Abstract
Papillary thyroid carcinoma (PTC) is characterized by proliferation of follicular cells with distinctive nuclear features such as ground glass appearance, nuclear groove and pseudoinclusion. From the proliferation pattern, PTC is divided into several histological subtypes; conventional histology is classified as papillary type, and there are also follicular and solid variants. PTC is heterogeneous in genetic alterations. PTC with BRAF mutation presents a histology of conventional PTC, and follows an aggressive clinical course. Most cases of PTC with RAS mutation show a follicular variant, and prognosis is favorable. RET/PTC1 is observed sporadically and in young cases, and prognosis is favorable. RET/PTC3 is associated with radiation exposure, and the solid variant is frequent. ETV6-NTRK3 may be associated with radiation exposure, and the clinical course is aggressive. Mutation in the telomerase reverse transcriptase promoter is observed in PTC cases involving elderly male patients. Tumor size is large, associated with distant metastasis and advanced stage. This mutation is found concomitantly with BRAF mutation, and the clinical course is aggressive. Genetic alterations form subsets of PTC with distinct clinicopathological features. Careful assessment of clinicopathological features is considered useful in predicting clinical course and when planning treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papillary thyroid carcinoma (PTC) is a malignant neoplasm of the thyroid gland. Incidence has been increasing worldwide [1]. Pathological features of PTC are distinct, and nuclear features are essential in the diagnosis of PTC.
Although PTC shows distinct histological features, genetic alterations are heterogeneous. Recent studies have revealed the presence of various mutations such as BRAF, RAS and in the promotor of telomerase reverse transcriptase (TERT), as well as gene rearrangement of RET/PTC and ETV6-NTRK. These genetic alterations are associated with the clinical and pathological characteristics of PTC. The correlation between clinicopathological characteristics and genetic alterations in PTC is reviewed.
Papillary thyroid carcinoma and morphological features
PTC is characterized by distinct nuclear features: pseudoinclusion (Fig. 1a), groove (Fig. 1b) and ground glass appearance (Fig. 1c). These nuclear features are essential for a definitive diagnosis of PTC.
The histological features of papillary thyroid cancer. Distinct nuclear features are nuclear pseudoinclusions (a), nuclear grooves (b), and ground glass appearance (c). Conventional papillary type of PTC is characterized by the papillary proliferation of cells with distinct nuclear features (d). Follicular variant is characterized by follicular pattern of proliferation (e). Solid variant shows a sheet-like proliferation of tumor cells (f). The tumor cells invade to the extrathyroidal tissue (g). Non-invasive follicular variant thyroid neoplasm with papillary nuclear features (NIFTP) is characterized by encapsulated follicular proliferation of tumor cells with distinct nuclear features (h, i)
Based on the proliferation pattern, PTC is categorized into several variants. The conventional type is characterized by papillary proliferation of cells with distinct nuclear features (Fig. 1d). The follicular variant is characterized by follicular proliferation of tumor cells (Fig. 1e), and the solid variant by a sheet-like proliferation of tumor cells (Fig. 1f).
Tumors usually have a thick fibrous capsule, and the tumor cells may invade into the fibrous capsule and infiltrate surrounding tissue and organs (Fig. 1g). Tumor cells may invade into lymph vessels.
When tumor cells have the distinct nuclear morphology of PTC but show encapsulated and follicular growth pattern, the tumor is diagnosed as non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) (Fig. 1h, i). It was suggested that the clinical course of NIFTP is favorable compared to that of PTC [2]. For a definitive diagnosis of NIFTP, careful histological evaluation of capsular and vascular invasion is required.
An overview of genetic alterations of PTC
Mutation of BRAF is observed in 36–83% of the cases of PTC [3]. RAS mutation is rare in conventional type PTC, but occurs in 25% of the cases of follicular variant PTC [3]. RET/PTC rearrangement is observed in 22–65% [4], ETV6-NTRK3 fusion gene in 2–14.5% [5], and TERT promotor mutation in 8% [6] of the cases of PTC.
Molecular pathways involved in genetic alteration in PTC are illustrated in Fig. 2. Mutation of BRAF and RAS and rearrangement of RET/PTC stimulate the MAPK pathway. ETV6-NTRK3 stimulates the insulin-like growth factor pathway and the PI3K/Akt pathway. These genetic alterations encourage cell growth, proliferation, and differentiation. TERT promotor mutation induces overexpression of TERT and promotes survival of tumor cells.
The molecules involved in the carcinogenesis of PTC. The mutation of BRAF and RAS and rearrangement of RET/PTC stimulate the MAPK pathway. ETV6-NTRK3 stimulate insulin-like growth factor pathway and the PI3K/Akt pathway. These genetic alterations enhance cell growth, proliferation, apoptosis and differentiation. TERT promotor mutation induces overexpression of TERT and promotes survival of tumor cells
Mutation of BRAF
Mutation of BRAF is observed in various malignant tumors, such as malignant melanoma, colon cancer and gastrointestinal stromal tumors [7]. Mutation of BRAF is the most common genetic alteration in PTC. While V600E mutation is the most frequent, other mutations of K601E, V600K601 delinsE and T599IV600_R603 del have also been reported [8].
PTC with BRAF mutation occurs in patients aged in their 50s [9]. Patients are predominantly male. Tumor size tends to be large [10] and tumor histology is that of conventional PTC [11]. Extrathyroidal extension, vascular invasion and lymph node metastasis are also frequent [12], and the pathological stage is usually advanced [13]. Recurrence is reported in 25% of patients [14]. Prognosis is worse than PTC with wild type of BRAF, and disease-specific death is 5% [15] (Table 1).
PTC with mutation of BRAF K601E is observed in 1.2% of the cases of PTC, and the follicular variant type is frequent. V600K601 delinsE occurs in 0.2%, which is associated with the solid variant type. BRAF T599IV600_R603 del is observed in 0.007% cases of PTC. PTC with these mutations follows a less aggressive clinical course than PTC with BRAF V600E [8].
Mutation of RAS
Among three isoforms, HRAS, NRAS, and KRAS, mutation of NRAS is the most frequent [16], and Q61R and Q61K are well reported.
Mutation of RAS is rarely observed in conventional PTC, but occurs in 25% of cases of follicular variant of PTC [3]. If the tumor is non-invasive, lymph node metastasis, distant metastasis, and recurrence are infrequent [17] (Table 1). However, prognostic significance of RAS mutation is not fully understood, due to the limited number of patients and follow-up period. Mutation of RAS is reported to be frequently observed in NIFTP [2].
RET rearrangement
RET is a receptor-type tyrosine kinase, and fusion with PTC1 and PTC3 has been reported.
RET/PTC1 is observed in cases of PTC involving young patients, less than 18 years of age [3, 18]. Correlation with radiation exposure is not observed. Conventional PTC is the most frequent histological subtype [19]. Clinical course is usually favorable.
RET/PTC3 is also observed in young patients, less than 18 years of age [20], and correlation with radiation exposure has been reported [21]. Both male and female patients are equally affected [20]. Tumors are usually in the advanced stage at the time of presentation. Regarding histological subtypes, the solid variant type is most frequent [22]. Despite advanced stage at diagnosis, survival is favorable, and tumors in this rearrangement are responsive to radioactive iodine therapy [3] (Table 1).
These rearrangements are not detected in poorly differentiated thyroid carcinoma and anaplastic thyroid carcinoma. PTC with these rearrangements is not considered to transform into these aggressive carcinomas [3].
ETV6-NTRK fusion gene
ETV6-NTRK3 results from translocation t(12;15) (p13;q25). ETV6 is a transcription factor of the ETS (E26 transformation specific) family, and NTRK3 is a receptor-type tyrosine kinase.
ETV6-NTRK3 was observed in various tumors, including mammary analog secretory carcinoma in salivary glands, infantile fibrosarcoma, chronic eosinophilic leukemia, acute myelogenous leukemia and gastrointestinal stromal tumors [23, 24].
This fusion gene may be associated with radiation exposure [5]. PTC with ETV6-NTRK3 presents a mixed pattern of follicular and papillary growth, and oncocytic cell or clear cell foci are frequently observed [5, 25]. Chronic lymphocytic thyroiditis may be present in the background, and some cases clinically show hypothyroidism [5, 25]. Extrathyroidal extension and lymph node metastasis are frequent [25] (Table 1). However, the prognostic value of this fusion gene is not fully understood due to the limited number of patients [4].
Telomerase reverse transcriptase (TERT) promoter mutations
TERT is an enzyme involved in the elongation of telomeres, the nucleoprotein complex at the end of a chromosome, preserving chromosome integrity and genomic stability [6]. Overexpression of TERT is caused by a mutation of its promotor. There are two hot spots for mutation located at the − 124 and − 146 base pairs upstream from the ATG codon. Mutations of the TERT promoter are observed in tumors of the central nervous system, bladder cancer, hepatocellular carcinoma, melanoma, and thyroid cancer [26]. Overexpression promotes survival of tumor cells.
TERT promoter mutations are observed in 8% of the cases of PTC [6], and occur along with other tumors originating from the follicular epithelium of the thyroid. These mutations are not detected in normal tissue, benign lesions, or medullary thyroid carcinomas [22].
TERT promotor mutations are found in cases of PTC involving older patients in their 50s and 60s [6, 22]. Patients are predominantly male [6, 22]. Tumor size tends to be large [22]. Association with specific histological subtypes has not been reported. Distant metastasis is found in 17% of the patients [12]. Disease is usually in the advanced stages, prognosis is usually unfavorable, and the 5-year disease-free survival rate is only 10% [22] (Table 1).
It should be noted that mutations of TERT promotor may be concomitantly present with BRAF mutations [12]. Concomitant TERT and BRAF mutations are reported in elderly male patients with PTC [27]. Recurrence is reported to be 68.6% in cases of PTC with both BRAF and TERT promoter mutations, whereas recurrence was only 8.7% in cases of PTC with either one mutation [12]. Coexistence of BRAF and TERT promotor mutations, and pathologically, a Ki-67 labeling index higher than 10% is reported as a promising marker to predict recurrence of PTC [28].
Pathological practice for papillary thyroid carcinoma
The most critical factor in the diagnosis of PTC is the identification of distinct nuclear features. Next, capsular and lymphovascular invasion must be carefully evaluated. When tumor cells demonstrate the distinct nuclear features of PTC, form a follicular pattern, and evidence of capsular or lymphovascular invasion is not present, the tumor is diagnosed as NIFTP.
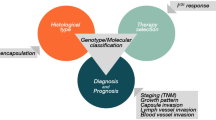
Genetic alterations may form subsets of PTC, with distinct clinical and pathological characteristics. Determining the genetic alteration in each case of PTC is not feasible. Instead, genetic alteration can be predicted by careful assessment of the clinical and pathological characteristics of PTC. Special attention should be given to age, sex, proliferation pattern and lymph node metastasis (Table 2).
References
La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E (2015) Thyroid cancer mortality and incidence: a global overview. Int J Cancer 136:2187–2195
Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, Barletta JA, Wenig BM, Al Ghuzlan A, Kakudo K, Giordano TJ, Alves VA, Khanafshar E, Asa SL, El-Naggar AK, Gooding WE, Hodak SP, Lloyd RV, Maytal G, Mete O, Nikiforova MN, Nose V, Papotti M, Poller DN, Sadow PM, Tischler AS, Tuttle RM, Wall KB, LiVolsi VA, Randolph GW, Ghossein RA (2016) Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2:1023–1029
Tavares C, Melo M, Cameselle-Teijeiro JM, Soares P, Sobrinho-Simoes M (2016) Endocrine Tumours: genetic predictors of thyroid cancer outcome. Eur J Endocrinol 174:R117–R126
Cordioli MI, Moraes L, Cury AN, Cerutti JM (2015) Are we really at the dawn of understanding sporadic pediatric thyroid carcinoma? Endocr Relat Cancer 22:R311–R324
Leeman-Neill RJ, Kelly LM, Liu P, Brenner AV, Little MP, Bogdanova TI, Evdokimova VN, Hatch M, Zurnadzy LY, Nikiforova MN, Yue NJ, Zhang M, Mabuchi K, Tronko MD, Nikiforov YE (2014) ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer 120:799–807
Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, Melo M, da Rocha AG, Preto A, Castro P, Castro L, Pardal F, Lopes JM, Santos LL, Reis RM, Cameselle-Teijeiro J, Sobrinho-Simoes M, Lima J, Maximo V, Soares P (2013) Frequency of TERT promoter mutations in human cancers. Nat Commun 4:2185
Kazakov VS, Demidchik EP, Astakhova LN (1992) Thyroid cancer after Chernobyl. Nature 359:21
Torregrossa L, Viola D, Sensi E, Giordano M, Piaggi P, Romei C, Materazzi G, Miccoli P, Elisei R, Basolo F (2016) Papillary thyroid carcinoma with rare Exon 15 BRAF mutation has indolent behavior: a single-institution experience. J Clin Endocrinol Metab 101:4413–4420
Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G, Fusco A, Santoro M, Fagin JA, Nikiforov YE (2003) BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 88:5399–5404
Kim TY, Kim WB, Rhee YS, Song JY, Kim JM, Gong G, Lee S, Kim SY, Kim SC, Hong SJ, Shong YK (2006) The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol 65:364–368
Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O’Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Clifton-Bligh R, Tallini G, Holt EH, Sykorova V (2015) Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol 33:42–50
Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, Pai S, Bishop J (2014) BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol 32:2718–2726
Yang LB, Sun LY, Jiang Y, Tang Y, Li ZH, Zhang HY, Bu H, Ye F (2015) The clinicopathological features of BRAF mutated papillary thyroid cancers in Chinese patients. Int J Endocrinol 2015:642046
Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, Romei C, Miccoli P, Pinchera A, Basolo F (2008) BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 93:3943–3949
Oishi N, Kondo T, Nakazawa T, Mochizuki K, Inoue T, Kasai K, Tahara I, Yabuta T, Hirokawa M, Miyauchi A, Katoh R (2017) Frequent BRAF V600E and absence of TERT promoter mutations characterize sporadic pediatric papillary thyroid carcinomas in Japan. Endocr Pathol 28:103–111
Howell GM, Hodak SP, Yip L (2013) RAS mutations in thyroid cancer. The Oncologist 18:926–932
Liu J, Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, Tuttle RM, Ghossein RA (2006) Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer 107:1255–1264
Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, Carson KA, Vasko V, Larin A, Tallini G, Tolaney S, Holt EH, Hui P, Umbricht CB, Basaria S, Ewertz M, Tufaro AP, Califano JA, Ringel MD, Zeiger MA, Sidransky D, Ladenson PW (2005) BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 90:6373–6379
Thomas GA, Bunnell H, Cook HA, Williams ED, Nerovnya A, Cherstvoy ED, Tronko ND, Bogdanova TI, Chiappetta G, Viglietto G, Pentimalli F, Salvatore G, Fusco A, Santoro M, Vecchio G (1999) High prevalence of RET/PTC rearrangements in Ukrainian and Belarussian post-Chernobyl thyroid papillary carcinomas: a strong correlation between RET/PTC3 and the solid-follicular variant. J Clin Endocrinol Metab 84:4232–4238
Su X, Li Z, He C, Chen W, Fu X, Yang A (2016) Radiation exposure, young age, and female gender are associated with high prevalence of RET/PTC1 and RET/PTC3 in papillary thyroid cancer: a meta-analysis. Oncotarget 7:16716–16730
Nikiforov YE, Rowland JM, Bove KE, Monforte-Munoz H, Fagin JA (1997) Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res 57:1690–1694
Melo M, da Rocha AG, Vinagre J, Batista R, Peixoto J, Tavares C, Celestino R, Almeida A, Salgado C, Eloy C, Castro P, Prazeres H, Lima J, Amaro T, Lobo C, Martins MJ, Moura M, Cavaco B, Leite V, Cameselle-Teijeiro JM, Carrilho F, Carvalheiro M, Maximo V, Sobrinho-Simoes M, Soares P (2014) TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab 99:E754–E765
Knezevich SR, McFadden DE, Tao W, Lim JF, Sorensen PH (1998) A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet 18:184–187
De Braekeleer E, Douet-Guilbert N, Morel F, Le Bris MJ, Basinko A, De Braekeleer M (2012) ETV6 fusion genes in hematological malignancies: a review. Leuk Res 36:945–961
Seethala RR, Chiosea SI, Liu CZ, Nikiforova M, Nikiforov YE (2017) Clinical and morphologic features of ETV6-NTRK3 translocated papillary thyroid carcinoma in an adult population without radiation exposure. Am J Surg Pathol 41:446–457
Capezzone M, Cantara S, Marchisotta S, Busonero G, Formichi C, Benigni M, Capuano S, Toti P, Pazaitou-Panayiotou K, Caruso G, Carli AF, Palummo N, Pacini F (2011) Telomere length in neoplastic and nonneoplastic tissues of patients with familial and sporadic papillary thyroid cancer. J Clin Endocrinol Metab 96:E1852–E1856
Jin L, Chen E, Dong S, Cai Y, Zhang X, Zhou Y, Zeng R, Yang F, Pan C, Liu Y, Wu W, Xing M, Zhang X, Wang O (2016) BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: a study of 653 patients. Oncotarget 7:18346–18355
Matsuse M, Yabuta T, Saenko V, Hirokawa M, Nishihara E, Suzuki K, Yamashita S, Miyauchi A, Mitsutake N (2017) TERT promoter mutations and Ki-67 labeling index as a prognostic marker of papillary thyroid carcinomas: combination of two independent factors. Sci Rep 7:41752
Acknowledgements
This work is supported by the Japanese Association of University Women and Children’s Cancer Association of Japan. The authors wish to thank Kiyoko Kawahara, Takenori Fujii, Kiyoshi Teduka, Yoko Kawamoto and Taeko Kitamura for their skillful assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kure, S., Wada, R. & Naito, Z. Relationship between genetic alterations and clinicopathological characteristics of papillary thyroid carcinoma. Med Mol Morphol 52, 181–186 (2019). https://doi.org/10.1007/s00795-019-00217-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-019-00217-6