Abstract
The outbreak of COVID-19 has caused great havoc and affected many parts of the world. It has imposed a great challenge to the medical and health fraternity with its ability to continue mutating and increasing the transmission rate. Some challenges include the availability of current knowledge of active drugs against the virus, mode of delivery of the medicaments, its diagnosis, which are relatively limited and do not suffice for further prognosis. One recently developed drug delivery system called nanoparticles is currently being utilized in combating COVID-19. This article highlights the existing methods for diagnosis of COVID-19 such as computed tomography scan, reverse transcription-polymerase chain reaction, nucleic acid sequencing, immunoassay, point-of-care test, detection from breath, nanotechnology-based bio-sensors, viral antigen detection, microfluidic device, magnetic nanosensor, magnetic resonance platform and internet-of-things biosensors. The latest detection strategy based on nanotechnology, biosensor, is said to produce satisfactory results in recognizing SARS-CoV-2 virus. It also highlights the successes in the research and development of COVID-19 treatments and vaccines that are already in use. In addition, there are a number of nanovaccines and nanomedicines currently in clinical trials that have the potential to target COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
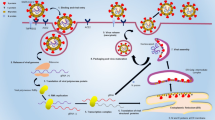
Viruses like Middle East Respiratory Syndrome (MERS), Zika, Ebola, Influenza A (H1N1), and Severe Acute Respiratory Syndrome (SARS) have spread around the world in the last 20 years. Health policy and economic relations have been profoundly affected by these pandemics (Boopathi et al. 2021). The most recent form of the virus is the novel coronavirus, which has been recognized globally as a thoroughly transmissible disease since 2019. It is commonly known as novel COVID-19 (SARS-CoV-2) (Lu and XZ 2020; Russell et al. 2020). The virus was firstly discovered in the Wuhan city of China. The infected patients exhibited unusual pneumonia-like symptoms that later spread globally (Russell et al. 2020). Coronavirus has caused great concerns due to its rapid diversity and a high number of new cases reported that resulted in huge economic loss worldwide. The structure of coronavirus consists of an envelope of spikes in its outer layer. Its total height is approximately 32 kb, with a single-stranded RNA (Benzigar et al. 2021). At the time of replication of polyprotein translations, it would act on the mRNA. Human SARS-CoV-2 has a spherical morphology with a dimension of around 125 nm (Fig. 1) (Daemi et al. 2021). The coronaviridae family and the nidovirales order comprise the virus evolutionary lineage (Lu et al. 2020; Su et al. 2016). Coronavirus is subdivided into four main categories, referred to as: alpha (α), beta (β), gamma (γ), and delta (δ) and all of which have been recognized to date. Some of the common coronavirus for human are HKU1, NL63, MERS-CoV, OC43, SARS-CoV, and 229E. Alpha coronavirus consists of NL63 and 229E, whereas the SARS-CoV, MERS-CoV, OC43, and HKU1 families are examples of a beta coronavirus (Li et al. 2020a, b). Four crucial structural proteins are encoded by the coronaviral genome, namely spike (S) protein, nucleocapsid (N) protein, membrane (M) protein, and the envelope (E) protein, that collectively constitute a complete viral entity (Masters 2006). Every protein plays a specialized function in the construction of the viral genome. These proteins have a central position in the replication process as well. The greatest predominant antigen in the viruses is the S glycoprotein, which is called the spikes of the virus (Jaimes et al. 2020). It has the ability to interact with the receptor and then fuse with the host membranes. The fused viral protein has branches in two different ways: first, it uses the S1 protein of the primary subunit to attach to the receptor. By reserving the cell membranes of the receptor, the S2 subunit would promote infusion. After targeting the virus, it would be structurally modified by the proteolytic enzymes. The peptide would then be incorporated into the cell membrane. Finally, irreversible cyclization would stress the S2 subunit of the fusion protein, which in turn would be mediated by ACE -2 (angiotensin-converting enzyme 2), allowing the viral genome to enter the cytoplasm (Fig. 2) (Shafiee et al. 2021; Varahachalam et al. 2021).
COVID-19 is considered a pandemic of the world’s most significant humanitarian catastrophes due to its uncontrollable spread worldwide, huge loss of life, and impact on the world’s economy. Huge efforts have been done to overcome the disease and prevail over the pandemic (Li et al. 2020a, b; Ziegler et al. 2020; Comentale et al. 2020). The most common symptoms among infected people are fever, loss of taste and smell, fatigue, and cough. In severe cases, several other symptoms are also reported, including difficulty breathing, pneumonia, and severe darkening of the lungs. Patients who present with such signs are evaluated by chest radiographs or computed tomography (Russell et al. 2020; Yang et al. 2020; Weiss et al. 2020). Officially, the coronavirus was termed as SARS-CoV-2 or 2019- nCoV (Lu et al. 2020; Munster et al. 2020; Liu et al. 2020; Habibi et al. 2020; Heymann and Shindo 2020; Wang et al. 2020).
Nanotechnology is pertinent in managing and treating a wide range of disorders. Applications of nanomedicine include immune engineering, drug delivery, nanosensors, cancer nanovaccines, and platform technologies (Chakravarty and Vora 2021). Since the existing antiviral agents have truncated bioavailability and a narrow spectrum, nanomaterials are crucial for antiviral treatment. ENMs (engineered nanomaterials) have easy tunable physicochemical, low cost, high efficiency, and efficacy of drug delivery with lesser side effects (Chen et al. 2020a, b). The cell–virus interactions can be adjusted, particularly their interaction with cell receptors or virus particles, by adjusting the shape, size, and surface of ENMs (Ye et al. 2015). Nanotechnologies can be used for rapid detection of COVID-19 to further improve the efficiency of diagnostic tests (Rabiee et al. 2020). Organisms with a higher incidence of susceptibility can also be diagnosed using nanomedicines, which act as a prophylactic and alternative means of diagnosing infectious diseases (Vahedifard and Chakravarthy 2021). Magnetic nanoparticles and quantum dots are the nanoparticles that are commonly employed to detect coronavirus. The protein corona sensor array technology, which would aid in identifying protein/biomolecule sequences at an early stage, is at the forefront of catastrophic COVID-19 (Misra et al. 2021). The design and implementation of nanotechnology facilitate the production of new nanovaccines with a higher efficacy. One such efficient delivery strategy for treating SARS-CoV-2 is a nanoloaded formulation that is currently being explored. In addition, novel nanovaccines and nanotherapeutics are being developed to address SARS-CoV-2 infection (Jan et al. 2022). Therefore, researchers are making tremendous efforts to discover novel nano-based technologies and develop relevant nanovaccines and create effective therapies for SARS-CoV-2 infections. This article focuses on the unique and innovative nanotechnology strategies, detection, and prevention against COVID-19 and the potential for future research.
Diagnosis of SARS-CoV-2 infestation
Computed tomography scan
A computed tomography (CT) scan is used to examine the internal organs. Chest radiographs and 3D cross-sectional images are utilized to identify the symptoms of COVID-19 (Shah et al. 2021). Patchy lung lesions of small size are seen in the early stages. One of the reports stated that CT scans are more likely to be tactful in the first stage of infection than RT-PCR (Law et al. 2015). CT scan helps to better understand the condition of the lung and the severity of the disease. It helps the physician to treat the patient appropriately depending on the severity (Bernheim et al. 2020). However, the usage of CT scans for COVID-19 has some challenges, including low specificity, which is only about 25%. As with asymptomatic patients, there are also opportunities for uncertainty with CT scans (Alafif et al. 2021).
Furthermore, the diagnostic images of CT scans could overlap with other diseases like pneumonia. Repeated radiation exposure also would inflict long-term negative effects (Wu et al. 2020a, b). Additionally, it is an expensive tool to utilize.
On chest X-rays, it is common to see recurring mottling, subsegmental, or segmental morning opacities in both lungs, and atypical findings are becoming more common (Kaur et al. 2020). However, CT radiation is associated with minimal carcinogenic risk. It is currently unclear how important CT is for the management of patients with COVID-19. Presently, no antiviral therapy is available for 2019-nCoV, which is not the case in pulmonary TB patients. Drug treatment for tuberculosis can be started without a positive pathogenic Mycobacterium tuberculosis smear or tuberculosis culture if a clinical diagnosis is made that is supported by imaging. In cases of COVID-19, CT may show symptoms similar to those of viral pneumonia, supporting the clinical diagnosis (Zhao et al. 2020). In contrast, a change from a suspicious to a clinically diagnosed COVID-19 case classification has no impact on clinical therapy (Wu et al. 2020a, b).
Reverse transcription-polymerase chain reaction (RT-PCR)
RT-PCR would establish the criteria for COVID-19 diagnosis. Although COVID-19 is based on the extent of chronic respiratory complication from viruses, the practical detection approach appears to be less reliable. The SARS-CoV-2 virus is a single-stranded RNA virus with a positive direction of infection. Therefore, the most accurate approach for identification is RT-PCR. This approach requires the conversion of RNA to its supporting DNA, followed by amplification for detection (Harvey and O’Regan 2020). The E, S, N protein, and the RNA-dependent RNA polymerase gene (RdRp) portions of the entire genome, and ORF1ab are the most common targets for RT-PCR-based detection. These have shown good sensitivity, with the exception of RdRp, as it has lower sensitivity due to reverse primer-template mismatches than the rest (Huang et al. 2020). The product of PCR amplification analysis is based upon CT (cycle threshold) value, i.e., the threshold value found in the duration of the amplification of the viral genome and control, i.e., negative control, standard control, and positive control. The positivity criteria of COVID-19 are analyzed based on the measurement of CT score. The CT score is a number that shows how long it took for the total number of PCR sessions to exceed the baseline fluorescence emission value. In the case of infection, the CT score cut-off is between 35 and 40 with a higher CT score indicating a lower viral load in the specimen. Thus, patients with a CT score of less than 35 would be classified as COVID-19 favorable, whereas patients with a CT score greater than 40 would be classified as clinically negative (Lai et al. 2020; Singh et al. 2021).
The virus accumulates in the nasopharynx, so a swab is taken from these body sites. Various biochemical reagents are used to remove impurities such as lipids and proteins to isolate only the RNA from the sample. The genetic information of the individual and, if applicable, the RNA of the virus are combined to form this RNA. A special enzyme is needed to convert the RNA into DNA. Then, researchers insert micro-DNA fragments that complement specific zones of replicated virulent DNA. When the virus is identified in the sample, these fragments bind to the target sites of the virulent DNA. During the amplification process, some parts of the genome are added to design the DNA sequence. On the other hand, DNA fragments are produced and attached to the sequences with labels to identify the virus. The device RT-PCR is then employed to test the sample (Udugama et al. 2020). The apparatus heats and cools the samples simultaneously, activating biochemical reactions that result in additional duplicated versions of the virulent DNA target regions (Craw and Balachandran 2012). The process is repeated infinitely to copy the viral DNA target regions. A conventional real-time system RT-PCR runs for 35 sessions, which means that each viral strand in the material will have produced approximately 35 billion additional versions of the viral DNA at the end of the process. As additional versions of the virulent DNA fragments are produced, the tags combine with the DNA sequences. The result is the release of a fluorescent dye that is tracked by the computer device and displayed on the monitor in real time. The computer device logs the amount of fluorescence in the sample at the end of each session. The prevalence of the virus is determined when a predetermined fluorescence threshold is exceeded (Rao et al. 2022). To assess the severity of the disease, experts also monitor the number of sessions required to do so: The fewer sessions required, the more severe the viral contamination (Mohammadniaei et al. 2021). The real-time PCR method is precise and accurate and can provide a reliable diagnosis within 3 h, whereas laboratory methods typically require 6–8 h. Real-time PCR (RT-PCR) is much faster and has less potential for inaccuracy than other known viral extraction methods because the entire process can be performed in a closed container (Wu et al. 2013).
Nucleic acid sequencing
Detection of SARS-CoV-2 can be sequence-specific based on nucleic acid sequencing. It is considered one of the most precise scientific methods for detecting COVID-19 as it can examine the whole genomic makeup of the virus (Lu et al. 2020). Sanger sequencing is a type of sequencing in which a patient's sample is recovered to recover viral RNA and spike-in control RNA that is different from viral RNA. Most of the diagnoses of COVID-19 are based on next-generation sequencing such as oxford nanopore sequencing and illumine sequencing. Unlike Sanger sequencing, the next-generation sequencing would concurrently target billions of target sequences (Quick et al. 2017). On the other hand, the amount of virus remaining in the specimen is measured and normalized based on the spiked RNA (Singh et al. 2021; Yu et al. 2021).
Assays based on nucleic acids usually take 12 to 24 h or longer and are thought to be the best way to identify microorganisms. On the other hand, nucleic acid-based assays offer a much shorter reaction time and excellent sensitivity and specificity, bringing them to the forefront of laboratory and, in some circumstances, field analysis. In the laboratory, nucleic acid sequencing has been effectively used to detect both nonpathogenic and pathogenic microorganisms. Ribosomal RNA techniques have been used to conduct similar studies of microbial diversity (Chandler-Brown et al. 2020).
Sequence-based ID with nucleic acid testing is a key part of any defense against biological agent attacks. It helps find out whether a biological agent attack has happened and, if so, what kinds of biological agents were used. Even if the test response time is slow—on the order of 15–30 min—this confirmation function is critical (Lim and Wang 2016).
The following is the sequence of processes that must occur within this response time: (1) collect the sample: prepare the sample for an assay. This includes the removal of assay inhibitors and lysis of target cells. This can be a serious problem with contaminated samples or when analyzing spore nucleic acids. (2) Performance of the actual assay: analyzing and reporting the assay results (Lim and Wang 2016).
Immunoassay
COVID-19 can be diagnosed indirectly by looking at the patients’ immunological response. Recent research has been conducted to determine serologic surveillance of COVID-19 by collecting various individual fluids (Rizzo and Buck 2012). The target for serological diagnosis is mainly blood samples instead of the nasopharyngeal swab. Detection of an immune response is the most important requirement for verifying transmission of the virus. Immunoassay is a bioanalytical method based on the interaction between antibody and antigen that allows rapid detection of COVID-19. Two prime serological detections based on immunoassay are enzyme-linked immunosorbent assay (ELISA) and lateral flow assay (LFA). Targeting specific antibodies and antigens is done for the diagnosis of COVID-19. According to studies, a minimum of 5 days is required for sufficient viral load. For antibodies, however, it usually takes at least 7 days, and the concentration decreases after the infection has progressed for 7 days (Alcoba-Florez et al. 2020). Thus, if the viral load is minimal, this will lead to misleading negative results.
Point-of-care test
Point-of-care (POC) testing is essential for disease management during a pandemic. For this reason, it is important to understand the causative agent of a virus before developing tools for diagnosis. It allows for early diagnosis by detecting the analyte, which could optimize the effectiveness of the therapy (Kaushik et al. 2020). The tools developed for the POC screening of COVID-19 are based on the detection of immunological components such as antibodies and antigen or viral RNA (Ma et al. 2020). Table 1 highlights the existing approaches for identifying SARS-CoV-2.
POC tests are procedures performed with a test strip or kit at home or at the healthcare facility where the person is being treated. The biosensor is the most important component of POC screening because it is required to perform molecular analysis to identify the virus. POC screening has the following advantages: (i) fewer storage and experimental facilities are needed; (ii) it is adaptable to a variety of medical problems; (iii) it is a significant test; and (iv) multiple sites can be used for evaluation (Asdaq et al. 2021).
Each POC test is approved for use with a specific type of specimen and can only be used with those specimens. Appropriate specimen procurement and handling are required for all COVID-19 assays, especially those performed in POC clinics. An erroneous or inaccurate test result may result from improper specimen submission or collection. Employees who collect specimens within 6 feet of persons must wear the required personal protective equipment (PPE). It consists of gloves, safety glasses, N95 protection, and a lab coat or robe. All personnel collecting specimens not actively involved in specimen collection should follow standard safety guidelines and not be more than one meter away from the patient (Dincer et al. 2017).
Detection from breath
For the objective of monitoring COVID-19, screening of expelled breath may be a less intrusive form of analysis (Corman et al. 2020; Broza et al. 2018). However, screening SARS-CoV-2 from exhaled air has proven to be quite complicated. These findings show that COVID-19 individuals breathed billions of RNA molecules of severe acute respiratory syndrome coronavirus every extra few minutes (Broza et al. 2015). According to scientific interpretation, exhaled air had a greater affirmative incidence (26.7%) than surface samples (5.4%) and air (3.8%). In addition, exhaled breath condensate (EBC), a unique technique for quickly identifying viruses from exhaled breath, was required to collect the sample for a longer period of time..
Furthermore, recent studies have shown that non-volatile components such as viruses, bacteria, DNA, and RNA, can be easily detected and observed by extracting and analyzing the aqueous fraction of expelled breath (Expelled Breath Aerosol (EBA) or EBC) (Ma et al. 2021). Depending on these two factors, an EBC system can effectively detect different droplets: (1) the percentage of the virus still alive after extraction and (2) the ratio between the percentage of extracted particles and the total percentage of particles in the air. For example, Haick and colleagues conducted a scientific investigation in March 2020 in Wuhan city of China, featuring screening with a breath scanner that follows the principle of chemoreceptive devices formed of gold nanoparticles combined with artificial intelligence algorithms (Corman et al. 2020; Lamote et al. 2020). In another experiment, researchers investigated preliminary markers of enhanced reactive oxygen species formation in swab samples. In this approach, swab samples were exposed to a biosensor nanostructured with carbon nanofibers, which provided 97% accurate confirmatory readings in about 30 s (Shan et al. 2020).
Nanotechnology-based biosensors for COVID-19
Biosensors are devices containing a physical transducer and a biorecognition element that can be used to diagnose the analytes present in solutions, liquids, and bodily fluids. A physical sensor is used to produce a response after an analyte coincides with a biological factor and transforms it into a measurable quantity. Biorecognition elements are glycoproteins (antibodies) or enzymes, nucleic acids such as RNA, DNA, biological receptors, organic receptors, tissues, and whole cells (Miripour et al. 2020). As per the specifications, enormous numbers of biosensors can be fabricated. Nanomaterials are beneficial as they provide the surface chemistry that would aid in molecular bioconjugation, powerful expanding effect on signals, and large surface energy. Metallic nanoparticles such as silver and gold nanoparticles, nanogels, carbon nanomaterials, i.e., graphene and nanotubes, microgels, and phonic crystals, are utilized in the application of biosensors. Meanwhile, a piezoelectric and electrochemical method based on nanocarbon particles has been developed for the detection of bacteria (Rocchitta et al. 2016).
A biosensor detects solutes in bodily fluids, liquids, and by combining a biological sensing device with a mechanical detector. When a solute interferes with a biological component, an output signal is generated, in which the physical detector then converts into a scientifically and quantitatively detectable component. Proteins such as antibodies or enzymes, nucleic acids such as RNA or DNA, physiological receptors, whole tissues, and cells are among the biological detector elements, while electrochemical, photonic, and piezoelectric devices are often used as physical detectors (Bandodkar and Wang 2014). Biosensor elements can be manufactured in large quantities to meet different requirements. Nanomaterials can be used as detectors due to their excellent resistance in a wide range of environments and bio-compatibility with biological systems (Sharma et al. 2020). Additionally, nanomaterials have high interfacial potential, robust signal amplification capability, and surface chemistry that can help bioconjugate biomolecules. The properties of photonic crystals, microgels, and magnetic nanostructures including gold nanostructures and silver, carbon-based nanoparticles (nanotubes and graphene), and nanocomposites have contributed to their use in biosensor technologies. The use of nanocarbon-based particles has facilitated microfluidic systems. These have already proven to be platforms for bacterial detection, using a range of strategies such as electrochemistry and piezoelectricity (Olvera and Monaghan 2021).
Development in nanotechnology-based COVID-19 diagnosis
When an infection occurs, immune globulin M (IgM) and immunoglobulin G (IgG) are produced. Initially, IgM antibodies appear, which are reduced to a minimum in general infection. Thereafter, IgG antibodies would develop and displace the IgM antibodies in the body, which remain in high concentration for a considerable time until they are identified after 12 weeks. In SARS-CoV-2 transmission, there is no latency between IgM and IgG confirmation, as both occur within 5–7 days of the onset of manifestations (Tarim et al. 2021). Several laboratory-based immunoassay methods, such as chemiluminescence immunoassay, can find antibodies in blood serum to the SARS-CoV-2 viral protein. The most commonly utilized method for detection is enzyme-linked immunosorbent assay. However, to identify contamination and conduct an on-site investigation, rapid and portable immunoassay detection is required. One promising, quick and portable platform for the point of care is lateral flow immunoassay, which uses colloidal gold nanoparticles (Deeks et al. 2020). Gold nanostructures are coupled to the SARS-CoV-2 protein and immobilized in the intracellular membrane in a lateral flow immunoassay. The testing for SARS-CoV-2 virus detection would take roughly 15 to 20 min. Due to this, cross-reactivity with other antibodies may result in false-positive outcomes (Han et al. 2019).
The rate at which antibodies to SARS-CoV-2 are produced is the most important immunologic factor determining susceptibility to infection. RT-PCR can be replaced with immunologic screening to validate SARS-CoV-2. The rate of unequivocal virus detection is significantly increased when immunologic testing and real-time PCR are coupled (Lustig et al. 2021). In most individuals, IgM levels rise in the first week resulting from exposed to SARS-CoV-2, continue to rise until levels reach a maximum after two weeks, and then begin to decline until levels are near reference values. After one week, IgG is visible and it remains in significant concentration for a longer period of time.
IgG, on the other hand, can be seen after only one week. Its levels stay high for a longer time, probably up to 48 days, and it may help prevent a second infection. Immunoglobulin A (IgA) responses begin to manifest about 4 and 10 days after infection. IgM, IgA, and IgG expression in blood plasma is a diagnostic factor. Different targets can describe the variability of antibodies to SARS-CoV-2. After exposure to infection, antibody titers may decrease for up to 7 days. Specific antibodies to SARS-CoV-2 were recently detected in saliva. Experts investigated whether there are differences in antibody concentrations between serum and saliva using multiplex antibody immunoassays for SARS-CoV-2. The antibodies indicate parallel compartmental humoral immune responses in saliva that correspond to those in serum (Pan et al. 2020).
Viral antigen detection
Prior biosensing strategy would utilize complementary DNA (cDNA) or antibodies to obtain viral RNA or antigen. The existence of SARS-CoV-2 infection is identified by utilizing graphene field-effect transistors (GFET) as a biosensor. It helps in determining the seriousness of the infection (Rocchitta et al. 2016). The graphite film serves as the reading region of the FET-based biosensor, which is then transferred to the Si/SiO2 substrate to successfully modify with the antibody of COVID-19. It is immobilized well on the surface of the graphene sheet by drop-casting. This instrument can detect COVID-19 antigen concentrations as low as 1 fg/ml in a phosphate buffer, which is substantially lower than the PCR/ELISA approach (Nguyen-Contant et al. 2020). The medium used is universal transport medium (UTM), with the assay used for biosensors for nasopharyngeal swab suspension.
A rapid assay method was developed to confirm the expression of the viral genome released by SARS-CoV-2 in samples from an infected person’s respiratory tract. In this experiment, the protein in the sample adheres to antibodies coupled with a sheet of paper in a glass container. Within half an hour, this reaction produces a detectable signal. The test could indicate intense or initial infection because the proteins found are expressed only when the pathogen is continuously replicating (Drobysh et al. 2022). In addition, Abbott has introduced a more powerful version of the COVID-19 instant diagnostic test, which screens for antibodies in infected human blood. Abbott's diagnostic devices can detect antibodies to SARS-CoV-2 and are available on the ARCHITECT i1000SR and i2000SR scientific instruments, which can perform 100–200 tests per hour. Antibodies to SARS-CoV-2 are produced after one week of infection. Age, nutritional status, the severity of the disease, concomitant diseases, and medications can influence the intensity of the antibody response (Seo et al. 2020).
Microfluidic device
Another possibility for experiments with real evidence is microfluidic devices. These microfluidic crisps are imprinted with micrometer-sized circuits and reactivity units made of polydimethyl sulfoxide and paper and offer advantages such as reduced sample size, compact dimensions, and rapid detection time. These microfluidic crisps work by blending and separating fluid materials employing electrokinetics and capillary pressure, which is their core principle (Ramachandran et al. 2021).
Mass screening of people is recognized as a crucial step in limiting the virus proliferation. Some countries in America and Africa reported fewer cases due to the shortage of tests done. Nowadays, quantitative reverse transcriptase polymerase chain reaction (qRTPCR) is applied and endorsed by WHO for case confirmation. qRTPCR (quantitative reverse transcriptase polymerase chain reaction) primer, thermocyclers, specific reagent, and qualified personnel are the most important requirements (Sen et al. 2022). Microfluidics can be used as an alternative to the time-consuming assay. The emergence of microfluid devices is enabled due to the microelectronics and micro-electromechanical systems as they can manipulate a small number of fluids.
Moreover, they can also retrieve information from the small sample volume. Microfluidic devices are used at the early stage of diagnosis as recent researchers have found that they can detect viruses (Kalita et al. 2021). The genetic code that forms virus consists of an envelope of proteins and a lipid ligand. Proteins are detected by Western blot, PCR, and amplified genetic material. Many approaches based on benchtop assays are transferred to the microfluidic device to minimize the amount of reagents needed and to speed up the reaction. The microfluidic device would elaborate a structure that can detect the biological samples. The detector that uses the amplification of genetic code requires a specific DNA probe design called primer to find a target sequence in the virus's genetic material (Maggi et al. 2019).
Magnetic nanosensor
Gold nanoislands (AuNIs) were utilized to build plasmonic photothermal (PT) biosensors that target the ORF1ab, RdRp, and E genes to pinpoint SARS-CoV-2 RNA sequences. Nanosensors based on magnetic nanoparticles (MNPs) generate heat as a result of their optical properties when aggregate vibrations in energy concentration take place at the interface of nanomaterial via conjugating to magnetic radiation. The increased frequency of collisions with lattice atoms caused by the enhanced motion of conduction electrons leads to the generation of PT-induced heat (Hofmann et al. 2008; Moabelo et al. 2021; Esbin et al. 2020).
Nanosensors based on magnetic nanoparticles (MNPs) are advantageous for viral pathogen identification (Islam and Ahsan 2020; Barnett et al. 2019). Tian et al. claim that nanosensors based on MNPs can be used for screening nucleic acids by combining uniform circle-to-circle amplification (UC2CA) and iron oxide NPs (IONPs). In this approach, the effect of an ambient magnetism allows both single and coupled IONPs to exhibit different optical properties (dispersion or absorption). When the recognition markers of the IONPs are paired with UC2CA, the coupled IONPs that produce the end amplicons (single-stranded DNA) are formed. Consequently, the configurations of the IONPs can be used to study the opto-magnetic parameters. This method has been shown to be accurate in distinguishing the genetic variants of SARS-CoV and SARS-CoV-2. In addition, it has been used to detect RdRp motifs for SARS-CoV-2 (Tian et al. 2020).
Nanomedicine
The discipline of medicine known as "nanomedicine" exploits nanotechnology to treat and prevent disease. It is utilized in various applications of living organisms, including diagnostics, delivery, and actuation. It is described as using manmade nanodevices and nanostructures to monitor, create, and regulate human biological systems at the molecular level (Zhang et al. 2022). The unique qualities of nanomedicines may hold the key to solving many of today's problems in cancer, cardiovascular and neurological illnesses, and sickness. The nanomedicine formulation imparts various beneficial properties such as lower side effects, molecular targeting by nanoengineered devices, better absorption with higher intestinal permeability, improved bioavailability, and prolonged and targeted medication delivery. The main benefit of nanomedicines in several current applications is improving drug delivery for therapy. The main mechanism of drug delivery based on nanomedicine called increased bioavailability, and retention is the example of selective medication tailoring (Patra et al. 2018a, b). It helps to increase absorbability and can be removed from the body waste before treatment, which can be effective. Nanomedicine is vital in improved efficacy, bioavailability, and targeting ability and is safer than conventional medicines. Nanomedicine influences all medicine sectors and is regarded as a significant tool for vaccinations, medical imaging, innovative diagnostics, nanotherapeutics, and the development of biomaterials for restorative medicine (Ledet and Mandal 2012). Surfactants (liquid crystals, nanoemulsions, and microemulsions), polymers (polymeric nanoparticles), proteins (protein nanoparticles), and lipids (liposomes, lipid–solid nanoparticles, and nanostructured lipid carriers) have all been employed in nanomedicine. The strength of interactions between biological/tissue molecules and nanoparticles is the foundation of their use in various medicinal applications (Flühmann et al. 2019). Soft nanomaterials are primarily used to enhance the pharmacokinetic, pharmacodynamics, and biopharmaceutical aspects of drug loading. The performance and tolerability of the therapy may be impacted by the use of nanoparticles to enhance selective therapeutic localization (active or passive) and optimized the release rate of the drug. Due to their antimicrobial qualities, metallic, and polymer-based nanoparticles were also applied in nanomedicine (antiviral, antifungal, antibacterial, and antiparasitic) (Patra et al. 2018a, b).
Nanomedicine-based drug delivery system
Numerous conditions, including diabetes, cancer, cerebrovascular disease, and neurodegenerative disorder, can be treated with drug delivery systems based on nanotechnology. Some nanomedicines are available in the market through parenteral or oral administration. Novel nanomedicines can be prepared through several preclinical and clinical trials, which can be administered through different routes such as nasal, ocular, vaginal, dermal and pulmonary. Several developed formulations based on nanomaterials are discussed below.
Liposomes
Alec Bangham developed liposomes in the year 1960. Cosmetics and pharmaceutical industries have utilized liposomes to transport drugs and other diverse molecules. Liposomes are considered a fixed formulation method to improve the drug delivery system. Liposomes are spherical-shaped vesicles, usually in the 50–450 nm, composed of steroids and phospholipids. They are regarded as a good vehicle for drug delivery due to their ability to incorporate drugs and possess an analogous membrane structure (Kupferschmidt and Cohen 2020). Liposomes would improve biodistribution and build stable compounds that allow them to utilize hydrophobic and hydrophilic drugs. There are four types of liposomes. The first is a lipid bilayer in which aqueous hydrophilic and hydrophobic substances are surrounded by cationic, anionic, or neutral phospholipids and cholesterol. The second variety is PEGylated liposomes, which consist of PEG (polyethylene glycol) inserted onto their surface to achieve steric equilibrium. The ligand-targeted type is the third kind in which ligands such as carbohydrates, antibodies, and peptides are attached to the surface of liposomes. The fourth form is the theranostic, a combination of the first three types addressed prior that consists of a nanoparticle with imaging, targeting, and therapeutic elements (Bozzuto and Molinari 2015).
Li et al. prepared and characterized a liposomal remdesivir inhalation solution for targeted pulmonary delivery for COVID-19 therapy. The results indicate that liposomal remdesivir shows rapid conversion of remdesivir to active nucleoside triphosphate and its Tmax is 1 h. This observation might be due to the fact that liposomes have high drug loading and are biocompatible with cell membranes. This would lead to faster uptake of remdesivir by cells and in larger amounts (Li et al. 2021). The central composite design was used to optimize the ritonavir proliposomes, and the results of the physicochemical properties were appropriate. The rats treated with the liposomal formulation exhibited a higher amount of drug in the lymphoid tissues compared with the pure drug. The results suggest that the targeting potential of biotin-coated proliposomes of ritonavir is present (Ahammed et al. 2017). Mice infected with either dead or live SARS-CoV-2 developed acute respiratory distress syndrome (ARDS). Treatment with tripterine liposomes significantly reduced the severity of pulmonary pathological changes and viral load. It was observed that tripterine liposomes reduced SARS-CoV-2 replication and hyperinflammation in mice and cells infected with the virus. Therefore, it is a potential option for a drug that could be used to treat ARDS caused by SARS-CoV-2 (Que et al. 2022).
Polymeric micelle
Micellar nanostructures known as polymeric micelles are composed of an amphiphilic block copolymer that has the ability to self-assemble into a core with a shell when introduced into an aquatic system. For example, docetaxel, camptothecin, and paclitaxel are hydrophobic drugs that can be loaded into the hydrophobic cavity. The hydrophilic coating would stabilize the core and make the system soluble in water when used. To avoid rapid excretion through the kidneys, polymeric micelles have a diameter of about 100 nm and have only a small volume of distribution. Therefore, they can be collected in tumor tissue due to their increased permeability and retention effect.
On the other hand, the hydrophobic medication can be integrated into the core structure, enhancing the drug's bioavailability and stability (Ahammed et al. 2017; Cho et al. 2015). These micelles can be synthesized by precipitating one block and adding a solvent and direct dissolution of polymer in a convenient solvent-based followed by dialysis (Cho et al. 2015; Xu et al. 2013). Micelle formation is affected by amphiphilic molecules in the hydrophobic chain, the concentration of amphiphiles, temperature, and solvent system (Kulthe et al. 2012).
Targeted drug delivery, nanoscopic core structure, minimal biodegradability, micellar coupling, biocompatibility, and much-improved longevity are all attributes of polymeric micelles that make them ideal for drug therapy (Chamundeeswari et al. 2019). Chaudhari and Handge reported the development, characterization, and validation of lopinavir-encapsulated polymeric micelles using co-solvent (Tween 80) and polymeric material (Pluronic F127 and Pluronic F68) (Chaudhari and Handge 2020). The novel drug formulation increased drug loading capacity, bioavailability, and encapsulation performance. Mahajan and Patil developed polymeric vitamin E-tocopheryl polyethylene glycol succinate micelles encapsulating lopinavir for oral therapy. The developed lopinavir polymeric micelles had a better dissolution rate. An in vivo pharmacokinetic study in New Zealand rabbits showed that the formulated drug had much higher bioavailability (3.17%) than lopinavir suspension (Mahajan and Patil 2020).
Dendrimers
Dendrimers are monodispersed 3-D entities that may be cleaved into two halves. They have a spherical shape whose surface can be uniformly functionalized, making them exceptional for drug delivery (Mahajan and Patil 2020; Kesharwani et al. 2015). The dendrimers can be synthesized in two ways: the dendrimers would start forming from the core and then outside of the dendrimers. The other method is convergent in which the formation would start from outside the dendrimers (Zhu and Shi 2013). There are various types of dendrimers based on their functionalization moieties such as polypropylene imine (PPI), polyamidoamine (PAMAM), core–shell, peptide, glycodendrimers, liquid crystalline, chiral and polyamidoamine-organosilicon (PAMAMOS). Since PAMAM is water-soluble and can penetrate epithelial tissues, it is mainly explored for oral drug delivery (Cheng et al. 2008). However, the applications of dendrimers are limited due to the presence of amino groups'. This group is cationic or positively charged, which makes them toxic. Therefore, the utilization of dendrimers requires some modifications to reduce their toxicities. Drugs are loaded into the dendrimers through electrostatic interaction, simple encapsulation, and covalent conjugation (Noriega-Luna et al. 2014). Drugs can be delivered in two ways. The first is in vivo delivery, where the degradation of the covalent bond of the dendrimers depends on the favorable environment and the enzymes available to cleave the bond. Ethynylene–perylene (EPer) would discharge the drug due to environmental changes such as temperature and pH (Tripathy and Das 2013). Drugs can be delivered orally, pulmonary, transdermally, ocularly, and targeted through dendrimers (Kesharwani et al. 2014).
Among the extensively documented studies on the application of dendrimer systems for therapeutic purposes COVID-19, numerous findings can be summarized. According to Itani et al., dendrimers can connect robustly with viruses and COVID-19 suppress invasion in target cells. This comprehensive analysis explored the prospective importance of nanoparticles such as theranostic dendrimers as suitable transporters for immune stimulators or cures to help combat COVID-19 (Prusty and Swain 2018). In an experiment comparable to Middle East coronavirus, Kandeel and co-workers investigated the antiviral potential of 16 different polyamidoamine dendrimers against SARS-CoV-2. In these intriguing experiments, polycationic dendrimers comprising the primary amine (-NH2-) class (grade 2/3/4 and/or 5) and three different categories of polyanionic dendrimers comprising the amido ethanol hydroxyl class (grade 2/3/4 and/or 5), the sodium carboxylate end group class (grade 1.5/2.5/3.5 and/or 4.5), succinic acid class (grade 2/3/4 and/or 5) were structured and assumed to have calcification inhibitory activity in SARS-CoV-2. The grade 1.5 CO2-Na polyamidoamine dendrimer inhibited SARS-CoV-2 calcification growth most strongly (inhibitory effect 40%), followed by the grade 5 polyamidoamine dendrimer with succinic acid (inhibitory effect 39.7%), which contains terminal grades 16 and 128. Vero cells showed a strong toxic effect in the presence of cationic dendrimers. The authors suggested that antiviral agents could be incorporated into these dendrimers for use as nanomedicines (Itani et al. 2020).
Inorganic nanoparticles
Inorganic nanoparticles, including gold, silver, silica, and iron oxide, possess exceptional potential applications. Gold and silver-like nanoparticles have properties like surface plasmon resonance (SPR), which is not possessed by dendrimers, liposomes, or micelles. They also have several advantages that include versatility and biocompatibility. Drug conjugation with gold nanoparticles can occur through covalent or ionic bonds. In addition, silver nanoparticles can exhibit antimicrobial activities. For example, a spongy and interconnected dextran/polyacrylamide nanohydrogel matrix covalently coupled with AuNPs could release the ornidazole with a 98.5% in vitro (Kandeel et al. 2020).
Experimental research with iron oxide (IO) nanoparticles targeting SARS-CoV-2 was explored. IO and metal oxide nanoparticles with the versatile properties of zinc oxide, titanium dioxide, and copper oxide are effective in antiviral diagnosis and treatment. Titanium dioxide nanoparticles destroy pathogens by generating reactive oxygen species. This is according to a recent paper that investigated the photocatalytic and antiviral properties of titanium dioxide. It tends to inhibit viruses, structurally destroy DNA, and degrade the phospholipid bilayer (Choudhury et al. 2005). Silver nanoparticles bind to viral proteins in two ways: (i) they attach to the outer membrane of the virus, preventing it from attaching to the cell surface receptor; and (ii) they attach to the viral nucleic acid (RNA or DNA), limiting transfection and viral replication within the host cell (Mahajan and Patil 2020). In addition, the entry of the antiviral transcription factors interferon regulatory factors (IRF)-7 into the nucleus of lung epithelial cells is suppressed by silver nanoparticles and mitochondrial cycling is disrupted (Halbus et al. 2017). According to a recent finding, SARS-CoV-2 may resist the antiviral effect of silver nanoparticles (Salleh et al. 2020).
Nanoemulsions
Nanoemulsions do not irritate or affect the skin. Therefore, they can effectively improve drug bioavailability (Villeret et al. 2018). In addition, nanoemulsions consist of small particles with a large surface area, which promotes absorbability (Abd Elkodous et al. 2021). To understand how some pathogenic viruses are affected by nanoemulsions of different essential oils, several experiments have been carried out. The majority of published studies assume that the nanoemulsions of various essential oils would interfere with the assembly of the virion envelope, thus blocking the attachment of viruses and their penetration into host cells (Naseema et al. 2021).
Nanoemulsions can compromise virion membrane integrity or obscure viral assembly, preventing SARS-CoV-2 penetration and adhesion into host cells. Furthermore, the encapsulated favipiravir nanoemulsion can suppress the viral polymerase enzyme of SARS-CoV-2, i.e., RNA-dependent RNA polymerase activity in infected cells. In conclusion, we predict that encapsulated favipiravir nanoemulsions could successfully prevent the adhesion of SARS-CoV-2 while disrupting its morphology (Franklyne et al. 2021).
Nanosuspensions
Nanosuspensions are submicron dispersions of hydrophobic drug particulates that have been stabilized with surfactants and possess a high affinity for target receptors (de M Ribeiro and Fonseca 2020). Nanosuspensions have high bioavailability and dissolution rate due to their small size and greater surface area (Jacob et al. 2020). In the recipient tissues, nanosuspensions can drastically limit viral replication and inhibit the virus from infiltrating host cells (Flühmann et al. 2019). Due to their extensive surface area and capacity to adhere various antigens to their interface, they are very often used in research. Furthermore, nanomaterials such as carbon quantum dots, gold nanoparticles, and carbon quantum dots have been characterized as both stimulating and preventing virus interaction in the host cells (Noyes and Whitney 1897).
Nanosuspensions are effective in delivering therapeutics that are lipid and water-insoluble (Szunerits et al. 2015). Additionally, they increase the drug solubility and rate of dissolution (Murtaza et al. 2020). Fucoxanthin (a carotenoid antioxidant) is readily soluble in organic solvents, i.e., acetone, and insoluble in water and exhibits excellent consistency and oxidative stability over a wide pH range. Acute toxicity was studied at a dose of 2000 mg/kg (LD50) and confirmed to be non-toxic. Conventional therapeutic administration is limited by the high dosage of fucoxanthin extract and its solubility properties. However, nanosuspension materials could easily overcome these limitations (Yousaf et al. 2021).
Quantum dots
Quantum dots have a size of 2–10 nm and are known as semiconductor nanocrystals. Their optical properties such as photoluminescence and absorption depend on their size (Muthuirulappan and Francis 2013). Nowadays, quantum dots have become very important in the field of nanomedicine. The emission of quantum dots is in the infrared range, i.e., below 650 nm. This is due to the lower scattering of light and low absorption in tissue, which makes them desirable for biomedical imaging (Muthuirulappan and Francis 2013; Volkov 2015).
Additionally, different quantum dot sizes and compositions can excite a similar source of light, culminating in different of emitting colors throughout a wide range of spectra (Liu et al. 2010; Xu et al. 2016). In this manner, quantum dots are highly captivating for multiplex imaging. Quantum dots are studied as sensors, target drug delivery, and bioimaging in the medicinal field. In literature, the in vivo imaging of the quantum dots is available for its application (Shi et al. 2015; Han et al. 2015).
Carbon-based quantum dots with a cationic external potential could be exploited to inactivate the SARS-CoV-2 S protein (Balaji et al. 2018). Furthermore, SARS-CoV-2 releases reactive oxygen species due to the binding of quantum dots with positive extrinsic characteristics to the virus-negative RNA motif (Ting et al. 2018). In a study conducted by Du et al., it was shown that carbon dots have antiviral properties against respiratory syndrome and pseudorabies virus (Dong et al. 2017). In addition, carbon dots trigger interferon-stimulated signaling pathways, including interferon-α formation, which limits viral replication. Integration of targeted substituents into quantum dots could also efficiently attach to the SARS-CoV-2 entry receptor and alter cellular reproduction (Du et al. 2016).
Protein and polysaccharide nanoparticles
Proteins and polysaccharides are natural biopolymers extracted from animals, plants, marine sources, and microorganisms (Iannazzo et al. 2018). Protein-based nanoparticles are usually metabolized, decomposable and facile for drug and other targeted ligand attachment. There are two ways to produce these nanoparticles. The first method is derived from water-soluble proteins such as human serum albumin and bovine serum albumin. The other option is to use insoluble proteins such as gliadin and zein. The usual synthesis is by desolvation or coacervation, complex coacervation, solvent/emulsion extraction, and electrospraying. These nanoparticles were chemically modified to integrate targeted compounds and localize tissues and cells to support the targeting mechanism (Bassas-Galia et al. 2017).
Polyguluronate sulfate, carrageenan, chitosan, and some derivatives of marine polysaccharides have potent inhibitory antagonistic potential against several viruses and provide a platform for conducting a study on coronaviruses (Lohcharoenkal et al. 2014). The most common cause of severe infectious diseases of the upper respiratory tract sometimes referred to as the common cold are respiratory viruses such as influenza viruses, coronaviruses, and rhinoviruses (Morokutti-Kurz et al. 2017; Tomas et al. 2022). Clinical studies have shown that using an iota carrageenan nasal spray to relieve the common cold can shorten the severity of the infection. The antiviral efficacy of carrageenan nasal spray was found to be significant for three categories of viruses: influenza A virus, human coronavirus, and human rhinovirus, with the greatest success observed in individuals with human coronavirus. In individuals with coronavirus treated with Carra-Gene, the severity of infection was 3 days shorter (p < 0.01), and the frequency of exacerbations was threefold lower (p < 0.01) than in untreated individuals (Morokutti-Kurz et al. 2017). In addition, sulfated polysaccharides such as sulfated rham-nan and fucoidan may impair the development and function of the epidermal growth factor receptor or decrease its signal transduction, which could improve the silencing function of coronavirus (Monto et al. 2001; Wang et al. 2017).
Role of nanomedicine in the management of COVID-19
One of the most pressing issues over the past pandemics is the absence of a viable therapy for viral diseases such as SARS-CoV-1, MERS-1, and SARS-CoV-2. As a result, repurposed therapeutics are more beneficial than newer drugs developed in treating COVID-19 outbreaks. A new vaccine typically takes a long time to produce, requiring multiple rounds of guidelines and trials. One of the major challenges in successfully treating COVID-19 is insufficient cellular uptake of the newly developed antiviral drugs. Their usage is also limited due to several factors such as low bioavailability, poor water solubility, increased toxicity, lower biological potential, and a high risk-to-benefit ratio (Wang et al. 2018; Ansari et al. 2020).
Consequently, these limitations would limit the prospects of new techniques to increase the efficacy of repurposed drugs and their better absorption into the body. To overcome this limitation, a variety of nanomedicine-based systems have been developed to enhance the therapeutic efficacy of newly developed antiviral drugs (Lembo et al. 2018). Antiviral drugs based on nanomedicine offer several opportunities to overcome the drawbacks of current antiviral therapeutics (Zazo et al. 2016). Several fundamental obstacles, such as low bioavailability and poor aqueous solubility, can be addressed through nanomedicine design. This involves modifying the pharmacokinetic and pharmacodynamic properties of drugs to achieve lower toxicity and lower dose, improve drug bioavailability, and inhibit viral spread (Sahakijpijarn et al. 2020; Ahmad et al. 2016). Additionally, nanomedicine can penetrate cellular membranes and concentrate therapeutic effects in viral persistence sites (Akhter et al. 2012). One of the most attractive approaches to minimize the adverse effects of viral rebound and patient compliance during the course of therapy is nanomedicine with intrinsically regulated or prolonged release capabilities (Yadav et al. 2019). Nanocarrier technology could thus be used to repurpose drugs and optimize treatment efficacy COVID-19 (Li et al. 2020a, b).
The upsides of nanocarriers include their high drug loading effectiveness, larger surface area, smaller in size, prolonged drug releasing pattern, and enhanced optimum performance of the loaded pharmaceuticals in such a novel drug delivery system (Fig. 3) (Sahakijpijarn et al. 2020; Ahmad et al. 2016; Wankar et al. 2020). Nanotechnology has a specific physicochemical advantage in medicine due to its unique hydrophobic or hydrophilic nature, nanosize, nanoshape, and the possibility of ligand conjugation on its membrane. These nanoscale physicochemical features would improve pharmaceutical applications' efficiency, specificity, functionality, and sensitivity (Kobayashi et al. 2014). Several nanomedicine groups could be repurposed to treat COVID-19 therapy, and they are further summarized below. Table 2 outlines the findings of nanomedicine as a strategy to augment the pharmacological output of several repurposed medications with the potential for COVID-19 treatment.
Role of nanomedicine drug delivery in COVID-19. Nanomedicine offers numerous characteristics that may be effectively used to transport therapeutic compounds to infected cells, and the tailored ligand-coupled nanoparticle selectively interacts with viral residues, resulting in virus destruction and inability to penetrate the cells
Liposomes targeted drug delivery system for COVID-19
The liposomes are the bilayers of lipid vesicles and are currently utilized to deliver aquaphobic drugs (Puri et al. 2009). The liposomes would function as stealth liposomes whereby the systemic circulation is long, targeted, and stimulus-responsive for drug delivery. As liposome is complex and biologically degraded in nature, it is one of the methods of choice for the carrier of drug formulation. Moreover, it is also a productive platform for developing a novel formulation of COVID-19 diseases. Studies have found that synthetic peptides loaded on the liposomes are potential venues for treating SARS coronavirus infection (Lokhande 2018). Liposomes that are chemically conjugated peptides have been used in the treatment of SARS because they are suitable for the cytotoxic T-lymphocyte strategy that reduces viral load. A novel hydroxychloroquine formulation administered to Sprague–Dawley rats had shown efficacy against COVID-19. Unlike the IV injection, the formulation is breathed and has a greater concentration of medication in the lungs, a longer half-life, and less exposure to other organs such as the heart. Liposome as a carrier system for the delivery of drugs in the treatment of COVID-19 has many advantages. Forty percentage of small molecule drugs used to treat cancer are poorly soluble in water. Therefore, a similar drug delivery method is needed immediately to encapsulate the drug and increase its water solubility (Wankar et al. 2020).
Cyclodextrin combination targeted drug delivery system for COVID-19
Cyclodextrin (a biocompatible natural polysaccharide) has α, β, and γ as essential components. According to reports, cyclodextrin nanoparticles are gaining importance due to numerous cyclodextrin components that absorb a greater amount of therapeutics than normal cyclodextrin (Sun et al. 2014). Cyclodextrin-based remdesivir nanoformulation has just received Food Drug Administration (FDA) approval for the treatment of COVID-19 (Khalaj‐Hedayati et al. 2020). In the clinical context, it has shown encouraging results in various viruses, including Middle East respiratory syndrome coronavirus and Nipah virus (Jones et al. 2020). In contrast to its function as a medical dispersant for therapeutics, which are primarily hydrophobic, cyclodextrin also possesses antiviral activities (De Wit et al. 2020). Therefore, cyclodextrin could be used as a delivery strategy for respiratory sprays for therapeutics that require targeted selectively to the pulmonary system (Khalaj‐Hedayati et al. 2020).
Inorganic and metallic-based nanocomposites targeted drug delivery system for COVID-19
Inorganic nanocomposites for therapeutics delivery can be developed using a variety of metallic nanoparticles, including platinum, silica, silver, gadolinium, gold, and its nanocrystal conglomerates (Kumari et al. 2017). Silver nanoparticles are known for their extensive antibacterial activity and are an essential ingredient in pharmaceutical formulations, e.g., silver sulfasalazine (a sulfa antibiotic), where silver particles support the antibacterial activity of the sulfasalazine. Silver nanoparticles are also being studied for their possible virucidal properties. It has been observed that SARS-CoV-2 attaches to cell membrane and persists there for up to 12 h, while the silver nanoparticle-based antibacterial film can eradicate the virus (Chaturvedi et al. 2020). This film can be sprayed on metal, glass, and ceramic surfaces to hamper the proliferation of SARS-CoV-2 in busy places such as universities, hospitals, and marketplaces (Prasad et al. 2015).
Polymeric solid colloidal nanoparticles targeted drug delivery system for COVID-19
Due to their unique properties, including targeted drug delivery, controlled drug release, improved tolerability, and efficiency, PN promotes stable in vivo delivery of therapeutic agents (De Wit et al. 2020; Balagna et al. 2020). Due to their nanosize, they also prevent large dose-dependent side effects and penetration through biological membranes. This favorable property of polymeric nanoparticles can be exploited for drug delivery to revive currently accessible therapeutics for the treatment of the SARS-CoV-2 outbreak. Zhang et al. formulated nanoparticles based on PLGA [poly (lactic-co-glycolic acid)] polymers to combat the infection rate of SARS-CoV-2. PLGA was used as the internal base component in a membrane-enveloped nanoparticle derived from human macrophages and human lung epithelial cells of the II type, which exhibit the same physiological processes as SARS-CoV-2 invading recipient cells (Verma et al. 2017). During the incubation period, these biological nanoparticles serve as a receptive target for SARS-CoV-2, where it is destroyed and can no longer enter recipient cells (Zhang et al. 2020a, b). Therefore, the polymer-based nanoparticles may provide a nanocarrier platform for the delivery of therapeutics against SARS-CoV-2 contamination.
Nano-based vaccines
The sudden appearance of COVID-19 has necessitated the immediate use of drugs to combat the virus. Health professionals have attempted various methods to manage the symptoms. Still, due to certain factors including low solubility, permeability, and the limitation of targeting competence, they could not be used as they were unable to provide the required therapeutic effect. To deliver the drug to the appropriate venue with the fewest adverse effects, nanotechnology has been essential (Kumari et al. 2018). They could transport the therapeutic molecule to the infected cells, and the targeted liganded nanoparticles would bind with the viral epitopes, deactivating the virus and preventing it from entering the cell. According to the WHO report on COVID-19 candidate vaccines, 52 possible vaccines are undergoing clinical trials, while 162 are in the preclinical stage of development throughout December 8, 2020 (Raj et al. 2021). The platforms include virus-like particles, DNA, protein subunits, RNA, inactivated viruses, and non-replicating viral vectors. With a pandemic like COVID-19, a huge amount of vaccines is needed, which is not made possible with the existing production of vaccines (Ashraf et al. 2021). In this case, the noble way of drug delivery has assisted, including reverse vaccinology, nanomedicine, next-generation sequencing, and human challenge studies. The nanoparticle-based vaccination has elicited a strong immunological response that is well-tolerated. Gold, silver sulfide, titanium oxide, zirconium, and graphene are some of the materials that have been considered (Haslberger et al. 2020). Drug delivery, cancer nanovaccines, immunological engineering, nanosensors, and platform technologies are some techniques employed by nanoresearchers.
In the case of the increasing number of pathogenic bacteria that are resistant to antimicrobial agents, there are several studies that have demonstrated the efficacy of nanotechnology-based antimicrobial therapy (Nikaeen et al. 2020). Targeted nanotechnology is a new resource for the treatment of antiviral diseases. The employment of nanoparticles in the battle against SARS-CoV-2 has created a mechanism that inhibits the virus's entry into the host cell until it is inactivated. The targeted nanoparticles specific to virus-expressed protein would minimize the internalization of the virus. Metal nanoparticles can prevent the virus from attaching to the surface of the cell and impair the virus's ability to replicate once it enters the cell (Kumar et al. 2018a, b). The size, shape, and surface charge of nanoparticles would determine how effective the treatment is. However, several safety precautions need to be taken to reduce host cell cytotoxicity. Well-established nanotechnology drug delivery would improve traditional therapies in medicine, as seen in the SARS-CoV-2 outbreak, but its use in viral diseases is still relatively unknown. By shortening PCR assays, reducing detection time, improving sensitivity, amplifying signals, and using them as adjuvants in vaccines, nanostructure technology could help diagnose disease. Nanotechnology has the potential to assist in the pathogenesis of COVID-19 in a variety of ways, including attenuation, possible membrane attachment and fusion upon viral entry, and protein fusion in infected cells. Activating intracellular pathways for reversible virus damage and deactivating viral transcription, translation, and replication may be more effective with nanocapsulated vaccines (Maji et al. 2020).
To increase half-life and longevity and to elicit adequate cellular and antibody responses, nanoparticles can serve as biologics or cargo for vaccination, sometimes referred to as nanovaccines, among other factors (Varahachalam et al. 2021). The basic principle of this approach is to integrate an epitope that triggers protective immunity into nanoparticles of 1–100 nm and establish an immunological storage that protects the body from emerging harmful microorganisms. Moreover, suitable protein nanoparticles from the architectural proteins of SARS-CoV-2, i.e., envelope (E), membrane (M), nucleocapsid (N), and spike (S), can be transported to target immune systems that would trigger immune feedback and confer durable resistance to the virus. Protein nanoparticles and biologics have already been used for Middle East Coronavirus 1 and Severe Acute Respiratory Syndrome, and they could also be developed for SARS-CoV-2 (Coleman et al. 2014; Xiang et al. 2006). Pimentel et al. developed a nanovaccine using a repetitive SARS-CoV-1 S protein antigen of around 25 nm to design a targeted antibody that can prevent contamination with SARS-CoV-1 (Pimentel et al. 2009). Matsuura et al. designed virus-like nanoparticles that stimulate a viral protein. We hypothesize that these nanoparticles can also be used to produce SARS-CoV-2 proteins for vaccination applications (Matsuura et al. 2010). Microparticles (25 nm) depending on epitope selectivity and adjuvant strategies have been utilized to enhance the transport of peptide/protein epitopes to dendritic cells to trigger both cellular and humoral immunological responses in rats (Matsuura et al. 2010). Numerous nanoparticles, including nanobioceramics, liposomes, cationic polymers, polymer-based particles, gold nanoparticles, virus-like nanoparticles, and peptide nanoparticles, can be used to stimulate lymphatic circulation and produce B lymphocytes (B cells) and T lymphocytes (T cells) for efficient clearance of SARS-CoV-2 (Reddy et al. 2008; Gregory et al. 2013; Wilson et al. 2018; Koshy et al. 2017; Chen et al. 2016). Stimulating CD8 + (cytotoxic) T lymphocyte cells or CD4 + (helper) T lymphocyte cells is essential for any proposed vaccination. In the case of coronavirus, T lymphocyte cells have shown the potential to prevent relapse and provide immunologic maintenance for up to 11 years after exposure to SARS-CoV-1 in recovered patients (Goodwin and Huang 2017; Tang et al. 2011).
Research shows that DNA vaccination also induces neutralization and preventive resistance to SARS-CoV-1 through upregulation of neutralizing antibodies, clusters of differentiation 4 (CD4 +), and clusters of differentiation 8 (CD8 +), suggesting that DNA vaccination can be delivered with appropriate nanoparticles. In addition, the antigens of B lymphocyte cells and T lymphocyte cells in the immunological domains of SARS-CoV-1 and SARS-CoV-2 were evaluated in a random analysis and were shown to be equivalent for both infections (Peng et al. 2006). This implies that SARS-CoV-1 vaccinations may be more effective at getting rid of SARS-CoV-2. Numerous properties may be considered for optimizing a SARS-CoV-1 vaccine against SARS-CoV-2, namely enhancing epitope integrity, cytokine production, specific target allocation, prolonged release of biologics and/or epitopes, sequestration of SARS-CoV-2 infections, and prevention of peripheral manifestations of infection (Ahmed et al. 2020; Rao et al. 2020). Table 3 provides an overview of some of the most promising COVID-19 vaccine alternatives that have been formally clinically evaluated (Vijayan et al. 2019).
Challenges in treatment with nano-based medicines
Nanovaccines and nanomedicine that have been utilized in novel medicines to fight against the continuous pandemic, like all current medications, must demonstrate their safety and be studied extensively before their release on the market. As the interaction between engineered nanomaterials (ENMs) with viral targets (envelope, capsid, and nucleic acid) is comprehended by many, the prediction and screening of ENM–virus interactions require a robust analytical framework. Toxicity relevant to inorganic nanoparticles is the limiting factor of their usage in various cases. Other beneficial biological properties can be made by changing the shape, size, and surface chemistry. Moreover, the physicochemical properties must be modified to reduce the side effects of the nanoparticles. ENMs should have a variable dose. Antiviral stability and bioavailability should be considered in many therapeutic applications. The main challenges of nanodelivery come in the form of physiological barriers, which are the mucosa and the alveolar fluid as they are utilized for intranasal as well as the reticuloendothelial structure and used for systemic delivery (Le et al. 2020). In the cell, the nanoparticle must be able to reject the endocytic degradation component. Certain studies also have shown that nanoparticles could cause damage to respiratory sites and affect lung function. The four key pathobiological aspects of nanoparticles, which include inflammation, genotoxicity, oxidative stress, and fibrosis, must be examined further. Certain studies have yet to prove whether a nasal or oral nanovaccine is safer and provides longer mucosal immunity and viral protection than others. That being said, the ability to provide adequate protection when administered to different age groups by different routes has yet to be confirmed. There are various nanomaterials ranging from polymers to dendrimers to nanoparticles, oligomers, liposomes and tiny molecules. However, once the virus chemical complex dissociates and the viruses are released, they lose their effectiveness and the cycle begins again. The reactive oxygen species formed by nanoparticles would lead to oxidative stress as it can cause a disturbance in mitochondrial respiration. This would cause harmful pathological processes in the cell that cause toxicity. In various studies, it was found that titanium dioxide could enter mice's lungs, causing nanoparticle accumulation to increase the level of lipid peroxidation and minimize the number of antioxidant cells treated by nanoparticles. Due to this, the level of the AP-1 transcription factor increased and affected the transcriptional process. DNA would interact with the carbon nanotubes (Zhang et al. 2021a, b). Although nanoparticles have gained tremendous importance in research because they are very effective, their toxicological aspects need to be considered. The way nanoparticles act in the human body is of critical importance. When deposited to the maximum, they can cause harmful side effects, especially with platinum-containing drugs (cisplatin, carbapenem) used in the treatment of lungs, colorectal, ovarian, head, neck, and bladder. Due to this, its use is limited as the prominent side effect of it is nephrotoxicity. The major challenge in translating nanodrugs is the first clearance by the RES that minimizes the efficacy and the increasing toxicity in the organs such as the spleen, liver, and kidney (Ting et al. 2018; Yan et al. 2020). Iron oxide particles have harmful factors both in vivo and in vitro as they generate reactive oxygen species (ROS).
Nevertheless, the iron oxide is coated with a polymer that would maximize cell viability. When hydroxyl group is added to the gadolinium fullerene particle, it prevents the formation of ROS and excessive release of pro-inflammatory cytokines, which can cause mortality and severe infection. Macrophages and monocyte lineage significantly control the cytokine-facilitated inflammatory response and innate and adaptive response (Zhang et al. 2021a, b; Shanley et al. 2021).
Future prospects
COVID-19 is a major global threat and the greatest challenge to humanity since World War II. The pandemic has caused many calamities that disrupted health and caused human casualties and other issues. Despite numerous attempts by all countries across the world, including extensive research and numerous clinical trials, no medication has yet been demonstrated to control the virus. This study has highlighted several traditional treatment strategies as well as the crucial function of nanomedicine in the new SARS-CoV-2. Drug substitution, such as remdesivir and chloroquine, is a quick way to achieve safe therapies. Furthermore, several relevant experimental types of research have revealed positive evidence against COVID-19. Nanometal vaccines for example, which are based on the antigenic properties of protein S, have been utilized to reduce viral load. However, a substantial number of studies that support the use of NP-based diagnostic and treatment techniques for SARS-CoV-2 have been published. As a result, all researchers across the world are encouraged to extend their studies in the field of NP-based therapy. There is a great requirement for adequate information on the virus's virulence and transmission to prevent and cure COVID-19 or any other viral pandemic. In the future, this will lead to the discovery of numerous unstructured encoded proteins, enzymes, and functional processes and enable us to understand viral transmission between species. As a result, we could use nanoparticles at the functional level to identify and establish therapeutic targets. As a result, a clinical trial to develop an effective nanovaccine can be conducted with the appropriate knowledge of the viral life cycle and host response. A universal NP-based vaccine with sufficient immunogenicity is the ultimate goal in overcoming the pandemic, as endorsed by researchers worldwide. With factors such as fast detection and mobility, microfluidics would play an essential role in the diagnosis of CoV.
Conclusion
Other than vaccines, there are no approved SARS-CoV-2 therapies being explored in clinical trials. In addition, the nano-based vaccine to treat SARS-CoV-2 has not been adequately studied. All efforts to combat the virus should be explored, and nanotechnology approaches should be prioritized because they may offer new perspectives, particularly in preventing or treating viral internalization compared with standard therapy. To develop a rational design for targeted therapies, further research is also required to comprehend the relationship between CoV and nanoparticles. As the pathophysiology of SARS-CoV-2 is unclear, nanotechnology might be a beneficial technique apart from other methods to produce excellent COVID-19 therapy results.
References
Abd Elkodous M, Olojede S, Morsi M, El-Sayyad GS (2021) Nanomaterial-based drug delivery systems as promising carriers for patients with COVID-19. RSC Adv 11(43):26463–26480
Agrawal U, Katikireddi SV, McCowan C, Mulholland RH, Azcoaga-Lorenzo A, Amele S, Fagbamigbe AF, Vasileiou E, Grange Z, Shi T, Kerr S, Moore E, Murray JLK, Shah SA, Ritchie L, O’Reilly D, Stock SJ, Beggs J, Chuter A, Torabi F, Akbari A, Bedston S, McMenamin J, Wood R, Tang RSM, de Lusignan S, Hobbs FDR, Woolhouse M, Simpson CR, Robertson C, Sheikh A (2021) COVID-19 hospital admissions and deaths after BNT162b2 and ChAdOx1 nCoV-19 vaccinations in 2·57 million people in Scotland (EAVE II): a prospective cohort study. Lancet Respir Med 9(12):1439–1449
Ahammed V, Narayan R, Paul J, Nayak Y, Roy B, Shavi GV, Nayak UY (2017) Development and in vivo evaluation of functionalized ritonavir proliposomes for lymphatic targeting. Life Sci 183:11–20
Ahmad MZ, Alkahtani SA, Akhter S, Ahmad FJ, Ahmad J, Akhtar MS, Mohsin N, Abdel-Wahab BA (2016) Progress in nanotechnology-based drug carrier in designing of curcumin nanomedicines for cancer therapy: current state-of-the-art. J Drug Target 24(4):273–293
Ahmed SF, Quadeer AA, McKay MR (2020) Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses 12(3):254
Akhter S, Ahmad MZ, Ahmad FJ, Storm G, Kok RJ (2012) Gold nanoparticles in theranostic oncology: current state-of-the-art. Expert Opin Drug Deliv 9(10):1225–1243
Alafif T, Tehame AM, Bajaba S, Barnawi A, Zia S (2021) Machine and deep learning towards COVID-19 diagnosis and treatment: survey, challenges, and future directions. Int J Environ Res Public Health 18(3):1117
Alcoba-Florez J, González-Montelongo R, Íñigo-Campos A, de Artola DGM, Gil-Campesino H, Team TMTS, Ciuffreda L, Valenzuela-Fernández A, Flores C (2020) Fast SARS-CoV-2 detection by RT-qPCR in preheated nasopharyngeal swab samples. Int J Infect Dis 97:66–68
Ansari MA, Almatroudi A, Alzohairy MA, AlYahya S, Alomary MN, Al-Dossary HA, Alghamdi S (2020) Lipid-based nano delivery of Tat-peptide conjugated drug or vaccine–promising therapeutic strategy for SARS-CoV-2 treatment. Expert Opin Drug Deliv 17(12):1671–1674
Arslan S, Delice O, Kahraman M, Yilmaz S, Aslan M (2021) Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in Turkey. Ann Clin Anal Med. pp 483–487.
Asdaq SMB, Ikbal AMA, Sahu RK, Bhattacharjee B, Paul T, Deka B, Fattepur S, Widyowati R, Vijaya J, Al Mohaini M, Alsalman AJ (2021) Nanotechnology integration for SARS-CoV-2 diagnosis and treatment: an approach to preventing pandemic. Nanomaterials 11(7):1841
Ashraf MU, Kim Y, Kumar S, Seo D, Ashraf M, Bae YS (2021) COVID-19 vaccines (revisited) and oral-mucosal vector system as a potential vaccine platform. Vaccines 9(2):171
Balagna C, Perero S, Percivalle E, Nepita EV, Ferraris M (2020) Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceram 1:100006
Balaji AB, Pakalapati H, Khalid M, Walvekar R, Siddiqui H (2018) Natural and synthetic biocompatible and biodegradable polymers. Biodegradable and biocompatible polymer composites. Woodhead Publishing, Duxford, pp 3–32
Bandodkar AJ, Wang J (2014) Non-invasive wearable electrochemical sensors: a review. Trends Biotechnol 32(7):363–371
Barnett JM, Monnier BM, Tyler S, West D, Ballantine-Dykes H, Regan E, Wraith P, Kiely J, Luxton R (2019) Initial trail results of a magnetic biosensor for the rapid detection of Porcine Reproductive and Respiratory Virus (PRRSV) infection. Sens Bio-Sens Res 27:100315
Barrett ADT, Titball RW, MacAry PA, Rupp RE, von Messling V, Walker DH, Fanget NVJ (2022) The rapid progress in COVID vaccine development and implementation. NPJ Vaccines 7(1):20
Bassas-Galia M, Follonier, S, Pusnik M, Zinn M (2017) Natural polymers: a source of inspiration. In Bioresorbable polymers for biomedical applications. Elsevier: pp 31–64
Benzigar MR, Bhattacharjee R, Baharfar M, Liu G (2021) Current methods for diagnosis of human coronaviruses: pros and cons. Anal Bioanal Chem 413(9):2311–2330
Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, Lin B, Zhu X, Li K, Li S (2020) Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology 295(3):685–691
Boopathi S, Poma AB, Kolandaivel P (2021) Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomol Struct Dyn 39(9):3409–3418
Bos R, Rutten L, van der Lubbe JE, Bakkers MJ, Hardenberg G, Wegmann F, Zuijdgeest D, de Wilde AH, Koornneef A, Verwilligen A, van Manen D (2020) Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. npj Vaccines 5(1):91
Bozzuto G, Molinari A (2015) Liposomes as nanomedical devices. Int J Nanomed 10:975
Broza YY, Mochalski P, Ruzsanyi V, Amann A, Haick H (2015) Hybrid volatolomics and disease detection. Angew Chem Int Ed 54(38):11036–11048
Broza YY, Vishinkin R, Barash O, Nakhleh MK, Haick H (2018) Synergy between nanomaterials and volatile organic compounds for non-invasive medical evaluation. Chem Soc Rev 47(13):4781–4859
Calucho E (2020) Lateral flow devices for COVID-19-related biomarkers. Biosens Pandemics. Vol. 29
Carter LJ, Garner LV, Smoot JW, Li Y, Zhou Q, Saveson CJ, Sasso JM, Gregg AC, Soares DJ, Beskid TR (2020) Assay techniques and test development for COVID-19 diagnosis. ACS Publications, Washington
Chakravarty M, Vora A (2021) Nanotechnology-based antiviral therapeutics. Drug Deliv Transl Res 11(3):748–787
Chamundeeswari M, Jeslin J, Verma ML (2019) Nanocarriers for drug delivery applications. Environ Chem Lett 17(2):849–865
Chandler-Brown D, Bueno AM, Atay O, Tsao DS (2020) A highly scalable and rapidly deployable RNA extraction-free COVID-19 assay by quantitative Sanger sequencing. Biorxiv
Chaturvedi VK, Yadav N, Rai NK, Ellah NHA, Bohara RA, Rehan IF, Marraiki N, Batiha GES, Hetta HF, Singh M (2020) Pleurotus sajor-caju-mediated synthesis of silver and gold nanoparticles active against colon cancer cell lines: a new era of herbonanoceutics. Molecules 25(13):3091
Chaudhari SP, Handge NM (2020) Formulation, development and evaluation of lopinavir loaded polymeric micelles. J Sci Technol 5:173–187
Chen HW, Huang CY, Lin SY, Fang ZS, Hsu CH, Lin JC, Chen YI, Yao BY, Hu CMJ (2016) Synthetic virus-like particles prepared via protein corona formation enable effective vaccination in an avian model of coronavirus infection. Biomaterials 106:111–118
Chen Y, Ma J, Xu M, Liu S (2020a) Antiviral nanoagents: more attention and effort needed? Nano Today 35:100976
Chen Z, Zhang Z, Zhai X, Li Y, Lin L, Zhao H, Bian L, Li P, Yu L, Wu Y (2020b) Rapid and sensitive detection of anti-SARS-CoV-2 IgG, using lanthanide-doped nanoparticles-based lateral flow immunoassay. Anal Chem 92(10):7226–7231
Cheng Y, Xu Z, Ma M, Xu T (2008) Dendrimers as drug carriers: applications in different routes of drug administration. J Pharm Sci 97(1):123–143
Cho H, Lai TC, Tomoda K, Kwon GS (2015) Polymeric micelles for multi-drug delivery in cancer. AAPS Pharm Sci Tech 16(1):10–20
Choudhury KR, Sahoo Y, Ohulchanskyy T, Prasad P (2005) Efficient photoconductive devices at infrared wavelengths using quantum dot-polymer nanocomposites. Appl Phys Lett 87(7):073110
Coleman CM, Liu YV, Mu H, Taylor JK, Massare M, Flyer DC, Glenn GM, Smith GE, Frieman MB (2014) Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine 32(26):3169–3174
Comentale G, Manzo R, Pilato E (2020) Sars-Cov-2 interference in HEME production: is it the time for an early predictive biomarker? J Mol Med 98(8):1053–1054
Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML (2020) Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 25(3):2000045
Craw P, Balachandran W (2012) Isothermal nucleic acid amplification technologies for point-of-care diagnostics: a critical review. Lab Chip 12(14):2469–2486
Daemi HB, Kulyar MF, He X, Li C, Karimpour M, Sun X, Zou Z, Jin M (2021) Progression and trends in virus from influenza A to COVID-19: an overview of recent studies. Viruses 13(6):1145
de Ribeiro LNM, Fonseca BB (2020) The role of pharmaceutical nanotechnology in the time of COVID-19 pandemic. Future Microbiol 15(16):1571–1582
De Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H (2020) Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci 117(12):6771–6776
Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, Adriano A, Beese S, Dretzke J, di Ruffano LF (2020) Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev 2020:CD013652
Dincer C, Bruch R, Kling A, Dittrich PS, Urban GA (2017) Multiplexed point-of-care testing–xPOCT. Trends Biotechnol 35(8):728–742
Dong X, Moyer MM, Yang F, Sun YP, Yang L (2017) Carbon dots’ antiviral functions against noroviruses. Sci Rep 7(1):1–10
Drobysh M, Ramanaviciene A, Viter R, Chen CF, Samukaite-Bubniene U, Ratautaite V, Ramanavicius A (2022) Biosensors for the determination of SARS-CoV-2 virus and diagnosis of COVID-19 infection. Int J Mol Sci 23(2):666
Du T, Liang J, Dong N, Liu L, Fang L, Xiao S, Han H (2016) Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon 110:278–285
Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, Aileni VK, Kanungo S, Rai S, Reddy P, Verma S, Singh C, Redkar S, Mohapatra S, Pandey A, Ranganadin P, Gumashta R, Multani M, Mohammad S, Bhatt P, Kumari L, Sapkal G, Gupta N, Abraham P, Panda S, Prasad S, Bhargava B, Ella K, Vadrevu KM, COVAXIN Study Group (2021) Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet (London, England) 398(10317):2173–2184
Esbin MN, Whitney ON, Chong S, Maurer A, Darzacq X, Tjian R (2020) Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID-19 detection. RNA 26(7):771–783
Flühmann B, Ntai I, Borchard G, Simoens S, Mühlebach S (2019) Nanomedicines: the magic bullets reaching their target? Eur J Pharm Sci 128:73–80
Franklyne JS, Gopinath PM, Mukherjee A, Chandrasekaran N (2021) Nanoemulsions: the rising star of antiviral therapeutics and nanodelivery system—Current status and prospects. Curr Opin Colloid Interface Sci 54:101458
Freitas RA Jr (2005) What is nanomedicine? Nanomedicine: nanotechnology. Biology Med 1(1):2–9
Garg B, Beg S, Kumar R, Katara O, Singh B (2019) Nanostructured lipidic carriers of lopinavir for effective management of HIV-associated neurocognitive disorder. J Drug Deliv Sci Technol 53:101220
Goodwin TJ, Huang L (2017) Investigation of phosphorylated adjuvants co-encapsulated with a model cancer peptide antigen for the treatment of colorectal cancer and liver metastasis. Vaccine 35(19):2550–2557
Gregory AE, Titball R, Williamson D (2013) Vaccine delivery using nanoparticles. Front Incell Infect Microbiol 3:13
Habibi R, Burci GL, De Campos TC, Chirwa D, Cinà M, Dagron S, Eccleston-Turner M, Forman L, Gostin LO, Mier BM (2020) Do not violate the international health regulations during the COVID-19 outbreak. The Lancet 395(10225):664–666
Halbus AF, Horozov TS, Paunov VN (2017) Colloid particle formulations for antimicrobial applications. Adv Coll Interface Sci 249:134–148
Han HS, Niemeyer E, Huang Y, Kamoun WS, Martin JD, Bhaumik J, Chen Y, Roberge S, Cui J, Martin MR (2015) Quantum dot/antibody conjugates for in vivo cytometric imaging in mice. Proc Natl Acad Sci 112(5):1350–1355
Han GR, Ki H, Kim MG (2019) Automated, universal, and mass-producible paper-based lateral flow biosensing platform for high-performance point-of-care testing. ACS Appl Mater Interfaces 12(1):1885–1894
Hardick J, Metzgar D, Risen L, Myers C, Balansay M, Malcom T, Rothman R, Gaydos C (2018) Initial performance evaluation of a spotted array Mobile Analysis Platform (MAP) for the detection of influenza A/B, RSV, and MERS coronavirus. Diagn Microbiol Infect Dis 91(3):245–247
Harvey CJ, O’Regan DP (2020) Radiological investigations and applications. Med MRCP 17:111
Haslberger A, Jacob U, Hippe B, Karlic H (2020) Mechanisms of selected functional foods against viral infections with a view on COVID-19: Mini review. Funct Foods Health Dis 10(5):195–209
Heymann DL, Shindo N (2020) COVID-19: what is next for public health? The Lancet 395(10224):542–545
Hofmann MA, Renzullo S, Mader M, Chaignat V, Worwa G, Thuer B (2008) Genetic characterization of toggenburg orbivirus a new bluetongue virus, from goats. Switzerland. Emerg Infect Dis 14(12):1855
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395(10223):497–506
Huang Z, Jiang Q, Wang Y, Yang J, Du T, Yi H, Li C, Li Y, Wu Z, Fan S, Liao Y, Zhang Y, Wang L, Jiang G, Tang D, Ye Y, Wang C, Li Z, Li Z, Zhang C, Ma K, Li Q (2021) SARS-CoV-2 inactivated vaccine (Vero cells) shows good safety in repeated administration toxicity test of Sprague Dawley rats. Food Chem Toxicol 152:112239
Iannazzo D, Pistone A, Ferro S, De Luca L, Monforte AM, Romeo R, Buemi MR, Pannecouque C (2018) Graphene quantum dots based systems as HIV inhibitors. Bioconjug Chem 29(9):3084–3093
Ishihara T, Kaneko K, Ishihara T, Mizushima T (2014) Development of biodegradable nanoparticles for liver-specific ribavirin delivery. J Pharm Sci 103(12):4005–4011
Islam A, Ahsan Z (2020) Plausible approach for rapid detection of SARS-CoV-2 virus by magnetic nanoparticle based biosensors. Am J Nanosci 6:6
Itani R, Tobaiqy M, Al Faraj A (2020) Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics 10(13):5932
Jacob S, Nair AB, Shah J (2020) Emerging role of nanosuspensions in drug delivery systems. Biomater Res 24(1):1–16
Jaimes JA, Millet JK, Stout AE, André NM, Whittaker GR (2020) A tale of two viruses: the distinct spike glycoproteins of feline coronaviruses. Viruses 12(1):83
Jan N, Madni A, Khan S, Shah H, Akram F, Khan A, Ertas D, Bostanudin MF, Contag CH, Ashammakhi N, Ertas YN (2022) Biomimetic cell membrane‐coated poly (lactic‐co‐glycolic acid) nanoparticles for biomedical applications. Bioeng Transl Med. p e10441
Javan F, Vatanara A, Azadmanesh K, Nabi-Meibodi M, Shakouri M (2017) Encapsulation of ritonavir in solid lipid nanoparticles: in-vitro anti-HIV-1 activity using lentiviral particles. J Pharm Pharmacol 69(8):1002–1009
Jiang M, Pan W, Arasthfer A, Fang W, Ling L, Fang H, Daneshnia F, Yu J, Liao W, Pei H (2020) Development and validation of a apid, single-step reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) system potentially to be used for reliable and high-throughput screening of COVID-19. Front Cell Infect Microbiol 10:331
Jones ST, Cagno V, Janeček M, Ortiz D, Gasilova N, Piret J, Gasbarri M, Constant DA, Han Y, Vuković L (2020) Modified cyclodextrins as broad-spectrum antivirals. Sci Adv 6(5):eaax9318
Kalita MJ, Dutta K, Hazarika G, Dutta R, Kalita S, Das PP, Sarma MP, Banu S, Idris M, Talukdar AJ (2021) In-house reverse transcriptase polymerase chain reaction for detection of SARS-CoV-2 with increased sensitivity. Sci Rep 11(1):1–12
Kandeel M, Al-Taher A, Park BK, Kwon HJ, Al-Nazawi M (2020) A pilot study of the antiviral activity of anionic and cationic polyamidoamine dendrimers against the Middle East respiratory syndrome coronavirus. J Med Virol 92(9):1665–1670
Katata-Seru L, Ojo BM, Okubanjo O, Soremekun R, Aremu OS (2020) Nanoformulated Eudragit lopinavir and preliminary release of its loaded suppositories. Heliyon 6(5):e03890
Kaur I, Sharma A, Jakhar D, Das A, Aradhya SS, Sharma R, Jindal V, Mhatre M (2020) Coronavirus disease (COVID-19): An updated review based on current knowledge and existing literature for dermatologists. Dermatol Ther 33(4):e13677
Kaushik AK, Dhau JS, Gohel H, Mishra YK, Kateb B, Kim NY, Goswami DY (2020) Electrochemical SARS-CoV-2 sensing at point-of-care and artificial intelligence for intelligent COVID-19 management. ACS Appl Bio Mater 3(11):7306–7325
Kesharwani P, Jain K, Jain NK (2014) Dendrimer as nanocarrier for drug delivery. Prog Polym Sci 39(2):268–307
Kesharwani P, Xie L, Banerjee S, Mao G, Padhye S, Sarkar FH, Iyer AK (2015) Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3, 4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloids Surf, B 136:413–423
Khalaj-Hedayati A, Chua CLL, Smooker P, Lee KW (2020) Nanoparticles in influenza subunit vaccine development: immunogenicity enhancement. Influenza Other Respir Viruses 14(1):92–101
Khan AA, Mudassir J, Akhtar S, Murugaiyah V, Darwis Y (2019) Freeze-dried lopinavir-loaded nanostructured lipid carriers for enhanced cellular uptake and bioavailability: statistical optimization, in vitro and in vivo evaluations. Pharmaceutics 11(2):97
Kobayashi K, Wei J, Iida R, Ijiro K, Niikura K (2014) Surface engineering of nanoparticles for therapeutic applications. Polym J 46(8):460–468
Koshy ST, Cheung AS, Gu L, Graveline AR, Mooney DJ (2017) Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv Biosyst 1(1–2):1600013
Kulthe SS, Choudhari YM, Inamdar NN, Mourya V (2012) Polymeric micelles: authoritative aspects for drug delivery. Des Monomers Polym 15(5):465–521
Kumar M, Curtis A, Hoskins C (2018a) Application of nanoparticle technologies in the combat against anti-microbial resistance. Pharmaceutics 10(1):11
Kumar S, Narayan R, Ahammed V, Nayak Y, Naha A, Nayak UY (2018b) Development of ritonavir solid lipid nanoparticles by Box Behnken design for intestinal lymphatic targeting. J Drug Deliv Sci Technol 44:181–189
Kumari P, Panda PK, Jha E, Kumari K, Nisha K, Mallick MA, Verma SK (2017) Mechanistic insight to ROS and apoptosis regulated cytotoxicity inferred by green synthesized CuO nanoparticles from Calotropis gigantea to embryonic zebrafish. Sci Rep 7(1):1–17
Kumari P, Panda PK, Jha E, Pramanik N, Nisha K, Kumari K, Soni N, Mallick MA, Verma SK (2018) Molecular insight to in vitro biocompatibility of phytofabricated copper oxide nanoparticles with human embryonic kidney cells. Nanomedicine 13(19):2415–2433
Kupferschmidt K, Cohen J (2020) Race to find COVID-19 treatments accelerates. Am Assoc Adv Sci 367:6485
Lai S, Ruktanonchai NW, Zhou L, Prosper O, Luo W, Floyd JR, Wesolowski A, Zhang C, Du X, Yu H (2020) Effect of non-pharmaceutical interventions for containing the COVID-19 outbreak: an observational and modelling study. MedRxiv 202003(03):20029843
Lamb YN (2021) BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs 81:495–501
Lamote K, Janssens E, Schillebeeckx E, Lapperre TS, De Winter BY, Van Meerbeeck JP (2020) The scent of COVID-19: viral (semi-) volatiles as fast diagnostic biomarkers? J Breath Res 14(4):042001
Law JWF, Ab Mutalib NS, Chan KG, Lee LH (2015) Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol 5:770
Le TT, Andreadakis Z, Kumar A, Román RG, Tollefsen S, Saville M, Mayhew S (2020) The COVID-19 vaccine development landscape. Nat Rev Drug Discov 19(5):305–306
Ledet G, Mandal TK (2012) Nanomedicine: emerging therapeutics for the 21st century. US Pharm 37(3):7–11
Lembo D, Donalisio M, Civra A, Argenziano M, Cavalli R (2018) Nanomedicine formulations for the delivery of antiviral drugs: a promising solution for the treatment of viral infections. Expert Opin Drug Deliv 15(1):93–114
Li H, Liu SM, Yu XH, Tang SL, Tang CK (2020a) Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents 55(5):105951
Li X, Geng M, Peng Y, Meng L, Lu S (2020b) Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 10(2):102–108
Li J, Zhang K, Wu D, Ren L, Chu X, Qin C, Han X, Hang T, Xu Y, Yang L, Yin L (2021) Liposomal remdesivir inhalation solution for targeted lung delivery as a novel therapeutic approach for COVID-19. Asian J Pharm Sci 16(6):772–783
Lim ES, Wang D. Pathogen discovery (2016) Molecular microbiology: diagnostic principles and practice. pp 80–91
Liu J, Lau SK, Varma VA, Moffitt RA, Caldwell M, Liu T, Young AN, Petros JA, Osunkoya AO, Krogstad T (2010) Molecular mapping of tumor heterogeneity on clinical tissue specimens with multiplexed quantum dots. ACS Nano 4(5):2755–2765
Liu YC, Liao CH, Chang CF, Chou CC, Lin YR (2020) A locally transmitted case of SARS-CoV-2 infection in Taiwan. N Engl J Med 382(11):1070–1072
Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y (2014) Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed Res Int
Lokhande SS (2018) Liposome drug delivery: an update review. Pharm Sci Monit 9(1)
Lu R, Zhao X (2020) Caracterización genómica y epidemiología del nuevo coronavirus 2019: implicaciones para los orígenes del virus y la unión al receptor. Lancet 395(10224):566–568
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet 395(10224):565–574
Lustig Y, Keler S, Kolodny R, Ben-Tal N, Atias-Varon D, Shlush E, Gerlic M, Munitz A, Doolman R, Asraf K (2021) Potential antigenic cross-reactivity between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and dengue viruses. Clin Infect Dis 73(7):e2444–e2449
Ma Q, Pan W, Li R, Liu B, Li C, Xie Y, Wang Z, Zhao J, Jiang H, Huang J (2020) Liu Shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-κB signaling pathway. Pharmacol Res 158:104850
Ma J, Qi X, Chen H, Li X, Zhang Z, Wang H, Sun L, Zhang L, Guo J, Morawska L (2021) Coronavirus disease 2019 patients in earlier stages exhaled millions of severe acute respiratory syndrome coronavirus 2 per hour. Clin Infect Dis 72(10):e652–e654
Maggi F, Pistello M, Antonelli G (2019) Future management of viral diseases: role of new technologies and new approaches in microbial interactions. Clin Microbiol Infect 25(2):136–141
Mahajan HS, Patil PH (2020) Central composite design-based optimization of lopinavir vitamin E-TPGS micelle: In vitro characterization and in vivo pharmacokinetic study. Colloids Surf, B 194:111149
Maji S, Biswas A, Mandal S, Sen DJ, Mahanti B (2020) Silver and copper nano particles in mask for Corona Virus-19 protection. Glob J Endrocrinol Metab 3:000556
Maniyar MG, Kokare CR (2019) Formulation and evaluation of spray-dried liposomes of lopinavir for topical application. J Pharm Investig 49:259–270.
Masters PS (2006) The molecular biology of coronaviruses. Adv Virus Res 66:193–292
Matsuura K, Watanabe K, Matsuzaki T, Sakurai K, Kimizuka N (2010) Self-assembled synthetic viral capsids from a 24-mer viral peptide fragment. Angew Chem Int Ed 49(50):9662–9665
Miripour ZS, Sarrami-Forooshani R, Sanati H, Makarem J, Taheri MS, Shojaeian F, Eskafi AH, Abbasvandi F, Namdar N, Ghafari H (2020) Real-time diagnosis of reactive oxygen species (ROS) in fresh sputum by electrochemical tracing; correlation between COVID-19 and viral-induced ROS in lung/respiratory epithelium during this pandemic. Biosens Bioelectron 165:112435
Misra N, Bhatt S, Arefi-Khonsari F, Kumar V (2021) State of the art in nonthermal plasma processing for biomedical applications: Can it help fight viral pandemics like COVID-19? Plasma Process Polym 18(7):2000215
Moabelo KL, Martin DR, Fadaka AO, Sibuyi NR, Meyer M, Madiehe AM (2021) Nanotechnology-based strategies for effective and rapid detection of SARS-CoV-2. Materials 14(24):7851
Mohammadniaei M, Zhang M, Ashley J, Christensen UB, Friis-Hansen LJ, Gregersen R, Lisby JG, Benfield TL, Nielsen FE, Henning Rasmussen J, Pedersen EB (2021) A non-enzymatic, isothermal strand displacement and amplification assay for rapid detection of SARS-CoV-2 RNA. Nat Commun 12(1):1–2
Monto AS, Fendrick AM, Sarnes MW (2001) Respiratory illness caused by picornavirus infection: a review of clinical outcomes. Clin Ther 23(10):1615–1627
Morokutti-Kurz M, Graf C, Prieschl-Grassauer E (2017) Amylmetacresol/2, 4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat. Int J General Med 10:53
Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E (2020) A novel coronavirus emerging in China—key questions for impact assessment. N Engl J Med 382(8):692–694
Murtaza A, Rehman T, Zafar Z, Noreen S, Iqbal M, Hassan S, Khawaish I, Tarar MHA (2020) Synthesis and characterization of herbal nano-suspensions and evaluation of their In-vivo antihypertensive potential with especial focus on piperine. ACE 1:23
Muthuirulappan S, Francis SP (2013) Anti-cancer mechanism and possibility of nano-suspension formulation for a marine algae product fucoxanthin. Asian Pac J Cancer Prev 14(4):2213–2216
Naseema A, Kovooru L, Behera AK, Kumar KP, Srivastava P (2021) A critical review of synthesis procedures, applications and future potential of nanoemulsions. Adv Coll Interface Sci 287:102318
Ndwandwe D, Wiysonge CS (2021) COVID-19 vaccines. Curr Opin Immunol 71:111–116
Negi JS, Chattopadhyay P, Sharma AK, Ram V (2014) Development and evaluation of glyceryl behenate based solid lipid nanoparticles (SLNs) using hot self-nanoemulsification (SNE) technique. Arch Pharmacal Res 37(3):361–370
Nguyen-Contant P, Embong AK, Kanagaiah P, Chaves FA, Yang H, Branche AR, Topham DJ, Sangster MY (2020) S protein-reactive IgG and memory B cell production after human SARS-CoV-2 infection includes broad reactivity to the S2 subunit. Mbio 11(5):e01991-e2020
Nikaeen G, Abbaszadeh S, Yousefinejad S (2020) Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomedicine 15(15):1501–1512
Noriega-Luna B, Godínez LA, Rodríguez FJ, Rodríguez A, Larrea GZLD, Sosa-Ferreyra CF, Mercado-Curiel RF, Manríquez J, Bustos E (2014) Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection. J Nanomater 2014:39–39
Noyes AA, Whitney WR (1897) The rate of solution of solid substances in their own solutions. J Am Chem Soc 19(12):930–934
Olvera D, Monaghan MG (2021) Electroactive material-based biosensors for detection and drug delivery. Adv Drug Deliv Rev 170:396–424
Pan Y, Li X, Yang G, Fan J, Tang Y, Zhao J, Long X, Guo S, Zhao Z, Liu Y, Hu H (2020) Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect 81(1):e28-32
Park GS, Ku K, Baek SH, Kim SJ, Kim SI, Kim BT, Maeng JS (2020) Development of reverse transcription loop-mediated isothermal amplification assays targeting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). J Mol Diagn 22(6):729–735
Patel G, Shelat P, Lalwani A (2016) Statistical modeling, optimization and characterization of solid self-nanoemulsifying drug delivery system of lopinavir using design of experiment. Drug delivery 23:3027–3042
Patel GM, Shelat PK, Lalwani AN (2017) QbD based development of proliposome of lopinavir for improved oral bioavailability. Eur J Pharm Sci 108:50–61
Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, Watkinson P, Khunti K, Harnden A, Coupland CAC, Channon KM, Mills NL, Sheikh A, Hippisley-Cox J (2022) Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med 28(2):410–422
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MdP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S (2018a) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 16(1):1–33
Patra JK, Das G, Kumar A, Ansari A, Kim H, Shin HS (2018b) Photo-mediated biosynthesis of silver nanoparticles using the non-edible accrescent fruiting calyx of physalis peruviana L. fruits and investigation of its radical scavenging potential and cytotoxicity activities. J Photochem Photobiol, B 188:116–125
Peng H, Lt Y, Ly W, Li J, Huang J, Zq Lu, Koup RA, Bailer RT, Cy Wu (2006) Long-lived memory T lymphocyte responses against SARS coronavirus nucleocapsid protein in SARS-recovered patients. Virology 351(2):466–475
Phan IQ, Subramanian S, Kim D, Murphy M, Pettie D, Carter L, Anishchenko I, Barrett LK, Craig J, Tillery L (2021) In silico detection of SARS-CoV-2 specific B-cell epitopes and validation in ELISA for serological diagnosis of COVID-19. Sci Rep 11(1):1–11
Pimentel TA, Yan Z, Jeffers SA, Holmes KV, Hodges RS, Burkhard P (2009) Peptide nanoparticles as novel immunogens: design and analysis of a prototypic severe acute respiratory syndrome vaccine. Chem Biol Drug Des 73(1):53–61
Prasad R, Pandey R, Barman I (2015) Engineering tailored nanoparticles with microbes: quo vadis. Wires Nanomed Nanobiotechnol. https://doi.org/10.1002/wnan.1363
Prusty K, Swain SK (2018) Nano silver decorated polyacrylamide/dextran nanohydrogels hybrid composites for drug delivery applications. Mater Sci Eng, C 85:130–141
Puga-Gómez R, Ricardo-Delgado Y, Rojas-Iriarte C, Céspedes-Henriquez L, Piedra-Bello M, Vega-Mendoza D, Pérez NP, Paredes-Moreno B, Rodríguez-González M, Valenzuela-Silva C, Sánchez-Ramírez B (2023) Open-label phase I/II clinical trial of SARS-CoV-2 receptor binding domain-tetanus toxoid conjugate vaccine (FINLAY-FR-2) in combination with receptor binding domain-protein vaccine (FINLAY-FR-1A) in children. Int J Infect Dis 126:164–173
Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, Blumenthal, R (2009) Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev™ Ther Drug Carr Syst 26(6)
Que H, Hong W, Lan T, Zeng H, Chen L, Wan D, Bi Z, Ren W, Luo M, Yang J, He C, Zhong A, Wei X (2022) Tripterin liposome relieves severe acute respiratory syndrome as a potent COVID-19 treatment. Signal Transduct Target Ther 7(1):399
Quick J, Grubaugh ND, Pullan ST, Claro IM, Smith AD, Gangavarapu K, Oliveira G, Robles-Sikisaka R, Rogers TF, Beutler NA, Burton DR (2017) Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat Protoc 12(6):1261–1276
Rabiee N, Bagherzadeh M, Ghasemi A, Zare H, Ahmadi S, Fatahi Y, Dinarvand R, Rabiee M, Ramakrishna S, Shokouhimehr M (2020) Point-of-use rapid detection of SARS-CoV-2: nanotechnology-enabled solutions for the COVID-19 pandemic. Int J Mol Sci 21(14):5126
Raj S, Khurana S, Choudhari R, Kesari KK, Kamal MA, Garg N, Ruokolainen J, Das BC, Kumar D (2021) In Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy, Seminars in cancer biology. Elsevier pp 166–177
Ramachandran AK, Das S, Joseph A (2021) Crosstalk between COVID-19 and associated neurological disorders: a review. Curr Neuropharmacol 19(10):1688–1700
Rao L, Xia S, Xu W, Tian R, Yu G, Gu C, Pan P, Meng QF, Ca X, Qu D (2020) Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines. Proc Natl Acad Sci 117(44):27141–27147
Rao PV, Krishna BS, Jeffree MS (2022) Coronaviruses: transmission, frontliners, nanotechnology and economy. Universiti Malaysia Sabah Press, Kota Kinabalu
Ravi PR, Vats R, Dalal V, Murthy AN (2014) A hybrid design to optimize preparation of lopinavir loaded solid lipid nanoparticles and comparative pharmacokinetic evaluation with marketed lopinavir/ritonavir coformulation. J Pharm Pharmacol 66(7):912–926
Ravi PR, Vats R, Dalal V, Gadekar N (2015) Design, optimization and evaluation of poly-ɛ-caprolactone (PCL) based polymeric nanoparticles for oral delivery of lopinavir. Drug Dev Ind Pharm 41(1):131–140
Reddy S, van der Vlies A, Simeoni E, Oneil C, Swartz M, Hubbell J (2008) Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Tissue Eng Part A 14:734–735
Rizzo JM, Buck M (2012) All things ChIP: ChIP-Chip, ChIP-Seq, ChIP-PCR. Epigenet Regul Epigenomics 2:41
Rocchitta G, Spanu A, Babudieri S, Latte G, Madeddu G, Galleri G, Nuvoli S, Bagella P, Demartis MI, Fiore V (2016) Enzyme biosensors for biomedical applications: strategies for safeguarding analytical performances in biological fluids. Sensors 16(6):780
Russell CD, Millar JE, Baillie JK (2020) Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet 395(10223):473–475
Russell RL, Pelka P, Mark BL (2021) Frontrunners in the race to develop a SARS-CoV-2 vaccine. Can J Microbiol 67(3):189–212
Sahakijpijarn S, Moon C, Koleng JJ, Christensen DJ, Williams RO (2020) Development of remdesivir as a dry powder for inhalation by thin film freezing. Pharmaceutics 12(11):1002
Salleh A, Naomi R, Utami ND, Mohammad AW, Mahmoudi E, Mustafa N, Fauzi MB (2020) The potential of silver nanoparticles for antiviral and antibacterial applications: a mechanism of action. Nanomaterials 10(8):1566
Sen AK, Nath A, Sudeepthi A, Jain SK, Banerjee U (2022) Microfluidics-Based Point-of-Care Diagnostic Devices. In: Advanced microfluidics-based point-of-care diagnostics. CRC Press pp. 99–120
Seo G, Lee G, Kim MJ, Baek SH, Choi M, Ku KB, Lee CS, Jun S, Park D, Kim HG (2020) Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano 14(4):5135–5142
Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S (2015) Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 6:286
Shafiee S, Cegolon L, Khafaei M, Gholami N, Zhao S, Khalesi N, Moosavian H, Fathi S, Izadi M, Ghadian A, Javanbakht M, Javanbakht A, Akhavan-Sigari R (2021) Gastrointestinal cancers, ACE-2/TMPRSS2 expression and susceptibility to COVID-19. Cancer Cell Int 21(1):431
Shah V, Keniya R, Shridharani A, Punjabi M, Shah J, Mehendale N (2021) Diagnosis of COVID-19 using CT scan images and deep learning techniques. Emerg Radiol 28(3):497–505
Shan B, Broza YY, Li W, Wang Y, Wu S, Liu Z, Wang J, Gui S, Wang L, Zhang Z (2020) Multiplexed nanomaterial-based sensor array for detection of COVID-19 in exhaled breath. ACS Nano 14(9):12125–12132
Shanley LC, Mahon OR, Kelly DJ, Dunne A (2021) Harnessing the innate and adaptive immune system for tissue repair and regeneration: Considering more than macrophages. Acta Biomater 133:208–221
Sharma A, Sharma N, Kumari A, Lee HJ, Kim T, Tripathi KM (2020) Nano-carbon based sensors for bacterial detection and discrimination in clinical diagnosis: a junction between material science and biology. Appl Mater Today 18:100467
Shi Y, Pramanik A, Tchounwou C, Pedraza F, Crouch RA, Chavva SR, Vangara A, Sinha SS, Jones S, Sardar D (2015) Multifunctional biocompatible graphene oxide quantum dots decorated magnetic nanoplatform for efficient capture and two-photon imaging of rare tumor cells. ACS Appl Mater Interfaces 7(20):10935–10943
Singh B, Datta B, Ashish A, Dutta G (2021) A comprehensive review on current COVID-19 detection methods: From lab care to point of care diagnosis. Sens Int 2:100119
Su S, Wong G, Shi W, Liu J, Lai AC, Zhou J, Liu W, Bi Y, Gao GF (2016) Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 24(6):490–502
Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y (2014) Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed 53(46):12320–12364
Sun S, He L, Zhao Z, Gu H, Fang X, Wang T, Yang X, Chen S, Deng Y, Li J, Zhao J, Li L, Li X, He P, Li G, Li H, Zhao Y, Gao C, Lang X, Wang X, Fei G, Li Y, Geng S, Gao Y, Wei W, Hu Z, Han G, Sun Y (2021) Recombinant vaccine containing an RBD-Fc fusion induced protection against SARS-CoV-2 in nonhuman primates and mice. Cell Mol Immunol 18(4):1070–1073
Szunerits S, Barras A, Khanal M, Pagneux Q, Boukherroub R (2015) Nanostructures for the inhibition of viral infections. Molecules 20(8):14051–14081
Tang F, Quan Y, Xin ZT, Wrammert J, Ma MJ, Lv H, Wang TB, Yang H, Richardus JH, Liu W (2011) Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol 186(12):7264–7268
Tarim EA, Karakuzu B, Oksuz C, Sarigil O, Kizilkaya M, Al-Ruweidi MK, Yalcin HC, Ozcivici E, Tekin HC (2021) Microfluidic-based virus detection methods for respiratory diseases. Emerg Mater 4(1):143–168
Tian B, Gao F, Fock J, Dufva M, Hansen MF (2020) Homogeneous circle-to-circle amplification for real-time optomagnetic detection of SARS-CoV-2 RdRp coding sequence. Biosens Bioelectron 165:112356
Ting D, Dong N, Fang L, Lu J, Bi J, Xiao S, Han H (2018) Multisite inhibitors for enteric coronavirus: antiviral cationic carbon dots based on curcumin. ACS Appl Nano Mater 1(10):5451–5459
To KK, Yip CC, Lai CY, Wong CK, Ho DT, Pang PK, Ng AC, Leung KH, Poon RW, Chan KH, Cheng VC (2019) Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect 25(3):372–378
Tomas M, Capanoglu E, Bahrami A, Hosseini H, Akbari-Alavijeh S, Shaddel R, Rehman A, Rezaei A, Rashidinejad A, Garavand F (2022) The direct and indirect effects of bioactive compounds against coronavirus. Food Front 3(1):96–123
Tripathy S, Das MK (2013) Dendrimers and their applications as novel drug delivery carriers. J Appl Pharm Sci 3(09):142–149
Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, Li VY, Chen H, Mubareka S, Gubbay JB, Chan WC (2020) Diagnosing COVID-19: the disease and tools for detection. ACS Nano 14(4):3822–3835
Vaezi A, Meysamie A (2021) COVID-19 vaccines cost-effectiveness analysis: a scenario for Iran. Vaccines 10(1):37
Vahedifard F, Chakravarthy K (2021) Nanomedicine for COVID-19: the role of nanotechnology in the treatment and diagnosis of COVID-19. Emerg Mater 4(1):75–99
Varahachalam SP, Lahooti B, Chamaneh M, Bagchi S, Chhibber T, Morris K, Bolanos JF, Kim NY, Kaushik A (2021) Nanomedicine for the SARS-CoV-2: state-of-the-art and future prospects. Int J Nanomed 16:539
Verma SK, Jha E, Sahoo B, Panda PK, Thirumurugan A, Parashar S, Suar M (2017) Mechanistic insight into the rapid one-step facile biofabrication of antibacterial silver nanoparticles from bacterial release and their biogenicity and concentration-dependent in vitro cytotoxicity to colon cells. RSC Adv 7(64):40034–40045
Vijayan V, Mohapatra A, Uthaman S, Park IK (2019) Recent advances in nanovaccines using biomimetic immunomodulatory materials. Pharmaceutics 11(10):534
Villeret B, Dieu A, Straube M, Solhonne B, Miklavc P, Hamadi S, Le Borgne R, Mailleux A, Norel X, Aerts J (2018) Silver nanoparticles impair retinoic acid-inducible gene I-mediated mitochondrial antiviral immunity by blocking the autophagic flux in lung epithelial cells. ACS Nano 12(2):1188–1202
Volkov Y (2015) Quantum dots in nanomedicine: recent trends, advances and unresolved issues. Biochem Biophys Res Commun 468(3):419–427
Wang W, Wu J, Zhang X, Hao C, Zhao X, Jiao G, Shan X, Tai W, Yu G (2017) Inhibition of influenza A virus infection by fucoidan targeting viral neuraminidase and cellular EGFR pathway. Sci Rep 7(1):1–14
Wang S, Wang W, Hao C, Yunjia Y, Qin L, He M, Mao W (2018) Antiviral activity against enterovirus 71 of sulfated rhamnan isolated from the green alga Monostroma latissimum. Carbohyd Polym 200:43–53
Wang C, Horby PW, Hayden FG, Gao GF (2020) A novel coronavirus outbreak of global health concern. The Lancet 395(10223):470–473
Wankar JN, Chaturvedi VK, Bohara C, Singh MP, Bohara RA (2020) Role of nanomedicine in management and prevention of COVID-19. Front Nanotechnol 2:589541
Weiss C, Carriere M, Fusco L, Capua I, Regla-Nava JA, Pasquali M, Scott JA, Vitale F, Unal MA, Mattevi C (2020) Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano 14(6):6383–6406
Wilson DR, Sen R, Sunshine JC, Pardoll DM, Green JJ, Kim YJ (2018) Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomed Nanotechnol Biol Med 14(2):237–246
Wu J, Ye F, Cui M, Shibata R, Xu R, Cheng L, Zhang DY (2013) Diagnostic methodology and technology in molecular genetic pathology. Molecular genetic pathology. Springer, New York, pp 211–300
Wu J, Liu J, Li S, Peng Z, Xiao Z, Wang X, Yan R, Luo J (2020a) Detection and analysis of nucleic acid in various biological samples of COVID-19 patients. Travel Med Infect Dis 37:101673
Wu K, Saha R, Su D, Krishna VD, Liu J, Cheeran MCJ, Wang JP (2020b) Magnetic-nanosensor-based virus and pathogen detection strategies before and during COVID-19. ACS Appl Nano Mater 3(10):9560–9580
Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, Plebanski M (2006) Pathogen recognition and development of particulate vaccines: does size matter? Methods 40(1):1–9
Xu G, Zeng S, Zhang B, Swihart MT, Yong KT, Prasad PN (2016) New generation cadmium-free quantum dots for biophotonics and nanomedicine. Chem Rev 116(19):12234–12327
Xu W, Ling P, Zhang T (2013) Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv
Yadav HK, Almokdad AA, Sumia I, Debe MS (2019) Polymer-based nanomaterials for drug-delivery carriers. In Nanocarriers for drug delivery. Elsevier pp 531–556
Yan X, Zhou M, Yu S, Jin Z, Zhao K (2020) An overview of biodegradable nanomaterials and applications in vaccines. Vaccine 38(5):1096–1104
Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8(5):475–481
Ye S, Shao K, Li Z, Guo N, Zuo Y, Li Q, Lu Z, Chen L, He Q, Han H (2015) Antiviral activity of graphene oxide: how sharp-edged structure and charge matter. ACS Appl Mater Interfaces 7(38):21571–21579
Yousaf SS, Isreb A, Khan I, Mewsiga E, Elhissi A, Ahmed W, Alhnan MA (2021) Impact of nanosizing on the formation and characteristics of polymethacrylate films: micro-versus nano-suspensions. Pharm Dev Technol 26(7):729–739
Yu CY, Chan KG, Yean CY, Ang GY (2021) Nucleic acid-based diagnostic tests for the detection SARS-CoV-2: an update. Diagnostics 11(1):53
Zazo H, Colino CI, Lanao JM (2016) Current applications of nanoparticles in infectious diseases. J Control Release 224:86–102
Zhang Q, Honko A, Zhou J, Gong H, Downs SN, Vasquez JH, Fang RH, Gao W, Griffiths A, Zhang L (2020b) Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett 20(7):5570–5574
Zhang F, Wang Z, Vijver MG, Peijnenburg WJ (2021a) Probing nano-QSAR to assess the interactions between carbon nanoparticles and a SARS-CoV-2 RNA fragment. Ecotoxicol Environ Saf 219:112357
Zhang J, Hu K, Di L, Wang P, Liu Z, Zhang J, Yue P, Song W, Zhang J, Chen T (2021b) Traditional herbal medicine and nanomedicine: Converging disciplines to improve therapeutic efficacy and human health. Adv Drug Deliv Rev 178:113964
Zhang X, Lu S, Wang SH, Yu X, Wang SJ, Yao L, Pan Y, Zhang YD (2022) Diagnosis of COVID-19 pneumonia via a novel deep learning architecture. J Comput Sci Technol 37(2):330–343
Zhang F, Abudayyeh OO, Gootenberg JS (2020a) A protocol for detection of COVID-19 using CRISPR diagnostics. A protocol for detection of COVID-19 using CRISPR diagnostics 8.
Zhao W, Zhong Z, Xie X, Yu Q, Liu J (2020) Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. Ajr Am J Roentgenol 214(5):1072–1077
Zhu J, Shi X (2013) Dendrimer-based nanodevices for targeted drug delivery applications. J Mater Chem B 1(34):4199–4211
Ziegler CG, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM (2020) SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181(5):1016–1035
Author information
Authors and Affiliations
Contributions
BB and RKS performed conceptualization; BB and AMAI did writing—original draft preparation; AF, SR, AM, AS, DK and JK contributed to writing—review and editing; RKS done supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhattacharjee, B., Ikbal, A.M.A., Farooqui, A. et al. Superior possibilities and upcoming horizons for nanoscience in COVID-19: noteworthy approach for effective diagnostics and management of SARS-CoV-2 outbreak. Chem. Pap. 77, 4107–4130 (2023). https://doi.org/10.1007/s11696-023-02795-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02795-3