Abstract
Our previous studies have showed that ginsenoside (GS)-Rd, a mono-compound isolated from traditional Chinese herb panax ginseng, has the neuroprotective effects following ischemic stroke. However, the underlying mechanisms are still largely unknown. Our latest study showed that GS-Rd could block calcium influx in cultured cortical neurons after excitotoxic injury, indicating that GS-Rd may act on cation channels. To explore this possibility, in this study, we used a rat middle cerebral artery occlusion (MCAO) model to examine the effects of GS-Rd on the expression of non-selective cation channels, including transient receptor potential melastatin (TRPM) and acid sensing ion channels (ASIC), and cation channels, including N-methyl-d-aspartate (NMDA) receptors, which all play essential roles in ischemic stroke. Our results showed that both TRPM and ASIC channels were expressed in the brain. At 24 h following MCAO insult, mRNA and protein expression levels of TRPM7, ASIC1a and ASIC2a were significantly increased. Pretreatment of 10 mg/kg GS-Rd attenuated MCAO-induced expression of TRPM7 and ASIC1a but promoted that of ASIC2a. In contrast, GS-Rd had no significant effects on the expression of NMDA receptors. Thus, our results suggest that GS-Rd neuroprotection following cerebral ischemia may be at least due to its effects on the expression of TRPM7, ASIC1a and ASIC2a.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Panax ginseng is a famous traditional Chinese herb, which has been used to treat a wide variety of diseases in Asian for thousands of years. The main molecular components contributive to the pharmacologic effects of panax ginseng are ginsenosides. At present, more than 50 ginsenosides have been identified, among which ginsenoside (GS)-Rd is one of the most active and efficient ingredients. Lines of evidence have implicated that GS-Rd presents antioxidant effects to ameliorate renal injury [1, 2] and aging process in senescence-accelerated mice [3]. Our previous studies also demonstrated that GS-Rd had significant neuroprotective effects. For example, GS-Rd can attenuate hydrogen peroxide-induced oxidative injury in PC12 cells [4], protect oxygen-glucose deprivation-injured cultured hippocampal neurons [5] and reduce the infarct size in rats after middle cerebral artery occlusion (MCAO) [6–9]. Moreover, our randomized, double-blind, placebo-controlled, phase II multicenter trial showed that GS-Rd was efficient and safe for the treatment of acute ischemic stroke [10]. However, the mechanisms underlying GS-Rd neuroprotection are still largely unknown. Our latest in vitro results showed that GS-Rd can block Ca2+ influx in cortical neurons after excitotoxic injury [11], indicating GS-Rd may have an effect on the expression or functions of cation channels on the neuronal membrane during cerebral ischemia.
Among the cation channels, glutamate receptors [e.g., N-methyl-d-aspartate (NMDA) receptor] are most well-studied following cerebral ischemia. Glutamate receptor-mediated Ca2+ overload which induces excitotoxicity is considered one of the most important causes of stroke-triggered cell death. However, clinical trials with agents blocking these receptors have shown little benefit [12–14]. Unfavorable experiences with mechanisms involving NMDA receptor resulted in redirection of attention to other receptor channels. Recently, growing evidence has implicated that a number of glutamate receptor-independent non-selective cation channels, such as transient receptor potential melastatin (TRPM) and acid sensing ion channels (ASIC) also play important roles in ischemic stroke. For example, among the TRPM channels, TRPM7 and TRPM2 have been found to be involved in delayed neuronal death after ischemia. TRPM7 channel activity was enhanced in oxygen/glucose deprivation-treated cortical neurons and TRPM7 knockdown by RNA interference in cultured neurons and hippocampi delayed anoxic cell death [15, 16]. Suppression of TRPM2 activity by poly(ADP-ribose) polymerase-1 inhibitors [17] or antisense oligonucleotide [18] protected the cells against oxidative stress-induced death. In addition, ASIC1a activation resulted in an increase in intercellular Ca2+, which was responsible for neuronal injury after ischemic stroke [19, 20]. By contrast, upregulation of ASIC2a expression after transient global ischemia implicated an endogenous neuroprotection against neuronal injury [21]. Therefore, targeting glutamate receptor-independent channels is likely to be a promising therapeutic strategy for cerebral ischemia.
In the present study, we focused on the effects of GS-Rd on the expression of non-selective cation channels (including various TRPM and ASIC channels) as well as cation channels (including NMDA receptors, NR1, NR2A, and NR2B) in a rat middle cerebral artery occlusion (MCAO) model by RT-PCR and western blot. Our results showed that GS-Rd pretreatment significantly attenuated MCAO-triggered expression of ASIC1a and TRPM7 but promoted that of ASIC2a.
Materials and methods
Focal cerebral ischemia and drug administration
Forty-eight male Sprague-Dawley rats weighing 250–300 g were used in the study. Transient MCAO was performed to induce focal cerebral ischemia as described previously [22, 23]. In brief, a 4-0 nylon suture coated with poly-l-lysine was used to achieve occlusion. After 2 h of occlusion, the suture was carefully removed to restore blood flow. During surgery and postoperative period, regional cerebral blood flow was monitored by laser Doppler flowmetry (PeriFlux 5000, Perimed AB, Sweden) and rectal temperature was maintained at 37.5°C by means of a feedback-controlled heating pad. The rats were suspended by the tail and those with left forelimb flexion were defined as a completed stroke model.
According to our previous study [24], a 10 mg/kg dose of GS-Rd (Tai-He Biopharmaceutical Co. Ltd., Guangzhou, China) was chosen and injected intraperitoneally 15 min before MCAO. The rats were randomly divided into the four groups: (1) Sham group (n = 12), with surgery but no occlusion; (2) Sham + GS-Rd group (n = 12), with GS-Rd administration to sham rats; (3) MCAO group (n = 12), with vehicle application before surgical occlusion; (4) MCAO + GS-Rd group (n = 12), with GS-Rd treatment before surgical occlusion. Animal protocols were approved by the Ethics Committee for Animal Experimentation of the Fourth Military Medical University.
Semi-quantitative and real-time RT-PCR
The rat brain tissues were collected at 24 h following MCAO, a time point at which the changes of ion channel expression were obvious [25–27]. Total RNA of brain tissues was extracted using Trizol reagent (Invitrogen Life Technologies, GA, USA) according to the manufacturer’s instructions. Reverse transcription was performed using 3 μg of total RNA. First strand cDNA was synthesized using RevertAid™ First Strand cDNA Synthesis Kit (Fermentas, Burlington, Canada). For PCR amplification, gene-specific primer sequences used were listed in Table 1. The amplified products were electrophoretically separated by 2.0 % agarose gels containing ethidium bromide. For real-time RT-PCR, the amplified reactions were conducted on an ABI 7901HT series PCR machine (Applied Biosystems, Foster City, CA, USA) using Power SYBR® Green PCR Master Mix (Takara, Shuzo, Japan) with a 95°C denaturation for 3 min followed by 45 cycles of 95°C for 5 s and 60°C for 45 s. In addition, data were normalized to β-actin expression and further normalized to the negative control. The following primer sequences for real-time RT-PCR were used: TRPM-7, F: 5′-AGGTGAGCCT GTCACAGTATA-3′, R: 5′-GTGCTCTGACCAGGTACA-3′; ASIC1a, F: 5′-GCCTCCGCCAAGTACCTG-3′, R: 5′-CACCCAACAGCCCTGCG ATCT-3′; ASIC2a, F: 5′-AAGTTGCTGCCTTACTTGGTG-3′, R: 5′-TCTTTGCC AAGCAGGTCTAAT-3′; GAPDH, F: 5′-ACCCATCACCATCTTCCAGGAG-3′, R: 5′-GAAGGGGCG GAGATGATGAC-3′.
Western analysis
The rat brains were collected at 24 h after the onset of MCAO. Total protein was extracted using RIPA lysis buffer (Beyotime, Beijing, China) according to the manufacturer’s instructions. In brief, the brain tissues were minced and lysed on ice in 100 μl lysis buffer for 30 min and then centrifuged at 12,000 rpm, 4°C for 30 min. Supernatants were collected from the lysates and protein concentrations were determined using the BCA protein assay kit (Beyotime, Beijing, China). Aliquots of the lysates (30 μg of protein) were boiled for 5 min and electrophoresed on 10% SDS-polyacrylamide gels. Proteins in the gels were transferred onto nitrocellulose membranes, which were incubated with anti-TRPM7 antibody (Alomone, Jerusalem, Israel), anti-ASIC1a (Alomone, Jerusalem, Israel) antibody, anti-ASIC2a (Alomone, Jerusalem, Israel) antibody or anti β-actin antibody (CoWin, Beijing, china). The membranes were then incubated with horseradish peroxidase-conjugated secondary antibody. Finally, protein bands were detected using an enhanced chemiluminescence western blotting detection kit (Pierce Biotechnology, Rockford, IL, USA).
Statistical analysis
All assays were repeated at least three times. The data were presented as mean ± SD. Differences between groups were evaluated using one-way ANOVA followed by Dunnett’s test. P < 0.05 was considered statistically significant.
Results
The effects of GS-Rd on the expression of TRPM after MCAO
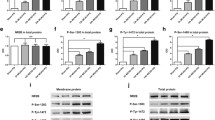
RT-PCR results showed that TRPM1-7 mRNAs were all expressed in brain tissue. GS-Rd pretreatment had no effect on the expression of TRPM channels in the sham rats (Fig. 1). At 24 h after MCAO, among seven TRPM channels, only TRPM7 expression was significantly upregulated in comparison with sham group (Fig. 1g), consistent with a previous report [28]. Pretreatment of GS-Rd attenuated TRPM7 expression induced by MCAO but did not affect the expression of other TRPM channels (Fig. 1g). Semi-quantitative analysis of TRPM1-7 mRNAs also showed the similar results (Fig. 1). Real-time RT-PCR assay further confirmed the effect of GS-Rd on TRPM7 expression after MCAO (Fig. 3a). Moreover, Western blot assay showed that the protein level of TRPM7 was also upregulated at 24 h after MCAO in comparison with sham group, and GS-Rd pretreatment attenuated MCAO-induced upregulation of TRPM7 expression (Fig. 3d).
The effects of GS-Rd on mRNA expression of TRPM after MCAO. Semi-quantitative RT-PCR analysis showed that TRPM1, TRPM2, TRPM3, TRPM4, TRPM5, TRPM6, and TRPM7 were expressed in rat brain and GS-Rd pretreatment did not affect the expression levels of TRPM channels (a–g). At 24 h following MCAO, mRNA levels of TRPM1-6 in MCAO groups were comparable to that of sham groups (a–f) while that of TRPM7 was significantly increased (g). GS-Rd pretreatment had no significant effects on the expression of TRPM1-6 (a–f) but downregulated MCAO-induced expression of TRPM7 (g). β-actin was an internal control. **P < 0.01, compared with sham group; *P < 0.05, compared with MCAO group
The effects of GS-Rd on the expression of ASIC after MCAO
In sham rats, ASIC1a and ASIC2a mRNAs were expressed in brain tissue while GS-Rd did not affect their expression levels (Fig. 2). MCAO insult significantly increased the expression levels of both ASIC1a and ASIC2a (Fig. 2), in consistence with a previous study [21]. GS-Rd pretreatment markedly decreased MCAO-induced ASIC1a expression (Fig. 2a) but further increased that of ASIC2a (Fig. 2b). Semi-quantitative (Fig. 2) and quantitative real-time RT-PCR results (Fig. 3b, c) further confirmed the changes in the expression of ASIC1a and ASIC2a observed above. Moreover, protein expression levels of ASIC1a and ASIC2a revealed by Western blot also showed that GS-Rd significantly attenuated the expression of ASIC1a induced by MCAO (Fig. 3e) but increased that of ASIC2a (Fig. 3f).
The effects of GS-Rd on mRNA expression of ASIC after MCAO. Semi-quantitative RT-PCR analysis showed that ASIC1a and ASIC2a were expressed in rat brain and GS-Rd pretreatment did not affect the expression levels of ASIC channels (a, b). At 24 h following MCAO, mRNA levels of both ASIC1a (a) and ASIC2a (b) in MCAO groups were significantly increased compared with the sham groups. GS-Rd pretreatment decreased the expression level of ASIC1a (a) while increased that of ASIC2a (b) compared with MCAO group. β-actin was an internal control. **P < 0.01, compared with sham group; *P < 0.05, compared with MCAO group
The changes in mRNA and protein levels of TRPM7, ASIC1a, and ASIC2a after MCAO. a–c Real-time quantitative RT-PCR analysis showed that mRNA levels of TRPM7, ASIC1a and ASIC2a in MCAO group were 3.3-, 2.8-, and 2.4-fold higher than that of sham group and GS-Rd markedly decreased the expression levels of TRPM7 and ASIC1a but further increased that of ASIC2a. d–f Western blot analysis showed that the protein levels of TRPM7, ASIC1a and ASIC2 underwent similar changes to those seen in mRNA levels. β-actin was an internal control. **P < 0.01, compared with sham group; *P < 0.05, compared with MCAO group
The effects of GS-Rd on the expression of NMDA receptors after MCAO
Finally, the expression of NMDA receptors, the key cation channels involving in cerebral ischemia, was examined by semi-quantitative RT-PCR analysis. Compared with MCAO group, GS-Rd pretreatment had no significant effects on the mRNA expression of NR1, NR2A and NR2B at 24 h after MCAO (Fig. 4).
Discussion
In this study, we examined the effects of GS-Rd on the expression of various TRPM and ASIC channels and NMDA receptors after focal cerebral ischemia in rats. Our results showed that GS-Rd pretreatment attenuated MCAO-induced TRPM7 and ASIC1a but promoted ASIC2a expression. These findings suggest that neuroprotective effects of GS-Rd as revealed by our previous studies [4, 5, 10], may be at least mediated via affecting the expression of TRPM7, ASIC1a or ASIC2a.
In consistence with a previous study [29], the present study also showed that TRPM channels were expressed in the central nervous system. At 24 h after focal cerebral ischemia, only TRPM7 expression was upregulated, and however, TRPM2 expression was unchanged, inconsistent with a previous study showing that TRPM2 mRNA expression was increased at 1 and 4 weeks following MCAO in rats [30]. This discrepancy may be explained by the dynamic expression pattern of TRPM2 following cerebral ischemia. That is, increased TRPM2 expression may be a relatively late event (1–4 week) while our observation was at an early time point (24 h). Among four genes ASIC1-4 encoding 7 subunits (ASIC1a, ASIC1b, ASIC1b2, ASIC2a, ASIC2b, ASIC3, ASIC4) in mammals, ASIC1a and ASIC2a are present in the brain [31]. Thus, our study focused on the expression of ASIC1a and ASIC2a in rats following MCAO. We found that both ASIC1a and ASIC2a expression was upregulated at 24 h after cerebral ischemia. Taken together, our results indicate that TRPM7, ASIC1a and ASIC2a channels may play essential roles in the ischemic process and should be targeted when treatment of cerebral stroke.
In our previous studies, we showed that GS-Rd, a mono-compound isolated from traditional Chinese herb panax ginseng, had significant neuroprotective effects. These studies revealed that GS-Rd can reduce the intracellular reactive oxygen species and malondialdehyde production, increase glutathione content, and enhance the antioxidant enzymatic activities of catalase, superoxide dismutase and glutathione peroxidase [4, 5, 8, 9]. We further found that GS-Rd can block Ca2+ influx in cultured cortical neurons after excitotoxic injury [11], suggesting GS-Rd may affect the expression and/or functions of neuronal cation channels. Our present in vivo study showed that 15 min of GS-Rd pretreatment significantly attenuated the expression of TRPM7 and ASIC1a while promoted ASIC2a expression following focal ischemia, but did not affect the expression of other non-selective cation channels and NMDA receptors. Therefore, we propose that GS-Rd may exert its neuroprotective effects at least by downregulating TRPM7 and ASIC1a expression and upregulating ASIC2a expression. On the other hand, GS-Rd might also function in inhibiting the activity of TRPM7 and ASIC1a channels. GS-Rd was reported to suppress the levels of reactive oxygen species [4, 5] and reduce the production of peroxynitrite, an activator for TRPM7 channel [16]. In addition, the antioxidative effects of GS-Rd can accelerate the decomposition of acid accumulation [4, 5] and consequently reduce the activity of ASIC1a channel. In contrast to TRPM7 and ASIC1a, we found that GS-Rd can upregulate the expression of ASIC2a, which was found to have a similar expression pattern to that of antiapoptotic proteins Bcl-2 and Bcl-w [21], implicating a protective role for this channel. Taken together, our results suggest that neuroprotective effects of GS-Rd following focal cerebral ischemia may be at least mediated by inhibiting the expression of TRPM7 and ASIC1a but promoting the expression of ASIC2a, a neuroprotective acid channel.
In addition to GS-Rd, other components of ginsenosides, such as GS-Rb and GS-Rg, were reported to have cell protective effects. For example, GS-Rg1 and GS-Rg2 can protect against glutamate-induced injury in lung [32] and PC12 cells [6], respectively; GS-Rb1, GS-Rc, and GS-Rg5 effectively protected striatal neurons from glutamate-induced apoptosis [7]; GS-Rg3 and GS-Rh2 were found to antagonize NMDA receptors in cultured rat hippocampal neurons [33–35]. All these evidence suggested that ginsenosides might act on glutamate receptors, such as NMDA receptors. However, our present results revealed that GS-Rd at least had no effects on the expression of NR1, NR2A, and NR2A following focal cerebral ischemia. Given our latest study demonstrating that GS-Rd can attenuate glutamate-/NMDA-induced Ca2+ influx [11], we propose that GS-Rd may affect the function but not expression of NMDA receptors to exert its protective effects.
In summary, the present study showed that GS-Rd could affect the expression of non-selective cation channels TRPM7, ASIC1a, and ASIC 2a, but not that of glutamate receptor ion channels. We still lack the knowledge about the mechanisms underlying the effects of GS-Rd on the expression of these non-selective cation channels. However, our present results provide new insights into the mechanisms of GS-Rd neuroprotection, which will benefit us to search for new treatment strategies for ischemic cerebrovascular disease.
References
Yokozawa T, Dong E (2001) Role of ginsenoside-Rd in cisplatin-induced renal injury: special reference to DNA fragmentation. Nephron 89(4):433–438. doi:10.1159/000046116
Yokozawa T, Liu ZW, Dong E (1998) A study of ginsenoside-Rd in a renal ischemia-reperfusion model. Nephron 78(2):201–206. doi:10.1159/000044911
Yokozawa T, Satoh A, Cho EJ (2004) Ginsenoside-Rd attenuates oxidative damage related to aging in senescence-accelerated mice. J Pharm Pharmacol 56(1):107–113. doi:10.1211/0022357022449
Ye R, Han J, Kong X, Zhao L, Cao R, Rao Z, Zhao G (2008) Protective effects of ginsenoside Rd on PC12 cells against hydrogen peroxide. Biol Pharm Bull 31(10):1923–1927. doi:10.1248/bpb.31.1923
Ye R, Li N, Han J, Kong X, Cao R, Rao Z, Zhao G (2009) Neuroprotective effects of ginsenoside Rd against oxygen-glucose deprivation in cultured hippocampal neurons. Neurosci Res 64(3):306–310. doi:10.1016/j.neures.2009.03.016
Li N, Liu B, Dluzen DE, Jin Y (2007) Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J Ethnopharmacol 111(3):458–463. doi:10.1016/j.jep.2006.12.015
Wu J, Jeong HK, Bulin SE, Kwon SW, Park JH, Bezprozvanny I (2009) Ginsenosides protect striatal neurons in a cellular model of Huntington’s disease. J Neurosci Res 87(8):1904–1912. doi:10.1002/jnr.22017
Ye R, Zhang X, Kong X, Han J, Yang Q, Zhang Y, Chen Y, Li P, Liu J, Shi M, Xiong L, Zhao G (2011) Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience 178:169–180. doi:10.1016/j.neuroscience.2011.01.007
Ye R, Yang Q, Kong X, Han J, Zhang X, Zhang Y, Li P, Liu J, Shi M, Xiong L, Zhao G (2011) Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem Int 58(3):391–398. doi:10.1016/j.neuint.2010.12.015
Liu X, Xia J, Wang L, Song Y, Yang J, Yan Y, Ren H, Zhao G (2009) Efficacy and safety of ginsenoside-Rd for acute ischaemic stroke: a randomized, double-blind, placebo-controlled, phase II multicenter trial. Eur J Neurol 16(5):569–575. doi:10.1111/j.1468-1331.2009.02534.x
Zhang C, Du F, Shi M, Ye R, Cheng H, Han J, Ma L, Cao R, Rao Z, Zhao G (2011) Ginsenoside Rd protects neurons against glutamate-induced excitotoxicity by inhibiting Ca(2+) influx. Cell Mol Neurobiol. doi:10.1007/s10571-011-9742-x
Davis SM, Albers GW, Diener HC, Lees KR, Norris J (1997) Termination of acute stroke studies involving selfotel treatment. Lancet 349(9044):32. doi:10.1016/S0140-6736(05)62166-6
Davis SM, Lees KR, Albers GW, Diener HC, Markabi S, Karlsson G, Norris J (2000) Selfotel in acute ischemic stroke: possible neurotoxic effects of an NMDA antagonist. Stroke 31(2):347–354. doi:10.1161/01.STR.31.2.347
Lees KR, Asplund K, Carolei A, Davis SM, Diener HC, Kaste M, Orgogozo JM, Whitehead J (2000) Glycine antagonist (gavestinel) in neuroprotection (GAIN International) in patients with acute stroke: a randomised controlled trial. Lancet 355(9219):1949–1954. doi:10.1016/S0140-6736(00)02326-6
Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M (2003) A key role for TRPM7 channels in anoxic neuronal death. Cell 115(7):863–877. doi:10.1016/S0092-8674(03)01017-1
Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, Kiyonaka S, Mori Y, Jones M, Forder JP, Golde TE, Orser BA, Macdonald JF, Tymianski M (2009) Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci 12(10):1300–1307. doi:10.1038/nn.2395
Fonfria E, Marshall IC, Benham CD, Boyfield I, Brown JD, Hill K, Hughes JP, Skaper SD, McNulty S (2004) TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol 143(1):186–192. doi:10.1038/sj.bjp.0705914
Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9(1):163–173. doi:10.1016/S1097-2765(01)00438-5
Xiong ZG, Chu XP, Simon RP (2006) Ca2+-permeable acid-sensing ion channels and ischemic brain injury. J Membr Biol 209(1):59–68. doi:10.1007/s00232-005-0840-x
Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP (2004) Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118(6):687–698. doi:10.1016/j.cell.2004.08.026
Johnson MB, Jin K, Minami M, Chen D, Simon RP (2001) Global ischemia induces expression of acid-sensing ion channel 2a in rat brain. J Cereb Blood Flow Metab 21(6):734–740. doi:10.1097/00004647-200106000-00011
Morin C, Zini R, Simon N, Tillement JP (2002) Dehydroepiandrosterone and alpha-estradiol limit the functional alterations of rat brain mitochondria submitted to different experimental stresses. Neuroscience 115(2):415–424. doi:10.1016/S0306-4522(02)00416-5
Sutherland BA, Shaw OM, Clarkson AN, Jackson DN, Sammut IA, Appleton I (2005) Neuroprotective effects of (−)-epigallocatechin gallate following hypoxia-ischemia-induced brain damage: novel mechanisms of action. FASEB J 19(2):258–260. doi:10.1096/fj.04-2806fje
Ye R, Kong X, Yang Q, Zhang Y, Han J, Li P, Xiong L, Zhao G (2011) Ginsenoside Rd in experimental stroke: superior neuroprotective efficacy with a wide therapeutic window. Neurotherapeutics 8(3):515–525. doi:10.1007/s13311-011-0051-3
Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP (2008) Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol 8(1):25–32. doi:10.1016/j.coph.2007.09.001
Besancon E, Guo S, Lok J, Tymianski M, Lo EH (2008) Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci 29(5):268–275. doi:10.1016/j.tips.2008.02.003
Simard JM, Tarasov KV, Gerzanich V (2007) Non-selective cation channels, transient receptor potential channels and ischemic stroke. Biochim Biophys Acta 1772(8):947–957. doi:10.1016/j.bbadis.2007.03.004
Jiang H, Tian SL, Zeng Y, Li LL, Shi J (2008) TrkA pathway(s) is involved in regulation of TRPM7 expression in hippocampal neurons subjected to ischemic-reperfusion and oxygen-glucose deprivation. Brain Res Bull 76(1–2):124–130. doi:10.1016/j.brainresbull.2008.01.013
Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S (2006) Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res 26(3):159–178. doi:10.1080/10799890600637506
Fonfria E, Mattei C, Hill K, Brown JT, Randall A, Benham CD, Skaper SD, Campbell CA, Crook B, Murdock PR, Wilson JM, Maurio FP, Owen DE, Tilling PL, McNulty S (2006) TRPM2 is elevated in the tMCAO stroke model, transcriptionally regulated, and functionally expressed in C13 microglia. J Recept Signal Transduct Res 26(3):179–198. doi:10.1080/10799890600637522
Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M (1997) A proton-gated cation channel involved in acid-sensing. Nature 386(6621):173–177. doi:10.1038/386173a0
Shen L, Han JZ, Li C, Yue SJ, Liu Y, Qin XQ, Liu HJ, Luo ZQ (2007) Protective effect of ginsenoside Rg1 on glutamate-induced lung injury. Acta Pharmacol Sin 28(3):392–397. doi:10.1111/j.1745-7254.2007.00511.x
Kim S, Ahn K, Oh TH, Nah SY, Rhim H (2002) Inhibitory effect of ginsenosides on NMDA receptor-mediated signals in rat hippocampal neurons. Biochem Biophys Res Commun 296(2):247–254. doi:10.1016/S0006-291X(02)00870-7
Kim S, Kim T, Ahn K, Park WK, Nah SY, Rhim H (2004) Ginsenoside Rg3 antagonizes NMDA receptors through a glycine modulatory site in rat cultured hippocampal neurons. Biochem Biophys Res Commun 323(2):416–424. doi:10.1016/j.bbrc.2004.08.106
Lee E, Kim S, Chung KC, Choo MK, Kim DH, Nam G, Rhim H (2006) 20(S)-ginsenoside Rh2, a newly identified active ingredient of ginseng, inhibits NMDA receptors in cultured rat hippocampal neurons. Eur J Pharmacol 536(1–2):69–77. doi:10.1016/j.ejphar.2006.02.038
Acknowledgments
The authors are grateful to Ms. Dongyun Feng for technical assistance. This work was supported by grants from the National Natural Science Foundation of China (No. 31170801).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Y. Zhang and L. Zhou contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, Y., Zhou, L., Zhang, X. et al. Ginsenoside-Rd attenuates TRPM7 and ASIC1a but promotes ASIC2a expression in rats after focal cerebral ischemia. Neurol Sci 33, 1125–1131 (2012). https://doi.org/10.1007/s10072-011-0916-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-011-0916-6