Abstract
This review addresses the impact of molecular alterations on the diagnosis and prognosis of differentiated thyroid carcinoma (DTC), including papillary, follicular, and well-differentiated carcinoma NOS, as well as oncocytic neoplasms. The molecular characterization of DTC is based upon the well-established dichotomy of BRAF-like and RAS-like designations, together with a remaining third group, less homogeneous, composed of non-BRAF-/non-RAS-like tumors. The role of BRAF V600E mutation in risk stratification is discussed in the clinico-pathological context, namely, staging and invasive features of classic papillary thyroid carcinoma (PTC) and histopathological variants carrying an excellent prognosis (microPTC) or a guarded prognosis, including the aggressive variants tall cell and hobnail cell PTCs. In follicular patterned tumors, namely, follicular thyroid carcinoma (FTC), with or without oncocytic features, the most prevalent molecular alteration are RAS mutations that do not carry prognostic significance. The only genetic alteration that has been proven to play a role in risk stratification of PTC and FTC is TERT promoter (TERTp) mutation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is the most frequent endocrine neoplasm, the tenth most prevalent cancer in both genders and the fifth in women [1], but it has very low disease-specific mortality. It is usually a treatable cancer with good/very good survival. Nevertheless, there are questions concerning prediction of disease persistence and recurrence. Up to 95% of malignant lesions of the thyroid originate in follicular cells and are designated differentiated thyroid carcinoma (DTC); about 85% of these are papillary thyroid carcinoma (PTC), and 10% are follicular thyroid carcinoma (FTC). DTCs have much higher survival rates than poorly differentiated and anaplastic thyroid carcinoma (PDTC and ATC, respectively); nevertheless, it is estimated that 10–15% of DTCs recur, persist, and/or metastasize to distant sites. Those are the ones that can cause death of DTC patients.
Several stratification systems have been developed to date, in an attempt to predict the behavior of patients with thyroid cancer [2]. Stratification systems have used clinico-pathological indicators, such as Age, Grade, Extent, and Size in the AGES system; distant Metastasis, Age, Completeness of resection, local Invasion, and tumor Size in the MACIS score; and Age, Metastases, Extent, and Size in the AMES system. In some of these systems, molecular data were added, as is the case of the DAMES system that includes DNA ploidy, besides Age, Metastases, Extent, and Size. None of the aforementioned systems was robust enough to be widely accepted and incorporated into clinical practice worldwide. The most accepted system is the staging system for differentiated and anaplastic thyroid cancer (AJCC/TNM, 8th edition) [3]; it is based on survival evaluation, despite the fact that survival is not the best indicator in a cancer with good/excellent survival figures, like DTC. Alternative scoring systems are needed, addressing not only survival but also recurrent/persistent disease, which is the main morbidity in patients with DTC.
It has been generally hoped that genetic characterization of these tumors will provide a better way to predict the outcome of thyroid cancer patients [4]. The first genetic alteration thought to be specifically associated with PTC was RET/PTC rearrangement, but it quickly became clear that this biomarker does not have prognostic value. We and others [5, 6] found that RET/PTC rearranged PTCs represented a subset of slow growing, less aggressive thyroid neoplasms. BRAF mutation was identified as another driver event in PTC carcinogenesis [7, 8], but the real value of BRAF mutation in predicting patient outcome is still controversial [9–11].
The genotype and molecular characterization of thyroid carcinomas are expected to improve several aspects of thyroid cancer patient management (Fig. 1). However, it is not realistic to assume that genetic features can replace robust prognostic features. Clinico-pathological features play a pivotal role in the prognosis of thyroid cancer, namely, size of tumor, pattern of growth (encapsulated or infiltrative), and/or presence of vascular invasion. In several studies these parameters are not taken into account in a systematic way, leading to variability in the clinico-pathological associations with molecular biomarkers and patient outcome (disease recurrence/persistence or mortality). In this review, we address the molecular risk stratification of DTC together with the gross and histopathologic features, dividing DTC into BRAF V600E-like, RAS-like, and non-BRAF-/ non-RAS-like tumors.
Molecular Classification of Differentiated Thyroid Carcinomas
With the advent of next-generation sequencing (NGS), the full genome of thyroid cancers was investigated, and a molecular classification of PTC was proposed [12]. The large-scale study of The Cancer Genome Atlas (TCGA) identified a 71-gene signature that divided PTCs into BRAF V600E-like and RAS-like tumors. BRAF V600E-like PTCs (that cluster together BRAF V600E with RET/PTC rearranged tumors) exhibit lower thyroid differentiation scores and represent predominantly classical PTCs with papillary architecture and including those with tall cell histology (tall cell PTC, TCPTC). On the other side of the spectrum, RAS-like PTCs are highly differentiated tumors enriched in follicular-patterned tumors (follicular variant PTCs, FVPTC) and usually displaying lower risk of recurrence. RAS-like PTCs are characterized by NRAS, HRAS, KRAS, BRAF non-V600E (namely BRAF K601E), and EIF1AX point mutations or rearrangements of PPARγ, FGFR2, and THADA genes. The two clusters are also distinct regarding their epigenomic and proteomic profiles [12].
In a subsequent study, Yoo et al. [13] expanded the molecular characterization of thyroid tumors focusing on follicular-patterned lesions: follicular adenoma (FA), FTC, and FVPTC. Besides corroborating the existence of BRAF V600E-like and RAS-like profiles, Yoo et al. identified a third molecular subtype, the non-BRAF-/non-RAS (NBNR group) with different tumor histotypes [13]. The latter cluster is enriched in FA and micro-invasive FTC (miFTC) and has been associated with DICER1, EIF1AX, IDH1, PTEN, SOS1, SPOP, and PAX8–PPARγ alterations. The authors also found a molecular signature related to mitochondrial biogenesis in oncocytic follicular thyroid tumors (ESRRA and PPARGC1A). In this study, the transcriptome of miFTC and encapsulated FVPTC was indistinguishable from that of FA, reinforcing the closeness of the subtypes; no clear association with clinico-pathological associations was found.
These NGS studies clarified the pathogenesis, at the molecular level, of thyroid carcinomas and reduced the fraction of cases with unknown oncogenic driver alterations to less than 4% [12, 13]. Furthermore, the results strength the “old” morphologic separation of thyroid carcinomas into papillary patterned lesions and follicular patterned lesions; however, the reclassification into three molecular groups did not provide clues for better risk stratification at the clinical level.
On histological grounds, using pattern of growth, differentiation, and invasion, we can identify lesions with very low potential such as the non-invasive follicular tumor with papillary-like nuclei (NIFTP) and so-called tumor of undetermined malignant potential tumors (UMP). Histology also allows the identification of aggressive variants that present at high stage or with signs of loss of differentiation. The most frequent challenge involves the “common” well-differentiated thyroid carcinomas that have uncertain risk of recurrence and progression. For those cases, one needs molecular stratification biomarkers (Fig. 2).
Molecular Risk Stratification of Papillary Thyroid Carcinomas (BRAF-Like)
BRAF-like thyroid carcinomas correspond mainly to classic variant PTC (CVPTC), including histopathological variants of PTC associated with excellent/very good prognosis (papillary microcarcinoma-PMC) and those with guarded prognosis, the aggressive variants of PTC. The challenge of improving molecular risk stratification is discussed in the following settings: TNM, PMC, and aggressive variants of PTC.
TNM—Classic Papillary Thyroid Carcinoma
It is known that the intrinsic risk of poor outcomes is not equal in all PTCs at AJCC TNM stage I, and it is sometimes difficult to decide the appropriate extent of surgical treatment [14]. It has been suggested that the inclusion of tumor mutational status might improve the discrimination of the existing scoring systems [15] (Table 1).
BRAF V600E mutation is the most prevalent (29–69%) point mutation identified in PTC [16, 17]. There is a strong association between the genotype, BRAF V600E mutation, and the PTC phenotype (classical PTC or any of the PTC variants, exception made for the FVPTC) [18]. Since BRAF V600E mutation prevalence in PTC can be as high as 70%, whereas a negative outcome is seen in only 10–15% of PTCs, the value of BRAF V600E mutation in prognosis is controversial [17]. Several authors have reported that BRAF V600E mutation was strongly associated with recurrence [19–21]. Xing et al. verified BRAF V600E mutation association with recurrence even in low-risk stage I or II disease and independent of other clinico-pathological risk factors [21] (Table 1); however, in a subsequent meta-analysis, the same group recognized that the risk was rather based on tumor classification with best prognosis in FVPTC, the worst in TCPTC, and intermediate in classical PTC [11]. In a different a meta-analysis, Vuong et al. described that BRAF mutation association with recurrence was only evident in short- and medium-term follow-up and was lost in long-term follow-up [22].
Patients with BRAF V600E-mutated PTCs are older than those with wild-type BRAF [23]. This association is interpreted by some authors as a synergistic interaction affecting PTC recurrence [21, 24], while others suggest that BRAF V600E mutation provides a less efficient tumorigenic stimulus leading to a longer and more indolent course [23].
BRAF mutation has been associated with the presence of LNM [19, 24, 25] (Table 1), and in general, there is a high concordance between the genotype of primary tumors and LNM [26]. It seems that the process of local metastasis does not implicate additional molecular alterations, indicating that BRAF V600E may play a role in the process of local spread [26]. Indeed, BRAF mutation was not robustly associated with the presence of distant metastases [27] and some studies even described a decreased frequency of distant metastases in BRAF V600E-mutated PTC [26] (Table 1). The association of BRAF mutation and PTC at advanced clinical stage (AJCC TNM stage III/IV) has been described [24, 25, 27]. It is also considered that risk stratification improves when BRAF V600E status is considered in conventional staging systems such as AMES, MACIS, TNM, and ATA Risk [28]. However, the predictive value of BRAF V600E is limited when histologic features are available to refine risk stratification [23]. The association of BRAF mutations with PTC-specific mortality is still debatable. BRAF mutation has been associated with disease-specific mortality in some studies [20, 21, 27], but it was evidenced that this association was dependent on several concurrent clinico-pathological features [20, 27]. Other authors did not find an association between BRAF mutation and increased risk of disease-specific mortality [22, 24]. To ascertain the real impact of BRAF mutation on prognosis, it is necessary to consider associated clinico-pathological features and other concomitant genetic alterations. The controversy likely reflects the limited data examined in some published studies [22].
The impact of TERTp mutation on the prognosis of PTC is more consistently established (Table 1). The frequency of TERTp mutation is low in PTC (11.3–12.3%) [10, 29], and its frequency increases significantly in poorly differentiated and undifferentiated carcinomas (up to 50%). The presence of TERTp mutation is correlated with the presence of distant metastases in PTC [30]. In a study that compared PTC with distant metastases to PTC without distant metastases, the frequency of TERTp mutations was 3.5-fold higher in the group with distant metastases [30]. TERTp-only mutation is the most frequent genetic alteration in distant metastases [26] and appears to be a key event in the process of distant dissemination, nominating TERTp mutation as a strong predictor of aggressiveness and metastasis of thyroid carcinoma [30]. TERTp mutations are associated with older age at diagnosis [18, 30]; thus, this mutation may be a major molecular mediator of the relationship between age and mortality in thyroid carcinoma [31]. TERTp mutations status improves prognostication for patients already stratified by conventional staging systems [19] and can be used as an independent and reliable marker for risk stratification and predicting outcomes [22, 32]. In PTCs, TERTp mutations are an independent and reliable molecular marker to predict persistence/recurrence and disease-specific mortality [18, 19, 22, 33] and this finding was independent of age and gender [34].
Coexistence of BRAF V600E and TERTp promoter mutations is a frequent event, and their interaction is a matter of debate. Some authors described a synergistic and independent role of coexisting BRAF V600E and TERTp mutations in PTC specific mortality [32, 35]. A strong independent and incremental role of this mutation duet in PTC specific mortality that remained strongly significant after multivariate adjustment for all the conventional clinico-pathological features has been reported [32]. It has been suggested that coexistence of BRAF V600E and TERTp mutations in PTC increases expression of TERT, likely providing oncogenic and tumor survival advantages that identify the small group of PTC patients with the highest mortality risk [32]. Other authors endorse that although the coexistence of BRAF V600E and TERTp mutations may be a stronger predictor of disease-specific mortality, the major factor driving mortality in those patients is the TERTp mutational status [30, 31]. A significant association of TERTp mutations and PTC specific mortality that was not affected by the presence of BRAF V600E mutation has been stressed in other studies [30, 31].
The phenotype of RAS-mutated PTC is more likely to be FVPTC with a low recurrence risk [24]. Differentiated thyroid cancer will most likely lack aggressiveness when harboring RAS mutations alone [22]. However, coexisting RAS and TERTp mutations may also confer increased aggressiveness in thyroid cancer, as shown in some studies [22, 32].
Classic PTC and TCPTC were the most common histologic subtypes associated with RET/PTC mutation [24]. RET/PTC mutation can also occur in cases of oncocytic variant of PTC [23]. Comparing with BRAF-mutated PTC, RET/PTC-positive patients had a high incidence of lateral LNM (35%) and distant metastasis (8%) at presentation, but the majority were disease free at last follow-up [24].
In relation to other molecular alterations identified through genomic studies in PTC (CHEK2, PTEN, PI3K, and others), the lower prevalence of the alterations precludes the establishment of robust associations with risk at this time. Some exceptions, associated with particular genes (EIF1AX, TP53, or DICER1) will be referred below.
Histopathology—Extremely Low Malignancy-microPTC
The term papillary microcarcinoma (PMC) defines any PTC measuring ≤ 1 cm [37]. PMC accounts for about half of all PTC diagnoses [38, 39] with an incidence of up to 33.8% and 35.6% in surgical specimens and autopsy series, respectively [40–46]. Although there is no solid biological basis indicating 10 mm in size as the boundary between extremely low and low risk PMCs [7], this definition has been found useful in operational terms to properly manage a significant percentage of PTCs. Asymptomatic (incidental) PMCs discovered during scans with different imaging techniques or after thyroidectomy performed for other reasons than PMC, have an excellent prognosis with nearly no risk of recurrence or death [47–49]. Incidentally discovered, PMCs are more commonly found in patients with chronic lymphocytic thyroiditis than multinodular goiter or Graves’ disease [42]. In contrast, clinically recognized (non-incidental) PMC is not different from conventional PTC in terms of lymph node metastases at presentation and/or loco-regional recurrences [49–51].
Numerous PMCs have been studied in an attempt to obtain prognostic markers (including molecular data) that able to identify the small subset of PMCs with potential aggressive behavior. The prevalence of RAS (KRAS, NRAS, and HRAS) mutations in PMCs is usually less than 5% [27, 52]; these mutations are associated with PMCs with a follicular growth pattern, as is the case with benign and malignant clinical tumors of follicular cells, and do not modify the management of these patients [53, 54].
In well-differentiated thyroid carcinomas, TERTp mutation is the best molecular marker of aggressiveness [55, 56]. Mutations in TERTp (3%) were exclusive to a series of 40 patients with PMC and lateral neck nodal metastases (pN1b), without evidence of mutations in the 71 patients with documented absence of nodal disease (pN0) [57]. TERTp mutations were not found in 15 PMCs that showed disease progression on active surveillance or in 10 PMCs without progression [58]. In another study, TERTp mutations were found in 4.7% of the 431 miPTCs analyzed, but no association between TERTp mutations and aggressive features or clinical outcome was found [53]. Consistent with all these data and the excellent prognosis of PMCs, no [57–61] or very low percentage [52, 62] of TERTp mutations have been detected in PMCs, indicating no [52, 53, 58] or a limited role in risk stratification in PMCs [62]. It remains unknown if the coexistence of BRAF and TERTp mutations could be associated with PMCs with a poor clinical outcome [63].
In a review of PMCs, the prevalence of BRAF V600E mutation was 57.4% [27]. Some studies have reported significant associations between BRAF V600E mutation and other indicators of poor prognosis such as male gender, older age, higher stage, larger size, and tumor recurrence [18], but in most of the publications, the association is not independent of numerous other clinico-pathological features of aggressiveness [64]. Therefore, it has been suggested that given the excellent prognosis of PMC, it is not realistic to suggest that PMCs with a BRAF mutation should be treated more aggressively [7]. Consequently, a combined molecular-pathologic score risk stratification model including BRAF status and three histopathologic features (superficial tumor location, intraglandular tumor spread/multifocality, and tumor fibrosis) was a better predictor of extrathyroidal spread of PMC than either mutation or histopathologic findings alone [65]. TCPMC is usually associated with aggressive features at presentation and with BRAF V600E mutation, and it should be distinguished from other PMCs [66]. Interestingly, a morphologic and molecular (BRAF, KRAS, HRAS, NRAS, and PIK3CA) study of 3 PMCs with fatal outcome suggested that morphology of the metastatic deposits, including tall cell features, poorly differentiated areas, and high-grade cytologic features, could be more useful than molecular data to predict the behavior of PMCs [67].
In a study of PTCs in the young population of Fukushima, among classic PTC and FVPTC detected on ultrasonographic screening of almost 300,000 individuals, the BRAF V600E mutation was significantly associated with smaller size of PMCs than tumors without this mutation [68]. These findings provide evidence that additional factors are probably important for tumor progression in pediatric PTCs. Morphological features such as the subcapsular location or the surface location of the PMC in the thyroid gland [69], tall cell morphology, extrathyroidal extension, and/or angioinvasion have recently been confirmed as predictive factors of lymph node metastasis in patients with PMC [70].
Summing up, genetic data in general, and BRAF V600E mutation in particular, do not provide a molecular risk stratification for PMCs. The possibility of using clinico-pathological and molecular data in order to identify a subset of PMCs that would fit the concept of papillary microtumor (PMT) is beyond the scope of this review [37, 71].
Histopathology—Aggressive variants of PTC
The group of PTC variants carrying a guarded prognosis encompasses diffuse sclerosing, diffuse/multinodular follicular variant, tall cell, columnar cell, and hobnail variants of PTC [39].
The main difference regarding the diffuse involvement of thyroid gland by a PTC concerns the association of diffuse sclerosing variant of PTC with BRAF-like features [72] and the association of diffuse/multinodular FVPTC with RAS-like features. The prognosis of these two variants is associated with usual clinico-pathological factors (Age, Gender, Staging, Lympho-vascular invasiveness) and, as far as we know, there is no evidence for molecular features in risk stratification. Regarding lympho-vascular invasion we recognize, nowadays the importance of the distinction of lymphatic from venous angioinvasion since it confers distinct biological meaning. Unfortunately, in the past years, the two parameters were not separated, and therefore, we kept the designation lympho-vascular invasion through the text and the tables.
In diffuse/multinodular FVPTC, one may occasionally find BRAF K601E mutation, as it may be detected in a small number of “common” FVPTCs [73]. The presence of the mutation does not have any prognostic significance.
Besides finding BRAF K601E in FVPTC, complex fusions of BRAF have been detected in some cases of solid variant [74, 75]. Again, this finding does not have prognostic value, although one has to realize that few cases have been diagnosed and followed-up to date. In most instances, the demonstration of rearrangements such as the BRAF fusions may be diagnostically important but the prognostic impact is not clear [76, 77]. The same applies to the detection of a number of other rearrangements including RET/PTC1, RET/PTC3, ETV6/NTRK3 PAX8/PPARγ, ROS1, and ALK [72, 76, 78–80].
In contrast, tall cell and columnar cell variant PTCs do not involve the whole thyroid nor even usually a whole lobe, but the most important prognostic factors remain clinico-pathological parameters (Tables 2 and 3). Concerning the putative prognostic importance of molecular data on aggressive variants of PTC one has to realize that in encapsulated, non-invasive carcinomas composed of tall cells, columnar cells, and perhaps, also hobnail cells, the prognosis is extremely low risk regardless of the molecular features [76, 81–83]. Besides acknowledging that BRAF V600E mutations are frequently detected in tall cell and columnar variants, it remains to be demonstrated that such mutation alone plays a significant role in risk stratification (Tables 2 and 3).
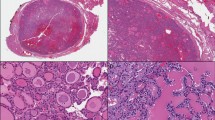
The hobnail variant of PTC (HVPTC) is a subtype of PTC defined by more than 30% of cells with hobnail features [39, 84]. This rare variant, which comprises about 0.3 to 2.7% of all PTCs [84–88], is associated with aggressive clinical behavior [84,89–93] (Fig. 3). Micropapillary and discohesive features in ≥ 20% of a PTC is associated with greater tumor recurrence and mortality [94, 95], and hobnail features, micropapillary pattern, and/or loss of cohesiveness/polarity in PTC are independent predictive factors for lymph node metastasis [96, 97]. Tumors with 10% hobnail/micropapillary features already predict aggressive behavior [90]. HVPTC has also been referred to as micropapillary variant [86, 91], but because the hobnail pattern is more consistent as a feature of aggressiveness, HVPTC is the preferred designation [93, [97, 98]. Oncocytic (oxyphilic) cells have been described in some cases of HVPTC [89], and the concomitant presence of tall cells, another feature associated with aggressiveness [39], has also been detected in cases of HVPTC [91, 92, 99]. This variant must be distinguished from the classic PTC with ischemic/degenerative atypia that simulates HV but has an indolent clinical behavior [99].
Compared with classic PTC, HVPTC has more high-risk pathological features, is radioactive iodine refractory, and has a higher mortality rate [84, 86, 100, 101]. Multifocality has been reported in about 35% of HVPTCs [87, 88, 101–103]. The presence of lympho-vascular invasion has been described in 62% of cases [85, 87, 88, 90–93, 101, 103–107], necrosis in 15.5% of cases [85, 87, 90, 91, 93, 102, 103, 105], and extrathyroidal extension in 50% of cases [85, 88, 90, 93, 99, 102–105, 107]. Lymph node metastases, which correlate with lymphatic invasion but do not alter mortality, have been reported 65.5% of cases [85, 87, 88, 90–93, 99, 101–104, 106, 107], in contrast, distant metastases to lung, bone, liver, brain, soft tissue, and/or nasopharynx, which reflect angioinvasion, have been reported in 23.2% of cases [85, 87, 88, 90–93, 99, 101, 102, 104, 106]. From 11 publications with appropriate follow-up, 10.5% of patients died of the disease [85, 87, 88, 91, 92, 99, 101–104, 106].
HVPTC is immunohistochemically characterized by its positivity for thyroglobulin and TTF1 [84]. Cyclin-D1 [85, 91], and common patched overexpression of p53 has been detected in more than 75% of cases [84, 85, 90–92, 103, 108]. The Ki-67 index ranged from 2 to 40% [90–92, 108, 109]. BRAF V600E is the most common mutation (> 70%) in HVPTC [85, 87, 88, 91, 92, 99, 101–103, 108, 110, 111], having also been detected in the encapsulated cases of HVPTC [104]. Because BRAF V600E is also present in classic PTC (even in PMC), it does not seem sufficient to explain the clinical aggressive behavior of HVPTC. On the other hand, mutation of TP53, which is the most frequently mutated gene in ATC, has been detected in 55.6% of a series of 18 cases of HVPTC, with seven of these cases harboring simultaneous BRAF V600E and TP53 mutations [108]. Other studies have also detected the simultaneous presence of BRAF and TP53 mutations, and even of TP53 and BRAF and TERTp mutations in HVPTC [88]. Hobnail pattern has been interpreted as evidence of high-grade transformation due to its greater association with poorly differentiated thyroid carcinoma (22%) compared with PTC (1.3%) [102]. Furthermore, synchronous [112] as well as metachronous transformation with progression to ATC has been reported in HVPTC [91].
TERTp (C228T) mutation was reported in 44.4% of a series of 18 HVPTC [108]. Concurrent BRAF V600E and TERTp mutations have been reported in one p53-positive HVPTC with progression to ATC [91]; concurrent TERTp, BRAF V600E, and TP53 mutations have also been detected in one HVPTC coexisting with ATC [112]. All these data support the important role of TERTp and/or TP53 mutations in both aggressiveness and tumor progression. PIK3CA (27.8%), CTNNB1 (16.7%), EGFR (11.1%), AKT1 (5%), and NOTCH1 (5.5%) mutations [108], as well as GNAS (31%) mutations [88], have also been reported in HVPTC. An increased risk of mortality has been reported in patients with HVPTC harboring concomitant BRAF mutation with TP53, TERTp, and/or PIK3CA mutations [88, 108].
RET/PTC1 rearrangements, which typically occur in small, slow growing PTCs [5], have only been detected in 7.6% (3/39) of HVPTC [87, 88, 92, 104, 111]. Neither RET/PTC1 nor RET/PTC3 rearrangements were detected in 4 HVPTCs that were negative for the BRAF V600E mutation [91, 108]. Neither NRAS, HRAS, KRAS, CDKN2A, nor PTEN mutations have been reported, nor were PAX8/PPARγ or ALK fusions in HVPTC [85, 88, 91, 101, 104, 108]. The major clinico-pathological and molecular characteristics of HVPTC are summarized in Table 4.
Molecular Risk Stratification in Follicular Patterned Lesions (RAS-Like)
The classification of follicular-patterned thyroid lesions has undergone major disruption recently due to changes in criteria and terminology, leading to changes in the prevalence of PTC and FTC [139]. From the simple dualistic diagnosis of FTC vs PTC, to the FTC vs PTC vs FVPTC, now we confront FTC vs PTC vs FVPTC vs NIFTP (Fig. 4). These changes clearly affect the relative prevalence of the tandem histology-genetic alterations in the various series on record as well as the putative prognostic indications.
In the classification of thyroid tumors, there was an initial dichotomy in which morphology (presence of papillae or follicles) was the distinctive feature; at that time, there was a predominance of FTC. In 1960, Lindsay [140] recognized the peculiar nuclear characteristics as the main distinctive feature of PTC. Later on, Chen and Rosai [141] identified thyroid carcinomas with PTC nuclei and follicular morphology and coined the term FVPTC. The application of these new criteria resulted in a predominance of PTC diagnosis and the demise of FTC [142]. The evidence that architectural and nuclear features were not enough to explain the diverse biological behaviors of these entities led to the return of invasive behavior as a pivotal factor in the differential diagnosis of follicular-patterned tumors. The entity designated as NIFTP was then introduced in order to avoid the over-diagnosis and overtreatment of the indolent and low grade non-invasive encapsulated FVPTC [143].
The genetic characterization of follicular lesions contributed also to progress in the classification of “follicular patterned thyroid tumors.” The genotyping of a growing number of FVPTCs reveals that these lesions have a genetic profile closer to FTC than to PTC. Higher frequency of RAS mutations and PAX8/PPARγ were found in these tumors, rather than the BRAF V600E mutation prevalent in classic PTC (Fig. 5). The NGS studies with multidimensional genomic and transcriptomic data also support the idea that a pathologic clarification of follicular-patterned thyroid lesions would lead to more precise surgical and medical therapy, especially with the introduction of targeted therapies in the management of thyroid cancer.
Adjustments are being made in the definition of NIFTP, namely, concerning histological aspects (degree of solid growth pattern and presence of oncocytic cells) and the presence/absence of genetic alterations. Initially, the criteria for NIFTP did not exclude cases harboring genetic alterations. It was advanced that NIFTP were enriched in RAS mutations and rarely harbored BRAF V600E mutation, but several studies reported NIFTP tumors with BRAF V600E mutation that displayed disease recurrence or lymph node involvement. Taking this into account and in order to avoid PTC misdiagnosis, the revised diagnostic criteria of NIFTP exclude the presence of mutations typical of classic PTC such as BRAF V600E or other BRAF V600E-like mutations (e.g., RET/PTC fusions) or other high-risk mutations (TERTp, TP53). In this setting the presence of these mutations is used as an exclusion criterion and triggers an exhaustive search for invasive features and papillary architecture in cases looking like NIFTP.
Due to persistence of diagnostic problems in NIFTP and FVPTC, we decided to focus on the molecular risk stratification in FTC (Table 5), a diagnosis that has been maintained relatively stable. RAS mutations have been consistently associated with follicular-patterned lesions; they can be present in benign and malignant lesions (FTA and FTC); therefore, they are not helpful in distinguishing benign from malignant tumors, but, as a general trend, RAS mutation rates are higher in FTC (40–50%) than in FTA (30–40%) displaying substantial overlapping in both conditions.
RAS mutations in FTC can affect all three RAS genes. NRAS 61 is the most frequent alteration found. Subtype-specific analysis of RAS mutation (HRAS, NRAS, KRAS) does not demonstrated significant differences in mutation rates between benign and malignant tumors. In some studies, an association between the presence of NRAS mutations and the presence of distant metastases and poor patient prognosis has been reported (Table 5) [144, 145]. Since most of these studies only look for RAS mutations, it remains unclear if other mutations can co-exist leading to a higher risk for such tumors. One of those high-risk mutations is the occasional existence of TERTp mutations. TERTp mutations are present in all types of malignant thyroid tumors as discussed previously, and their presence has been reported in 15–30% cases of FTC. The presence of TERTp mutations is consistently associated with age, distant metastases, advanced TNM stage, persistence/recurrence, and disease-specific mortality [84, 146–148].
Other genes were recently reported in follicular-patterned lesions. EZH1 mutations were first reported in the TCGA study [12]; other studies confirm these mutations in benign and malignant follicular-patterned lesions; EZH1 mutations are rarely present in RAS-mutated tumors and appear to be associated with low grade tumors, FTA or minimally invasive FTC [148].
PAX8/PPARγ rearrangement has been described in FTA, in FTC and in FVPTC, thus clearly pointing to the fact that it plays a role in follicular morphology but not associated with clinico-pathological features or patient outcome.
Mitochondrion-Rich Tumors–Hürthle Cell Carcinoma
The mutational profile of HCCs seems to be different from that of other types of well-differentiated thyroid cancer such as PTC and FTC [149–151]. There are virtually no BRAF V600E mutations (with the exception of oncocytic classical PTCs), and the frequency of RAS mutations is much lower than that of non-oncocytic FTC (~ 45% in FTCs vs. ~ 10% in HCCs) [150, 151]. EZH1 mutations are relatively frequent in benign and malignant oncocytic tumors (20% of Hürthle cell adenoma and 10% of Hürthle cell carcinoma). On the other hand, mutations in TERT promoter are frequent (~ 30%), being more common in widely invasive HCC than in minimally invasive HCC (32 vs. 10%, respectively) [150, 151].
Admittedly, the most characteristic genetic alteration of oncocytic tumors are mutations in mtDNA, and consistently, these mtDNA mutations are specifically enriched in disruptive mutations in genes encoding components of complex I [149–151]. However, not all oncocytic tumors present these mutations, nor are those associated with worse or better prognosis. Regarding changes in microRNAs, oncocytic tumors also seem to be different from conventional follicular tumors (FTCs) [152]. In particular, the miR-885-5p was found to be strongly up-regulated (> 40-fold) in oncocytic FTCs but not in conventional FTCs and, although without prognostic significance, it appears to be a good diagnostic marker for HCC.
Interestingly, in studies where a thorough analysis of the genetics of tumors is carried out, widespread chromosomal losses are a hallmark feature of HCC, being associated with a poorer prognosis, suggesting that widespread chromosomal losses contributed to more aggressive disease [149–151, 153].
Molecular Risk Stratification in Other Thyroid Lesions (Non-BRAF/Non-RAS)
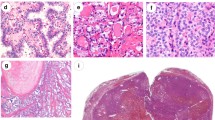
The less frequent follicular-derived tumors in the group non-BRAF-/non-RAS-like neoplasms are often difficult to classify in terms of nuclear features, and they may include sporadic thyroid tumors as well as tumors arising in the setting of germline molecular alterations. The prismatic example of the latter is the cribriform-morular thyroid carcinoma, previously considered a variant of PTC [144]. This tumor is classically associated with familial adenomatous polyposis and activating germline APC mutations in the WNT/ β-catenin pathway but may also occur in the sporadic setting. In cribriform-morular carcinoma, the β-catenin intracellular levels increase and when they accumulate in the nuclei, they tend to induce the expression of genes involved in cell proliferation and loss of differentiation. Nuclear and cytoplasmic staining for β-catenin is the hallmark of this tumor type. Consistent with Knudson’s two-hit model, additional APC somatic mutations have been found in about 50% of thyroid carcinomas associated with familial adenomatous polyposis [148]. In the sporadic cribriform-morular thyroid carcinoma, somatic APC gene mutations or combinations of somatic mutations in phenotypically equivalent genes such as CTNNB1 and AXIN1 are involved in the constitutive activation of the WTN/β-catenin pathway [148].
Another example of this non-BRAF-/non-RAS-like group of tumors that also encompasses a familial setting is the DICER1 syndrome [155]. The DICER1 syndrome is an autosomal dominant condition arising from loss of functioning germline variants of the DICER1 gene. The DICER1 syndrome is characterized by the development of pleuropulmonary blastoma, cystic nephroma, Sertoli-Leydig tumors, botryoid-type embryonal rhabdomyosarcoma of the cervix, and tumors occurring in other locations, including thyroid. The expression of the thyroid disease in the setting of DICER1 germline mutations can vary from a benign multinodular goiter to thyroid carcinoma. The thyroid carcinomas diagnosed in patients with DICER1 syndrome are not easy to classify. They include cases reported as PTC, cases reported as FTC and cases that were classified as well differentiated carcinoma, not otherwise specified (WDC, NOS), in which the nuclear classification is more difficult [156]. The presence of DICER1 somatic mutations have also been reported in the sporadic setting, namely, in poorly differentiated thyroid carcinomas of childhood and adolescence [157] and in a recently described aggressive tumor, the so-called thyroblastoma [158]. The thyroblastoma of the thyroid is an exceedingly rare malignant teratoid tumor that affects predominantly young patients and has a unique triphasic phenotype characterized by solid nests of small primitive monomorphic cells embedded in a cellular immature stroma and primitive teratoid epithelial tubules. The so-called malignant thyroid teratomas that affect predominantly adults and elderly, according to the few published cases, can also harbor DICER1 mutation [159]. This small cell, very aggressive thyroblastoma of the thyroid that occurs predominantly in young patients must be distinguished from the small cell carcinomas of the thyroid with Ewing family tumor elements (CEFTE) that frequently harbor a favorable prognosis [160]. CEFTEs are rare tumors that are typically associated with PTC foci and disclose EWSR1/FLI1 rearrangements. The EWSR1 rearrangement is a frequent event in PTC, mainly in the classic type [161], favoring the origin of CEFTE from PTC (Fig. 6). Nevertheless, the EWSR1 rearrangements appear to have no association with the clinical or biological behavior of PTC.
Another molecular alteration recently described aids in the differential diagnosis between PTC and hyalinizing trabecular tumor, two tumors with similar nuclear morphological alterations, including clearing, grooving and pseudoinclusions. The PAX8/GLIS3 and the PAX8/GLIS1 rearrangements were identified in hyalinizing trabecular tumors and were not detected in PTCs [162] representing a potential diagnostic tool to prevent overtreatment of patients with hyalinizing trabecular tumor, that present usually an excellent prognosis, although we must consider the existence of exceptional cases of hyalinizing trabecular tumors with metastases [163].
Another rare tumor that may occur in the thyroid that is also characterized by a typical rearrangement is the mammary analog secretory carcinoma (MASC). MASC is a salivary-gland like tumor that resembles secretory carcinoma of the breast, expresses S100 protein and GATA3, and harbors a balanced chromosomal translocation t(12;15)(p13;q25) that leads to the gene fusion ETV6-NTRK3 [164].
Summing up, there is no evidence supporting molecular risk stratification in non-BRAF-/non-RAS-like cases of DTC regardless of being sporadic or familial, although we must recognize that some are rare forms of thyroid carcinoma with only a few cases reported.
Integrating Molecular Risk Into Clinico-Pathological and Dynamic Risk Stratification
From the clinical standpoint, risk stratification in thyroid cancer has evolved in recent years to a dynamic process [165]. Even though response to therapy has always been considered in the follow-up of patients to reassess risk, the proposal to classify response to therapy into four categories and to continuously reassess risk was introduced in clinical practice and is now recommended in most recent guidelines [166,167]. Risk stratification is now an active process that begins when a suspicious thyroid nodule is found and continues until last follow-up; during this time, dynamic risk stratification will mainly drive the initial treatment approach and predict the risk of recurrence, disease-specific mortality, and the most likely response to initial therapy.
After surgery and facing a confirmed diagnosis of DTC, risk stratification involves predicting the risk of recurrence and the risk of disease-specific mortality. The former is usually evaluated with the ATA risk system [166] (Table 6) and the latter with UICC/AJCC staging system [3]. Considering the low risk of mortality of most patients with DTC, two key issues will drive the decision to alter the initial treatment approach with completion thyroidectomy (if lobectomy has been performed) and radioiodine: the ATA risk of recurrence category and postsurgical thyroglobulin values, in combination with imaging studies in selected cases [168]. More aggressive treatment, namely, radioiodine, should be considered in intermediate-risk patients and is recommended in high-risk patients. In the 2015 guidelines, the ATA has integrated for the first-time molecular alterations in its prognostic staging system for recurrence [166]. It was emphasized that appropriate molecular risk stratification requires the integration of the genetic factor into the clinical context, proposing a risk continuum based on the combination of clinico-pathological and molecular features. Additional clinical value was for BRAF mutation (in combination with several other factors) and TERTp mutations. Of note, apart from mPTC, the single presence of TERTp mutations seems to be associated with an intermediate or high risk of recurrence and with higher disease-specific mortality [33, 34, 169170]. The role of combined mutations (TERTp, BRAF or RAS) in risk stratification is an issue under debate [9, 10].
After the initial treatment approach, patients are classified according to their response to therapy into four classes: excellent response, with no clinical, biochemical, or structural evidence of disease; biochemical incomplete response, with persistent abnormal thyroglobulin values or rising anti-thyroglobulin antibody levels in the absence of localizable disease; structural incomplete response, with persistent or newly identified local or distant disease; and indeterminate response, when non-specific biochemical or structural findings are present [166]. We think there is now evidence enough to purpose the value of TERTp mutations in refining dynamic risk stratification in prognostic groups for DTC. This evidence has also shown better prediction of outcomes when TERTp mutations were incorporated into the different categories of response to therapy [171]. Regarding BRAF, no added benefit was found in predicting response to therapy [172].
In conclusion, there are two genetic alterations that can now be used to improve risk stratification: BRAF V600E and TERTp mutations. If BRAF V600E, integrated in the specific clinico-pathological context, may be helpful in initial risk stratification for the risk of recurrence, the prognostic value of TERTp mutations has been repeatedly demonstrated in different clinico-pathological contexts, for different outcomes and in different time points during follow-up.
Conclusion
The diagnostic and prognostic meaning of molecular data depend on its integration in the clinico-pathological context of DTC. The detection of RAS mutation does not allow the diagnosis of FTC or another malignant tumor. RAS mutations do not have prognostic significance per se although it is known that RAS mutations are associated with poor prognosis in the setting of poorly differentiated thyroid carcinomas, thus demonstrating the crucial role played by histopathology and staging [173]. The same applies to the detection of BRAF V600E mutation in the prognostic evaluation of thyroid tumors.
The most important prognostic factors in DTC are related to the size of the tumors as well as other TNM factors together with the presence of signs of extrathyroidal and blood vessel invasion. At present, TERTp mutation is the only molecular feature contributing significantly to risk stratification in DTC.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424, 2018.
Lang BH, Lo CY, Chan WF, Lam KY, Wan KY, Staging systems for papillary thyroid carcinoma: A review and comparison. Ann Surg 245: 366-378, 2007.
Tuttle M, Morris L, Haugen Bet al., Thyroid-differentiated and anaplastic carcinoma (Chapter 73) In: Amin MB, Edge SB, Greene F, et al., editors. AJCC Cancer Staging Manual. 8th., New York City: Springer International Publishing, 2017.
Trovisco V, Soares P, Preto A, Castro P, Maximo V, Sobrinho-Simões M, Molecular genetics of papillary thyroid carcinoma: Great expectations. Arq Bras Endocrinol Metabol 51: 643-653, 2007.
Soares P, Fonseca E, Wynford-Thomas D, Sobrinho-Simões M, Sporadicret-rearranged papillary carcinoma of the thyroid: A subset of slow growing, less aggressive thyroid neoplasms? The Journal of Pathology 185: 71-78, 1998.
Tallini G, Santoro M, Helie Met al., RET/PTC oncogene activation defines a subset of papillary thyroid carcinomas lacking evidence of progression to poorly differentiated or undifferentiated tumor phenotypes. Clin Cancer Res 4: 287-294, 1998.
Soares P, Sobrinho-Simoes M, Cancer: Small papillary thyroid cancers--is BRAF of prognostic value? Nat Rev Endocrinol 7: 9-10, 2011.
Soares P, Trovisco V, Rocha ASet al., BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene 22: 4578-4580, 2003.
Melo M, da Rocha AG, Vinagre J, Sobrinho-Simoes M, Soares P, Coexistence of TERT promoter and BRAF mutations in papillary thyroid carcinoma: Added value in patient prognosis? J Clin Oncol 33: 667-668, 2015.
Xing M, Liu R, Liu Xet al., BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol 32: 2718-2726, 2014.
Shi X, Liu R, Basolo Fet al., Differential Clinicopathological Risk and Prognosis of Major Papillary Thyroid Cancer Variants. J Clin Endocrinol Metab 101: 264-274, 2016.
Cancer Genome Atlas Research Network, Integrated genomic characterization of papillary thyroid carcinoma. Cell 159: 676-690, 2014.
Yoo SK, Lee S, Kim SJet al., Comprehensive Analysis of the Transcriptional and Mutational Landscape of Follicular and Papillary Thyroid Cancers. PLoS Genet 12: e1006239, 2016.
Huang Y, Qu S, Zhu Get al., BRAF V600E Mutation-Assisted Risk Stratification of Solitary Intrathyroidal Papillary Thyroid Cancer for Precision Treatment. J Natl Cancer Inst 110: 362-370, 2018.
Prescott JD, Sadow PM, Hodin RAet al., BRAF V600E status adds incremental value to current risk classification systems in predicting papillary thyroid carcinoma recurrence. Surgery 152: 984-990, 2012.
Yip L, Nikiforova MN, Yoo JYet al., Tumor genotype determines phenotype and disease-related outcomes in thyroid cancer: A study of 1510 patients. Ann Surg 262: 519–525; discussion 524–515, 2015.
Soares P, Celestino R, Melo M, Fonseca E, Sobrinho-Simões M, Prognostic biomarkers in thyroid cancer. Virchows Arch 464: 333-346, 2014.
Tavares C, Melo M, Cameselle-Teijeiro JM, Soares P, Sobrinho-Simoes M, ENDOCRINE TUMOURS: Genetic predictors of thyroid cancer outcome. Eur J Endocrinol 174: R117-126, 2016.
Patel KN, Yip L, Lubitz CCet al., The American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in Adults. Ann Surg 271, 2020.
Melo M, da Rocha AG, Vinagre J, Sobrinho-Simões M, Soares P, Coexistence of TERT promoter and BRAF mutations in papillary thyroid carcinoma: Added value in patient prognosis? J Clin Oncol 33: 667-668, 2015.
Xing M, Alzahrani AS, Carson KAet al., Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol 33: 42-50, 2015.
Vuong HG, Duong UN, Altibi AMet al., A meta-analysis of prognostic roles of molecular markers in papillary thyroid carcinoma. Endocr Connect 6: R8-r17, 2017.
Trovisco V, Soares P, Preto Aet al., Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients' age but not with tumour aggressiveness. Virchows Arch 446: 589-595, 2005.
Yip L, Nikiforova MN, Yoo JYet al., Tumor genotype determines phenotype and disease-related outcomes in thyroid cancer: A study of 1510 patients 262: 519, 2015.
Tufano RP, Teixeira GV, Bishop J, Carson KA, Xing M, BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: A systematic review and meta-analysis. Medicine 91: 274-286, 2012.
Melo M, Gaspar da Rocha A, Batista Ret al., TERT, BRAF, and NRAS in Primary Thyroid Cancer and Metastatic Disease. The Journal of Clinical Endocrinology & Metabolism 102: 1898–1907, 2017.
Rodrigues AC, Penna G, Rodrigues E, Castro P, Sobrinho-Simoes M, Soares P, The Genetics of Papillary Microcarcinomas of the Thyroid: Diagnostic and Prognostic Implications. Curr Genomics 18: 244-254, 2017.
Patel KN, Yip L, Lubitz CCet al., The American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in Adults. Ann Surg 271: e21-e93, 2020.
Liu R, Xing M, TERT promoter mutations in thyroid cancer. Endocr Relat Cancer 23: R143-R155, 2016.
Gandolfi G, Ragazzi M, Frasoldati A, Piana S, Ciarrocchi A, Sancisi V, TERT promoter mutations are associated with distant metastases in papillary thyroid carcinoma. Eur J Endocrinol 172: 403-413, 2015.
Melo M, Gaspar da Rocha A, Cancela EPG, Sobrinho-Simões M, Soares P, Age-Associated Mortality Risk in Papillary Thyroid Cancer: Does BRAF Make a Real Difference? J Clin Oncol 36: 1455-1456, 2018.
Liu R, Bishop J, Zhu G, Zhang T, Ladenson PW, Xing M, Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer: Genetic Duet of BRAF and TERT Promoter Mutations in Thyroid Cancer Mortality. JAMA Oncol 3: 202-208, 2017.
Yang J, Gong Y, Yan S, Chen H, Qin S, Gong R, Association between TERT promoter mutations and clinical behaviors in differentiated thyroid carcinoma: a systematic review and meta-analysis. Endocrine 67: 44-57, 2020.
Melo M, da Rocha AG, Vinagre Jet al., TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab 99: E754-E765, 2014.
Moon S, Song YS, Kim YAet al., Effects of Coexistent BRAF(V600E) and TERT Promoter Mutations on Poor Clinical Outcomes in Papillary Thyroid Cancer: A Meta-Analysis. Thyroid 27: 651-660, 2017.
Prescott JD, Sadow PM, Hodin RA et al., BRAFV600E status adds incremental value to current risk classification systems in predicting papillary thyroid carcinoma recurrence 152: 984–990, 2012.
Rosai J, LiVolsi VA, Sobrinho-Simoes M, Williams ED, Renaming papillary microcarcinoma of the thyroid gland: The Porto proposal. Int J Surg Pathol 11: 249-251, 2003.
Aliyev E, Ladra-Gonzalez MJ, Sanchez-Ares Met al., The Authors Reply PMC and PMT: Real Medicine and Not Just Biology. Am J Surg Pathol Publish Ahead of Print, 2020.
Rosai J, Albores Saavedra J, Asioli Set al. : WHO Classification of Tumours of Endocrine Organs. 4th ed., Lyon: IARC, 2017.
Furuya-Kanamori L, Bell KJL, Clark J, Glasziou P, Doi SAR, Prevalence of Differentiated Thyroid Cancer in Autopsy Studies Over Six Decades: A Meta-Analysis. J Clin Oncol 34: 3672–3679, 2016.
Miyauchi A, Kudo T, Ito Yet al., Natural history of papillary thyroid microcarcinoma: Kinetic analyses on tumor volume during active surveillance and before presentation. Surgery 165: 25-30, 2019
Paparodis RD, Karvounis E, Bantouna Det al., Incidentally Discovered Papillary Thyroid Microcarcinomas Are More Frequently Found in Patients with Chronic Lymphocytic Thyroiditis Than with Multinodular Goiter or Graves' Disease. Thyroid 30: 531-535, 2020.
Rego-Iraeta A, Perez-Mendez LF, Mantinan B, Garcia-Mayor RV, Time trends for thyroid cancer in northwestern Spain: True rise in the incidence of micro and larger forms of papillary thyroid carcinoma. Thyroid 19: 333-340, 2009.
Soares P, Celestino R, Gaspar da Rocha A, Sobrinho-Simoes M, Papillary thyroid microcarcinoma: how to diagnose and manage this epidemic? Int J Surg Pathol 22: 113-119, 2014.
Sobrinho-Simoes MA, Sambade MC, Gonçalves V, Latent thyroid carcinoma at autopsy:A study from Oporto, Portugal. Cancer 43: 1702-1706, 1979.
Wartofsky L, Management of papillary microcarcinoma: primum non nocere? J Clin Endocrinol Metab 97: 1169-1172, 2012.
Sugitani I, Fujimoto Y, Symptomatic versus asymptomatic papillary thyroid microcarcinoma: A retrospective analysis of surgical outcome and prognostic factors. Endocr J 46: 209-216, 1999.
Ahn HS, Kim HJ, Welch HG, Korea's thyroid-cancer "epidemic"--screening and overdiagnosis. N Engl J Med 371: 1765-1767, 2014.
Barbaro D, Simi U, Meucci G, Lapi P, Orsini P, Pasquini C, Thyroid papillary cancers: microcarcinoma and carcinoma, incidental cancers and non-incidental cancers - are they different diseases? Clin Endocrinol (Oxf) 63: 577-581, 2005.
Karatzas T, Vasileiadis I, Kapetanakis S, Karakostas E, Chrousos G, Kouraklis G, Risk factors contributing to the difference in prognosis for papillary versus micropapillary thyroid carcinoma. Am J Surg 206: 586-593, 2013.
Pazaitou-Panayiotou K, Capezzone M, Pacini F, Clinical features and therapeutic implication of papillary thyroid microcarcinoma. Thyroid 17: 1085-1092, 2007.
Song YS, Kang BH, Lee Set al., Genomic and Transcriptomic Characteristics According to Size of Papillary Thyroid Microcarcinoma. Cancers (Basel) 12: 1345, 2020.
de Biase D, Gandolfi G, Ragazzi Met al., TERT Promoter Mutations in Papillary Thyroid Microcarcinomas. Thyroid 25: 1013–1019, 2015.
Rosario PW, Ward LS, Graf H, Vaisman F, Mourao GF, Vaisman M, Thyroid nodules </= 1 cm and papillary thyroid microcarcinomas: Brazilian experts opinion. Arch Endocrinol Metab 63: 456-461, 2019.
Melo M, Gaspar da Rocha A, Batista Ret al., TERT, BRAF, and NRAS in Primary Thyroid Cancer and Metastatic Disease. J Clin Endocrinol Metab 102: 1898–1907, 2017.
Penna GC, Pestana A, Cameselle JMet al., TERTp mutation is associated with a shorter progression free survival in patients with aggressive histology subtypes of follicular-cell derived thyroid carcinoma. Endocrine 61: 489-498, 2018.
Perera D, Ghossein R, Camacho Net al., Genomic and Transcriptomic Characterization of Papillary Microcarcinomas With Lateral Neck Lymph Node Metastases. J Clin Endocrinol Metab 104: 4889-4899, 2019.
Yabuta T, Matsuse M, Hirokawa M, Yamashita S, Mitsutake N, Miyauchi A, TERT Promoter Mutations Were Not Found in Papillary Thyroid Microcarcinomas That Showed Disease Progression on Active Surveillance. Thyroid 27: 1206-1207, 2017.
Vinagre J, Almeida A, Populo Het al., Frequency of TERT promoter mutations in human cancers. Nat Commun 4: 2185, 2013.
Sama MT, Grosso E, Mele Cet al., Molecular characterisation and clinical correlation of papillary thyroid microcarcinoma. Endocrine, 2020.
Yu FX, Hu MX, Zhao HXet al., Precise Detection of Gene Mutations in Fine-Needle Aspiration Specimens of the Papillary Thyroid Microcarcinoma Using Next-Generation Sequencing. Int J Endocrinol 2019: 4723958, 2019.
Kim SY, Kim T, Kim K, Bae JS, Kim JS, Jung CK, Highly prevalent BRAF V600E and low-frequency TERT promoter mutations underlie papillary thyroid carcinoma in Koreans. J Pathol Transl Med 54: 310-317, 2020.
Liu J, Liu R, Shen X, Zhu G, Li B, Xing M, The Genetic Duet of BRAF V600E and TERT Promoter Mutations Robustly Predicts Loss of Radioiodine Avidity in Recurrent Papillary Thyroid Cancer. J Nucl Med 61: 177-182, 2020.
Xing M, Alzahrani AS, Carson KAet al., Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 309: 1493-1501, 2013.
Niemeier LA, Kuffner Akatsu H, Song Cet al., A combined molecular-pathologic score improves risk stratification of thyroid papillary microcarcinoma. Cancer 118: 2069-2077, 2012.
Bernstein J, Virk RK, Hui Pet al., Tall cell variant of papillary thyroid microcarcinoma: clinicopathologic features with BRAF(V600E) mutational analysis. Thyroid 23: 1525-1531, 2013.
Piana S, Ragazzi M, Tallini Get al., Papillary thyroid microcarcinoma with fatal outcome: Evidence of tumor progression in lymph node metastases: Report of 3 cases, with morphological and molecular analysis. Hum Pathol 44: 556-565, 2013.
Mitsutake N, Fukushima T, Matsuse Met al., BRAF(V600E) mutation is highly prevalent in thyroid carcinomas in the young population in Fukushima: A different oncogenic profile from Chernobyl. Sci Rep 5: 16976, 2015.
Tallini G, De Leo A, Repaci Aet al., Does the Site of Origin of the Microcarcinoma with Respect to the Thyroid Surface Matter? A Multicenter Pathologic and Clinical Study for Risk Stratification. Cancers (Basel) 12: 246, 2020.
Medas F, Canu GL, Cappellacci Fet al., Predictive Factors of Lymph Node Metastasis in Patients With Papillary Microcarcinoma of the Thyroid: Retrospective Analysis on 293 Cases. Front Endocrinol (Lausanne) 11: 551, 2020.
Aliyev E, Ladra-Gonzalez MJ, Sanchez-Ares Met al., Thyroid Papillary Microtumor: Validation of the (Updated) Porto Proposal Assessing Sex Hormone Receptor Expression and Mutational BRAF Gene Status. Am J Surg Pathol 44: 1161-1172, 2020.
Coca-Pelaz A, Shah JP, Hernandez-Prera JCet al., Papillary Thyroid Cancer-Aggressive Variants and Impact on Management: A Narrative Review. Adv Ther 37: 3112-3128, 2020.
Trovisco V, Vieira de Castro I, Soares Pet al., BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J Pathol 202: 247–251, 2004.
Trovisco V, Soares P, Soares R, Magalhaes J, Sa-Couto P, Sobrinho-Simoes M, A new BRAF gene mutation detected in a case of a solid variant of papillary thyroid carcinoma. Hum Pathol 36: 694-697, 2005.
Baloch ZW, LiVolsi VA, Special types of thyroid carcinoma. Histopathology 72: 40-52, 2018.
Rivera M, Ricarte-Filho J, Patel Set al., Encapsulated thyroid tumors of follicular cell origin with high grade features (high mitotic rate/tumor necrosis): A clinicopathologic and molecular study. Hum Pathol 41: 172-180, 2010.
Lu Z, Zhang Y, Feng D, Sheng J, Yang W, Liu B, Targeted next generation sequencing identifies somatic mutations and gene fusions in papillary thyroid carcinoma. Oncotarget 8: 45784-45792, 2017.
Chou A, Fraser S, Toon CW et al., A detailed clinicopathologic study of ALK-translocated papillary thyroid carcinoma. Am J Surg Pathol 39: 652–659, 2015
Perot G, Soubeyran I, Ribeiro Aet al., Identification of a recurrent STRN/ALK fusion in thyroid carcinomas. PLoS One 9: e87170, 2014
Ritterhouse LL, Wirth LJ, Randolph GW et al., ROS1 Rearrangement in Thyroid Cancer. Thyroid 26: 794–797, 2016.
Chen J-H, Faquin WC, Lloyd RV, Nosé V, Clinicopathological and molecular characterization of nine cases of columnar cell variant of papillary thyroid carcinoma. Modern Pathology 24: 739-749, 2011.
Evans HL, Columnar-Cell Carcinoma of the Thyroid: A Report of Two Cases of an Aggressive Variant of Thyroid Carcinoma. American Journal of Clinical Pathology 85: 77-80, 1986.
Wenig BM, Thompson LDR, Adair CF, Shmookler B, Heffess CS, Thyroid papillary carcinoma of columnar cell type. Cancer 82: 740-753, 1998.
Asioli S, Erickson LA, Sebo TJet al., Papillary thyroid carcinoma with prominent hobnail features: A new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical, and molecular study of eight cases. Am J Surg Pathol 34: 44–52, 2010.
Lee YS, Kim Y, Jeon S, Bae JS, Jung SL, Jung CK, Cytologic, clinicopathologic, and molecular features of papillary thyroid carcinoma with prominent hobnail features: 10 case reports and systematic literature review. Int J Clin Exp Pathol 8: 7988-7997, 2015.
Donaldson LB, Yan F, Morgan PF et al., Hobnail variant of papillary thyroid carcinoma: A systematic review and meta-analysis. Endocrine, 2020.
Ieni A, Barresi V, Cardia Ret al., The micropapillary/hobnail variant of papillary thyroid carcinoma: A review of series described in the literature compared to a series from one southern Italy pathology institution. Rev Endocr Metab Disord 17: 521-527, 2016.
Teng L, Deng W, Lu Jet al., Hobnail variant of papillary thyroid carcinoma: Molecular profiling and comparison to classical papillary thyroid carcinoma, poorly differentiated thyroid carcinoma and anaplastic thyroid carcinoma. Oncotarget 8: 22023-22033, 2017.
Albores-Saavedra J, Papillary thyroid carcinoma with prominent hobnail features: A new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical, and molecular study of 8 cases. Am J Surg Pathol 34: 913; author reply 914, 2010.
Asioli S, Erickson LA, Righi A, Lloyd RV, Papillary thyroid carcinoma with hobnail features: Histopathologic criteria to predict aggressive behavior. Hum Pathol 44: 320-328, 2013.
Cameselle-Teijeiro JM, Rodriguez-Perez I, Celestino Ret al., Hobnail Variant of Papillary Thyroid Carcinoma: Clinicopathologic and Molecular Evidence of Progression to Undifferentiated Carcinoma in 2 Cases. Am J Surg Pathol 41: 854-860, 2017.
Lubitz CC, Economopoulos KP, Pawlak AC et al., Hobnail variant of papillary thyroid carcinoma: An institutional case series and molecular profile. Thyroid 24: 958-965, 2014.
Motosugi U, Murata S, Nagata K, Yasuda M, Shimizu M, Thyroid papillary carcinoma with micropapillary and hobnail growth pattern: A histological variant with intermediate malignancy? Thyroid 19: 535-537, 2009.
Bai Y, Kakudo K, Li Yet al., Subclassification of non-solid-type papillary thyroid carcinoma identification of high-risk group in common type. Cancer Sci 99: 1908-1915, 2008.
Liu Z, Kakudo K, Bai Yet al., Loss of cellular polarity/cohesiveness in the invasive front of papillary thyroid carcinoma, a novel predictor for lymph node metastasis; possible morphological indicator of epithelial mesenchymal transition. J Clin Pathol 64: 325-329, 2011.
Chung YJ, Lee JS, Park SYet al., Histomorphological factors in the risk prediction of lymph node metastasis in papillary thyroid carcinoma. Histopathology 62: 578-588, 2013.
Yue C, Zhang Y, Xing Let al., [Clinicopathological factors in risk prediction of lymph node metastasis in papillary thyroid carcinoma]. Zhonghua Yi Xue Za Zhi 94: 3637-3641, 2014.
Lino-Silva LS, Dominguez-Malagon HR, Caro-Sanchez CH, Salcedo-Hernandez RA, Thyroid gland papillary carcinomas with "micropapillary pattern," a recently recognized poor prognostic finding: clinicopathologic and survival analysis of 7 cases. Hum Pathol 43: 1596-1600, 2012.
Wong KS, Chen TY, Higgins SE et al., A potential diagnostic pitfall for hobnail variant of papillary thyroid carcinoma. Histopathology 76: 707-713, 2020.
Ambrosi F, Righi A, Ricci C, Erickson LA, Lloyd RV, Asioli S, Hobnail Variant of Papillary Thyroid Carcinoma: A Literature Review. Endocr Pathol 28: 293-301, 2017.
Watutantrige-Fernando S, Vianello F, Barollo Set al., The Hobnail Variant of Papillary Thyroid Carcinoma: Clinical/Molecular Characteristics of a Large Monocentric Series and Comparison with Conventional Histotypes. Thyroid 28: 96-103, 2018.
Amacher AM, Goyal B, Lewis JS, Jr., El-Mofty SK, Chernock RD, Prevalence of a hobnail pattern in papillary, poorly differentiated, and anaplastic thyroid carcinoma: A possible manifestation of high-grade transformation. Am J Surg Pathol 39: 260-265, 2015.
Asioli S, Maletta F, Pagni Fet al., Cytomorphologic and molecular features of hobnail variant of papillary thyroid carcinoma: Case series and literature review. Diagn Cytopathol 42: 78-84, 2014.
Agarwal S, Sadiq Q, Ortanca I, Hobnail cells in encapsulated papillary thyroid carcinoma: Report of 2 cases with immunohistochemical and molecular findings and literature analysis. Pathol Res Pract 216: 152678, 2020.
Lilo MT, Bishop JA, Ali SZ, Hobnail variant of papillary thyroid carcinoma: A case with an unusual presentation. Diagn Cytopathol 45: 754-756, 2017.
Liu J, Brown R, Rubenfeld S, Karni R, Papillary thyroid carcinoma with prominent hobnail features diagnosed preoperatively by fine needle aspiration and demonstrating constitutive activation of mTOR signaling pathway: a case report. J Clin Exp Pathol 3: 2161–0681.10001, 2013.
Mehrotra S, Lapadat R, Barkan GA, Pambuccian SE, "Teardrop," "comet," and "bowling-pin" cells in a hobnail variant of papillary thyroid carcinoma fine needle aspirate. Diagn Cytopathol 47: 839-842, 2019.
Morandi L, Righi A, Maletta Fet al., Somatic mutation profiling of hobnail variant of papillary thyroid carcinoma. Endocr Relat Cancer 24: 107-117, 2017.
Ito Y, Hirokawa M, Hayashi Tet al., Case report: exceptionally rapid growth character of hobnail variant of papillary thyroid carcinoma: A report of four cases. Endocr J 67: 1047-1053, 2020.
Al-Yahri O, Abdelaal A, El Ansari Wet al., First ever case report of co-occurrence of hobnail variant of papillary thyroid carcinoma and intrathyroid parathyroid adenoma in the same thyroid lobe. Int J Surg Case Rep 70: 40-52, 2020.
Bellevicine C, Cozzolino I, Malapelle U, Zeppa P, Troncone G, Cytological and molecular features of papillary thyroid carcinoma with prominent hobnail features: A case report. Acta Cytol 56: 560-564, 2012.
Ragazzi M, Torricelli F, Donati Bet al., Coexisting well-differentiated and anaplastic thyroid carcinoma in the same primary resection specimen: immunophenotypic and genetic comparison of the two components in a consecutive series of 13 cases and a review of the literature. Virchows Arch, 2020.
Oh WJ, Lee YS, Cho Uet al., Classic Papillary Thyroid Carcinoma with Tall Cell Features and Tall Cell Variant Have Similar Clinicopathologic Features. Korean Journal of Pathology 48: 201, 2014.
Wang X, Cheng W, Liu C, Li J, Tall cell variant of papillary thyroid carcinoma: Current evidence on clinicopathologic features and molecular biology. Oncotarget 7: 40792-40799, 2016.
Villar-Taibo R, Peteiro-González D, Cabezas-Agrícola JMet al., Aggressiveness of the tall cell variant of papillary thyroid carcinoma is independent of the tumor size and patient age. Oncology Letters 13: 3501-3507, 2017.
Leung AK-C, Chow S-M, Law SCK, Clinical Features and Outcome of the Tall Cell Variant of Papillary Thyroid Carcinoma. The Laryngoscope 118: 32–38, 2008.
Morris LGT, Shaha AR, Tuttle RM, Sikora AG, Ganly I, Tall-Cell Variant of Papillary Thyroid Carcinoma: A Matched-Pair Analysis of Survival. Thyroid 20: 153-158, 2010.
Min HS, Lee C, Jung KC, Correlation of Immunohistochemical Markers and BRAF Mutation Status with Histological Variants of Papillary Thyroid Carcinoma in the Korean Population. Journal of Korean Medical Science 28: 534, 2013.
Ghossein RA, Leboeuf R, Patel KNet al., Tall Cell Variant of Papillary Thyroid Carcinoma without Extrathyroid Extension: Biologic Behavior and Clinical Implications. Thyroid 17: 655-661, 2007.
Rüter A, Dreifus J, Jones M, Nishiyama R, Lennquist S, Overexpression of p53 in tall cell variants of papillary thyroid carcinoma. Surgery 120: 1046-1050, 1996.
Nikiforova MN, Kimura ET, Gandhi Met al., BRAF Mutations in Thyroid Tumors Are Restricted to Papillary Carcinomas and Anaplastic or Poorly Differentiated Carcinomas Arising from Papillary Carcinomas. The Journal of Clinical Endocrinology & Metabolism 88: 5399-5404, 2003.
Xing M, BRAF mutation in thyroid cancer. Endocrine Related Cancer 12: 245-262, 2005.
Basolo F, Torregrossa L, Giannini Ret al., Correlation between theBRAFV600E Mutation and Tumor Invasiveness in Papillary Thyroid Carcinomas Smaller than 20 Millimeters: Analysis of 1060 Cases. The Journal of Clinical Endocrinology & Metabolism 95: 4197-4205, 2010.
Qasem E, Murugan AK, Al-Hindi Het al., TERT promoter mutations in thyroid cancer: A report from a Middle Eastern population. Endocrine-Related Cancer 22: 901-908, 2015.
Wong KS, Higgins SE, Marqusee E, Nehs MA, Angell T, Barletta JA, Tall Cell Variant of Papillary Thyroid Carcinoma: Impact of Change in WHO Definition and Molecular Analysis. Endocrine Pathology 30: 43-48, 2018.
Basolo F, Giannini R, Monaco Cet al., Potent Mitogenicity of the RET/PTC3 Oncogene Correlates with Its Prevalence in Tall-Cell Variant of Papillary Thyroid Carcinoma. The American Journal of Pathology 160: 247-254, 2002.
Shimizu M, Hirokawa M, Manabe T, Tall cell variant of papillary thyroid carcinoma with foci of columnar cell component. Virchows Archiv 434: 173-175, 1999.
Bronner MP, LiVolsi VA, Spindle cell squamous carcinoma of the thyroid: an unusual anaplastic tumor associated with tall cell papillary cancer. Mod Pathol 4: 637-643, 1991.
Ganly I, Ibrahimpasic T, Rivera Met al., Prognostic Implications of Papillary Thyroid Carcinoma with Tall-Cell Features. Thyroid 24: 662-670, 2014.
Vuong HG, Long NP, Anh NHet al., Papillary thyroid carcinoma with tall cell features is as aggressive as tall cell variant: A meta-analysis. Endocrine Connections 7: R286-R293, 2018.
Wang S, Xiong Y, Zhao Q, Song H, Yi P, Liu C, Columnar cell papillary thyroid carcinoma prognosis: Findings from the SEER database using propensity score matching analysis. Am J Transl Res 11: 6262-6270, 2019.
Sobrinho-Simões M, Nesland JM, Johannessen JV, Columnar-Cell Carcinoma: Another Variant of Poorly Differentiated Carcinoma of the Thyroid. American Journal of Clinical Pathology 89: 264-267, 1988.
Enriquez ML, Baloch ZW, Montone KT, Zhang PJ, LiVolsi VA, CDX2 Expression in Columnar Cell Variant of Papillary Thyroid Carcinoma. American Journal of Clinical Pathology 137: 722-726, 2012.
Sujoy V, Pinto A, Nosé V, Columnar Cell Variant of Papillary Thyroid Carcinoma: A Study of 10 Cases with Emphasis on CDX2 Expression. Thyroid 23: 714-719, 2013.
Krasner JR, Alyouha N, Pusztaszeri Met al., Molecular mutations as a possible factor for determining extent of thyroid surgery. J Otolaryngol Head Neck Surg 48: 51, 2019.
Akslen LA, Varhaug JE, Thyroid Carcinoma with Mixed Tall-Cell and Columnar-Cell Features. American Journal of Clinical Pathology 94: 442-445, 1990.
Putti TC, Bhuiya TA, Mixed columnar cell and tall cell variant of papillary carcinoma of thyroid: A case report and review of the literature. Pathology 32: 286-289, 2000.
Tranchida P, Bernacki E, Budev H, Giorgadze T, Preoperative cytologic diagnosis of papillary thyroid carcinoma with mixed columnar cell and tall cell features. Diagnostic Cytopathology 40: E4-E7, 2011.
Tallini G, Tuttle RM, Ghossein RA, The History of the Follicular Variant of Papillary Thyroid Carcinoma. J Clin Endocrinol Metab 102: 15-22, 2017.
Lindsay S, Carcinoma of the thyroid gland: A clinical and pathologic study of 293 patients at the University of California hospital, chapter VI. Pathologic Study of Thyroid Carcinoma: Classification of Thyroid Carcinoma. Springfield, IL: Charles C Thomas: 30–65, 1960.
Chen KT, Rosai J, Follicular variant of thyroid papillary carcinoma: A clinicopathologic study of six cases. Am J Surg Pathol 1: 123-130, 1977.
LiVolsi VA, Asa SL, The demise of follicular carcinoma of the thyroid gland. Thyroid 4: 233-236, 1994.
Nikiforov YE, Seethala RR, Tallini Get al., Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol 2: 1023-1029, 2016.
Cameselle-Teijeiro JM, Peteiro-Gonzalez D, Caneiro-Gomez Jet al., Cribriform-morular variant of thyroid carcinoma: A neoplasm with distinctive phenotype associated with the activation of the WNT/beta-catenin pathway. Mod Pathol 31: 1168-1179, 2018.
Fukahori M, Yoshida A, Hayashi Het al., The associations between RAS mutations and clinical characteristics in follicular thyroid tumors: New insights from a single center and a large patient cohort. Thyroid 22: 683-689, 2012.
Song YS, Lim JA, Min HSet al., Changes in the clinicopathological characteristics and genetic alterations of follicular thyroid cancer. Eur J Endocrinol 177: 465-473, 2017.
Jang EK, Song DE, Sim SYet al., NRAS codon 61 mutation is associated with distant metastasis in patients with follicular thyroid carcinoma. Thyroid 24: 1275-1281, 2014.
Cameselle-Teijeiro JM, Sobrinho-Simoes M, Cribriform-morular variant of thyroid carcinoma. Pathologica 111: 1-3, 2019.
Cavadas B, Pereira JB, Correia Met al., Genomic and transcriptomic characterization of the mitochondrial-rich oncocytic phenotype on a thyroid carcinoma background. Mitochondrion 46: 123-133, 2019.
Ganly I, Makarov V, Deraje Set al., Integrated Genomic Analysis of Hurthle Cell Cancer Reveals Oncogenic Drivers, Recurrent Mitochondrial Mutations, and Unique Chromosomal Landscapes. Cancer Cell 34: 256–270 e255, 2018.
Gopal RK, Kubler K, Calvo SEet al., Widespread Chromosomal Losses and Mitochondrial DNA Alterations as Genetic Drivers in Hurthle Cell Carcinoma. Cancer Cell 34: 242–255 e245, 2018.
Dettmer M, Vogetseder A, Durso MBet al., MicroRNA expression array identifies novel diagnostic markers for conventional and oncocytic follicular thyroid carcinomas. J Clin Endocrinol Metab 98: E1-7, 2013.
Erickson LA, Jalal SM, Goellner JRet al., Analysis of Hurthle cell neoplasms of the thyroid by interphase fluorescence in situ hybridization. Am J Surg Pathol 25: 911-917, 2001.
Jung CK, Kim Y, Jeon S, Jo K, Lee S, Bae JS, Clinical utility of EZH1 mutations in the diagnosis of follicular-patterned thyroid tumors. Hum Pathol 81: 9-17, 2018.
Khan NE, Bauer AJ, Schultz KAPet al., Quantification of Thyroid Cancer and Multinodular Goiter Risk in the DICER1 Syndrome: A Family-Based Cohort Study. J Clin Endocrinol Metab 102: 1614-1622, 2017.
Gullo I, Batista R, Rodrigues-Pereira Pet al., Multinodular Goiter Progression Toward Malignancy in a Case of DICER1 Syndrome: Histologic and Molecular Alterations. Am J Clin Pathol 149: 379-386, 2018.
Chernock RD, Rivera B, Borrelli Net al., Poorly differentiated thyroid carcinoma of childhood and adolescence: A distinct entity characterized by DICER1 mutations. Mod Pathol 33: 1264-1274, 2020.
Agaimy A, Witkowski L, Stoehr Ret al., Malignant teratoid tumor of the thyroid gland: An aggressive primitive multiphenotypic malignancy showing organotypical elements and frequent DICER1 alterations-is the term "thyroblastoma" more appropriate? Virchows Arch 477: 787-798, 2020.
Rooper LM, Bynum JP, Miller KPet al., Recurrent DICER1 Hotspot Mutations in Malignant Thyroid Gland Teratomas: Molecular Characterization and Proposal for a Separate Classification. Am J Surg Pathol 44: 826-833, 2020.
Eloy C, Oliveira M, Vieira J, Teixeira MR, Cruz J, Sobrinho-Simoes M, Carcinoma of the thyroid with ewing family tumor elements and favorable prognosis: Report of a second case. Int J Surg Pathol 22: 260-265, 2014.
Oliveira G, Polonia A, Cameselle-Teijeiro JMet al., EWSR1 rearrangement is a frequent event in papillary thyroid carcinoma and in carcinoma of the thyroid with Ewing family tumor elements (CEFTE). Virchows Arch 470: 517-525, 2017.
Nikiforova MN, Nikitski AV, Panebianco Fet al., GLIS Rearrangement is a Genomic Hallmark of Hyalinizing Trabecular Tumor of the Thyroid Gland. Thyroid 29: 161-173, 2019.
Sambade C, Franssila K, Cameselle-Teijeiro J, Nesland J, Sobrinho-Simoes M, Hyalinizing trabecular adenoma: A misnomer for a peculiar tumor of the thyroid gland. Endocr Pathol 2: 83-91, 1991.
Skalova A, Vanecek T, Sima Ret al., Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 34: 599-608, 2010.
Tuttle RM, Alzahrani AS, Risk Stratification in Differentiated Thyroid Cancer: From Detection to Final Follow-up. J Clin Endocrinol Metab 104: 4087-4100, 2019.
Haugen BR, Alexander EK, Bible KCet al., 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26: 1-133, 2016.
Filetti S, Durante C, Hartl Det al., Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol 30: 1856-1883, 2019.
Melo M, Vicente N, Ventura M, Gaspar Da Rocha A, Soares P, Carrilho F, The role of ablative treatment in differentiated thyroid cancer management. Expert Review of Endocrinology & Metabolism 12: 109-116, 2017.
Chen B, Shi Y, Xu Y, Zhang J, The predictive value of coexisting BRAFV600E and TERT promoter mutations on poor outcomes and high tumour aggressiveness in papillary thyroid carcinoma: A systematic review and meta-analysis. Clin Endocrinol (Oxf), 2020.
Liu R, Xing M, TERT promoter mutations in thyroid cancer. Endocrine-related cancer 23: R143-155, 2016.
Kim TH, Ki CS, Kim HSet al., Refining Dynamic Risk Stratification and Prognostic Groups for Differentiated Thyroid Cancer With TERT Promoter Mutations. J Clin Endocrinol Metab 102: 1757-1764, 2017.
Kowalska A, Walczyk A, Kowalik Aet al., Response to therapy of papillary thyroid cancer of known BRAF status. Clin Endocrinol (Oxf) 87: 815-824, 2017.
Garcia-Rostan G, Zhao H, Camp RLet al., ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol 21: 3226-3235, 2003.
Funding
This work was financed by FEDER—Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020—Operacional Programme for Competitiveness and Internationalization (POCI), Portugal 2020, and by Portuguese funds through FCT—Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Inovação in the framework of the project “Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274). Additional funding by the European Regional Development Fund (ERDF) through the Operational Programme for Competitiveness and Internationalization—COMPETE2020, and Portuguese national funds via FCT, under project POCI-01-0145-FEDER-016390: CANCEL STEM and from the FCT under the project POCI-01-0145-FEDER-031438: The other faces of telomerase: Looking beyond tumor immortalization (PDTC/MED_ONC/31438/2017). Additional funding through the Sociedade Portuguesa de Endocrinologia, Diabetes e Metabolismo—Bolsa SPEDM para projecto de investigação (2017). JV is supported by FCT with a research contract (CEECIND/00201/2017). This work was supported in part (JMC-T) by Grant ISCIII-PI19/01316-FEDER from Instituto de Salud Carlos III, Ministry of Science and Innovation, Spain. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soares, P., Póvoa, A.A., Melo, M. et al. Molecular Pathology of Non-familial Follicular Epithelial–Derived Thyroid Cancer in Adults: From RAS/BRAF-like Tumor Designations to Molecular Risk Stratification. Endocr Pathol 32, 44–62 (2021). https://doi.org/10.1007/s12022-021-09666-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-021-09666-1