Abstract
Chronic cerebral hypoperfusion is associated with cognitive decline in aging and age-related neurodegenerative disease. Epigenetic mechanisms are involved in the maintenance of long-term hypoxia-adapted cellular phenotypes. In the present study, the epigenetic signatures such as DNA methylation and histone acetylation, as well as S-adenosylmethionine (SAM) cycle using chronic cerebral hypoperfusion rat model were explored. Chronic cerebral hypoxia-induced global DNA hypermethylation associated with the increase of DNA methyltransferase (DNMT) 3A as well as alteration of SAM cycle. Meanwhile, an enhanced level of global histone H4 acetylation accompanied with the upregulation of histone acetyltransferase, p300/CREB-binding protein (CBP), and the downregulation of histone deacetylases (HDACs), was also observed. SAM could improve spatial capacity through the upregulation of acetylcholine and brain-derived neurotrophic factor (BDNF) rather than alteration of DNA methylation levels. In conclusion, we have demonstrated a genome-wide adjustment of DNA methylation and histone acetylation under chronic cerebral hypoxic conditions in a rat’s brain. These epigenetic signatures may represent an additional mechanism to promote and maintain a hypoxic-adapted cellular responds with a potential role in memory deficits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral hemodynamic disorders are the causes of or factors contributing to numerous neurological illnesses. A sudden disruption of the blood supply may lead to stroke, while a moderate but persistent reduction in cerebral blood flow (CBF) involves memory processes and contributes to the development and progression of dementia [1, 2]. Hypoxia, constantly encountered by living cells, is a common stress condition occurring during development and physiological processes. In order to adapt to this inevitable condition, living cells have to develop various mechanisms to cope with it and survive. In addition to the activation of transcriptional regulators (hypoxia-inducible factors, HIF), other epigenetic mechanisms should be used in gene regulation. These epigenetic mechanisms include changes in DNA methylation status, histone modification, transcriptional coregulators, and chromatin-modifying complexes [3, 4]. These epigenetic mechanisms may play a very important role in a more substantial way to maintain the long-term hypoxia-adapted cellular phenotypes in the absence of HIF [5, 6].
In recent years, more and more information has been reported regarding the connection between homocysteine (HCY) metabolism and cognitive function, from mild cognitive decline to vascular dementia and Alzheimer’s disease (AD). AD may manifest hyperhomocysteinemia, low B12 and folate in the blood, and changes of methylation levels, indicating that dysregulation in S-adenosylmethionine (SAM) cycle is involved in epigenetic regulation through DNA methylation [7–9]. SAM cycle refers to the reaction to produce, consume, and regenerate SAM. Firstly, SAM is produced from methionine by methionine adenosyltransferase (MAT) through adenosine triphosphate (ATP). DNA methyltransferases (DNMTs) use SAM (methyl donor) as the substrate in a reaction that transfers methyl groups to cytosines on DNA, thus leading to the production of S-adenosylhomocysteine (SAH). SAH is quickly hydrolyzed to HCY and adenosine (Ado) by SAH hydrolase. Then, HCY can be remethylated to methionine using vitamin B12 and folate or trans-sulfurated to cystathionine using vitamin B6 as the cofactor [10, 11]. SAM cycle plays an important role in transferring of methyl groups to a wide range of molecules, and such methylation alteration will have impact on the biological activity of molecules, such as reproduction, growth, and cellular homeostasis.
Our previous studies have revealed that in vitro oxidative stress can induce an imbalance between DNA methylation/demethylation and histone acetylation/deacetylation [12, 13]. Since hypoxia is a major cause for the generation of oxidative stress, whether hypoxia has impact on DNA methylation and histone acetylation as well as SAM circle in vivo have attracted our extensive interest. Here, we show for the first time a genome-wide adjustment of DNA methylation and histone acetylation under chronic cerebral hypoxia. SAM could improve spatial capacity through the upregulation of acetylcholine and brain-derived neurotrophic factor (BDNF) rather than alteration of DNA methylation.
Materials and Methods
For all experiments, male Sprague–Dawley (SD) rats of 23 weeks (Beijing Vital River Experimental Animal Technology Co., Ltd) were used. Animals were housed in Experimental Animal Center of the Capital Medical University under standard conditions. All experimental procedures were complied with the Guidance for the Care and Use of Laboratory Animals formulated by the Ministry of Science and Technology of China. The rats were randomly divided into 10 groups (18 rats per group). The rats from six groups were subjected to two-vessel occlusion (2-VO) surgery. Among them, one group was selected as SAM-treated group, and another one group as the control. The rats from other four groups were subjected to sham surgery.
Animal Surgery and Treatment
Surgical operation of permanent bilateral carotid occlusion through 2-VO was carried out as previously described [14]. Briefly, rats were anesthetized by intraperitoneal injection of chloral hydrate (350 mg/kg). The bilateral common carotid arteries were separated carefully from the cervical sympathetic and vagal nerves through a ventral cervical incision. Then, the arteries were doubly ligated with silk sutures. The animals received the same surgical operation without carotid artery ligation used as the sham-operated controls. Two days after 2-VO surgery, the rats were treated with intraperitoneal injection of either SAM (10 mg/kg, Sigma Chemical Co., St. Louis, MO, USA) or saline every other day for 90 days.
Morris Water Maze Test
Morris water maze test [15] was used to estimate spatial memory of rats as previously described [16]. Rats were subjected to Morris water maze test for 6 days consecutively. In the first 2 days (training time), the rats were given four trials per day to search the hidden platform. Each rat was placed facing the wall at the quadrant opposite to the platform. The rats who failed to find the platform within 120 s were placed on the platform for 10 s for reinforcement. There was a 10-min recovery period between two trials. The escape latency (time to reach the platform) and the length of the path (the routes rats swam to the platform) were recorded by a videocassette recorder and an Image Analysis Computer System (Mobile Datum, V2.2.0, Shanghai, China). On the sixth day, the platform was removed, and the time that the rats spent in the training quadrant where the platform was previously placed was recorded.
Global Methylation Analysis
Global DNA methylation was tested with the MethyFlash™ Methylated DNA Quantification Kit (Epigentek, NY, USA) according to the manufacturer’s instructions. Genomic DNA was extracted from cortex using Wizard® SV Genomic DNA Purification System (Promega, WI, USA). The integrity and purity of DNA were spectrophotometrically examined according to its A260/A280 absorption. In this assay, the methylated fractions of DNA were identified by an anti-5-methylcytosine (5-mc) antibody and tested by using an enzyme-linked immunosorbent assay-like reaction and quantified through a colorimetric reaction by a microplate reader at the wavelength of 450 nm after complete mixing (EXL800UV, Bio-Tek Inc, USA). The mean value was obtained in each group. The experiment was repeated for three times. The methylation rate was calculated by using the following equation:
APP and BACE1 Genes Methylation Analysis
The sequences with 3,000 bp in APP (GenBank accession number NC-005110) and BACE1 (GenBank accession number NC-005107) genes around transcription start site were analyzed by using GENSCAN (http://genes.mit.edu/GENSCAN.html) and CpGPlot (http://www.ebi.ac.uk/emboss/cpgplot/index.html), and the CpG position was obtained. A total of 200 ng genomic DNA from each sample was bisulfite-treated with the Methylamp DNA Modification Kit (Epigentek, NY, USA). The quality of bisulfite conversion was examined by using PCR products without methyl groups as the control. Sequenom MassARRAY platform (CapitalBio, Beijing, China) was employed, which consisted of matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry and RNA base-specific cleavage (MassCLEAVE). PCR primers were designed by Methprimer (http://epidesigner.com). An additional T7 promoter tag for in vivo transcription was attached to each reverse primer, whereas a 10-mer tag was integrated into the forward primer in order to correspondingly adjust the melting temperature. Table 1 lists all primers used in this study. The methylation ratios were generated by Epityper software version 1.0 (Sequenom, San Diego, CA, USA). The generated data were put into the EPI 3.1 Database (EpiData Association, Odense, Denmark) and analyzed with SPSS-11.5 software (McGraw-Hill Inc, New York, NY, USA; Table 1).
High-Performance Liquid Chromatography Analysis
Parietal cortex tissues were weighed and prepared by sonication at a concentration of 4 μL PBS/mg tissue on ice. Following a centrifugation at 12,000 rpm for 15 min at 4 °C, the supernatant was collected, and then added 100 % trichloroacetic acid (TCA) at a 1:10 ratio, mixed and incubated on ice for 30 min. After centrifugation at 12,000 rpm for 20 min at 4 °C, the supernatant was filtered to be subjected to high-performance liquid chromatography (HPLC) analysis. Totally, 40 μL sample was injected into the HPLC column (4.6 mm × 150 mm, 5 μm; ZORBAX Eclipse C18 column; Agilent, CA, USA), and the measurements were carried out using Agilent 1100 HPLC system monitored spectrophotometrically at 254 nm. Calculation of peak areas were performed via a computer-based data system (Varian Star 5.3). For SAM and SAH analysis, the mobile phases were 10 % methanol and 90 % 0.04 M ammonium phosphate containing 10 mM heptane sulfonate (pH 4.0) at a flow rate of 1 mL/min. For ATP and Ado analysis, gradient elution mode was used at a flow rate of 0.9 mL/min. The mobile phases were 0.03 M potassium dihydrogen phosphate (pH 5.85) and methanol. The percentage of methanol is 2 % at 0–4 min, 15 % at 4–20 min, 80 % at 20–26 min, and 2 % at 26–27 min; post time 5 min.
Plasma samples were collected for HCY assay. Approximately 30 μL tris-(2-carboxylethyl)-phosphine hydrochloride (TCEP, 40 g/L) was added to 60 μL plasma samples. After mixing and incubating at 37 °C for 10 min, the mixture was then added 50 μL TCA (100 g/L, containing 4 mM EDTA-2 K) with mix and centrifugation at 12,000 rpm for 5 min. Totally, 50 μL supernatant was collected to add 10 μL NaOH (1.5 mM), 125 μL boric acid buffer (0.125 M, containing 4 mM EDTA-2 K, pH 9.5), 50 μL 7-fluorobenzo-2-oxa-1, 3-diazole-4-sulfonate (SBD-F, 1 g/L), and then mixed and incubated at 70 °C for 10 min. The reaction was stopped on ice and mixed with 10 μL 0.8 % phosphoric acid. Finally, 10 μL sample was injected into the HPLC column (3.9 mm × 150 mm, 5 μm, Symmetry ShieldTM RP18 column, Waters, MA, USA), and the measurements were carried out using Waters LC2695 system (automatic sampler, Empower chromatographic work station, 2475 fluorescence detector). The excitation wavelength was 390 nm and emission wavelength was 470 nm. The mobile phases were 2 % methanol and 98 % 0.08 M sodium acetate (pH 4.5) at a flow rate of 0.8 mL/min.
Histone Acetylation Assay
The acetylation of histone H3 and H4 was detected by EpiQuick Global Histone H3/H4 acetylation assay kit (Epigentek Group Inc) according to the manufacturer’s instructions as described previously [13]. The experiment was repeated for three times. The acetylation rate was calculated by using the following equation:
Western Blot Analysis
Tissue of hippocampus and cortex of the rats were dissected and homogenized. Routine procedures were carried out as described previously [16]. The primary antibodies used were as follows: rabbit anti-APP polyclonal antibody (1:1,000, Cell Signaling, MA, USA), rabbit anti-HDAC3 monoclonal antibody (1:1,000, Cell Signaling), rabbit anti-MBD2 (methyl-CpG binding domain 2) polyclonal antibody (1:1,000, Santa Cruz, CA, USA), rabbit anti-DNMT1 monoclonal antibody (1:1,000, Cell Signaling), rabbit anti-DNMT3a polyclonal antibody (1:1,000, Cell Signaling), rabbit anti-BACE1 polyclonal antibody (1:1,500, Abcam, London, UK), mouse anti-p300/CBP (p300/CREB-binding protein) monoclonal antibody (1:250, Abcam), rabbit anti-BDNF polyclonal antibody (1:5,000, Abcam), and mouse anti-GAPDH monoclonal antibody (1:500, Sigma, MO, USA). The signal quantification was carried out using a Gel-Doc Image Scanner (Bio-Rad, CA, USA) and Quantity One software program version 4.2.1 (Bio-Rad).
Immunohistochemistry Staining
The rats were anaesthetized by intraperitoneal injection of chloral hydrate (350 mg/kg) and perfused through heart with 0.01 M phosphate-buffered saline (pH 7.6) and then with 4 % paraformaldehyde. Brain tissues were removed and fixed in 4 % paraformaldehyde for 3 days and then transferred to in 0.1 M PBS containing 30 % sucrose for incubating at 4 °C overnight. The brain tissues were cryosectioned in the thickness of 10 μm and the sections at the bregma of 3.60 mm according to the Paxinos and Watson rat brain atlas [17] from all groups were selected for immunohistochemical staining.
The sections were pretreated with 1 % H2O2 for 10 min, blocked with 5 % fetal calf serum in PBS for 30 min and incubated with primary antibody (rabbit anti-BDNF polyclonal antibody (1:100, Abcam) at 4 °C overnight. After washing, sections were incubated with secondary antirabbit/goat IgG antibodies (1:300) for 2 h. The immunoreactivity was visualized by the avidin-biotin-peroxidase method (ABC kit, Vector, Burlingame, CA, USA) combined with 0.05 % 3,3′-diaminobenzidine (DAB) and 0.01 % H2O2 for 5 min. Negative control staining was performed by replacing the primary antibody with PBS.
Histochemistry Staining
Sections at the bregma 0.60 mm according to the Paxinos and Watson rat brain atlas from each group were selected for acetylcholinesterase (AChE) staining to label acholinergic neurons. Sections were incubated at 37 °C for 75 min in a mixture containing 5 mg acetylthiocholine iodine, 6.5 mL 0.1 M acetate buffer, 0.5 mL 0.1 M sodium citrate, 1 mL 30 mM copper sulfate, and 1 mL 5 mM potassium ferricyanide. Before and after the incubation period, the sections were washed in distilled water, and finally mounted, dehydrated, and coverslipped.
All images were obtained with Nikon digital sight DS-Ri1 for Professional Microscopy (Nikon Eclipse 80i, Japan). Total number of acholinergic neurons in one field (400×) was counted. The cell density was obtained by cell numbers/field area (4.66 × 105 μm2). The average cell density was obtained from 10 non-overlapping fields (400×) of each section (n = 3). The counts were performed by a computer-assisted image analysis system (Leica Qwin Analysis software V2.8, Germany).
Statistical Analysis
Data are presented as means ± standard deviation (SD). Statistical analysis was performed using two sample Student’s t test or one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test when appropriate. Statistical significance was defined as p < 0.05. The SPSS 11.5 system (SPSS, Inc. Chicago, IL, USA) was utilized for the statistical analysis.
Results
Chronic Cerebral Hypoperfusion Induces the Global DNA Hypermethylation Associated with the Increase of DNMT3A
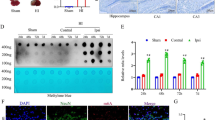
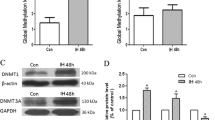
Global DNA methylation levels were examined by methylated DNA Quantification Kit. As shown in Fig. 1a, acute ischemia/hypoxia (2-VO surgery for 10 days) revealed a decrease of the global DNA methylation level, while the opposite results were achieved by long-term cerebral hypoperfusion. By the time of 2-VO surgery for 90 days, the global DNA methylation level had increased by 54 % compared with the sham, and it was increased by twofold of the sham after 2-VO surgery for 180 days. The changes of methylation level and memory deficit (see below) were significant in 2-VO rats after surgery for 90 days, the time point of SAM treatment for 90 days was selected to explore the effect of SAM. SAM treatment for 90 days did not have significant effect on the global DNA methylation levels. Since DNA methylation is completed by DNA DNMTs, it is highly necessary to investigate whether the change of DNMTs was involved in DNA methylation under long-term cerebral hypoperfusion. In the present study, DNMT3a were significantly upregulated after 2-VO surgery for 90 days and this upregulation could be reversed by SAM. No significant change of DNMT1 was observed. MBD2 protein acts as transcriptional repressors of demethylase, and can transform methylated cytosine to cytosine [18]. An increase trend was observed in MBD2 under chronic cerebral hypoperfusion, and SAM had no effect on the expression of MBD2 (Fig. 1b, c). These data implied that chronic cerebral hypoperfusion could induce the upregulation of DNMT3A responsible for global DNA hypermythelation.
Methylation levels of global DNA and change of DNA methylation-related enzymes. a Methylation levels of global DNA were evaluated by MethyFlash™ Methylated DNA Quantification Kit from the samples of sham group and 2-VO rats after surgery. DNA methylation level was calculated as the methylation rate (%). The data were expressed as mean ± SD (n = 3). b and c Western blot analysis of DNA methyltransferase (DNMT1, DNMT3a, and MBD2). Homogenates of brain tissue from the sham group, 2-VO rats after surgery with or without SAM treatment for 90 days were analyzed. The blots were densitometrically quantified, and the data after being normalized with the GAPDH blots were shown in mean ± SD (n = 6). *p < 0.05; #p < 0.01 vs. sham group
Chronic Cerebral Hypoperfusion Induces the Hypomethylation of APP and BACE1 genes
In order to explore the effect of chronic hypoxia on specific gene methylation status, amyloid precursor protein (APP) gene and beta-site APP-cleaving enzyme 1 (BACE1) gene were selected. APP and BACE1 were amplified from the samples of sham, and 2-VO rats after surgery as well as 2-VO rats treated with SAM for 90 days after the bisulfite conversion of genomic DNA. Figure 2 shows the mean methylation levels, which were evaluated by Student’s t test. For APP gene, a transitorily increase of methylation level was observed in 2-VO rats after surgery for 10 days, then sustained decrease was remained and strengthened after surgery for 180 days (decrease to 52.7 % of the sham). The mean methylation level of BACE1 gene revealed a decreasing trend till the surgery for 180 days (decrease to 45.83 % of the sham). However, SAM had no influence on mean methylation level of each gene. We also evaluated the methylation level of each CpG site within both genes. A total of 24 CpG sites in APP were divided into 11 CpG sites, and 17 CpG sites in BACE1 promoter were divided into 9 CpG sites (Fig. 3). The methylation levels revealed a large variation at different CpG sites.
Methylation levels of APP and BACE1 genes. Schematic diagram of amyloid precursor protein (APP) (a) and β-site APP-cleaving enzyme 1 (BACE1) (c). The transcripts were composed of a 5′-untranslated region (UTR) with internal promoter activity, open reading frame (ORF), and a 3′-UTR. a The sequence represents a 240-base pair (bp) fragment (bps between 26 and 266) in APP gene. c The sequence represents a 250-bp fragment (bps between −318 and −58) in BACE1 gene. Numbers refer to the locations of cytosine-guanine (CpG) sites and underlying highlights of CpG sites include more than 1 CpG site tested at the same time. b, d Mean methylation levels of CpG sites in APP and BACE1 gene. Methylation levels of all CpG sites in each gene were compared among brain samples from the sham group, 2-VO rats after surgery and 2-VO rats treated with SAM for 90 days. The Sequenom MassARRAY platform was used for the quantitative methylation analysis. b CpG sites were numbered as 1-24 from the 5′ end to 3′ end in APP gene. d CpG sites were numbered as 1–17 from the 5′ end to the 3′ end in BACE1 gene. The CpG sites that were not shown did not exhibit obvious methylation signals. The data were expressed as mean ± SD. *p < 0.05; #p < 0.01 vs. sham group (n = 3)
Since APP and BACE1 gene hypomethylation were induced under chronic cerebral hypoperfusion, the expression of APP and BACE1 were examined. As shown in Fig. 4, both APP and BACE1 were upregulated under chronic hypoxia condition. SAM had strong reverse effect on the expression of APP, while no significant effect on the expression of BACE1. Chronic cerebral hypoperfusion could induce the hypomethylation of both specific genes which was not consistent with the changes of global DNA methylation levels.
Effect of cerebral hypoperfusion and SAM on the expression of APP and BACE1. Homogenates of brain tissues from the sham group, 2-VO rats after surgery with or without SAM treatment for 90 days were analyzed. The blots were densitometrically quantified, and the data after being normalized with GAPDH blots were shown in mean ± SD (n = 6). *p < 0.05 vs. sham group
Chronic Cerebral Hypoperfusion Alters the SAM Cycle
Since cerebral hypoperfusion could influence the status of DNA methylation, and SAM circle is the major source of methyl-donor molecule SAM, the change of metabolites involved in this cycle should be considered. HPLC results revealed that SAH revealed the enhancement by threefold when compared with the sham group, while SAM exhibited the decrease by 15 % compared with the sham (Fig. 5a, b). SAM/SAH ratio, also termed as methylation potential (MP), is considered as an indicator of methylation potential of a biological system [10]. In this experiment, SAM/SAH ratio decreased to 12 when compared to normal ratio of 42. In order to determine whether the change in energy metabolism plays an important role in the hypoxic regulation of SAM/SAH metabolism, we next investigated the influence of hypoxia on ATP concentration. Ado is product and inhibitor of SAH hydrolysis, we determined Ado concentration by HPLC because transmethylation and energy metabolism are linked by SAH hydrolysis. Approximately 30 % decrease of ATP level was observed in 2-VO rats after surgery for 90 days, but Ado was increased (Fig. 5c, d). HCY is a sulfur-containing and non-protein amino acid produced in the SAM cycle. As shown in Fig. 5e, f, chronic cerebral hypoperfusion could increase plasma HCY by 1.2-fold compared with the sham group, and SAM revealed 20 % decrease compared with the 2-VO rats after surgery for 90 days. These data suggested that SAM circle was disturbed under chronic cerebral hypoperfusion and resulted in high plasma HCY.
Effect of chronic cerebral hypoperfusion on metabolites involved in the SAM cycle. Brain samples from sham group and 2-VO rats after surgery for 90 days were analyzed by HPLC. a, b SAM and SAH separation curve and concentration. c, d ATP and Ado separation curve and concentration. Plasma samples from the sham group, 2-VO rats after surgery with or without SAM treatment for 90 days were analyzed by HPLC. e, f HCY separation curve and concentration. The data were expressed as mean ± SD. *p < 0.05 vs. sham group (n = 6)
Chronic Cerebral Hypoperfusion-Induced Global Histone H4 Hyperacetylation and SAM may have Reverse Effect in an Enzyme-Dependent Manner
In order to further investigate epigenetic mechanisms, the acetylation level of global histone H3/H4 was examined by using histone H3/H4 acetylation assay kits. As shown in Fig. 6a, the acetylation level of global histone H4 was markedly increased in 2-VO rats after surgery for 90 days; however, no significant change in acetylation level of histone H3 was detected. Histone acetyltransferases (HATs) catalyze the acetylation of core histones. Co-activators p300 and CBP have been identified as HATs. Deacetylation catalyzed by histone deacetylases (HDACs) also plays an important role in turning off or maintaining genes in a repressed state. Therefore, we examined the expression level of p300/CBP and HDAC3 by Western blot. As shown in Fig. 6b, c, the expression of p300/CBP was upregulated in samples from 2-VO rats after surgery for 90 days when compared with that in the sham samples, while the expression of HDAC3 revealed a decrease by 66.67 % when compared with the sham. SAM could reverse the expression of these two proteins. These results implied that enzyme-dependent hyperacetylation of histone H4 was involved in gene regulation under chronic cerebral hypoperfusion so that SAM might reverse this process in an enzyme-dependent manner.
Acetylation levels of histone H3 and H4 and change of acetylation-related enzymes. a Acetylation levels of histone H3/H4 were evaluated by EpiQuick Global Histone H3/H4 acetylation assay kit and the acetylation levels were calculated as the acetylation rate (%). The data are expressed as mean ± SD (n = 6). b, c Western blot analysis of p300/CBP and histone deacetylase (HDAC3). Homogenates of brain tissues from the sham group, 2-VO rats after surgery with or without SAM treatment for 90 days were analyzed. The blots were densitometrically quantified, and the data after being normalized with GAPDH blots were shown in mean ± SD (n = 6). *p < 0.05 vs. sham group
SAM Improves Spatial Memory Deficits Induced by Chronic Cerebral Hypoperfusion
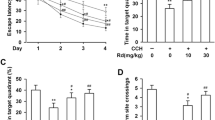
The reduction of spontaneous motion and approach-withdrawal capacity in animals subjected to 2-VO surgery, especially at the beginning of 1–3 days, were observed. However, no change in animals subjected to sham surgery was observed. The mortality rates were 25.5 % in the 2-VO group and 13.1 % in the sham group. The effect of cerebral hypoperfusion on spatial learning was examined by using the hidden-platform water maze. No significant difference in the escape latency and the length of the path among the rats from 2-VO and sham groups after surgery for 10 days was observed. However, the rats from the 2-VO group exhibited deficient learning after surgery for 30 days. The deficits remained or strengthened after surgery for 180 days. However, the 2-VO rats with SAM treatment for 90 days showed a significant decrease in escape latency and long time spending in the target quadrant when compared with the rats after surgery for 90 days (Fig. 7a, c). Swimming speed was not different among groups (Fig. 7b).
Effect of cerebral hypoperfusion and SAM on spatial learning. Sham, 2-VO rats after surgery and 2-VO rats treated with SAM for 90 days were subjected to Morris water maze test on a designed time for five consecutive days, mean latency (a) for finding the escape platform, swimming speed (b) and probe trial (c) are shown as mean ± SD (n = 12). *p < 0.05; #p < 0.01 vs. sham group
As SAM can improve spatial memory deficits induced by chronic cerebral hypoperfusion, the neurotransmitters such as acetylcholine and BDNF have important function in brain plasticity-related processes, and are necessarily to be further investigated. The basal nucleus of Meynert characterized by a collection of large, cholinergic neurons [19] is a major part of the magnocellular basal forebrain system including the vertical and horizontal nuclei of the diagonal band (VDB and HDB) [20]. The number of cholinergic neurons in VDB labeled by AChE histochemistry staining was counted and the cell density was shown in Table 2. Chronic cerebral hypoperfusion could induce the decrease of cholinergic cell density, while SAM had reverse effect on it (Fig. 8a–d). BDNF immunohistochemistry staining revealed that, either in cortex or hippocampus, SAM could reverse BDNF downregulation induced by chronic cerebral hypoperfusion (Fig. 8e–j). This result was confirmed by western blot analysis (Fig. 8k–l). These results indicated that chronic cerebral hypoperfusion could impair learning and memory capability of the rats and SAM had the reverse potential through upregulation of acetylcholine and BDNF.
Effect of cerebral hypoperfusion and SAM on the expression of acetylcholine and BDNF. Microphotographs show histochemistry staining of AChE for cholinergic neurons in the nucleus of the vertical limb of the diagonal band (VDB) of basal nucleus of Meynert (a–d). a Low magnification shows the selected study position. b–d High magnification of labeled box in a from the sham group (b), 2-VO rats after surgery for 90 days (c) and 2-VO rats treated with SAM for 90 days (d), respectively. The arrows indicate neuron bodies. Scale bar 20 μM. BDNF immunohistochemical staining in cortex (e–g) and CA1 area of hippocampus (h–j). BDNF positive immunoreactivity was located in the cytoplasm. e, h Sham, f, i 2-VO rats after surgery for 90 days, g, j 2-VO rats treated with SAM for 90 days. Scale bar 50 μM. k, l Western blot analysis of BDNF in hippocampus. Homogenates of hippocampus tissues from the sham group, 2-VO rats after surgery with or without SAM treatment for 90 days were analyzed. The blots were densitometrically quantified, and the data after being normalized with GAPDH blots were shown in mean ± SD (n = 6). *p < 0.05 vs. sham group
Discussion
In 2-VO rats, the blood vessel occlusion is permanent and long-lasting to ensure no reperfusion injury occurring. This kind of cerebral hypoperfusion is global, and a distinct ischemic core and penumbra region cannot be found [21]. No infarctions or hemorrhage in either gray matter regions or white matter regions were observed after 2-VO surgery by hematoxylin-eosin staining (data not shown). The damage to the nervous tissue is less severe, and there are no obvious signs of motor dysfunction or seizures. After the acute phase (2-VO surgery for a maximum of 2-3 days), a phase of chronic hypoperfusion is followed, which may last for 8 weeks to 3 months. This hypoperfusion is considered to sustain chronic, moderate hypoglycemia, corresponding with oligemia. This phase closely resembles the condition of reduced CBF in human aging and dementia [21, 22]. In our experiment, the changes of DNA methylation level and memory deficit were observed in 2-VO rats after surgery for 30–90 days, while the same changing trend in 2-VO rats after surgery for 180 days was observed. From the consideration of saving animals and shortening experiment duration, 2-VO rats after surgery for 90 days were preferred for the further studies.
It is well known that acute hypoxia initiates a cascade of hypoxic response through HIF-1 pathways; however, only a small number of hypoxia-induced gene expression changes can be accounted for by the direct effects of HIF-1 [23]. Meanwhile, since the transient feature of HIF-1 expression, other mechanisms must be involved in the chronic hypoxic response to accomplish and maintain an adaptive phenotype. Environmental factors are the common cause to alter the fundamental epigenetic programming of the human genome. Tissue hypoxia that usually occurs in the aging organs [24, 25] could equally have an impact on such global epigenetic programming. In this study, we have identified a global DNA hypermethylation and histone H4 hyperacetylation in response to chronic cerebral hypoxia which may signify a new epigenetic signature indicative of the altered gene expression profile of a hypoxia-adapted cell.
Usually, cancer cell lines are used in the studies of hypoxia on global DNA methylation and reveal the inconsistent result. Shahrzad et al. [26] demonstrated that the exposure to hypoxia could induce 15–20 % reduction of CpG methylation in human colorectal and melanoma cell lines. Prostate cells permanently maintained with 1 % oxygen manifested an increase of DNA methylation [27]. Moreover, in support of our findings of global hypermethylation, we have identified a significant increase in DNMT3a expression and a decrease in the levels of SAM, which may be related with increased use of SAM during the addition of new methyl groups. DNA methylation accomplished by DNMTs has two patterns. DNMT1 appears to be primarily attributed in maintenance methylation of hemimethylated DNA after DNA replication, whereas DNMT3a and DNMT3b play important role in de novo DNA methylation responsible for establishing new patterns of DNA methylation [28]. So we can infer that chronic hypoxia-induced global DNA hypermethylation in brain is caused by increase new sites of DNA methylation. Watson et al. [27] have reported that chronic hypoxia can induce sustained enhancement of global DNA methylation, which is associated with the significant increase in the expression of DNMT3b. Recent studies have suggested that microRNAs are important modulators of DNMT3a and DNMT3b. The increased expression of DNMT3 is correlated with the decreased expression of miR-29 family in lung cancer [29]. As the expression of miR-29 drops significantly in the ischemic heart [30], it is reasonable to speculate that miR-29 regulates DNMT3a expression under hypoxia condition.
As we know, the change in global DNA methylation level was not completely consistent with the specific genes. For example, age-dependent global DNA hypomethylation is accompanied with hypermethylation of tumor suppressor gene in tumor patients. APP and BACE1 are two important genes related to Aβ production which play a key role in AD pathogenesis. Our former study revealed that in vitro oxidative stress can induce hypomethylation of APP and BACE1 genes [12, 13]. In the present study, we found that chronic cerebral hypoperfusion could induce progressive decrease in the methylation of APP and BACE1 genes with upregulation of both proteins. Age-dependent hypomethylation of a number of specific genes related to AD has been reported. For example, the methylation of cytosines in APP promoter, particularly GC-rich segment from approximately −270 to −182, is significantly lower in autopsy cases with the age more than 70 years old [31]. In vitro, the expression of BACE1 and PS1 genes is increased after folate deprivation-induced hypomethylation [7]. Here is the first time to demonstrate the change in methylation level of global DNA- and AD-related specific genes under chronic hypoxia in vivo. As we know, DNA methylation is accomplished by DNMTs; however, the role of this enzyme is not specific. The exact underlining mechanisms are not clear. From our experiment, we can infer that the methylation level of global DNA cannot represent the methylation status of a specific gene. Many efforts are needed to explore the specific mechanisms to uncover the relationship between disease and DNA methylation.
A connection between the disturbance of SAM cycle leading to high HCY and cognitive function has been reported. Dietary deficiency of folic acid, vitamin B12, and vitamin B6 leading to hyperhomocysteinemia is thought to be the main cause [32, 33]. Besides this poor lifestyle, poor renal function, age, sex, drug, and genetic factors have to be considered [34]. However, as the high risk factor of AD, whether chronic hypoxia has influence on this cycle? Our present study has revealed that chronic cerebral hypoperfusion can disturb of SAM cycle with decrease MP and high plasma HCY. ATP is the necessary energy supply in the production of SAM from methionine [7]. Chronic hypoxia can induced the downregulation of SAM accompanied with the decrease in ATP, suggesting that low energy impede the synthesis of SAM. Meanwhile, the inactivation of MAT enzyme may be deduced. MP is referred to as the methylation index within cells, which determines the level of methylation. In this experiment, MP reduction from 42 to 12 is mainly due to the increased SAH level. One explanation of increase SAH in ischemic brain tissue could be the inhibition of SAH hydrolysis by Ado. In addition, an enhanced rate of SAH synthesis from Ado and HCY is also one of the reasons [35, 36]. The reaction from SAH to HCY is reversible, and the thermodynamic equilibrium of this reaction is on the side of SAH synthesis. With ischemic kidney, Kloor et al. [35] have found that the contents of Ado and SAH are obviously increased with a dramatic decrease of MP. The application of exogenous Ado did not change the accumulation of SAH, while when HCY was infused into the ischemic kidney, the SAH was increased by fivefold compared with the control level. The elevation of SAH results in the inhibition of SAH hydrolysis and HCY is the rate-limiting factor for SAH synthesis [37, 38]. SAH is also a potent inhibitor of methyltransferases with high affinity in a SAM-dependent manner. Since the central nerve system (CNS) cells can not export SAH from cells, it is the only way for CNS cells to decrease the level of SAH by the extracellular transport of HCY [39], thus leading to the increase of plasma HCY.
Histone acetylation is one of the well studied among the histone modifications. Acetylation is a dynamic modification, being added by HATs and removed by HDACs. For most of the genes, acetylation of histones correlates with transcription activation and a more open chromatin structure. On the other hand, removal of acetylation relates with transcriptional repression and heterochromatin. In this study, we found that chronic cerebral hypoperfusion induced an enhanced level of global histone H4 acetylation accompanied with the upregulation of HAT and the downregulation of HDAC. Studies with different subjects under hypoxia conditions demonstrate the conflicting results. A significant increase in global levels of H3K9 histone acetylation in PwR-1E benign prostate epithelial cells was observed under severe chronic hypoxia (10 % O2 for 7 weeks, then 3 % O2 for 4 weeks, and, finally, 1 % O2 for 3 weeks) [27]. Under high-altitude long-term hypoxia, the global histone 4 acetylation in fetus pulmonary arteries was reduced [40]. Several lung carcinoma studies have reported a global hypoacetylation in response to hypoxia [41–43].
Some preclinical and clinical information have reported that dietary supplementation with SAM alleviates many risk factors and hallmarks associated with AD [44, 45]. In the present study, SAM could improve spatial memory deficits induced by chronic cerebral hypoperfusion associated with the upregulation of acetylcholine and BDNF. According to the report by Chan et al. [46], SAM-dependent increase in N-methyl nicotinamide is accounted for the increase of acetylcholine, which is associated with the maintenance of normal cognition. It has been reported that the expression of BDNF is related to the oxidative stress [47]. Zou and Crews [48] have shown that oxidative stress can result in the glutamate-induced neurotoxicity as well as reduced expression of BDNF mRNA. Hypoxia and HCY provoke oxidative damage by the activation of glutamatergic receptors with consequence of reactive species generation [49, 50]. In consistent with the previous report, BDNF downregulation induced by chronic cerebral hypoperfusion either in cortex or hippocampus was observed in the present study. However, SAM has the reverse effect of BDNF expression. With the rat model of ischemia–reperfusion, Villalobosa et al. [51] have found that SAM can reduce oxidative damage in rat brain tissues. This antioxidative function of SAM may keep BDNF in a normal level even under the hypoperfusion condition. However, as the methyl donor, SAM seemed to have no significant effect on the alteration of either global DNA methylation level or specific gene methylation levels under the current chronic hypoxia condition. This result implied that DNA methylation is a more complex process involving each segment in SAM circle, not only dependent on methyl donor. Although the effect of SAM on the expression of APP and histone acetylation-related enzyme is reverse, the exact mechanisms of these results are not clear and need further studies in the future. The reverse effect of SAM on high HCY might also contribute to the rescue memory deficits.
Conclusions
In the present study, we have reported that chronic cerebral hypoperfusion in vivo resulted in an imbalance between DNA methylation and demethylation as well as histone acetylation and deacetylation. Meanwhile, SAM circle is disturbed which might be the cause or consequence in this pathophysiological process. Providing SAM in the early stage may reduce the risk of cognitive diseases.
References
Bakker FC, Klijn CJM, Jennekens-Schinkel A, Kappelle LJ (2000) Cognitive disorders in patients with occlusive disease of the carotid artery: a systematic review of the literature. J Neurol 247:669–676
Sekhon LHS, Morgan MK, Spence I, Weber N (1997) Chronic cerebral hypoperfusion: pathological and behavioral consequences. Neurosurgery 40:548–556
Tsai YP, Wu KJ (2013) Epigenetic regulation of hypoxia-responsive gene expression: focusing on chromatin and DNA modifications. Int J Cancer. doi:10.1002/ijc.28190
Watson JA, Watson CJ, McCann A, Baugh J (2010) Epigenetics, the epicenter of the hypoxic response. Epigenetics 16:293–296
Kim SH, Jeong JW, Park JA, Lee JW, Seo JH, Jung BK, Bae MK, Kim KW (2007) Regulation of the HIF-1 alpha stability by histone deacetylases. Oncol Rep 17:647–651
Kenneth NS, Mudie S, van Uden P, Rocha S (2008) SWI/SNF regulates the cellular response to hypoxia. J Biol Chem 284:4123–4131
Fuso A, Seminara L, Cavallaro RA, D'Anselmi F, Scarpa S (2005) S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol Cell Neurosci 28:195–204
Tchantchou F, Shea TB (2008) Folate deprivation, the methionine cycle, and Alzheimer’s disease. Vitam Horm 79:83–97
Quadri P, Fragiacomo C, Pezzati R, Zanda E, Forloni G, Tettamanti M, Lucca U (2004) Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am J Clin Nutr 80:114–122
Chiang PK, Gordon RK, Tal J, Zeng GC, Doctor BP, Pardhasaradhi K, McCann PP (1996) Sadenosylmethionine and methylation. FASEB J 10:471–480
Medina MA, Urdiales JL, Amores-Sanchez MI (2001) Roles of homocysteine in cell metabolism: old and new function. Eur J Biochem 268:3871–3882
Gu X, Sun J, Li S, Wu X, Li L (2013) Oxidative stress induces DNA demethylation and histone acetylation in SH-SY5Y cells: potential epigenetic mechanisms in gene transcription in Aβ production. Neurobiol Aging 34:1069–1079
Guo X, Wu X, Ren L, Liu G, Li L (2011) Epigenetic mechanisms of Aβ production in anisomycin-treated SH-SY5Y cells. Neurosci 194:271–281
Ni J, Ohta H, Matsumoto K, Watanabe H (1994) Progressive cognitive impairment following chronic cerebral hypoperfusion induced by permanent occlusion of bilateral carotid arteries in rats. Brain Res 653:231–236
Sun MK, Xu H, Alkon DL (2002) Pharmacological protection of synaptic function, spatial learning, and memory from transient hypoxia in rats. J Pharmacol Exp Ther 300:408–416
Liu H, Xing A, Wang X, Liu G, Li L (2012) Regulation of β-amyloid level in the brain of rats with cerebrovascular hypoperfusion. Neurobiol Aging 33(826):e31–e42
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates. Academic Press, London
Bhattacharya SK, Ramchandani S, Cervoni N, Szyf M (1999) A mammalian protein with specific demethylase activity for mCpG DNA. Nature 397:579–583
Mesulam MM, Geula C (1988) Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetytransferase. J Comp Neurol 275:216–240
Heimer L (2003) The legacy of the silver methods and the new anatomy of the basal forebrain: implications for neuropsychiatry and drug abuse. Scand J Psychol 44:189–201
Farkas E, Luiten PG, Bari F (2007) Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 54:162–180
de la Torre JC, Stefano GB (2000) Evidence that Alzheimer’s disease is a microvascular disorder: the role of constitutive nitric oxide. Brain Res Rev 34:119–136
Xia X, Lemieux ME, Li W, Carroll JS, Brown M, Liu XS, Kung AL (2009) Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci U S A 106:4260–4265
Tanaka T, Kato H, Kojima I, Onse T, Son D, Tawakami T, Yatagawa T, Inagi R, Fujita T, Nangaku M (2006) Hypoxia and expression of hypoxia-inducible factor in the aging kidney. J Gerontol A Biol Sci Med Sci 61:795–805
Aalami O, Fang TD, Song HM, Nacamuli RP (2003) Physiological features of aging persons. Arch Surg 138:1068–1076
Shahrzad S, Bertrand K, Minhas K, Coomber BL (2007) Induction of DNA hypomethylation by tumor hypoxia. Epigenetics 2:119–125
Watson JA, Watson CJ, McCrohan AM, Woodfine K, Tosetto M, McDaid J, Gallagher E, Betts D, Baugh J, O'Sullivan J, Murrell A, Watson RW, McCann A (2009) Generation of an epigenetic signature by chronic hypoxia in prostate cells. Hum Mol Genet 18:3594–3604
Hermann A, Gowher H, Jeltch A (2004) Biochemistry and biology of mammalian DNA methyltransferases. Cell Mol Life Sci 61:2571–2587
Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C, Volinia S, Guler G, Morrison CD, Chan KK, Marcucci G, Calin GA, Huebner K, Croce CM (2007) MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A 104:15805–15810
van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN (2008) Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A 105:13027–13032
Tohgi H, Utsugisawa K, Nagane Y, Yoshimura M, Genda Y, Ukitsu M (1999) Reduction with age in methylcytosine in the promoter region −224 approximately −101 of the amyloid precursor protein gene in autopsy human cortex. Brain Res Mol Brain Res 70:288–292
Fuso A, Nicolia V, Cavallaro RA, Ricceri L, D'Anselmi F, Coluccia P, Calamandrei G, Scarpa S (2008) B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-beta deposition in mice. Mol Cell Neurosci 37:731–746
Fuso A, Nicolia V, Ricceri L, Cavallaro RA, Isopi E, Mangia F, Fiorenza MT, Scarpa S (2012) S-adenosylmethionine reduces the progress of the Alzheimer-like features induced by B-vitamin deficiency in mice. Neurobiol Aging 33(1482):e1–e16
Miller AL (2003) The methionine-homocysteine cycle and its effects on cognitive diseases. Altern Med Rev 8:7–19
Kloor D, Delabar U, Mühlbauer B, Luippold G, Osswald H (2002) Tissue levels of S-adenosylhomocysteine in the rat kidney: effects of ischemia and homocysteine. Biochem Pharmacol 63:809–815
Hermes M, von Hippel S, Osswald H, Kloor D (2005) S-adenosylhomocysteine metabolism in different cell lines: effect of hypoxia and cell density. Cell Physiol Biochem 15:233–244
Liu Q, Liu L, Zhao Y, Zhang J, Wang D, Chen J, He Y, Wu J, Zhang Z, Liu Z (2011) Hypoxia induces genomic DNA demethylation through the activation of HIF-1α and transcriptional upregulation of MAT2A in hepatoma cells. Mol Cancer Ther 10:1113–1123
Kloor D, Hermes M, Fink K, Schmid H, Klingel K, Mack A, Grenz A, Osswald H (2007) Expression and localization of S-adenosylhomocysteine-hydrolase in the rat kidney following carbon monoxide induced hypoxia. Cell Physiol Biochem 19:57–66
West RL, Lee JM, Maroun LE (1995) Hypomethylation of the amyloid precursor protein gene in the brain of an Alzheimer’s disease patient. J Mol Neurosci 6:141–146
Yang Q, Lu Z, Ramchandran R, Longo LD, Raj JU (2012) Pulmonary artery smooth muscle cell proliferation and migration in fetal lambs acclimatized to high-altitude long-term hypoxia: role of histone acetylation. Am J Physiol Lung Cell Mol Physiol 303(11):L1001–L1010. doi:10.1152/ajplung.00092.2012
Chen H, Yan Y, Davidson TL, Shinkai Y, Costa M (2006) Hypoxic stress induces dimethylated histone H3 lysine 9 through histone methyltransferase G9a in mammalian cells. Cancer Res 66:9009–9016
Costa M, Davidson TL, Chen H, Ke Q, Zhang P, Yan Y, Huang C, Kluz T (2005) Nickel carcinogenesis: epigenetics and hypoxia signalling. Mutat Res 592:79–88
Johnson AB, Denko N, Barton MC (2008) Hypoxia induces a novel signature of chromatin modifications and global repression of transcription. Mutat Res 640:174–179
Shea TB, Chan A (2008) S-adenosyl methionine: a natural therapeutic agent effective against multiple hallmarks and risk factors associated with Alzheimer’s disease. J Alzheimers Dis 13:67–70
Lee S, Lemere CA, Frost JL, Shea TB (2012) Dietary supplementation with S-adenosyl methionine delayed amyloid-β and tau pathology in 3xTg-AD mice. J Alzheimers Dis 28:423–431
Chan A, Tchantchou F, Graves V, Rozen R, Shea TB (2008) Dietary and genetic compromise in folate availability reduces acetylcholine, cognitive performance and increases aggression: critical role of S-adenosyl methionine. J Nutr Health Aging 12:252–261
Kapczinski F, Frey BN, Andreazza AC, Kauer-Sant’Anna M, Cunha AB, Post RM (2008) Increased oxidative stress as a mechanism for decreased BDNF levels in acute manic episodes. Rev Bras Psiquiatr 30:243–245
Zou J, Crews F (2006) CREB and NF-kappaB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cell Mol Neurobiol 26:385–405
Gao L, Zeng XN, Guo HM, Wu XM, Chen HJ, Di RK, Wu Y (2012) Cognitive and neurochemical alterations in hyperhomocysteinemic rat. Neurol Sci 33:39–43
Matté C, Pereira LO, Dos Santos TM, Mackedanz V, Cunha AA, Netto CA, Wyse AT (2009) Acute homocysteine administration impairs memory consolidation on inhibitory avoidance task and decreases hippocampal brain-derived neurotrophic factor immunocontent: prevention by folic acid treatment. Neurosci 163:1039–1045
Villalobos MA, De La Cruz JP, Cuerda MA, Ortiz P, Smith-Agreda JM, Sánchez De La Cuesta F (2000) Effect of S-adenosyl-l-methionine on rat brain oxidative stress damage in a combined model of permanent focal ischemia and global ischemia-reperfusion. Brain Res 883:31–40
Acknowledgments
This work was supported by National Natural Science Foundation (Nos. 81070926 and 81271310) and China 973 Preprogram (2011CB512109).
Conflict of interest statement
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, X., Sun, J., Zhang, X. et al. Epigenetic Signature of Chronic Cerebral Hypoperfusion and Beneficial Effects of S-adenosylmethionine in Rats. Mol Neurobiol 50, 839–851 (2014). https://doi.org/10.1007/s12035-014-8698-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8698-5