis after behavior test.
Cognitive Test (Morris Water Maze Test)
Spatial learning and memory performance was measured in the Morris water maze (MWM) (n = 6/group) as previously described.
Apparatus
The water maze test was performed in a circular tank (122 cm in diameter and 51 cm in height) filled with opaque water which was placed in a warm and quiet behavioral room with ample surrounding visual cues. Water was filled to a depth of 37.5 cm with the temperature at 22–25 °C. A platform (diameter 10 cm) was submerged 1–1.5 cm beneath the water surface in the center of the target quadrant. The swimming path of the mouse was recorded by a video-computerized tracking system (Dig-Behav, Jiliang Co., Ltd., Shanghai, China).
Procedure
The MWM protocol and treatment regimens were based on previous studies [19] with some modifications. Briefly, mice experienced a learning trails phase with the platform hidden in the target quadrant and a probe test phase without the platform. At the beginning of each trial, the mice were released into the water facing the tank wall from one of the four fixed entry points randomly. Individual trials were 1 min, and the interval was 15 s. A trial finished when the mouse climbed onto the platform and remained on it for 10 s; any mouse that failed to locate the platform within 60 s was helped to place on the platform for 15 s. After a trial finished, the mice were immediately dried and kept in their home cages for at least 10–15 min until the next trial. All mice experienced four trails per day for four consecutive days. The probe test was carried out 24 h after the last learning trail for 60 s. The trials were recorded and analyzed by an automated analyzing system. Latency to the platform site, platform site crossings, time in target quadrant, and time in platform site were recorded to evaluate the animal’s learning and reference memory capacities. Data were expressed as the average of four trails on the training days. Different mice were used in each of the following experiments.
Memory Consolidation
To assess the effects of GSRd on spatial memory consolidation, mice experienced four trials per day for four consecutive days (day 1–day 4) with the platform hidden in the target quadrant. Immediately after the last trail on day 4, a 60-s probe test was carried out on day 5 with the platform removed. The locomotor activity was calculated as the average swimming speed of training trials in the first day by using an automatic tracking system.
Open Field Test
The open field test was conducted as described before [20]. The animal was placed in an opaque open field (30 cm × 30 cm × 30 cm), in which the middle and inner areas (15 cm × 15 cm) were termed the “central” region. Each test session was initiated by placing the mice in the central area, and the mice were then allowed to move freely and explore the environment for 10-min period. Assessments of voluntary locomotor activity were made using an automated video-tracking system (Jiliang, Shanghai, China). A video tracking program was used to measure the total track length (total distance traveled) and the time spent in the central region of the open field.
Hematoxylin and Eosin Staining
After behavior evaluation, the brains (n = 6 in each group) were perfused with cold 4 % paraformaldehyde in 0.05 M phosphate-buffered saline (PBS). Brain blocks containing dorsal hippocampus and prefrontal cortex (PFC) area were embedded in paraffin. Coronal sections (6 μm) were cut using a Leica CM1800 Cryostat (Leica Microsystems, Wetzlar, Germany). Six sections, 200 μm apart from each other, from each animal were selected for hematoxylin and eosin (H&E) staining of the hippocampus and PFC. The sections were observed under light microscopy (Olympus, Japan) after staining to evaluate the morphological changes of the hippocampus and PFC.
Western Blot Analysis
The samples were harvested at designed time points and prepared for Western blot analysis. The protein contents were quantified by a BCA kit, and equal amounts of protein (30 μg) were separated and electrotransferred onto a PVDF membranes. The membranes were blocked in 5 % fat-free milk in Tris-phosphate buffer containing 0.05 % Tween 20 (TBST) for 1 h at room temperature. It was further incubated overnight at 4 °C with primary antibodies including anti-acetyl histone H3 (1:5000), anti-HDAC2 (1:5000), anti-BDNF (1:2000), anti-caspase-3 (1:1,000), anti-CBP (1:1000), and anti-β-actin (1:10,000) served as a loading control. The membranes were then incubated with HRP-conjugated secondary antibodies for 1–2 h at room temperature after three washes for 10 min with TBST. The target protein signal was detected by ECL chemiluminescence. The staining luminescence was recorded, and the integrated density of each band was quantified using ImageJ software. Western blot analysis was repeated three times, and qualitatively similar results were obtained.
Chromatin Immunoprecipitation Assay
The chromatin immunoprecipitation (ChIP) assay was performed as previously described [12], with some modifications. Briefly, the hippocampal tissues were cut into 1-mm3 cubes immediately after the behavioral tests and fixed in 1 % formaldehyde for 15 min at room temperature. Fixation was stopped by adding glycine (125-mM final concentration). Tissue was washed in PBS and the pellet suspended in sodium dodecyl sulfate (SDS) lysis buffer (50 mM Tris, pH 8.1, 10 mM EDTA, 1 % SDS). Chromatin was solubilized and sonicated on ice 6 × 10 s to obtain fragments of approximately 300–400 bp. A tenth of the lysates was saved as “input” DNA for ChIP normalization. The polyclonal antibody against acetylated histone H3 or HDAC2 antibody was added to each sample and incubated overnight at 4 °C with gentle mixing, and rabbit or mouse IgG was used as the negative control.
Immune complexes were incubated for 4–6 h with magnetic beads (Life Technologies) and sequentially washed with 150 mM NaCl low-salt buffer and 500 mM NaCl high-salt buffer, resuspended in 120 ml of 1 % SDS in 0.1 M NaHCO3, and incubated at 65 °C overnight. De-crosslinked samples were treated with RNase and proteinase K (Sigma-Aldrich), and DNA was purified by QIAquick PCR Purification Kit (Qiagen). To quantitate histone-associated gene promoters, immunoprecipitated DNA samples were subjected to quantitative real-time PCR as described earlier. Real-time PCR was performed to amplify fragments (about 200 bp) within the transcriptional control region of target genes. Primer sets were used as following: bdnf exon IV (5′-TCAGGAGTACATATCGGCCACCA-3′, 5′-GTAGGCCAAGTTGCCTTGTCCGT-3′) and gapdh (5′-AGACAGCCGCATCTTCTTGT-3′, 5′-CTGCGGGAGAAGAAAGTCAG-3′). The ChIP signal was analyzed as the following method in the handbook provided by the manufacturer: ΔCt[normalized ChIP] = (Ct[ChIP] − (Ct[Input] − Log2(input dilution factor))), and ChIP/Input ratio (ChIP%) was calculated as 2(−ΔCt[normalized ChIP]).
Primary Neuronal Cell Culture
Animal care and procedures were approved by the institutional animal care and use committee in full compliance with international rules and policies. Every effort was made to minimize the number of animals used and their suffering. Primary hippocampal cultures were prepared from the brain of E17-E18 C57BL/6 mouse embryos (obtained from the Experimental Animal Center of the Fourth Military Medical University, Xi’an, China). Neurons from each embryo hippocampus were isolated and seeded into multiple well plates with equal cell numbers (1.5 × 105 cells/well in 24-well plates, 6 × 105 cells/well in a six-well plate). The neurons were maintained in Neurobasal medium with 2 % B27, 0.5 mM glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin. Medium was half changed every 3 days. The purity of neurons is more than 90 % judged by β-tubulin III immunostaining. Cultures were used for experiments 7 to 10 days after seeding.
Oxygen–Glucose Deprivation and Reoxygenation
To mimic ischemia, neurons were subjected to oxygen–glucose deprivation/reoxygenation (OGD/R) as described previously [21, 22]. In brief, cells were washed with PBS and incubated with DMEM (no glucose), and culture plates were placed in an anaerobic chamber containing 5 % CO2 and 95 % N2 (< 1 % O2) at 37 °C to initiate OGD. Three hours later, the OGD was terminated by bringing the cultures back to the prewarmed Neurobasal medium supplemented with 2 % B27 and 0.5 mM glutamine as before and placing them in a normoxic chamber for 24 h of reoxygenation. Neuron cultures were terminated to run tests after OGD procedures. To determine the effect of GSRd, cultured cortical neurons were pretreated with 0.1, 1.0, and 10 μM GSRd for 2 h prior to OGD and presented during the whole experiment of OGD/R. Cells of control group were treated identically except that they were not exposed to OGD. To investigate the involvement of epigenetic modulation of BDNF in GSRd-mediated neuroprotection, MS-275 (1 μM) was added to the medium 30 min before OGD. For concurrent treatment, GSRd was present in the culture medium during OGD and reoxygenation; for control, only vehicle (saline) was added to the culture medium.
Cell Viability Analysis and Cytotoxicity Assay
Cell viability was evaluated by MTT assay as described before [23]. For GSRd-mediated protection assay, neurons were pretreated with GSRd (0, 1, and 10 μM) 2 h before being subjected to OGD/R injury. At the end of each treatment, the culture medium was replaced with fresh medium containing 0.5 mg/ml MTT for 4 h at 37 °C. At the end of incubation, 100 μl/well DMSO was added for 30 min to resolve the formazan crystals. The plates were shaken vigorously to ensure complete solubilization of formazan crystals. The optical density was measured at 570 nm (630 nm as a reference) using a microplate reader. To confirm hippocampal neuron injury, lactate dehydrogenase (LDH) activity in the medium after OGD/R was determined according to the manufacturer’s protocol. Since the enzyme is released from cells with damaged membranes, the efflux of LDH is closely related to the extent of damaged or destroyed neurons [23]. Briefly, the culture medium was collected at the desired time point and treated with LDH assay reaction mixture for 30 min at 25 °C in the dark. The absorbance was measured with a microplate reader at 490 nm.
Enzyme-Linked Immunosorbent Assay
Secreted BDNF levels were measured using ELISA kit. Brain extracts were obtained from the hippocampus and prefrontal cortex (PFC) from both control and CCH mice with or without GSRd treatment (10, 30 mg/kg). Tissue blocks were dissected on ice, and wet weight was rapidly measured. Protein content was determined by BCA assay. BDNF levels measured were normalized to the total amount of proteins loaded. The supernatants from treated and untreated cell cultures were collected at the end of reoxygenation. The levels of BDNF from both the brain samples and supernatant of cultured neurons were subjected to immunoassay for BDNF according to the manufacturer’s instructions. The ELISA results of BDNF showed as nanogram per milligram of tissue (ng/mg) or picogram per milliliter of supernatant (pg/ml) and were expressed as mean values ± standard error of the mean (SEM).
Statistical Analysis
Statistical analyses for differences between various groups were performed using the two-sample Student’s t test, one- or two-way analysis of variance (ANOVA). All data are expressed as the mean ± SEM, and P values <0.05 were considered statistically significant.
Results
Ginsenoside Rd Administration Improved Spatial Learning and Memory in Chronic Cerebral Hypoperfusion Mice
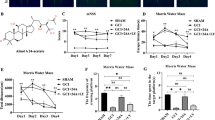
CCH contributes to a persistent or progressive cognitive and neurological dysfunction [1–3]. The model of CCH was established as described previously, and the cognitive function was assessed using MWM test. Three weeks after GSRd treatment (0, 10, 30 mg/kg), the animals were trained and the latency traveled to find the platform is shown in Fig. 1a. All of four groups over the four training days exhibited significant, substantial reductions in their latencies to find the platform. However, compared to the control group, CCH group spent significantly longer time to find the platform, implying an impairment of spatial learning process, and this impairment occurred from training day 2 onward (P < 0.01). The escape latency in GSRd-treated CCH mice was significantly less than CCH group.
The effects of GSRd on chronic cerebral hypoperfusion (CCH)-induced deficits in spatial learning and memory as measured with the Morris water maze. Mice were given saline (10 ml/kg) or GSRd (10, 30 mg/kg) intraperitoneally for 21 days at 15 days post bilateral carotid artery stenosis (BCAS), which was established as model of chronic cerebral hypoperfusion (CCH). a Mean daily escape latencies (time from the starting to the hidden platform). b Time spent in the target quadrant during the probe trials. c Distance in target quadrant during the probe trials. d Numbers of crossing platform site spent in target quadrant during the probe trials. All values were expressed as the mean ± the SEM. *P < 0.05, **P < 0.01 compared to the sham group; #P < 0.05, ##P < 0.01 compared to the CCH group
In the probe trial, the CCH mice spent significantly less time in target quadrants (Fig. 1b) compared with control group (P < 0.01). However, GSRd treatment increased the time spent in the target quadrant, which was significantly more than CCH group. Accordingly, distance in target quadrant from GSRd group was much longer than CCH mice (Fig. 1c). The crossing time of CCH mice was significantly less than the control group (0.40 ± 0.24 for CCH and 4.25 ± 0.41 for control, correspondingly, P < 0.01). The crossing times of GSRd-treated CCH mice were 3.13 ± 0.51 (10 mg/kg GSRd group) and 4.25 ± 0.41 (30 mg/kg GSRd group), respectively, which were significantly higher than the CCH group (P < 0.01) and had no significant difference between 30 mg/kg GSRd group and control group (Fig. 1d). In order to exclude the possibility that the results were due to the impairments of visual and motor function in mice, a visible platform test was performed after the probe trials. There was no statistical difference in the escape latencies among all groups in the visible platform test, and there was no significant difference in swimming speeds (data not shown). The results confirmed that the alteration of all parameters in the hidden platform tests and probe trials did not result from changes in visual or motor abilities of mice.
Ginsenoside Rd Administration Improved Neuron Survival in the Chronic Cerebral Hypoperfusion Mice
GSRd ameliorated the impairment of learning and memory in CCH mice; we then evaluated the ability of GSRd to reduce ischemic damage in the CCH model. H&E staining was performed to determine the neuroprotective effects of GSRd. The normal neurons in the hippocampus and PFC of the sham group were packed tightly and orderly with clear nuclei in sham group. However, it exhibited obvious pathological abnormalities with loosed arranged neurons, pyknotic nuclei and loss, or dark color staining in the hippocampus and PFC in the CCH group (Fig. 2a). The numbers of the normal neurons were decreased to 28.93 ± 2.57 and 32.82 ± 2.17 % in the hippocampus and the PFC after CCH, respectively (P < 0.01 compared with 90.30 ± 2.70 and 85.60 ± 2.38 % in the hippocampus and PFC of control group). By contrast, these histopathological alterations were dramatically reduced after GSRd administration. Normal cells were significantly increased to 74.60 ± 2.84 % in the hippocampus and 76.81 ± 2.10 % in the PFC in the GSRd-treated group (P < 0.01 compared with CCH) as shown in Fig. 2b. This result indicated that GSRd prevented loss of neurons after CCH injury, and the promotion of neuron survival after GSRd administration may be responsible for the promoted learning and memory capacities after CCH damage.
GSRd increased the neural survival after bilateral carotid artery stenosis (BCAS). a Morphological evaluation in the hippocampus and prefrontal cortex (PFC) by hematoxylin and eosin staining in sham, CCH, and CCH + GSRd groups. For the CCH + GSRd group, mice were given saline (10 ml/kg) or GSRd (10, 30 mg/kg) intraperitoneally for 21 days at day 15 post bilateral carotid artery stenosis (BCAS), a model of chronic cerebral hypoperfusion (CCH). b The percentage of healthy neurons in total neurons for sham, CCH, CCH + GSRd groups. Six different fields (containing about 50–60 cells each) were counted per slice in three separate experiments. Each value in b represented the mean ± SEM of three independent experiments (n = 3, **P < 0.01 versus sham, and #P < 0.05, ##P < 0.01 versus CCH group)
The Levels of Brain-Derived Neurotrophic Factor Increased After Ginsenoside Rd Treatment in Chronic Cerebral Hypoperfusion Mice and Neurons Under Oxygen–Glucose Deprivation/Reoxygenation
BDNF plays critical roles in neuronal development, function, and survival and modulates neural and behavioral plasticity to injury, as well as response to drug treatment [9]. To further assay the possible contributing factor of GSRd-mediated neuroprotection after CCH, the levels of BDNF were determined both in vivo and in vitro. The brain samples were collected after behavior tests and subjected to real-time PCR for bdnf mRNA; the level of bdnf mRNA in CCH model decreased dramatically to 0.45 ± 0.18 from 1.00 ± 0.13 of control group; the bdnf mRNA was reversed to 0.63 ± 0.18 and 0.88 ± 0.12 after GSRd administration for a month as shown in Fig. 3a. ELISA assay data revealed that the level of BDNF in CCH model decreased to 70.31 ± 3.76 from 152.08 ± 3.63 ng/mg of control group, and the BDNF was reversed to 110.32 ± 4.54 and 138.89 ± 3.99 ng/mg after GSRd administration (Fig. 3b). It indicated that GSRd-mediated neuroprotection was accompanied by a significant induction of the BDNF level in the brain. In order to determine the mechanisms of GSRd-mediated neuroprotection, OGD/R for cultured neurons was established which mimics ischemic condition as described [21, 22]. Consistent with the ELISA results from the brain tissue, the BDNF levels were also significantly raised in GSRd-treated cultured neurons compared to controls 24 h after OGD/R as shown in Fig. 3c. This data suggested that GSRd promoted BDNF levels under hypoxia condition both in vivo and in vitro.
Effects of GSRd on BDNF expression. a Real-time PCR for bdnf mRNA expression levels and b ELISA assay for BDNF production in sham, CCH, and CCH + GSRd groups. For the CCH + GSRd group, mice were given saline (10 ml/kg) or GSRd (10, 30 mg/kg) intraperitoneally for 21 days at 15 days post bilateral carotid artery stenosis (BCAS). c Real-time PCR for bdnf mRNA expression levels in cultured neurons. Primary cultures of mouse neurons were treated with GSRd at 0.1, 1, and 10 μM for 2 h followed by exposure to OGD/R for 24 h. Each value in b represented the mean ± SEM of three independent experiments (n = 3, *P < 0.05, **P < 0.01 versus sham, and #P < 0.05, ##P < 0.01 versus CCH group)
Ginsenoside Rd Promoted Cultured Neuron Survival upon Oxygen–Glucose Deprivation/Reoxygenation Injury In Vitro
Although ischemia cannot be reproduced in neuronal culture, OGD/R mimics ischemic condition and provides a convenient model to test pharmacological compounds for neuroprotective potency. In order to determine the mechanisms of GSRd-mediated neuroprotection, we evaluated the effects of GSRd on cultured neurons by measuring MTT induction and LDH release as well as apoptosis. As expected, our results indicated that OGD/R decreased cell viability. Pretreatment with GSRd at 1 and 10 μM for 2 h showed effective neuroprotection against OGD/R injury as measured by MTT assay (Fig. 4a). OD value in OGD/R decreased to 0.31 ± 0.08 from 0.52 ± 0.04 in control (P < 0.01 versus control), and GSRd treatment reversed to 0.38 ± 0.04 (1 μM, P < 0.05 versus control and P < 0.05 versus OGD/R) and 0.42 ± 0.03 (10 μM, P < 0.01 versus OGD/R). We also determined the level of LDH, which is an indicator of the integrity of cell membrane; the results showed that GSRd attenuated the release of LDH from neurons upon OGD/R challenge as indicated as Fig. 4b. LDH in 1 and 10 μM GSRd-treated groups decreased to 67.65 ± 3.12 % and 47.29 ± 2.11 U/L from 87.32 ± 2.83 U/L in the OGD/R group. The similar tendencies were observed in percentage of cell apoptosis and the expression level of caspase-3. OGD/R resulted in the increased cell apoptosis and caspase-3 expression, while GSRd treatment will ameliorate the cell injury by inhibiting apoptosis as shown in Fig. 4c, d.
GSRd protected mouse hippocampal neurons from OGD/R-induced cell injury. a Effects of GSRd on the neuron viability after exposure to OGD/R were determined by MTT method, b LDH release from injured cells was determined using LDH assay kit, c quantitative assessment of apoptotic cell death, and d the expression level of caspase-3 by Western blot. Cultured cortical neurons were treated with GSRd at different concentrations for 30 min followed by exposure to OGD/R for 24 h. Each value represented the mean ± SEM of three independent experiments (n = 3, *P < 0.05, **P < 0.01 versus control, and #P < 0.05, ##P < 0.01 versus OGD/R group)
The Effects of Ginsenoside Rd Treatment on p300/CREB-Binding Protein and Histone Deacetylase 2 After Chronic Cerebral Hypoperfusion Injury
It is evidenced that BDNF expression and release are altered by genetic and epigenetic variations especially histone acetylation which is regulated by two classes of enzyme, histone deacetylases (HDACs) and histone acetyltransferases (HATs) [16]. The data presented in Fig. 3 indicated that BDNF signaling was decreased both in CCH mice and in OGD/R-treated neurons; notably, BDNF facilitates neuroplasticity for learning and memory. We then investigated the p300/CBP and HDAC2 activity after GSRd treatment (Fig. 5). Western blot analysis showed that p300/CBP and HDAC2 were expressed in non-injured hippocampal neurons; significant changes in the levels of p300/CBP (0.42 ± 0.20-fold of control group, P < 0.01 versus control group; Fig. 6a, b) and HDAC2 (3.15 ± 0.12-fold of control group, P < 0.01 versus control group; Fig. 6c, d) were observed in CCH hippocampus. However, 10 and 30 mg/kg GSRd administration exhibited an epigenetic modulation by increasing p300/CBP (1.10 ± 0.13-, 0.99 ± 0.14-fold of control group, P < 0.01 versus CCH group; Fig. 6a, b) and inhibiting HDAC2 level (1.68 ± 0.15-, 1.45 ± 0.13-fold of control group, P < 0.01 versus CCH group; Fig. 6c, d). Considering the primary role of BDNF signaling in activity-dependent synaptic plasticity, the data presented here suggested that some of the beneficial effects of CBP gene transfer on learning and memory may be linked to a BDNF-mediated increase in epigenetic modulation signaling.
Effects of GSRd on epigenetic signal protein expression were determined by Western blot analysis. a Western blot results showing the expression level of P300/CBP. b summary of GSRd administration on the expression level of P300/CBP. c Western blot results showing expression level of HDAC2 and d summary of GSRd administration on the expression level of HDAC2 protein. CCH mice were treated with GSRd at 0, 10, and 30 mg/kg for 21 days at day 15 post bilateral carotid artery stenosis (BCAS). Data b, d were expressed as mean ± SEM of three independent experiments (n = 3, **P < 0.01 versus control, and ##P < 0.01 versus CCH group)
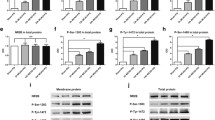
The effect of GSRd on histone acetylation and deacetylation in the hippocampal neurons. a Western blot analysis of the levels of histone acetylation and deacetylation (Ac-H3 and HDAC2) after OGD/R for 1, 12, and 24 h, and β-actin served as the loading control. b The densities were normalized to β-actin, and the bars represented the mean relative optical densities ± the SEMs (n = 3). c The induction of BDNF in cultured neurons was determined by ELISA assay. Hippocampal neurons were subjected to OGD/R for 1, 12, and 24 h and treated with GSRd at 10 μM followed OGD/R for 24 h. d Western blot results showing expression levels of Ac-H3 and HDAC2 after GSRd administration upon OGD/R challenge. e Summary of the expression levels of Ac-H3 and HDAC2 protein in d expressed as mean ± SEM of three independent experiments (n = 3, *P < 0.05, **P < 0.01 versus control, and #P < 0.05, ##P < 0.01 versus OGD/R group)
Histone Acetylation Levels Were Reduced in Hippocampal Neurons After Injurious Ischemia
Chromatin remodeling via histone acetylation is a key biological mechanism used for controlling gene transcription. To investigate whether acetylation of histones in neurons is affected by ischemia, we exploited an established in vitro model of ischemic cell death as OGD/R. In this assay, mouse primary hippocampal neurons were exposed to injurious OGD for 3 h as before and cells were collected at different time points (0, 1, 12, 24 h) after reoxygenation. The cell lysates were analyzed for the expression levels of Ac-H3 and HDAC2 by Western blotting. The results showed that OGD/R induced decreased acetylation levels of histone H3 to 0.75 ± 0.17- and 0.46 ± 0.25-fold of control at 12 and 24 h of OGD/R (P < 0.01 versus control group, Fig. 6a, b); meanwhile, OGD/R increased levels of HDAC2 to 1.55 ± 0.18- and 2.34 ± 0.21-fold of control at 12 and 24 h of OGD/R (P < 0.01 versus control group, Fig. 6a, b). In OGD control cultures, histone acetylation and deacetylation levels did not change. At 0, 1, 12, and 24 h after the termination of OGD, supernatants from different groups were collected and subjected to BDNF ELISA assay. Accordingly, the BDNF concentration decreased from 112.12 ± 4.11 to 85.14 ± 7.21 pg/ml after OGD/R at 12 h and 65.44 ± 11.06 pg/ml at 24 h; GSRd administration reversed BDNF concentration to 98.20 ± 8.68 pg/ml (Fig. 6c). Treatment of neurons with GSRd for 24 h reversed the decreased level of histone H3 acetylation and increased HDAC2 upon OGD/R challenge (Fig. 6d, e). This result showed that injurious ischemia caused a reduction in bulk acetylation levels of histone H3 and induction of HDAC2 in neurons, which may affect BDNF induction upon OGD/R.
Ginsenoside Rd Induced Prevention of Oxygen–Glucose Deprivation/Reoxygenation-Mediated Neuron Injury via Modulation of Histone Deacetylase 2
It is clear that epigenetic modulation contributes to BDNF regulation in certain conditions. To further characterize the cellular mechanisms of GSRd-mediated neuroprotection upon OGD/R, we treated cultured neurons with GSRd in the presence or absence of an HDAC2 inhibitor, MS-275. Pretreatment cultured neurons with MS-275 enhanced the induction of BDNF mediated by GSRd administration under OGD/R (Fig. 7a). Accordingly, in the presence of MS-275, neuron injury was significantly attenuated following GSRd administration upon OGD/R challenge measured by LDH assay (Fig. 7b) while MS-275 treatment alone had no effect in neurons (data not shown). ChIP analysis demonstrated significant increase in HDAC2 (Fig. 7c) and decreases in acetylated H3 at the bdnf promoters of exons IV after OGD/R (Fig. 7d), and these selective decreases were consistent with downregulation of bdnf mRNA expression as shown in Fig. 3c. The increase of acetylated H3 at the bdnf promoters of exons IV induced by GSRd was enhanced markedly in the presence of MS-275 as shown in Fig. 7d. These results indicated that GSRd promoted neuron survival upon OGD/R mediated by BDNF which was modulated by Ac-H3 and HDAC2.
Inhibition of HDAC2 enhanced GSRd-mediated neuroprotection. Mouse hippocampal neurons were pretreated with or without MS-275 for 2 h and then subjected to GSRd for 30 min followed by exposure to OGD/R for 24 h. a BDNF production was determined by ELISA assay upon GSRd treatment. b The release of LDH from injured neurons for control, GSRd alone, OGD/R, GSRd + OGD/R, and GSRd + OGD/R + MS-275 groups was determined by ELISA assay. Each value represented the mean ± SEM of three independent experiments (n = 3 experiments, *P < 0.05, **P < 0.01 versus control, #P < 0.05, ##P < 0.01 versus OGD/R group). c ChIP analysis of HDAC2 at the bdnf promoters of exons IV upon OGD/R challenge for 24 h. d ChIP analysis of acetyl-H3 at the bdnf promoters of exons IV after GSRd treatment in the presence or absence of MS-275, an inhibitor of HDAC2, upon OGD/R challenge
Discussion
In the present study, we found that GSRd administration ameliorated CCH-induced cognitive dysfunction in mice, and induction of BDNF was responsible for GSRd-mediated neuroprotection both CCH in vivo and OGD/R in vitro. A marked reduction of H3 acetylation and induction of HDAC2 at the bdnf gene promoter were observed in neurons upon OGD/R, which appeared to be time-dependent. Moreover, MS-275 (an HDAC2 inhibitor) enhanced the upregulation of BDNF induced by GSRd administration and thus promoted neuron survival upon OGD/R injury.
Sufficient cerebral blood flow is critical for normal brain functions. A sudden disruption of the blood supply may lead to stroke, while CCH is assumed to be associated with the cognitive deficits in several types of neurodegenerative disorders including Alzheimer’s disease and vascular dementia patients [3]. The model of BCAS is recently used to investigate the effects of CCH on neurodegenerative diseases, in which the microcoils were twined by rotating around both CCAs in mice [18]. We measured the cognitive behaviors after BCAS was established and found that this model led to cognitive deficit in mice, and GSRd treatment ameliorated the impairment of learning and memory in CCH mice (Fig. 1). It indicated that GSRd was beneficial for CCH mice cognition by promoting neuron survival (Fig. 2) and BDNF level (Fig. 3). It has been demonstrated that GSRd significantly ameliorated neurological outcome after transient middle cerebral artery occlusion (MCAO) in rats [24] via inhibition of ion channels or modulation of vasospasm [25].
We further investigated the mechanisms involved in GSRd-mediated upregulation of BDNF. It is well demonstrated that BDNF plays pivotal roles in synaptic plasticity, neurogenesis, and cell survival [8]. However, these regulatory mechanisms may have been compromised. Recent studies have suggested that epigenetic mechanisms including DNA methylation and histone acetylation influence gene expression, playing pivotal roles in several human disorders [9, 16, 26]. Histone acetylation is responsible for transcriptional activation, while deacetylation of histone is linked with transcriptional repression. HATs and HDACs are the main enzymes in this process. HATs are known to interact with various transcription factors, such as p300 and CBP, to regulate gene transcription. HDACs, classified into four families, are sequence-specific to genetic loci leading to transcriptional repression. HDAC2 is one of the most highly expressed class I HDACs throughout the brain, including the hippocampus [27]. Elevated levels of HDAC2, a class I HDAC, may accompany with the cognitive decline of the human neurodegenerating brain [28, 29], and HDAC2 also negatively regulates memory and synaptic plasticity in the healthy mouse brain [29, 30]. The present study revealed significant declines in H3 acetylation and increase in HDAC2 during cerebrovascular hypoperfusion, and GSRd administration reversed the alterations (Fig. 5), suggesting that CCH condition modified the status of histone acetylation. Additionally, significant decreases in acetylated H3 at the bdnf promoters after CCH were consistent with downregulation of bdnf mRNA and protein expression (Fig. 3); this is consistent with other evidence that BDNF was regulated by epigenetic pathway [16, 31, 32]. To gain insight into the mechanisms underlying the increase in HDAC2, we exposed primary cultured neurons to neurotoxic stimuli characteristic of CCH-related neurodegeneration. As revealed by Western blot analysis, treatment with OGD/R was sufficient to increase HDAC2 and reduced H3 acetylation upon OGD/R in vitro accompanied with decline of BDNF as well, and this effect was rescued by exposure to GSRd (Fig. 6), suggesting the involvement of transcriptional mechanisms.
Accordingly, pharmacological treatments aimed at increasing histone acetylation have shown promising results in reversing cognitive deficits by the use of non-selective HDAC inhibitors [33, 34]. Our data suggested that elevated HDAC2 levels are causally involved in reduction of BDNF in brain of CCH mice, application of MS-275 to block HDAC2, enhanced expression of bdnf mRNA and protein, and concurrently rescued memory capacities after GSRd treatment.
Hence, our results suggest that CCH led to a persistent inhibition of BDNF signaling in the hippocampus by decreasing H3 acetylation and induction of HDAC2, and this effect seems to be altered by GSRd administration. These findings have partially revealed the molecular mechanisms underlying neuroprotection of GSRd. However, we could not exclude the possibility that GSRd may undergo neuroprotective activities through other pathways. The recruitment of epigenetic neuroprotective pathways might represent a possible target for therapeutic interventions for CCH-related diseases.
Abbreviations
- CCH:
-
Chronic cerebral hypoperfusion
- BCAS:
-
Bilateral carotid artery stenosis
- BDNF:
-
Brain-derived neurotrophic factor
- GSRd:
-
Ginsenoside Rd
- Ac-H3:
-
Acetylated histone H3
- HDAC2:
-
Histone deacetylase 2
- HATs:
-
Histone acetyltransferases
- OGD/R:
-
Oxygen–glucose deprivation/reoxygenation
- CNS:
-
Central nervous system
- CBP:
-
p300/CREB-binding protein
- MWM:
-
Morris water maze
- ChIP:
-
Chromatin immunoprecipitation
- ELISA:
-
Enzyme-linked immunosorbent assay
References
Dong YF, Kataoka K, Toyama K et al (2011) Attenuation of brain damage and cognitive impairment by direct renin inhibition in mice with chronic cerebral hypoperfusion. Hypertension 58:635–642
Shibata M, Yamasaki N, Miyakawa T et al (2007) Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke 38:2826–2832
Iadecola C (2004) Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5:347–360
Lin T, Liu Y, Shi M et al (2012) Promotive effect of ginsenoside Rd on proliferation of neural stem cells in vivo and in vitro. J Ethnopharmacol 142:754–761
Wang B, Feng G, Tang C et al (2013) Ginsenoside Rd maintains adult neural stem cell proliferation during lead-impaired neurogenesis. Neurol Sci 34:1181–1188
Ong WY, Farooqui T, Koh HL et al (2015) Protective effects of ginseng on neurological disorders. Front Aging Neurosci 7:129
Ye R, Li N, Han J et al (2009) Neuroprotective effects of ginsenoside Rd against oxygen-glucose deprivation in cultured hippocampal neurons. Neurosci Res 64:306–310
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14:7–23
Tsankova NM, Berton O, Renthal W et al (2006) Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9:519–525
Phillips HS, Hains JM, Armanini M et al (1991) BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron 7:695–702
Scalzo P, Kummer A, Bretas TL et al (2010) Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J Neurol 257:540–545
Koppel I, Timmusk T (2013) Differential regulation of Bdnf expression in cortical neurons by class-selective histone deacetylase inhibitors. Neuropharmacology 75:106–115
Barichello T, Generoso JS, Simoes LR et al (2015) Sodium butyrate prevents memory impairment by re-establishing BDNF and GDNF expression in experimental pneumococcal meningitis. Mol Neurobiol 52:734–740
Valor LM, Pulopulos MM, Jimenez-Minchan M et al (2011) Ablation of CBP in forebrain principal neurons causes modest memory and transcriptional defects and a dramatic reduction of histone acetylation but does not affect cell viability. J Neurosci 31:1652–1663
Petrij F, Giles RH, Dauwerse HG et al (1995) Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature 376:348–351
Caccamo A, Maldonado MA, Bokov AF et al (2010) CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 107:22687–22692
Lopez JP, Mamdani F, Labonte B et al (2013) Epigenetic regulation of BDNF expression according to antidepressant response. Mol Psychiatry 18:398–399
Kitaguchi H, Tomimoto H, Ihara M et al (2009) Chronic cerebral hypoperfusion accelerates amyloid beta deposition in APPSwInd transgenic mice. Brain Res 1294:202–210
Hwang CJ, Yun HM, Park KR et al (2015) Memory impairment in estrogen receptor alpha knockout mice through accumulation of amyloid-beta peptides. Mol Neurobiol 52:176–186
Guo YY, Liu SB, Cui GB et al (2012) Acute stress induces down-regulation of large-conductance Ca2+-activated potassium channels in the lateral amygdala. J Physiol 590:875–886
Chen M, Sun HY, Hu P et al (2013) Activation of BKca channels mediates hippocampal neuronal death after reoxygenation and reperfusion. Mol Neurobiol 48:794–807
Yu P, Wang L, Tang F et al (2016) Resveratrol pretreatment decreases ischemic injury and improves neurological function via sonic hedgehog signaling after stroke in rats. Mol Neurobiol
Wang M, Li YJ, Ding Y et al (2015) Silibinin prevents autophagic cell death upon oxidative stress in cortical neurons and cerebral ischemia-reperfusion injury. Mol Neurobiol
Ye R, Kong X, Yang Q et al (2011) Ginsenoside rd in experimental stroke: superior neuroprotective efficacy with a wide therapeutic window. Neurotherapeutics 8:515–525
Zhang Y, Zhou L, Zhang X et al (2012) Ginsenoside-Rd attenuates TRPM7 and ASIC1a but promotes ASIC2a expression in rats after focal cerebral ischemia. Neurol Sci 33:1125–1131
Chouliaras L, Rutten BP, Kenis G et al (2010) Epigenetic regulation in the pathophysiology of Alzheimer’s disease. Prog Neurobiol 90:498–510
Broide RS, Redwine JM, Aftahi N et al (2007) Distribution of histone deacetylases 1–11 in the rat brain. J Mol Neurosci 31:47–58
Graff J, Rei D, Guan JS et al (2012) An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 483:222–226
Guan JS, Haggarty SJ, Giacometti E et al (2009) HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459:55–60
Akhtar MW, Raingo J, Nelson ED et al (2009) Histone deacetylases 1 and 2 form a developmental switch that controls excitatory synapse maturation and function. J Neurosci 29:8288–8297
Wu X, Sun J, Zhang X et al (2014) Epigenetic signature of chronic cerebral hypoperfusion and beneficial effects of S-adenosylmethionine in rats. Mol Neurobiol 50:839–851
Sen A, Nelson TJ, Alkon DL (2015) ApoE4 and Abeta oligomers reduce BDNF expression via HDAC nuclear translocation. J Neurosci 35:7538–7551
Kazantsev AG, Thompson LM (2008) Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov 7:854–868
Yasuda S, Liang MH, Marinova Z et al (2009) The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry 14:51–59
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China No. 81371322 and 81571328 (to Dr. Wu) and Scientific Research Foundation for the Returned Overseas Chinese Scholars No. HG3402 (to Dr. Wu).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there have no conflicts of interest.
Additional information
Qun Wan and Xue Ma contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wan, Q., Ma, X., Zhang, ZJ. et al. Ginsenoside Reduces Cognitive Impairment During Chronic Cerebral Hypoperfusion Through Brain-Derived Neurotrophic Factor Regulated by Epigenetic Modulation. Mol Neurobiol 54, 2889–2900 (2017). https://doi.org/10.1007/s12035-016-9868-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9868-4