Abstract
SARS-CoV-2 (COVID-19) pandemic has been an unpredicted burden on global healthcare system by infecting over 700 million individuals, with approximately 6 million deaths worldwide. COVID-19 significantly impacted all sectors, but it very adversely affected the healthcare system. These effects were much more evident in the resource limited part of the world. Individuals with acute conditions were also severely impacted. Although classical COVID-19 diagnostics such as RT-PCR and rapid antibody testing have played a crucial role in reducing the spread of infection, these diagnostic techniques are associated with certain limitations. For instance, drawback of RT-PCR diagnostics is that due to degradation of viral RNA during shipping, it can give false negative results. Also, rapid antibody testing majorly depends on the phase of infection and cannot be performed on immune compromised individuals. These limitations in current diagnostic tools require the development of nanodiagnostic tools for early detection of COVID-19 infection. Therefore, the SARS-CoV-2 outbreak has necessitated the development of specific, responsive, accurate, rapid, low-cost, and simple-to-use diagnostic tools at point of care. In recent years, early detection has been a challenge for several health diseases that require prompt attention and treatment. Disease identification at an early stage, increased imaging of inner health issues, and ease of diagnostic processes have all been established using a new discipline of laboratory medicine called nanodiagnostics, even before symptoms have appeared. Nanodiagnostics refers to the application of nanoparticles (material with size equal to or less than 100 nm) for medical diagnostic purposes. The special property of nanomaterials compared to their macroscopic counterparts is a lesser signal loss and an enhanced electromagnetic field. Nanosize of the detection material also enhances its sensitivity and increases the signal to noise ratio. Microchips, nanorobots, biosensors, nanoidentification of single-celled structures, and microelectromechanical systems are some of the most modern nanodiagnostics technologies now in development. Here, we have highlighted the important roles of nanotechnology in healthcare sector, with a detailed focus on the management of the COVID-19 pandemic. We outline the different types of nanotechnology-based diagnostic devices for SARS-CoV-2 and the possible applications of nanomaterials in COVID-19 treatment. We also discuss the utility of nanomaterials in formulating preventive strategies against SARS-CoV-2 including their use in manufacture of protective equipment, formulation of vaccines, and strategies for directly hindering viral infection. We further discuss the factors hindering the large-scale accessibility of nanotechnology-based healthcare applications and suggestions for overcoming them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is already influencing disease identification, care, and prevention, allowing for initial disease detection and diagnosis and accurate and successful treatment [1]. Due to their nanoscale sizes, nanoparticles have been easily incorporated in biological units such as DNA, RNA, and ribosomes in living cells and have thereby aided in screening, detection of any defect and infection. Diagnosis of infectious diseases depends largely on laboratory methods since the clinical diagnosis of infectious diseases especially viral infections is difficult. The specialty of nanodiagnosis is that it can be carried out on a nanoscale, leading to a trend of the use of handheld devices which are portable, user friendly, and easy to market. Most important aspect is that they are simple designs, inexpensive, rapid, accurate, and can be used in point of care testing. Some of the implications of nanotechnology include surgical techniques, cancer detection and therapy, implant technologies, molecular imaging, tissue engineering, drug, protein, gene, and radionuclide delivery systems [2]. These materials are better and crucial in many fields of human interest due to their unique size-dependent properties. Several nanoparticles have shown promise in detecting illness symptoms, pre-cancerous cells, viral fragments, particular proteins, antibodies, and other disease indicators, addressing the potential use of nanotechnology in medical diagnosis. Many advancements have already emerged and will have a significant impact on diagnostics in the future years [3]. Nanotechnology and biology have the potential to tackle a wide range of biomedical problems and revolutionize the healthcare industry [4]. Imaging technology, for example, is using nanotechnology to investigate the deepest corners of medical science targeted medication delivery [5], use of sensors, and gene delivery mechanisms [6, 7], and artificial implantation method [8, 9]. Nanoparticles consisting of polymers, metals, or ceramics are the new era of medications, and they can fight cancer and dangerous germs in people [10, 11]. One example of novel therapeutic techniques is the development of targeted transport vehicles that enable drug delivery to specific cells or cellular structures. The use of bioengineered nanoparticles to deliver diagnostic or therapeutic agents is of keen interest. The various areas of usage of nanotechnology in medicine are represented in Fig. 1.
The various arenas of usage of nanotechnology in medicine. Applications and goals of nanomedicine in different sphere of biomedical research are offering numerous exciting possibilities in healthcare. Three important aspects of nanomedicine, diagnostic approach, therapeutic approach, and vaccine development
During the COVID-19 pandemic, there has been a tremendous application of nanotechnology beginning from the fabrication of personnel-protective equipment and devices/kits aiding COVID-19 diagnosis to the formulation of treatment/prevention strategies. Nanofibers have been used for improving the efficiency of filtering masks [12]. In addition, nanotechnology has also been used in making protective gloves [13] and metallic nanoparticle-based disinfectants [14]. The COVID-19 diagnosis has also been aided using gold nanoparticles, magnetic nanoparticles, and carbon-based nanomaterials [15]. The earliest vaccines that received approval for use in the USA developed by Pfizer and Moderna were lipid nanoparticle-based mRNA vaccines [16]. In this article, we have discussed in-detail the various applications of nanotechnology in SARS-CoV-2 diagnosis, prevention, and treatment. We further discuss the possible hurdles for large-scale application of nanotechnology in healthcare and suggestions for overcoming them.
Advancements and challenges in SARS-CoV-2 detection
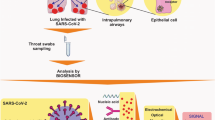
Since 2020, specific detection and analysis of SARS-CoV-2 have been a critical part of the fight against the pandemic. It was a challenge for all biologists globally to track the right number of infected individuals taking into consideration the different variants and mutations that are occurring in the virus genome. Molecular and serological markers were taken into consideration for PCR as well as antigen-based rapid tests respectively. Reverse transcription polymerase chain reaction (RT-PCR)-based diagnostic tests (which detect viral nucleic acids) are considered the gold standard for detecting current SARS-CoV-2 infection. More recently, Nucleic Acid Amplification Tests (NAATs) have included isothermal amplification platforms, e.g., nicking endonuclease amplification reaction (NEAR), loop-mediated isothermal amplification (LAMP), and transcription-mediated amplification (TMA). Laboratory-based NAATs generally have higher sensitivity than point-of-care tests [17]. The window period of NAATs is 7 days while serological tests need 21 days as they are dependent on the generation of an antibody response. Since the start of SARS-CoV-2 detection procedures, more than 80 serological factors are in consideration by FDA for Emergency Use Authorizations (EUA) only [18]. Antigen tests can yield false positive results due to incomplete adherence to the instructions for antigen test performance. Serologic assays may detect IgM, IgG, or IgA antibodies, or certain combinations of these antibodies. Serologic assays that detect IgG and total antibodies have a higher specificity to detect past infection than assays that detect IgM and/or IgA antibodies or a combination of IgM and IgG antibodies. Cross-reactivity among different viral disease antibodies may also lead to false positive response [19].
The probability that the PCR test detects infection peaked at 77% (54–88%) 4 days after infection, decreasing to 50% (38–65%) by 10 days after the infection. While testing every other day would detect 57% (33–76%) of symptomatic cases prior to onset and 94% (75–99%) of asymptomatic cases within 7 days if test results were returned within a day [20].
The challenges associated with rapid antibody testing and RT-PCR-based testing to detect SARS-CoV-2 infections are summarized in Table 1.
Nanoparticles
Nanoparticles are a recent breakthrough that can transform many medicines, biological systems, cancer diagnosis, and treatment. Its applications in medicine have exploded in recent years, aimed towards preventing and treating human diseases [27]. Nanomedicine (health applications of nanotechnology) can provide promising solutions to many diseases, raising high hopes for many patients for safer, more effective, and accessible healthcare. Moreover, it is a subset of nanotechnology that involves highly targeted medical intermediation at the molecular level to treat disease or restore damaged tissues like bone [28], nerve, muscle, and coronary artery disease [29, 30]. From diagnosing diseases to discovering new drugs, nanoparticle-based technologies have shown to have a lot of potential in the medical sector [8].
Nanoparticle-based diagnostic methods have been used in various areas of the medical field. A combination of plasma, nano, and digital technologies was used in the form of a PillCam which has aided in diagnosis of the different gastrointestinal diseases [31,32,33]. Nanodiagnostics has also been used to detect the biomarkers for cardiovascular diseases such as C-reactive protein [34, 35]. Another study has depicted the utility of surface enhanced Raman scattering in the assessment of hypertension-related blood autoantibodies [36] and microalbuminuria in urine [36] for the detection vascular or endothelial dysfunction [37]. Paramagnetic iron oxide nanoparticles have facilitated the detection of lymph node cancer while polymeric nanoparticles have been used in detection of prostate cancer [38,39,40]. Nanotechnology has also aided the detection of brain disorders such as Alzheimer’s disease as in a recent study in which fluorescent cyclic peptide nanoparticles were used to detect amyloid-beta aggregates in cerebrospinal fluid and serum [41].
Established nanoparticle-based technologies must overcome several challenges before they can be used in clinical settings in addition to nanoparticle delivery in a selective manner, possible cytotoxicity, nanoparticle imaging, and the efficacy of real-time therapeutic evaluation. Since nanomaterials are similar in size to most biological molecules, they can be employed in vivo and in vitro for biological research and applications [4].
Despite nanotechnology being a relatively emerging field, there are various nanomaterial-based devices that are currently being developed a few of which are summarized below:
-
1. Nanobots: Nanobots, also called as nanorobots, are made up of nanosize material which can perform function of sensing and controlled operations from a distance, which are generally regulated by a program. These devices are micro/nanosized machines that can convert various external stimuli such as light field, magnetic field, and sound field into motion [42]. Recently, a plasmonic-magnetic nanorobot-based detection assay for SARS-CoV-2 was developed with the basic block of Fe3O4/Au/Ag nanoparticles by sequential chemical reduction method. The DNA probes used for detection in these nanorobots were coated on the Ag surface and the process of hybridization of viral RNA with the probes resulted in the release of the complex. The quantification of the remnant probe based on the electrochemical properties of the nanorobots was then used to estimate the target viral RNA [43].
-
The main advantage of nanobots is that continuous monitoring of body functions can be performed with minimal invasive procedure [44]. Nanobots have been widely used in diagnostic applications as well as targeted delivery of drug at the tumor site [45,46,47,48]. Based on their ability to monitor body functions with minimal invasion, nanobots can be efficiently utilized for in vivo sensing of COVID-19 viral infection or targeted drug delivery.
-
2. Microchips: In structure, microchips have a simple design in which a fluidics system allows the analyte sample to flow to the sensor system thereby generating a detectable signal. The advantage of microchips is that they are very sensitive to detect the samples in nanoliter range. These lab-on chips are very sensitive and have been utilized for detecting the very small number of analytes in the biological fluids [49]. Two recent studies have devised microchip-based detection systems for SARS-CoV-2. The first study used chitosan-glutaraldehyde cross-linked SARS-CoV-2 N protein on a paper-based microfluidic chip with a sandwich ELISA-based detection method [50]. A second kit used a microchip-based RT-PCR technique for SARS-CoV-2 detection. The microchip was loaded with primers targeting the N gene of the virus and reduced the necessity of a larger sample volume required by conventional RT-PCR assays while being able to detect as low as 1 copy per sample [51].
-
3. Biosensor: Biosensors achieve the detection of a specific analyte using a biological molecule, for example, a protein, an enzyme, antibodies, or nucleic acid [52]. Depending on the signal that is used to assessment of the target, the biosensors can be classified as optical biosensors, electrochemical biosensors, piezoelectric biosensors, and thermal biosensors [53]. Several different biosensors have been recently studied for SARS-CoV-2 detection [52] and are discussed in the proceeding section.
-
4. Microelectromechanical systems (MEMS): This is a unique type of diagnostic system which relies on light emitting diode and metal oxide semiconductor camera encapsulated for non-invasiveness [54]. Microelectromechanical systems are currently being studied for their use in COVID-19 diagnostics. Muhsin et al. have developed a microelectro-mechanical system-based impedance biosensor for precise detection of SARS-CoV-2 [55].
-
5. Nanoidentifiers of single-celled structures: Nanoparticles combined with specific receptors can be used to identify and block the activity of SARS-CoV-2 virus. For example, a study on ACE2 nanodecoys derived from human lung spheroid cells has shown that these particles could identify and neutralize SARS-CoV-2 and protect host lung cells from infection. Inhalation of the nanodecoys by mice also resulted in effective clearance of SARS-CoV-2 mimics [56].
The above nanoparticle-based diagnosis approaches are important new pursuits in addition to other recent advances in COVID-19 treatment based on in silico analysis of potential candidates against SARS-CoV-2 and development of vaccine strategies [57].
Diagnostic devices, contrast agents, analytical procedures, physical therapy applications, and drug delivery vehicles have all profited from the merging of nanomaterials with biology. To generate a quantifiable signal, size-dependent features of nanoparticles, mainly optical and magnetic parameters, can be modified. When a nanoparticle label or probe binds to a target biomolecule, a detectable signal is generated that is specific to that biomolecule [58]. Furthermore, nanoparticles are being used as one-of-a-kind intravascular or cellular probes for diagnostic and therapeutic applications, such as medication or gene delivery, which could lead to new discoveries and play an essential role in medicine [59]. There are various biosensors used to execute the applications.
Biosensors
A biosensor is three-part analytical instrument that includes a bioreceptor, a transducer for detection, and a signal processing unit [60,61,62]. “Self-contained devices capable of giving quantitative or semi-quantitative information through a combination of bio-receptor and transducer that are directly linked with one another and transform detected biological events into a measured signal,” according to the International Union of Pure and Applied Chemistry (IUPAC).
Nanobiosensors are useful in medical diagnosis because nanomaterials are chemically and biologically sensitive and can identify biomolecules, cells, or some areas of the body. Using florescence properties of quantum dots of some metals such as cadmium selenide and zinc sulfide, tumors within the body could be located by finding the fluoresced nanodot that was earlier injected in patient’s body. Enzymes, receptors, entire microbial cells, parts of nucleic acid, parts of antibodies, plant/animal tissues, or polysaccharides may all be used as bioreceptors and serve as recognition elements in biosensors. The transducer can detect the various physio-chemical changes such as current, electric potential, density, temperature, viscosity, conductance, and impedance. Biosensors are categorized into two groups: the first one is affinity biosensors are those in which the bioreceptor forms a complex with the analyte, and another one is catalytic biosensors are those in which the bioreceptor interacts with the analyte [63]. DNA or geno-sensors, immunosensors, and receptor sensors are the three types of affinity biosensors mentioned. As bioreceptors, DNA or geno-sensors use natural or synthetic nucleic acids [64]. Peptide nucleic acids, DNA and RNA aptamers, synthetic nucleic acids, and denatured single-stranded DNA (ssDNA) as bioreceptors have all been identified as DNA sensors [65, 66]. Other immunosensors are rapid detection systems that use immobilized antigens or antibodies as bioreceptors. Catalytic biosensors, including enzyme-based biosensors, use enzymes as bioreceptors that detect substrates and read the resulting formation of the product. To date, various enzyme-based catalytic biosensors have been reported [67].
Types of biosensors
The transducers in biosensors are used to classify them into various categories. A transducer is a component/device of a comprehensive system that changes a signal from chemical, physical, or biological form to an electrical signal with great sensitivity [67]. A variety of transducer systems have been developed, and more are in the works. Optical, piezoelectric, calorimetric, and electrochemical biosensors are among the most prevalent transducer systems used in biosensors. Metal nanoparticles, quantum dots, carbon nanotubes, nanowires, dendrimers, nanobots, and lab on chips are among examples of commonly used and potentially useful diagnostic nanostructures [58] and the most popular nanostructured materials used in biosensors [68], and the various probes are listed in Table 2.
Nanotechnology in SARS-CoV-2 detection
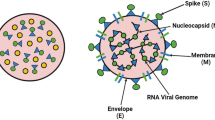
The diagnostic tools for COVID-19 can be classified into three different categories depending on the analytes being measured: (1) SARS-CoV-2 RNA detection; (2) antigen/antibodies; (3) whole virus-based detection methods [80]. Of the three categories, detection of SARS-CoV-2 RNA, antigens, or the whole virus can aid in early detection of the virus since the generation of antibodies against the SARS-CoV-2 occurs at least after a week post-viral infection. The advantages of using nanomaterial-based viral biosensors are higher specificity, reduced size, faster generation of results, and ease of performing the tests anywhere [81, 82]. Discussed below are the various nanomaterial-based diagnostic tools which have recently been introduced for detection of SARS-CoV-2.
Detection of SARS-CoV-2 RNA.
Nucleic acid-based biosensors are prominently used as in a recent study; the plasmonic photothermal (PPT) effect and localized surface plasmon resonance (LSPR) sensing transduction were coupled in a dual-functional plasmonic biosensor for the detection of nucleic acid from SARS-CoV-2. This COVID-19 detection without any sophisticated instruments comprises of colorimetric detection, which uses thiol-modified antisense oligonucleotides (ASOs)-coated with gold NPs. It was observed that thiol-modified ASOs aggregated in presence of SARS-CoV-2 target RNA sequence. There was also a distinct change in surface plasmon resonance [83].
Another group used reverse transcription loop-mediated isothermal amplification (RT-LAMP) in combination with nanoparticle-based biosensors to diagnose COVID-19 [84]. Detection of SARS-CoV-2 envelope (E) and RNA-dependent RNA polymerase (RdRP) genes has also been shown by electrochemical measurements from signals detected by a screen-printed carbon electrode (SPCE) decorated with Au nanostars (AuNSs) and coupled to a battery operated thin film heater based on the LAMP technique [85].
SARS-CoV-2 viral RNA was also recently extracted using magnetic nanoparticles for RT-PCR-based detection [86]. A study has devised a rapid electrochemical biosensor in which MXene nanosheets (Ti3C2Tx) and carbon platinum (Pt/C) were used for signal amplification to detect SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) gene region [87]. Probes targeting N and ORF1a have been designed with Au NPs@Ta2C-M-modified gold-coated tilted fiber Bragg grating (TFBG) sensors to achieve high signal transduction efficiency and high sensitivity [88].
Antibody/antigen-based diagnostic tests.
Antibody-based biosensors, such as biosensing devices based on field-effect transistors (FETs), are also particularly effective at detecting SARS-CoV-2 in clinical samples. Gold NPs can also be used for rapid SARS-CoV-2 detection. An assay integrating Au-nanoparticles on a nitrocellulose strip can identify biomarkers like IgG and IgM in COVID-19 patients. When COVID-19 biomarkers interact with antibodies present on the strip, color change is observed. Detection using gold NPs via rapid kit is sensitive, reliable, quick, and specific [89]. Additionally, a particular antibody against the SARS-CoV-2 spike protein was coated onto FET graphene sheets to form the sensor. The sensor performance was evaluated using antigen protein, cultured virus, and nasopharyngeal swab samples from COVID-19 patients, which does not require sample preparation [90]. A fluorescent immunochromatographic assay has been developed with a unique two-dimensional Ti3C2-QD immunoprobe generated by the adsorption of dense quantum dots (QDs) on a light green monostromatic Ti3C2 MXene surface. The assay results in a light green colorimetric and superior fluorescence signal and has been shown to have good sensitivity and stability [91].
A new LFIA platform to detect SARS-CoV-2 spike-S1 protein using mouse monoclonal antibodies costumed with quantum dot (QD)-loaded dendritic mesoporous silica nanoparticles that were modified further for -COOH group surface coating (Q/S-COOH nanospheres) has been created by a recent study and it was found to higher sensitivity in comparison to traditional AuNP LFIA- and ELISA-based detection methods [92].
Detection of SARS-CoV-2 nucleocapsid protein was also recently achieved using an electrochemical immunosensor with a novel polyaniline functionalized NiFeP nanosheet array and Au/Cu2O nanocubes as a signal amplifier. The coating of polyaniline on NiFeP was attained by electropolymerization and this helped the antibody loading process. This electrochemical sensor was found to have a wide detection range and efficient analytical performance [93].
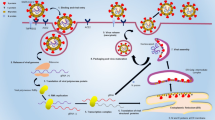
Along with graphene, gold nanourchins have also been used as sensors to detect the shape specificity of virus internalization [94]. CNTs have been used for detecting viruses including SARS-CoV-1 and SARS-CoV-2 in respiratory tract. CNT size-tunable enrichment microdevice (CNT-STEM) increases virus detection sensitivity and isolation rate. It was helpful in detecting avian influenza virus strain; hence, it can be modified for SARS-CoV-2 RNA or proteins [95]. In in vitro diagnostic kits, CNT was associated with electrochemical reaction based on reactive oxygen species/H2O2 system [89]. Aspects relating nanotechnology to SARS-CoV-2 are shown in Fig. 2.
Approaches based on nanotechnology to counter COVID-19. Various nanoparticles can be used for the detection, prevention, treatment, and vaccination processes against SARS-CoV-2 infection. Prevention: NPs can be used in the making of face masks, face shields, hand gloves, gowns, shoe covers, and head covers to deactivate viruses on the very first contact. Detection: NPs can be used for detecting the SARS-CoV-2 infection. For example, spike-protein detection method, nucleic acid-based PPT effect detection technique, RT-LAMP method, gold NP–coated strips which changes color in contact with infected sample. Treatment: NPs can assist direct and safe drug delivery to specific target like alveolar cells. Vaccination: NP-mediated biological molecules can act as vaccine against SARS-CoV-2 infection. NPs fit the purpose because of their scalability, safety, and long-lasting effects
Fast diagnosis and inhibition of coronavirus using spike antibody attachment
In HEK293T cells that express angiotensin-converting enzyme 2 (ACE2), an enzyme by which SARS-CoV-2 penetrates human cells, anti-spike antibody-attached gold nanoparticles reduce SARS-CoV-2 infection. Antibody-coated gold nanoparticles bind to the SARS-CoV-2 spike protein, preventing the virus from attaching to cell receptors and so stopping virus infection and propagation [96]. Chloroquine prevents cells from absorbing nanoparticles, and high dosages of the drug reduce the aggregation of synthetic nanoparticles in cell lines and the mononuclear phagocyte system of mice. Because synthetic nanoparticles and SARS-CoV-2 are the same size and shape, nanoparticles can aid in drug research for COVID-19 prevention and therapy. The researcher designed NP-based peptides that mimic the virus-binding region of the ACE protein, which aids viral entrance, resulting in a customized NP mimetic (antagonist) that prevents the virus from infecting cells. According to this, inhalers containing the mimic would be effective in preventing virus activation in the lungs [97]. Scientists are working on nanoparticles to help in inflammation management. Loaded lipid-based nanoparticles with immunomodulating and antioxidant molecules (adenosine and α-tocopherol, respectively) can preferentially deliver their therapeutic cargo to the areas of acute inflammation, modifying oxidative stress and cytokine response in the process. Clinical trials related to usage of nanotechnology in COVID-19 diagnostic and medical interventions as per clinical trial data [98] shown in Table 3 and as per WHO report in Table 4 [99].
Nanodiagnostic tools that have gained popularity are the ones which are based on optical biosensors. These diagnostic tools are rapid and have very high sensitivity [100]. However, Au nanoparticle–based lateral flow-immunoassay (LFIA) has a limitation that the signal produced is majorly qualitative in nature but is not able to generate the quantitative estimation. For quantitative estimation, electrochemical biosensors have been widely developed, but these are limited by development of specialized equipment for data collection and analysis. Label-based biosensors enhance sensitivity and selectivity as compared to SPR-based biosensors [101]. Nanodiagnostic tools have emerging potential, but it needs better understanding of the properties of the matter to be successfully used as a comprehensive diagnostic tool.
Nanomaterials are being used to treat SARS-CoV-2 symptoms
Pneumonia is the most common symptom of COVID-19 infection, and nanotechnology could be utilized to treat it [62]. From using nanomaterials to deliver anti-inflammatory drugs to developing inhalation methods, fabricating platelet-derived nanomaterials that are actively targeted to inflammatory sites and allowing for controlled drug release, for using oxygen-generation nanomaterials, such methodologies may not only provide timely solutions but also stimulate future research. To manage the cytokine storm and detect the location of pneumonia, platelet-derived nanoparticles can be encapsulated with [5-(p-fluorophenyl)-2-ureido] thiophene-3-carboxamide (TPCA-1) [102]. Furthermore, antioxidant nanomaterials, such as cerium dioxide nanoparticles, can also be employed to eliminate reactive oxygen species (ROS) from the inflammatory site [103]. Other technologies could be used with nanotechnology to make COVID-19 treatments easier and more effective. Artificial intelligence, for example, has been investigated for COVID-19 diagnosis [104] and the validation of drugs that could be retooled to treat COVID-19 [105] should also be considered to aid in the isolation of drug/vaccine-loaded nanoparticles, therapeutic nanomaterials, and nanomaterials that could alleviate pneumonia. We hope and believe that as our knowledge of SARS-CoV-2 and related research grows, nanotechnology will enable faster and more effective methods for dealing with SARS-CoV-2 and other emerging viruses in the future.
Nanotechnology in SARS-CoV-2 prevention
Blocking viral entry into the host
A FET biosensor for SARS-CoV-2 was developed; this device was a COVID-19 immunological diagnostic approach that did not require any sample preparation or labeling. Many HSPG mimicking materials, like zinc oxide nanoparticles, have been highlighted for their antiviral efficacy, and studying their anti-SARS-CoV-2 potential could give an early therapeutic treatment for COVID-19. Balagna et al. coated face masks with a silver nanocluster/silica combination to prevent contamination. When tested for SARS-CoV-2, this coating successfully lowered virus titers on the mask. To protect against SARS-CoV-2 viral contamination, such coatings can be employed on regularly exposed surfaces in public settings [106].
Virus inhibition without external stimulus
Graphene and graphene-related materials (GRMs) are suitable candidates for designing and creating high-performance components and systems for the COVID-19 pandemic and other future disasters due to their unique physicochemical, electrical, optical, antiviral, antibacterial, and other characteristics. Graphene and GRMs’ potential were examined in healthcare applications, as well as how they can aid in the fight against cancer. COVID-19 is a viral disease that can be treated with the same strategy [107].
Delivery of drugs and vaccines
The phase 3 clinical studies for the mRNA-1273 SARS-CoV-2 vaccine are presently underway to investigate its safety and efficacy in preventing COVID-19 for up to 2 years following the second dose. Another study increased SARS-CoV-2 antigen production by modifying the endogenous untranslated regions (UTRs) of mRNAs. Furthermore, when it came to delivering mRNA vaccines, TT3 nanoparticles outperformed FDA-approved lipid nanoparticles [108]. Aside from vaccines, nanotechnology has the potential to build vaccine adjuvants that boost antibody production. Since traditional aluminum hydroxide (alum) adjuvants, which seem to be plate-like microgels with a positive charge, are more likely to link to the membrane than being internalized by dendritic cells, an oil/water interphase of particulate alum via Pickering emulsion (instead of just a surfactant-stabilized emulsion) was generated that not only absorbed but also promoted antigen uptake [109].
Antiviral nanomaterials
In addition to functioning as a delivery platform for antiviral medications or vaccines, nanomaterials can directly combat viruses. To begin, a thorough understanding of the virus reproductive cycle is required to develop an effective antiviral strategy. While more research into viral life cycles may yield new insights, the current model comprises attachment, entry, biosynthesis, virus assembly, and release, with viral suppression achievable at each step. The immense promise of nanomaterials in this field has only recently begun to be realized. DNA origami technology has added to the original nanomaterial library, despite its early stages of application in antiviral research.
DNA origami can provide a frame to transform nanoparticles into desirable morphological materials [110]. Dengue virus activity can be suppressed by using spatial pattern interaction to design a DNA nanoarchitecture with a specific star configuration [111]. MUS acid-modified cyclodextrins have also been investigated for their antiviral capabilities [112], as the modified MUS acid can imitate HSPG to cause a virucidal response. Bacteriophage capsids with ligands that strongly bind the influenza virus have recently been introduced as part of a multivalent strategy to limit the virus’ invasion [113]. These “nanodecoys” trap the Zika virus and prevent it from infecting its intended targets. Nanosponges made from human macrophage cell membranes or plasma membranes made from human lung epithelial type II cells have recently been employed to mimic the host cell surface and trap SARS-CoV-2 for neutralization [64]. The spread of SARS-CoV-2 viral particle was not well understood by aerosol transmission. A systematic study has simulated the aerosols produced by the human patients [114]. In this study, the authors developed a vibrating mesh nebulizer which has replicated the production of aerosol containing nanoparticle (CorNPs). The study highlighted the drying impact on aerosol and evaluated the impact of environmental pollution and other factors in spread of infection.
We have high aspirations for the future of nanotechnology in antiviral research, as evidenced by the examples above.
Application of nanotechnology in viral infections
SARS, dengue fever, Asian flu, and other bacterial and viral infectious agents spread rapidly and cause high morbidity and mortality disease outbreaks [115]. Nanotheranostics is a revolutionary approach to diseases including cancer and neurological disorders in which a single multifunctional nanoplatform combines therapeutic and diagnostic functions [116]. Theranostic nanoparticles (NPs) have lately acquired popularity for aiding the targeted delivery of active therapeutic compounds such as drugs and vaccines. They also allow non-invasive imaging approaches to image and track both the infection and treatment mechanisms [117]. Three factors must be considered while developing a prospective theranostics-based nanoplatform: nanocarriers, the therapeutic drug, and imaging agent [118]. Multifunctional NPs are regarded as promising theranostic agents due to their physicochemical properties such as size, charge, solubility, ease of synthesis, targeted drug delivery, biocompatibility, biodistribution biodegradability, and enhanced retention inside tissues of interest [119]. Biosynthesized NPs containing therapeutic compounds and imaging agents were shown to have theranostics properties [120]. Due to their remarkable qualities, gold NPs and magnetic NPs are the most often utilized nanotheranostics agents. MNPs can be utilized as contrast agents or drug carriers in magnetic resonance imaging [121]. Plaque tests, -galactosidase assays, confocal imaging assays, transmission electron microscopy (TEM), Western blot assays, flow cytometry, and RT-PCR are now used to assess the antiviral action of NPs [122]. However, due to their high cost, lack of precision and sensitivity, time consumption, and the necessity for well-equipped laboratory facilities and highly experienced technicians, most of these procedures are inappropriate for clinical usage or point-of-care diagnostics [123]. This analysis examines various CoV therapeutic methods, emphasizing the use of nanomedicine to contain COVID-19 and other pathogenic CoVs shown in Fig. 3.
Nanomedicine, with its physicochemical properties, may be a promising therapeutic strategy to defeat CoVs and their host cells. SARS-CoV-2 and any re-emerging CoV could be treated with nanoparticles (NPs) studded with viral antigens or antibodies. This analysis examines various CoV therapeutic methods, with an emphasis on the use of nanomedicine to contain COVID-19 and other pathogenic CoVs. Acronyms: VLPs, virus-like particles
Development of a novel vaccine strategy
A glycoprotein-based vaccine production strategy has also been considered, during which the Spike S protein is glycosylated by the virus. The glycosylation of viral spikes hides the immunogenic areas identified by the host immune system. As a result, thereby enabling the invasion of the virus in the immune system of the host. This method is used by many viruses. However, the SARS-glycan CoV-2 profile revealed that the glycosyl density on the spikes is insufficient to successfully protect the virus from host immune systems. This weakness in the virus’ glycan coating could be useful in the generation of powerful neutralizing antibodies, and hence in vaccine development [124]. Both humoral and cell-mediated immunity performs a protective role in the SARS-CoV-2 infection. Nanoparticles have shown their ability to target.
Immunomodulation is another way for developing antiviral vaccine. Physical and chemical properties of nanomaterials can prompt immune response in case of SARS-CoV-2 infection. Nanoproducts based on their physicochemical properties can be developed combining nanoenabled product with desired-immunity design concept. Nanoproducts can act as immuno stimulators, immunosuppressors, and vaccines. Upon pathogen interaction, nanoparticles generate both humoral and cell-mediated immune response. Nanoparticles activate macrophages and dendritic cells along with B and T cells. Nanoparticles stimulate apoptosis in APC cells, which causes immunosuppression. Nanoparticles can also adsorb cytokines in response to cytokine-storm in COVID-19 patients. A virus-like or lipid nanoparticle can be used for vaccination [59]. Uptake of naked mRNA will be subjected to lysosomal digestion; therefore, mRNA-loaded lipid nanoparticles can be used. Nucleic acid-based nanovaccines come with its own perks like can be easily altered for its size and shape, safety, and prolonged-expression of antigens. These lipid NPs are internalized in host cells and undergo endosome escape and mRNA is released for protein formation and desired immune response is achieved [125]. Both Moderna and Pfizer-BioNTech mRNA vaccines are based on lipid nanoparticle [126]. In the emergence of SARS-CoV-2 variant, new nanotechnology-driven strategies are developed [127]. Examples of such nanotechnology-based molecules are listed in Table 5.
Challenges and prospects of nanotechnology
In this article, we have summarized the applications of nanotechnology in healthcare, especially with respect to its role in diagnosis and protection against SARS-CoV-2 infections. There are, however, several factors that hinder the translation of nanotechnological research to the industry. The cost-effectiveness of nanotechnology-based healthcare applications is an important factor that prevents its widespread adoption. The process of development of nanomaterial-based applications needs thorough analysis of the physical and chemical properties, toxicological properties, and pharmacokinetic properties of the nanomaterials being used [144, 145].
Assessment of these factors needs specialized machinery and devices and further manpower with specialized training for their operation. The process of manufacturing the nanomaterial also slows the pace at which it reaches the market, due to the structural and physio-chemical complexity of the nanomaterials. The inherent high cost of nanomaterials and that incurred during the process of making the nanomedical devices contributes to the roadblock [144, 145].
The high expense of nanotechnology-based healthcare applications eventually gets transferred to the patients, making its access to low-income groups much more difficult, especially in systems where healthcare is governed by insurance-based health systems [146, 147]. Large-scale manufacture of nanomaterial is also associated with issues related to the constituting particle’s toxicological features, in vivo biodegradability, and the difficulty in achieving balance between different constituents at a large scale [148].
The regulatory factors governing nanomaterials such as government policies, safety and quality control, and patent protection also affect the commercialization process of nanomedical research. The absence of defined guidelines for the regulation and safety of the development of nanomaterial-based healthcare products further increases the transition time to the industry [145, 149,150,151].
In addition to the inequality in access to nanomedical technology due to economic factors, inequity in access also exists due to geographical barriers. Developed nations such as the Republic of Korea, Canada, and Germany are at the forefront in nanomedical research as per the UNESCO Science Report [152]. This has led to the accumulation of the knowledge and know-how related to the nanotechnology-based healthcare applications in these nations, a lot of which is due the exclusivity granted by their high number of patents applications [147]. This limits the access of a lot of the life-saving nanotechnology applications to the developed world as there is again a huge cost of purchasing such products which is a barrier in the developing world. For example, mRNA-based COVID-19 nanovaccines BNT162b1 and mRNA-1273 were produced by Pfizer-BioNTech and Moderna respectively and were globally the first ones to receive approval for administration [16]. However, due to the high cost of these nanovaccines and additional cost of their low temperature storage requirement, economically backward countries could purchase, and much later, the low-cost adenovirus vector bases vaccine ChAdOx1 nCoV-19 (AZD1222, Oxford-AstraZeneca) [153, 154]. In addition, people in developing countries, due to the associated economic constraints, have a reduced access to nanochemical drugs and nanomedical devices [155, 156].
To overcome the inequality in access to nanotechnology in healthcare applications, efforts need to be taken globally to promote better exchange of ideas and technology and promote a way to make the nanomedical technology available at a lower cost to the patients in developing countries. A framework by the European nations called as the Responsible Research and Innovation (RRI) framework has been suggested as possible strategy to overcome the barrier in equal access to nanomedical technology. This framework fosters collaborative efforts between various stakeholders developing novel technologies throughout the process. While this framework is restricted geographically, it has been suggested that its principles can be used for a broader global application [157]. It has also been suggested that incorporating a priority towards the large-scale benefit to humanity in the regulatory policies can also help to reduce the inequality in access to healthcare applications of nanotechnology [147].
The initiative of COVID-19 Vaccines Global Access (COVAX) which was developed by Coalition for Epidemic Preparedness Innovations (CEPI), Global Alliance for Vaccines and Immunizations (GAVI), and WHO to allow for an equal distribution of COVID-19 vaccines globally is a good example of the collaborative strategies that can overcome the hurdle of unequal access to healthcare-related nanotechnology applications worldwide [158]. Another good example is of the group which developed SARS-CoV-2 protective garments containing copper oxide nanoparticles and graphene nanosheets for antiviral activity without patenting the technology. This allowed for free and easy sharing of production technology and has been a great step in breaking barrier in technology access [153, 159].
To increase demand for nano-based formulations, the FDA should concentrate on three issues: (a) developing methods for characterization and quantification of nanomaterials’ toxicological effects, (b) dealing with consumer acceptance and understanding through a public awareness campaign, and (c) product labeling [160]. Furthermore, the global marketing of nano-based formulations is restricted [112]. Future study should focus on (i) establishing the mechanism of action of nano-based formulations, (ii) comprehending the behavior and interaction of nanomaterials with the human body, (iii) assessing the possible toxicity and residual direct and indirect effects of nanomaterials, (iv) creating new nanomaterials, and (v) developing universal regulations [161]. Finally, it is an alarming time to understand and appreciate nanotechnology’s enormous potential for the production of new and more effective tools and products. It is vital that continued research incorporates academics, corporations, research institutions, and government organizations to turn this technology into a commercial reality and offer alternatives for the identification and control of various diseases.
Conclusion
Nanotechnology in life sciences has become a revolution by providing many useful tools that can be applied for the detection of biomolecules and analytes especially for early disease diagnosis. Early diagnosis has been the goal for prompt arrest and management of various health conditions especially so in the diagnosis of infectious diseases. Antibody detection is not very useful in the concurrent diagnosis of infections except those infections with prolonged incubation period and chronic infection. Moreover, auto fluorescence and optical absorption of either the matrix of biological samples or reagents is a major limiting factor of ELISA technique. Using the principles of antigen antibody interaction, any combination of pathogen-specific antigen or antibody can be immobilized onto the surface of NPs for the diagnosis of infectious diseases. Use of microchips, biosensors, nanorobots, nanoidentification of single-celled structures, and microelectromechanical systems are current techniques being developed for use in nanodiagnostics. These newer platforms have made diagnostics less cumbersome and more sensitive because of their physical properties. Recent advances of this technology have now aided in integrating them into simple devices which can used even outside the laboratory with safety.
Despite the potential benefits of nanomaterials in COVID-19 diagnostics and treatment as discussed in the review, there are certain associated drawbacks. Due to the nanoscale size of these materials, they have increased reactivity with other molecules, leading to oxidative stress. Nanomaterials are also associated with toxicity in the body, as clearance of these foreign materials is not well understood. Nanoparticles can get accumulated at the site of delivery to cause chronic local or systemic inflammation. One report observed serious lung injury due to nanoparticles which can be deadly during SARS-CoV-2 infection [162]. Additionally, due to lack of knowledge about their interaction within the environment, it is very crucial to diligently study the proper disposal ways of diagnostic tools [154]. Most importantly, including the nanodiagnostics in the patient of care treatment can be very expensive because of its production cost. It will be a financial burden to access the comparable level of diagnostics for everyone. We need to invest extra efforts to reduce the cost of its production to be available to everyone. Another crucial ethical concern for nanodiagnostics is the informed decision during clinical trial to the participant.
Altered physio-chemical properties make engineered nanoparticles commercially attractive but, at the same time, they also raise concerns regarding potential risk to environmental and human health through consumer use, occupational, and environmental exposure. In addition to occupational exposure, direct human exposures through medicinal application and ambient air pollution are major concerns [163]. Ultrafine particles (UFPs) or nanoparticles (NPs) by inhalation exposure translocate through nasal, tracheobronchial, olfactory, and trigeminal nerves and then these nerves release tachykinins and catecholamine which have direct effect on cardiac autonomic function. These nanoparticles also evade phagocytosis which is the defense mechanism of our body resulting in inflammation, pulmonary endothelial injury, upregulation of ICAM-1 and VCAM-1, impairment in arteriolar dilation that causes increase in systemic blood pressure, and an increased risk for heart failure. When they translocate to systemic circulation, they reach various organs like the liver, the heart, the kidney, and the bone marrow and cause a predisposition to clot formation and cardiopulmonary events and may also lead to cancer due to the release of C-reactive protein, fibrinogen, ROS, cytokines, and chemokines [164].
Although the usage of nanoparticles has been proven to be tremendously advantageous for both diagnostic and therapeutic purposes, it is important to discuss about the associated safety and potential toxicity concerns. To measure the potential hazardous risks associated with nanoparticles, physiology-based pharmacokinetic (PBPK) models and nanoquantitative structure–activity relationship (QSAR) models have been proposed. These proposed models help in analyzing in vitro and in vivo generated datasets using nanomedicine and understanding OMICS data in nanotoxicology [165, 166].
Outlook
Extensive research has been conducted on various aspects of SARS-CoV-2 diagnosis, treatment, and prevention. Prevention had been adopted as a first strategy to combat the disease during pandemic. Preventive measures relied on two major factors: (1) reducing the viral spread by contact (social distancing) and (2) maintaining the individual hygiene and health (face covering, handwashing). Early diagnosis has played a key role to stop the spread of infection. Despite RT-PCR being the gold standard for testing the infection, it has its limitations such as expensive instruments and the requirement of trained personnel. Efforts are still being put in diagnosis of SARS-CoV-2 viral infection. The diagnostic techniques can be divided into four categories based on the biomolecule tested: (1) nucleic acid based (RT-PCR, digital PCR, isothermal PCR); (2) serological (ELISA, lateral flow, antigen detection, quantum dots, protein chip); (3) biosensor based (SPR, LSPR, colorimetric); and (4) radiology (X-ray and chest imaging) [167]. These diagnostic techniques are still improving to become mainstream testing options. Artificial intelligence (AI) and machine learning (ML) have also been utilized in diagnosis of COVID-19 [168]. IKONOS, an AI tool developed by Gomes et al. [169], can differentiate which can differentiate COVID-19 from pneumonia by using machine learning algorithm on chest X-ray data [170]. In another study, ultra-low dose of CT scan examination has been performed to predict the data from normal dose of CT scan examination by utilizing deep learning methods [171].
A deep learning ResNet-101 system has also been developed which utilizes methods to identify COVID-19 with 99% accuracy in CT scans [172]. These AI and ML algorithm have also been utilized to predict the efficacy and toxicity of nanomaterials which is crucial before delivering it in clinical trials, and an excellent review has summarized this aspect of nanomedicine [165].
OMICS science and digital transformation has changed the diagnostic medicine in post-COVID-19 era. Digital platforms have been developed to device nanobiosensors that can diagnose presence of nanomaterials in human use products to assess the safety measurements. One such nanobiosensor can detect SARS-CoV-2 in speech nanoparticle-sized aerosol/droplets from infected patients that causes transmission of COVID-19 [173, 174] We therefore need to carefully evaluate various aspect of nanomedicine for future use.
Post the diagnosis of SARS-CoV-2 infection, treatment also played a crucial role in saving the lives of patients. Therapeutic options can be divided into three categories: (1) antiviral drugs (remdesivir, baricitinib, dexamethasone) [175, 176]; (2) antibody-based therapy (convalescent plasma therapy, monoclonal antibody therapy, intravenous immunoglobulins therapy) [177,178,179,180]; and (3) cell therapy for COVID-19 treatment [181,182,183,184]. The pandemic has taught individuals, policymakers, and healthcare workers to be better prepared for any upcoming health disasters. Policy makers and healthcare workers have the key responsibility to develop strategies such as controlling the infection beforehand, making an action plan to control future infections, and developing the facilities where any kind of future pandemic can be controlled.
A survey conducted for nanotechnology usage in the area of medicine showed positive attitude among stakeholders [185]. The US Food and Drug Administration (FDA) formed nanotechnology task force to accelerate collaboration among researchers and stakeholders to expand usage of nanomaterial in public health. A few examples of such collaboration include FDA’s partnership with the National Institute of Standards and Technology (NIST) and the National Cancer Institute (NCI) to form the Nanotechnology Characterization Laboratory (NCL). It also collaborated with Johns Hopkins University and Houston-based Alliance for NanoHealth (ANH) along with its eight member institutions to conduct multi-disciplinary research in medical field [186].
Nanotechnology is often referred to as “future technology” that can solve many problems. It will present new opportunities to make stuff of life better and cheaper, using fewer raw materials. Through wearable fitness technology, we can monitor our health by strapping gadgets to ourselves. By scaling down nanotechnology, this technology opens up the possibility of implanting or injecting tiny sensors inside our bodies. The properties of nanoparticles can be used to improve early diagnosis and treatment of neurodegenerative disease and cancer. There are endless possibilities ranging from monitoring inflammation and post-surgery recovery to more exotic applications whereby electronic devices actually interfere with our body signals for controlling organs. Nanomaterials and concepts are also currently being developed that show potential for producing energy from movement, light, variations in temperature, glucose, and other sources with high conversion efficiency [187].
In this study, we have provided an overview of the recent advances of nanotechnology against the COVID-19 disease. The discussion is based on the three important strategies that have been used for combating COVID-19, i.e., prevention, efficient diagnosis, and treatment. The nanodiagnostic techniques discussed in this article include novel strategies such as nanobots, microchips, and MEMS. Recent work on the various types of biosensors for COVID-19 detection such as RT-LAMP-based biosensors, tilted fiber Bragg grating (TFBG) sensors, quantum dot-based immunochromatographic assays, and electrochemical immunosensors has also been summarized in our study. The properties of nanomaterials being used in biosensor-based diagnostic devices and the associated advantages and disadvantages are important points that have also been covered in this study. We have also outlined the novel nanoparticle-based vaccine formulations that are currently being developed, strategies by which nanomaterials can be used in preventing the viral infections and further discuss the challenges for the widespread application of nanotechnology. While the different applications of nanotechnology in controlling COVID-19 are important advances, an area that needs further research is the study of toxicological properties of the nanomaterials, in vivo availability, and biodegradability. Furthermore, AI and machine learning-based techniques can aid in the vaccine design process, toxicological analysis of nanomaterials, and design of diagnostic devices. A limitation of our study is the lack of in-depth discussion of the synthesis process for the different formulations of nanomaterials.
Nanotechnology has displayed tremendous potential in the control of COVID-19 by aiding the diagnosis, prevention, and treatment processes. The very first mRNA-based vaccine formulations utilized lipid-nanoparticles, which were used to provide enhanced stability and efficient delivery. Such formulation strategies can therefore be used in future for developing immunity against other viral diseases. Furthermore, nanomaterials with antiviral properties play an important role in the manufacture of personnel protective equipment and this application of nanotechnology too has broader future implications. The rapid point-of-care SARS-CoV-2 diagnostic kits have numerous advantages such as ease of use and detection, compactness, high sensitivity, and low cost in comparison to traditional RT-PCR-based techniques. The design strategies of such kits can also be utilized for other virus-based infections, thus extending reach of nanotechnology in combating viral diseases.
References
Buxton DB, et al. Recommendations of the National Heart, Lung, and Blood Institute Nanotechnology Working Group. Circulation. 2003;108(22):2737–42.
Jain KK. Nanodiagnostics: application of nanotechnology in molecular diagnostics. Expert Rev Mol Diagn. 2003;3(2):153–61.
Yezhelyev MV, et al. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 2006;7(8):657–67.
Curtis A, Wilkinson C. Nantotechniques and approaches in biotechnology. Trends Biotechnol. 2001;19(3):97–101.
Langer R. Drugs on Target. Science. 2001;293(5527):58–9.
Roy K, et al. Oral gene delivery with chitosan–DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5(4):387–91.
Gemmati D, et al. Host genetics impact on SARS-CoV-2 vaccine-induced immunoglobulin levels and dynamics: the role of TP53, ABO, APOE, ACE2, HLA-A, and CRP genes. Front Genet. 2022;13:1028081.
Vaseashta A, Dimova-Malinovska D. Nanostructured and nanoscale devices, sensors and detectors. Sci Technol Adv Mater. 2005;6(3):312–8.
Sachlos E, Gotora D, Czernuszka JT. Collagen scaffolds reinforced with biomimetic composite nano-sized carbonate-substituted hydroxyapatite crystals and shaped by rapid prototyping to contain internal microchannels. Tissue Eng. 2006;12(9):2479–87.
Farokhzad OC, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103(16):6315–20.
Panáček A, et al. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B. 2006;110(33):16248–53.
O'Dowd K, et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials (Basel). 2020;13(15):3363.
Aydemir D, Ulusu NN. Correspondence: Angiotensin-converting enzyme 2 coated nanoparticles containing respiratory masks, chewing gums and nasal filters may be used for protection against COVID-19 infection. Travel Med Infect Dis. 2020;37:101697.
Campos EVR, et al. How can nanotechnology help to combat COVID-19? Opportunities and urgent need. Journal of Nanobiotechnology. 2020;18(1):125.
Rasmi Y, et al. Recent progress in nanotechnology for COVID-19 prevention, diagnostics and treatment. Nanomaterials. 2021;11(7):1788.
WHO. In: Interim recommendations for use of the Pfizer–BioNTech COVID-19 vaccine, BNT162b2, under emergency use listing. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-BNT162b2-2021.1. Accessed 2 July 2023.
Centers for Disease Control and Prevention. In: Interim guidelines for collecting and handling of clinical specimens for COVID-19 testing. 2019. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html. Accessed June 22 2023.
FDA. In: Emergency use authorization medical device and serology test. 2019. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance. Accessed June 1 2023.
Guo L, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. 2020;71(15):778–85.
Hellewell J, et al. Estimating the effectiveness of routine asymptomatic PCR testing at different frequencies for the detection of SARS-CoV-2 infections. BMC Med. 2021;19(1):106.
Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15(3):155–66.
Binnicker MJ. Challenges and controversies to testing for COVID-19. J Clin Microbiol. 2020;58(11):e01695-20.
Li Y, et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020;92(7):903–8.
Abootalebi S, et al. Call to Action: SARS-CoV-2 and CerebrovAscular DisordErs (CASCADE). J Stroke Cerebrovasc Dis. 2020;29(9): 104938.
Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20(5):453–4.
Liu G, Rusling JF. COVID-19 antibody tests and their limitations. ACS Sens. 2021;6(3):593–612.
Angeli E, et al. Nanotechnology applications in medicine. Tumori Journal. 2008;94(2):206–15.
Mahony O, Jones JR. Porous bioactive nanostructured scaffolds for bone regeneration: a sol-gel solution. Nanomedicine (Lond). 2008;3(2):233–45.
Campbell GR, et al. The peritoneal cavity as a bioreactor for tissue engineering visceral organs: bladder, uterus and vas deferens. J Tissue Eng Regen Med. 2008;2(1):50–60.
Schoenhagen P, Conyers JL. Nanotechnology and atherosclerosis imaging: emerging diagnostic and therapeutic applications. Recent Pat Cardiovasc Drug Discov. 2008;3(2):98–104.
Hale MF, Sidhu R, McAlindon ME. Capsule endoscopy: current practice and future directions. World J Gastroenterol. 2014;20(24):7752–9.
Liu D, Szili EJ, Ostrikov KK. Plasma medicine: opportunities for nanotechnology in a digital age. Plasma Process Polym. 2020;17(10):2000097.
Deng S, et al. Application of nanotechnology in the early diagnosis and comprehensive treatment of gastrointestinal cancer. J Nanobiotechnology. 2022;20(1):415.
Vilian ATE, et al. Efficient electron-mediated electrochemical biosensor of gold wire for the rapid detection of C-reactive protein: a predictive strategy for heart failure. Biosens Bioelectron. 2019;142:111549.
Vashist SK, Schneider EM, Luong JH. Surface plasmon resonance-based immunoassay for human C-reactive protein. Analyst. 2015;140(13):4445–52.
Li X, et al. Autoantibody profiling on a plasmonic nano-gold chip for the early detection of hypertensive heart disease. Proc Natl Acad Sci U S A. 2017;114(27):7089–94.
Smith BR, Edelman ER. Nanomedicines for cardiovascular disease. Nature Cardiovascular Research. 2023;2(4):351–67.
Jazrawi A, et al. A comparison of skin staining after sentinel lymph node biopsy in women undergoing breast cancer surgery using blue dye and superparamagnetic iron oxide nanoparticle (spio) tracers. Cancers (Basel). 2022;14(23):6017
Martin DT, et al. Targeting prostate cancer with Clostridium perfringens enterotoxin functionalized nanoparticles co-encapsulating imaging cargo enhances magnetic resonance imaging specificity. Nanomedicine. 2022;40: 102477.
Tiwari H, et al. Recent advances in nanomaterials-based targeted drug delivery for preclinical cancer diagnosis and therapeutics. Bioengineering (Basel, Switzerland). 2023;10(7):760.
Sun L, et al. Fluorescent peptide nanoparticles to detect amyloid-beta aggregation in cerebrospinal fluid and serum for Alzheimer’s disease diagnosis and progression monitoring. Chem Eng J. 2021;405: 126733.
Zhang Y, et al. Micro/Nanorobots for medical diagnosis and disease treatment. Micromachines (Basel). 2022;13(5):648.
Kim J, et al. Plasmonic-magnetic nanorobots for SARS-CoV-2 RNA detection through electronic readout. Appl Mater Today. 2022;27: 101402.
Freitas RA. Nanomedicine, Volume I: Basic capabilities. 1st ed. Routledge: Taylor and Francis group. 1999.
Wang W, Zhou C. A journey of nanomotors for targeted cancer therapy: principles, challenges, and a critical review of the state-of-the-art. Adv Healthcare Mater. 2021;10(2):2001236.
Schmidt CK, et al. Engineering microrobots for targeted cancer therapies from a medical perspective. Nat Commun. 2020;11(1):5618.
Soto F, et al. Medical micro/nanorobots in precision medicine. Advanced Science. 2020;7(21):2002203.
Gupta SL, et al. B-cell-based immunotherapy: a promising new alternative. Vaccines. 2022;10(6):879.
Rao BS, Uda H. Microfluidic photomask design using CAD software for application in lab-on-chip biomedical nano diagnostics. Adv Mater Res. 2013;795:388–392.
Sun M, et al. Paper-based microfluidic chip for rapid detection of SARS-CoV-2 N protein. Bioengineered. 2022;13(1):876–83.
Cojocaru R, et al. Microchip RT-PCR detection of nasopharyngeal SARS-CoV-2 samples. J Mol Diagn. 2021;23(6):683–90.
Samson R, Navale GR, Dharne MS. Biosensors: frontiers in rapid detection of COVID-19. Biotech. 2020;10(9):385.
Saylan Y, et al. An alternative medical diagnosis method: biosensors for virus detection. Biosensors (Basel). 2019;9(2):65.
Chircov C, Grumezescu AM. Microelectromechanical systems (MEMS) for biomedical applications. Micromachines (Basel). 2022;13(2):164.
Muhsin SA, et al. A microfluidic biosensor architecture for the rapid detection of COVID-19. Anal Chim Acta. 2023;1275: 341378.
Li Z, et al. Cell-mimicking nanodecoys neutralize SARS-CoV-2 and mitigate lung injury in a non-human primate model of COVID-19. Nat Nanotechnol. 2021;16(8):942–51.
Barghash RF, et al. In silico modeling as a perspective in developing potential vaccine candidates and therapeutics for COVID-19. Coatings. 2021;11(11):1273.
West JL, Halas NJ. Engineered nanomaterials for biophotonics applications: improving sensing, imaging, and therapeutics. Annu Rev Biomed Eng. 2003;5:285–92.
Singh P, et al. Insights from nanotechnology in COVID-19: prevention, detection, therapy and immunomodulation. Nanomedicine. 2021;16(14):1219–35.
Bhalla N, et al. Introduction to biosensors. Essays Biochem. 2016;60(1):1–8.
Mascini M, Tombelli S. Biosensors for biomarkers in medical diagnostics. Biomarkers. 2008;13(7):637–57.
Gupta SL, Basu S. Smart nanosensors in healthcare recent developments and applications. In: Kaushik S, Soni V, Skotti E, editors. Nanosensors for futuristic smart and intelligent healthcare systems. Routledge: Taylor and Francis; 2022; Edition 1; pp. 3–18.
Patel PD. (Bio)sensors for measurement of analytes implicated in food safety: a review. TrAC, Trends Anal Chem. 2002;21(2):96–115.
Zhang D, et al. Label-free electrochemical DNA biosensor array for simultaneous detection of the HIV-1 and HIV-2 oligonucleotides incorporating different hairpin-DNA probes and redox indicator. Biosens Bioelectron. 2010;25(5):1088–94.
Qureshi A, Gurbuz Y, Niazi JH. Label-free detection of cardiac biomarker using aptamer based capacitive biosensor. Procedia Engineering. 2010;5:828–30.
Dai H, et al. Biocompatible electrochemiluminescent biosensor for choline based on enzyme/titanate nanotubes/chitosan composite modified electrode. Biosens Bioelectron. 2010;25(6):1414–9.
Lowe CR. Overview of Biosensor and Bioarray Technologies. In: Marks RS, Cullen DC, Karube I, Lowe CR, Weetall HH, editors. Handbook of biosensors and biochips. John Wiley & Sons, Ltd; 2008. https://doi.org/10.1002/9780470061565.hbb003.
Khan R, et al. Two-dimensional nanostructures for electrochemical biosensor Sensors. 2021;21(10):3369.
Dykman LA, Khlebtsov NG. Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Naturae. 2011;3(2):34–55.
Abdellatif AAH, et al. Biomedical applications of quantum dots: overview, challenges, and clinical potential. Int J Nanomedicine. 2022;17:1951–70.
Shen H, et al. Biomedical applications of graphene. Theranostics. 2012;2(3):283–94.
Simon J, et al. Overview of carbon nanotubes for biomedical applications. Materials (Basel, Switzerland). 2019;12(4):624.
Mukhtar A, et al. Magnetic nanowires in biomedical applications. Nanotechnology. 2020;31(43): 433001.
Svenson S, Tomalia DA. Dendrimers in biomedical applications–reflections on the field. Adv Drug Deliv Rev. 2005;57(15):2106–29.
Chis AA, et al. Applications and limitations of dendrimers in biomedicine. Molecules (Basel, Switzerland). 2020;25(17):3982.
Li J, et al. Micro/nanorobots for biomedicine: delivery, surgery, sensing, and detoxification. Sci Robot. 2017;2(4):eaam6431.
Li M, et al. An overview of recent progress in micro/nanorobots for biomedical applications. Advanced Materials Technologies. 2023;8(11):2201928.
Kim EM, Jeong HJ. Liposomes: biomedical applications. Chonnam Med J. 2021;57(1):27–35.
Nakhaei P, et al. Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front Bioeng Biotechnol. 2021;9:705886.
Preethi M, et al. Outlook of various diagnostics and nanodiagnostic techniques for COVID-19. Biosens Bioelectron X. 2022;12:100276.
Zhang N, et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020;92(4):408–17.
Galindo-Hernandez O, et al. Elevated concentration of microvesicles isolated from peripheral blood in breast cancer patients. Arch Med Res. 2013;44(3):208–14.
Moitra P, et al. Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano. 2020;14(6):7617–27.
Zhu X, et al. Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosens Bioelectron. 2020;166:112437.
Kim HE, et al. Gold nanostructures modified carbon-based electrode enhanced with methylene blue for point-of-care COVID-19 tests using isothermal amplification. Talanta. 2023;265:124841.
Palestino G, et al. Can nanotechnology help in the fight against COVID-19? Expert Rev Anti Infect Ther. 2020;18(9):849–64.
Bolourinezhad M, et al. Design of a rapid electrochemical biosensor based on MXene/Pt/C nanocomposite and DNA/RNA hybridization for the detection of COVID-19. Talanta. 2023;265: 124804.
Yang W, et al. Ultra-sensitive and specific detection of pathogenic nucleic acids using composite-excited hyperfine plasma spectroscopy combs sensitized by Au nanoarrays functionalized with 2D Ta(2)C-MXene. Biosens Bioelectron. 2023;235:115358.
Tavares JL, et al. Nanotechnology and COVID-19: quo vadis? J Nanopart Res. 2022;24(3):62.
Seo G, et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14(4):5135–42.
Liu Y, et al. Development of a fluorescent immunochromatographic assay based on quantum dot-functionalized two-dimensional monolayer Ti(3)C(2) MXene nanoprobes for the simultaneous detection of influenza A virus and SARS-CoV-2. ACS Appl Mater Interfaces. 2023;15(30):35872–83.
Tao S, et al. SARS-Cov-2 spike-S1 antigen test strip with high sensitivity endowed by high-affinity antibodies and brightly fluorescent QDs/silica nanospheres. ACS Appl Mater Interfaces. 2023;15(23):27612–23.
Bai L, et al. A polyaniline functionalized NiFeP nanosheet array-based electrochemical immunosensor using Au/Cu(2)O nanocubes as a signal amplifier for the detection of SARS-CoV-2 nucleocapsid protein. Analyst. 2023;148(14):3359–70.
Singh AV, et al. Interfacial water in the SARS spike protein: investigating the interaction with human ACE2 receptor and in vitro uptake in A549 cells. Langmuir. 2022;38(26):7976–88.
Yeh Y-T, et al. Tunable and label-free virus enrichment for ultrasensitive virus detection using carbon nanotube arrays. Sci Adv. 2016;2(10):e1601026.
Pramanik A, et al. The rapid diagnosis and effective inhibition of coronavirus using spike antibody attached gold nanoparticles. Nanoscale Adv. 2021;3(6):1588–96.
Han Y, Král P. Computational design of ACE2-based peptide inhibitors of SARS-CoV-2. ACS Nano. 2020;14(4):5143–7.
National Library of Medicine. In: Clinical trials.gov. www.clinicaltrial.gov. Accessed April 2023.
World Health Organization. (2020). Clinical management of COVID-19: interim guidance. World Health Organization. https://apps.who.int/iris/handle/10665/332196. Accessed June 1 2023.
Damborský P, Švitel J, Katrlík J. Optical biosensors. Essays Biochem. 2016;60(1):91–100.
Daniels JS, Pourmand N. Label-free impedance biosensors: opportunities and challenges. Electroanalysis. 2007;19(12):1239–57.
Ma Q, et al. Calming cytokine storm in pneumonia by targeted delivery of TPCA-1 using platelet-derived extracellular vesicles. Matter. 2020;3(1):287–301.
Serebrovska Z, et al. Anti-inflammatory and antioxidant effect of cerium dioxide nanoparticles immobilized on the surface of silica nanoparticles in rat experimental pneumonia. Biomed Pharmacother. 2017;92:69–77.
Mei X, et al. Artificial intelligence–enabled rapid diagnosis of patients with COVID-19. Nat Med. 2020;26(8):1224–8.
Ke YY, et al. Artificial intelligence approach fighting COVID-19 with repurposing drugs. Biomed J. 2020;43(4):355–62.
Balagna C, et al. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceramics. 2020;1:100006–100006.
Srivastava AK, et al. Potential of graphene-based materials to combat COVID-19: properties, perspectives, and prospects. Mater Today Chem. 2020;18:100385.
Elechiguerra JL, et al. Interaction of silver nanoparticles with HIV-1. Journal of Nanobiotechnology. 2005;3(1):6.
Peng S, et al. Particulate alum via pickering emulsion for an enhanced COVID-19 vaccine adjuvant. Adv Mater. 2020;32(40):2004210.
Dwivedi M, et al. Self-assembly of DNA-grafted colloids: a review of challenges. Micromachines. 2022;13(7):1102.
Kwon PS, et al. Designer DNA architecture offers precise and multivalent spatial pattern-recognition for viral sensing and inhibition. Nat Chem. 2020;12(1):26–35.
Jones W, et al. Nanomaterials in construction – what is being used, and where? Proceedings of the Institution of Civil Engineers - Construction Materials. 2019;172(2):49–62.
Lauster D, et al. Phage capsid nanoparticles with defined ligand arrangement block influenza virus entry. Nat Nanotechnol. 2020;15(5):373–9.
Singh AV, et al. Coronavirus-mimicking nanoparticles (CorNPs) in artificial saliva droplets and nanoaerosols: influence of shape and environmental factors on particokinetics/particle aerodynamics. Sci Total Environ. 2023;860: 160503.
Soni V, et al. Genomic surveillance of bacterial pathogens: expanding horizons. In: Rajesh P, editor. Genomic surveillance and pandemic preparedness. Academic Press; 2023;71–117.
Kundu P, et al. Cancer nanotheranostics: a nanomedicinal approach for cancer therapy and diagnosis. Anti Cancer Agents Med Chem. 2020;20(11):1288–1299.
Itani R, Tobaiqy M, Al Faraj A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics. 2020;10(13):5932–42.
Madamsetty VS, et al. Tumor selective uptake of drug-nanodiamond complexes improves therapeutic outcome in pancreatic cancer. Nanomedicine: Nanotechnology. Biol Med. 2019;18:112–21.
Singh R. Nanotechnology based therapeutic application in cancer diagnosis and therapy. 3 Biotech. 2019;9(11):415–415.
Vemuri R, et al. Effect on structural and magnetic properties of Mg2+ substituted cobalt nano ferrite. Results in Physics. 2019;12:947–52.
Singh A, Sahoo SK. Magnetic nanoparticles: a novel platform for cancer theranostics. Drug Discovery Today. 2014;19(4):474–81.
Chen L, Liang J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater Sci Eng, C. 2020;112.
Justino CI, Duarte AC, Rocha-Santos TA. Immunosensors in clinical laboratory diagnostics. Adv Clin Chem. 2016;73:65–108.
Watanabe Y, et al. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369(6501):330–3.
Gao Y, et al. Nanotechnology-enabled COVID-19 mRNA vaccines. Encyclopedia. 2021;1(3):773–80.
Gupta SL, et al. An assessment of the strategy and status of COVID-19 vaccination in India. Immunol Res. 2023;71(4):565–577.
Huang X, et al. Nanotechnology-based strategies against SARS-CoV-2 variants. Nat Nanotechnol. 2022;17(10):1027–37.
Tenchov R, et al. Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–7015.
Hou X, et al. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6(12):1078–94.
Wilson B, Geetha KM. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J Drug Deliv Sci Technol. 2022;74:103553.
Goepfert PA, et al. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo-controlled, phase 1–2, dose-ranging study. Lancet Infect Dis. 2021;21(9):1257–70.
Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21(2):73–82.
Huo J, et al. A potent SARS-CoV-2 neutralising nanobody shows therapeutic efficacy in the Syrian golden hamster model of COVID-19. Nat Commun. 2021;12(1):5469.
Zupancic JM, et al. Engineered multivalent nanobodies potently and broadly neutralize SARS-CoV-2 variants. Adv Ther (Weinh). 2021;4(8):2100099.
Koenig PA, et al. Structure-guided multivalent nanobodies block SARS-CoV-2 infection and suppress mutational escape. Science. 2021;371(6530):eabe6230.
Liu H, et al. Two pan-SARS-CoV-2 nanobodies and their multivalent derivatives effectively prevent Omicron infections in mice. Cell Rep Med. 2023;4(2):100918.
Rao L, et al. Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines. Proc Natl Acad Sci U S A. 2020;117(44):27141–7.
Rao L, et al. Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines. Proc Natl Acad Sci. 2020;117(44):27141–7.
Chakraborty A, et al. Mechanism of antiviral activities of nanoviricide’s platform technology based biopolymer (NV-CoV-2). AIMS Public Health. 2022;9(2):415–22.
Salamończyk GM. A fast and convenient synthesis of new water-soluble, polyanionic dendrimers. Molecules. 2021;26(16):4754.
Nie C, et al. Spiky nanostructures for virus inhibition and infection prevention. Smart Mater Med. 2020;1:48–53.
Zhang J, et al. Spatially patterned neutralizing icosahedral DNA nanocage for efficient SARS-CoV-2 blocking. J Am Chem Soc. 2022;144(29):13146–53.
Chauhan N, et al. Net-shaped DNA nanostructures designed for rapid/sensitive detection and potential inhibition of the SARS-CoV-2 virus. J Am Chem Soc. 2022. https://doi.org/10.1021/jacs.2c04835.
Đorđević S, et al. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv Transl Res. 2022;12(3):500–25.
Hua S, et al. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front Pharmacol. 2018;9:790.
Resnik DB, Tinkle SS. Ethics in nanomedicine. Nanomedicine. 2007;2(3):345–50.
Wasti S, et al. Ethical and legal challenges in nanomedical innovations: a scoping review. Front Genet. 2023;14:1163392.
Paliwal R, Babu RJ, Palakurthi S. Nanomedicine scale-up technologies: feasibilities and challenges. AAPS PharmSciTech. 2014;15(6):1527–34.
Gaspar R. Regulatory issues surrounding nanomedicines: setting the scene for the next generation of nanopharmaceuticals. Nanomedicine. 2007;2(2):143–7.
Tinkle S, et al. Nanomedicines: addressing the scientific and regulatory gap. Ann N Y Acad Sci. 2014;1313(1):35–56.
Sainz V, et al. Regulatory aspects on nanomedicines. Biochem Biophys Res Commun. 2015;468(3):504–10.
Brownsword R. Regulating nanomedicine—the smallest of our concerns? NanoEthics. 2008;2(1):73–86.
Uskoković V. Nanomedicine for the poor: a lost cause or an idea whose time has yet to come? Nanomedicine (Lond). 2021;16(14):1203–18.
Kwatra Shubhika. Nanotechnology and medicine – The upside and the downside. International Journal of Drug Development and Research. 2013;5(1):1–10.
Meetoo D. Nanotechnology: is there a need for ethical principles? British Journal of Nursing. 2009;18(20):1264–8.
Fisher E, et al. Responsible healthcare innovation: anticipatory governance of nanodiagnostics for theranostics medicine. Expert Rev Mol Diagn. 2012;12(8):857–70.
Schroeder D, et al. Responsible, inclusive innovation and the nano-divide. NanoEthics. 2016;10:177–88.
Global alliance for vaccines and immunization. The COVAX facility: interim distribution forecast – Latest as of February 3 2021. http://www.gavi.org/sites/default/files/covid/covax/COVAX-Interim-Distribution-Forecast.pdf. Accessed 20 June 2023.
Ahmed MK, Afifi M, Uskoković V. Protecting healthcare workers during COVID-19 pandemic with nanotechnology: a protocol for a new device from Egypt. J Infect Public Health. 2020;13(9):1243–6.
Hua R, et al. A sensitive potentiometric resolved ratiometric photoelectrochemical aptasensor for Escherichia coli detection fabricated with non-metallic nanomaterials. Biosens Bioelectron. 2018;106:57–63.
Parisi C, Vigani M, Rodríguez-Cerezo E. Agricultural nanotechnologies: what are the current possibilities? Nano Today. 2015;10(2):124–7.
Li C, et al. PAMAM nanoparticles promote acute lung injury by inducing autophagic cell death through the Akt-TSC2-mTOR signaling pathway. J Mol Cell Biol. 2009;1(1):37–45.
Owen R, Depledge M. Nanotechnology and the environment: risks and rewards. Mar Pollut Bull. 2005;50(6):609–12.
Gwinn MR, Vallyathan V. Nanoparticles: health effects–pros and cons. Environ Health Perspect. 2006;114(12):1818–25.
Singh AV, et al. Artificial intelligence and machine learning disciplines with the potential to improve the nanotoxicology and nanomedicine fields: a comprehensive review. Arch Toxicol. 2023;97(4):963–79.
Singh AV, et al. Artificial intelligence and machine learning in computational nanotoxicology: unlocking and empowering nanomedicine. Adv Healthcare Mater. 2020;9(17):1901862.
Sharma A, et al. COVID-19 diagnosis: current and future techniques. Int J Biol Macromol. 2021;193(Pt B):1835–44.
Chandrasekar V, et al. Investigating the use of machine learning models to understand the drugs permeability across placenta. IEEE Access. 2023;11:52726–39.
Gomes JC, et al. IKONOS: an intelligent tool to support diagnosis of COVID-19 by texture analysis of X-ray images. Research on Biomedical Engineering. 2022;38(1):15–28.
de Santana MA, et al. An intelligent tool to support diagnosis of COVID-19 by texture analysis of computerized tomography X-ray images and machine learning. In: Pani SK, et al., editors. Assessing COVID-19 and other pandemics and epidemics using computational modelling and data analysis. Cham: Springer International Publishing; 2022. p. 259–82.
Shiri I, et al. Ultra-low-dose chest CT imaging of COVID-19 patients using a deep residual neural network. Eur Radiol. 2021;31(3):1420–31.
Ardakani AA, et al. Application of deep learning technique to manage COVID-19 in routine clinical practice using CT images: results of 10 convolutional neural networks. Comput Biol Med. 2020;121: 103795.
Singh AV, et al. Digital transformation in toxicology: improving communication and efficiency in risk assessment. ACS Omega. 2023;8(24):21377–90.
Singh AV, et al. Integrative toxicogenomics: advancing precision medicine and toxicology through artificial intelligence and OMICs technology. Biomed Pharmacother. 2023;163: 114784.
Nitulescu GM, et al. Comprehensive analysis of drugs to treat SARS-CoV-2 infection: mechanistic insights into current COVID-19 therapies (review). Int J Mol Med. 2020;46(2):467–88.
Williamson BN, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020;585(7824):273–6.
Garraud O, et al. Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow. Transfus Clin Biol. 2016;23(1):39–44.
Gunn BM, et al. A role for Fc function in therapeutic monoclonal antibody-mediated protection against Ebola virus. Cell Host Microbe. 2018;24(2):221–233.e5.
van Erp EA, et al. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol. 2019;10:548.
Saini P, et al. Siglec-9 restrains antibody-dependent natural killer cell cytotoxicity against SARS-CoV-2. MBio. 2023;14(1):0339322.
Golchin A. Cell-based therapy for severe COVID-19 patients: clinical trials and cost-utility. Stem Cell Rev Rep. 2021;17(1):56–62.
Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Reviews and Reports. 2020;16(3):427–33.
Gupta SL, et al. Children’s SARS-CoV-2 infection and their vaccination. Vaccines. 2023;11(2):418.
Gupta SL, Jaiswal RK. Relevant of neutralizing antibody during SARS-CoV-2 infection and their therapeutic usage. Mol Biol Rep. 2022;49(10):10137–40.
van Dijk H, et al. Determinants of stakeholders’ attitudes towards a new technology: nanotechnology applications for food, water, energy and medicine. J Risk Res. 2017;20(2):277–98.
FDA. In: Nanotechnology partnerships at FDA. 2018. https://www.fda.gov/science-research/nanotechnology-programs-fda/nanotechnology-partnerships-fda. Accessed 15 June 2022.
Sadiku M, et al. Future of nanotechnology. International Journal Of Scientific Advances. 2021;2(2);131–134.
Author information
Authors and Affiliations
Contributions
A.D., S.L.G, P.S, A.K., K.S., R.T., P.S., and R.K.J designed, wrote, and edited the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
No animal has been used in this study.
Informed consent
All authors are agreeing to publish this manuscript in your valuable journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhar, A., Gupta, S.L., Saini, P. et al. Nanotechnology-based theranostic and prophylactic approaches against SARS-CoV-2. Immunol Res 72, 14–33 (2024). https://doi.org/10.1007/s12026-023-09416-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-023-09416-x